Abstract
AbstractAs a cancer predisposition syndrome, individuals with neurofibromatosis type 1 (NF1) are at increased risk for the development of both benign and malignant tumors. One of the most common locations for these cancers is the central nervous system, where low-grade gliomas predominate in children. During early childhood, gliomas affecting the optic pathway are most frequently encountered, whereas gliomas of the brainstem and other locations are observed in slightly older children. In contrast, the majority of gliomas arising in adults with NF1 are malignant cancers, typically glioblastoma, involving the cerebral hemispheres. Our understanding of the pathogenesis of NF1-associated gliomas has been significantly advanced through the use of genetically engineered mice, yielding new targets for therapeutic drug design and evaluation. In addition, Nf1 murine glioma models have served as instructive platforms for defining the cell of origin of these tumors, elucidating the critical role of the tumor microenvironment in determining tumor growth and vision loss, and determining how cancer risk factors (sex, germline NF1 mutation) impact on glioma formation and progression. Moreover, these preclinical models have permitted early phase analysis of promising drugs that reduce tumor growth and attenuate vision loss, as an initial step prior to translation to human clinical trials.
Keywords: brain tumor, brainstem, glioma, NF1, optic pathway, RAS
Children and adults with neurofibromatosis type 1 (NF1) are genetically predisposed to the development of benign and malignant cancers of the central nervous system (CNS).1–5 The types of tumors encountered in children and adults with NF1 differ in terms of brain location, age at presentation, and clinical behavior.4,6–8 In this regard, low-grade gliomas (LGGs) predominate in children, while high-grade gliomas (malignant gliomas; glioblastoma) are more commonly seen in young adults with NF1.7 Herein, we discuss the clinical features of gliomas encountered in children and adults with NF1, the role of the NF1 protein (neurofibromin) in tumor growth regulation, and the use of preclinical animal models to better understand NF1 glioma pathobiology and therapeutic targeting relevant to the management of patients.
Gliomas in Children and Adults with NF1
The vast majority of the brain tumors encountered in individuals with NF1 are histologically classified as gliomas (astrocytomas).1–4 However, tumor location, age of onset, symptomatology, and clinical behavior can be quite heterogeneous in this population of at-risk patients. In general, gliomas in children most commonly are localized to the optic pathway and brainstem6,7; however, recent studies have shown that gliomas in other locations are also frequently observed.6–14 Lastly, while far less common, high-grade (malignant) gliomas involving the cerebral hemispheres may arise in young adults.3,7,9,15
Optic Pathway Gliomas
In early childhood (mean age, 4.5 y), the most common brain tumor is a glioma of the optic pathway (optic pathway glioma; OPG).6,7,16–22 These tumors can affect any segment of the optic pathway, including the optic nerves, chiasm, tracts, and radiations (Figure 1A).6,7,16–22 While neuroimaging is not an element of routine medical screening of children, the proportion of children with NF1 and OPG has been estimated at ~15%.6,16,18,20,21 While most OPGs diagnosed in children with NF1 are asymptomatic or nonprogressive, as many as 50% of children with NF1-OPG will experience ophthalmologic (vision loss, proptosis)6,7,16–26 or endocrinologic (precocious puberty)6,7,16–22,26–28 signs or symptoms. This is in striking contrast to OPGs arising in children without NF1, who generally have a less favorable course.17,19,29,30
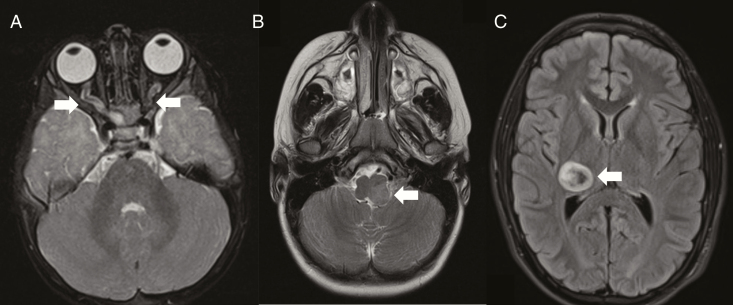
Figure 1.
Brain tumors in children with NF1. (A) Bilateral optic nerve gliomas with nerve thickening and tortuosity. (B) Left-sided brainstem glioma. (C) Right thalamic glioma. Arrows denote the tumors in each magnetic resonance imaging study.
Currently, children with NF1 are screened annually using age-appropriate visual acuity measures, including Teller, Lea, HOTV, and Snellen acuity cards, for at least the first decade of life.31 While these tests can provide accurate assessments of vision, they are often limited by patient cooperation, which can be problematic in children with NF1 and concurrent attention or cognitive deficits.32 For this reason, ocular coherence tomography (OCT) is emerging as an objective measure of visual acuity. OCT provides ultrasound quantification of the retinal fiber nerve layer (RFNL) and ganglion cell layers, but requires sedation (general anesthesia) in young children.33
Since routine neuroimaging is not performed and visual assessments can be challenging in children with NF1, risk factors for OPG development and progression have been sought. To date, several risk factors for OPG development have been postulated. First, there is evidence for genotype–phenotype association in NF1-OPG, where individuals with mutations in the 5′ end of the NF1 gene more often develop gliomas than individuals with mutations located elsewhere in the gene.34–37 Second, NF1-OPGs are more prevalent in Caucasian children than in those from other races and ethnicities; however, race had no impact on clinical progression.26,38 The observation that ethnicity/race modifies glioma risk, which could relate to genomic variations seen in different ethnic groups or races. This notion has been further explored by examining single nucleotide polymorphisms, where variants in the adenylate cyclase-8 (AC8) gene in individuals with NF1 are associated with different risks of low-grade glioma formation.39 Third, children with NF1 who have co-existing atopic conditions (eczema, asthma) are less likely to harbor an OPG.40
With respect to vision loss, three additional risk factors have been described, including involvement of the posterior optic pathway (tracts and radiations),24,41,42 young age at presentation (<2 y),24 and sex (female).24,41 While boys and girls with NF1 have the same incidence of OPGs, females harbor a 3- to 5-fold greater risk of vision loss.43,44 At this time, each of these risk factors lacks sufficient sensitivity and specificity to be incorporated into clinical decision making, but their integration into future risk assessment algorithms might help to stratify children into high and low risk groups.
Treatment is typically initiated when there is evidence of progressive vision loss (2-line decrement in visual acuity).31,45 While investigational treatments (e.g., MEK and mTOR inhibitors) are being evaluated in clinical trials, the standard first line treatment is carboplatin/vincristine chemotherapy.23,24,26,28,46,47 Those children who fail upfront therapy are usually treated with vinblastine24 or a combination of irinotecan and bevacizumab.48,49 Surgical resection is reserved for uncommon indications, such as atrophy of the eye,31,47 and radiation therapy is avoided due to the risk of secondary malignant tumor formation in children with this cancer predisposition syndrome.31,50 Unfortunately, in most cases, successful anti-tumoral treatment does typically not result in improved visual acuity.17,20,23,25
Brainstem Gliomas
While less common than OPGs, children with NF1 can also develop brainstem gliomas (BSGs; Figure 1B), with a mean age at diagnosis of ~7 years.51–53 These tumors occur in fewer than 10% of individuals with NF1,6,7,53 are more indolent than those observed in the general population,51 and are usually low-grade gliomas.53 Within the brainstem, they most frequently involve the midbrain and medulla.51,53 Unlike NF1-OPGs, NF1-BSG progression is not influenced by sex53; however, older children more often require treatment.53 While many patients with NF1-BSGs are asymptomatic7,51–53; some tumors can cause obstructive hydrocephalus6,51,52 as a result of aqueductal stenosis or lead to other neurologic signs/symptoms, such as headache, nausea/vomiting, cranial neuropathies and ataxia or gait instability.6,7,10,51–54 Due to their indolent behavior, a conservative approach to treatment is typically recommended, with hydrocephalus managed by cerebrospinal fluid (CSF) diversion (eg, ventricular shunt) and continued tumor growth with chemotherapy or less commonly surgery.6,7,51–53 It should be noted that progression-free survival was 3 years shorter for children with NF1-BSG receiving tumor-directed therapy relative to those who received no treatment or CSF diversion.53
Gliomas Arising in Other Locations
It is not uncommon for individuals with NF1 to harbor more than one CNS tumor, particularly those outside of the optic pathway or brainstem. These gliomas are typically located in the temporal lobe, cerebellum, thalamus, basal ganglia or spinal cord,7–10,13 and many are asymptomatic.7,13 Compared to NF1-OPGs and NF1-BSGs, less is known about the natural history of these tumors. When symptomatic or increasing in size on neuroimaging, patients with these tumors are managed with surgical resection, chemotherapy, and/or CSF diversion, depending upon the location and specific clinical indications.7,8,13 Those tumors involving the thalamus (Figure 1C) tend to have a poor prognosis.8
In addition, rosette-forming glioneuronal tumors have been found to harbor mutations in the NF1 gene.55 This rare low-grade brain neoplasm most typically arises in the fourth ventricle and cerebellum of young adults, and has been reported to occur in rare individuals with NF1.56
High-Grade (Malignant) Gliomas
High-grade (malignant) gliomas are uncommon in children with NF1, but increase in prevalence in early adulthood.3,7,9,10,15 Most frequently, these tumors arise in the cerebral hemispheres; however, the rarity of these neoplasms has limited our ability to identify clinical patterns. Based on epidemiologic studies, high-grade gliomas are encountered more often than would be predicted, with estimates of >50-fold increased risk relative to the general population.12,14,54,57 Molecular analyses of NF1-associated high-grade gliomas (anaplastic astrocytoma and glioblastoma)15 and anaplastic astrocytomas with piloid features58,59 have revealed mutational and genomic alterations similar to those observed in their sporadic counterparts, including mutations in the ATRX, TP53, and CDKN2A genes, as well as in genes whose proteins function within the phosphoinositol-3 kinase (PI3K) pathway. Importantly, NF1-associated high-grade gliomas lack the IDH and histone H3 mutations commonly observed in sporadic malignant gliomas.15,59,60 Current therapies are similar to those used to treat glioblastoma arising in adults without NF1.
The NF1 Tumor Suppressor Gene
The NF1 gene encodes a tumor suppressor protein (neurofibromin) that largely functions as a negative regulator of cell growth through suppression of RAS activation.61–63 Neurofibromin contains three putative structural domains: (a) a cysteine/serine-rich domain (CSD), (b) a GTPase activating protein (GAP) related domain (GRD), and (c) a domain with homology to the lipid-binding domain of the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec 14p (Sec14p).64,65 While mutations within the Sec1465 and CSD66 have been reported, their functional relevance remains to be elucidated. In contrast, mutations within the GRD are hypothesized to lead to increased RAS activation, resulting in increasing cell growth. Whereas all patients are born with a germline mutation in the NF1 gene (creating a non-functional NF1 allele), tumor formation requires somatic inactivation of the second NF1 allele (loss of heterozygosity), leading to loss of neurofibromin expression and function.67–70 As such, neurofibromin loss in Schwann cells,71–73 astrocytes,74,75 and myeloid cells76,77 is associated with high levels of activated RAS.73,78 Consistent with this mechanism of tumor growth regulation, increased RAS activation has also been observed in both human74 and mouse75NF1-associated gliomas.
RAS hyperactivation induces cell growth by inducing activation of downstream signaling intermediates (Figure 2), including Mitogen Activated Protein Kinases (MEK/ERK),79–81 Phosphoinositide-3-Kinase (PI3K),74,81–83 and cyclic AMP (cAMP).84 Additionally, ERK and PI3K/Protein Kinase-B (AKT) phosphorylation both lead to mechanistic target of rapamycin (mTOR) activation.74,81,82 While less is known about the mechanism of cAMP regulation by neurofibromin in neural progenitors and astroglial cells, neurofibromin controls cAMP homeostasis in a RAS-dependent manner through the activation of the atypical protein kinase C-zeta (PKCζ) in neurons.85 Each of these effector molecules serve as logical targets for therapeutic drug design (see below section on “Preclinical drug identification and evaluation”).
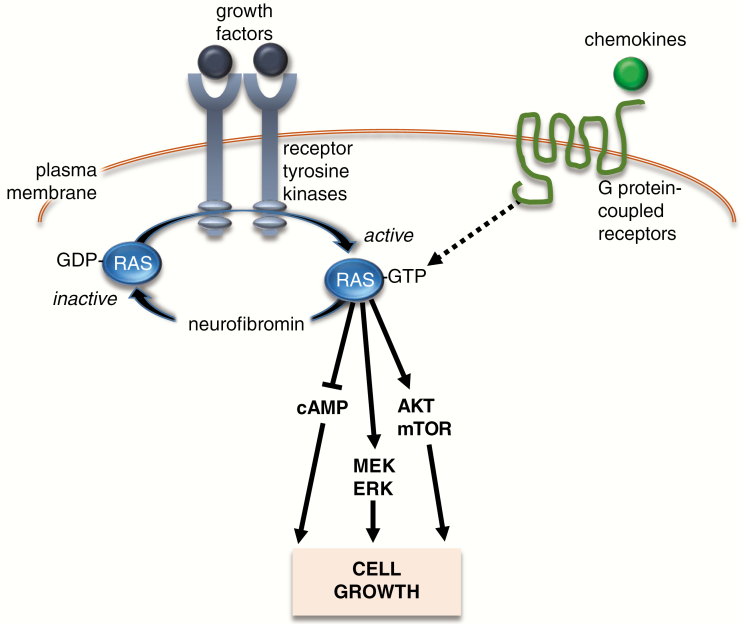
Figure 2.
Schematic representation of RAS signaling cascade. Growth factors activate receptor tyrosine kinases, which accelerate the conversion of inactive GDP-bound RAS to its active GTP-bound form. Impaired neurofibromin expression, as a result of NF1 mutation, leads to elevated RAS activity. In addition, chemokines can directly activate RAS-GTP through binding to G protein-coupled receptors. Activated RAS initiates signal transduction through the activation (phosphorylation) of AKT and MEK/ERK, which each can converge on the mechanistic target of rapamycin (mTOR), to increase cell growth (proliferation/survival). Activated RAS can also suppress cyclic AMP (cAMP) levels through atypical protein kinase C signaling to regulate cell growth.
Modeling NF1-Gliomas in Mice
Since human tumors are not routinely biopsied as part of routine clinical care and have not been successfully maintained as patient-derived xenografts, much of our understanding of the pathobiology of these tumors has resulted from the use of genetically engineered mouse models. Even though there are striking differences between humans and rodents in terms of brain structure and organization, these preclinical experimental platforms have revealed important insights into the role of genetic and genomic factors (germline mutation, sex), the tumor microenvironment, and systemic disease in the formation and progression of NF1-associated gliomas.
Optic Gliomas
NF1-OPGs rarely harbor additional somatic mutations,86–89 and are genetically characterized by bi-allelic NF1 inactivation.70,89,90 For this reason, murine Nf1-OPGs have been modeled by combining an inactivating germline Nf1 gene mutation with somatic Nf1 loss using conditional Cre-Lox technology. Whereas neither mice heterozygous for a germline mutation in the Nf1 gene (Nf1+/− mice) nor those with bi-allelic Nf1 loss in neuroglial progenitors develop OPGs, the combination of these two genetic events is sufficient to generate optic gliomas in mice. As such, >95% of these mice develop low-grade gliomas involving the prechiasmatic optic nerves and chiasm by 3 months of age. Similar to their human counterparts, they exhibit low proliferative indices (<1% Ki67+ cells), increased microglia infiltration, and localized enlargement of the prechiasmatic optic nerves and chiasm.91 These tumors can be visualized by small-animal magnetic resonance imaging (MRI),92 and are associated with progressive axonal damage, retinal ganglion cell (RGC) death, and reduced visual acuity.93,94 Moreover, like other human brain tumors, they contain a small population of CD133+ cancer stem cells, which can generate low-grade gliomas following transplantation into the brainstems of naïve recipients.95,96
Malignant Glioma
In contrast to low-grade gliomas in individuals with NF1, their malignant counterparts harbor additional genetic alterations, including mutations in the TP53, EGFR, and RB1 genes.15,90 Based on the frequent cooccurrence of NF1 and TP53 mutations in human NF1-associated malignant glioma,15,90 murine glioblastoma models have focused on combining Nf1 and Trp53 (p53 gene) loss. In this regard, mice carrying heterozygous germline mutations in Nf1 and Trp53 on the same copy of chromosome 11 (NPcis mice) develop brain tumors following loss of the wild-type Nf1 and Trp53 genes. The astrocytomas encountered range in tumor grade from low-grade diffuse astrocytoma to high-grade glioblastoma, with 100% tumor penetrance observed in mice older than 6 months. These tumors have elongated astrocytic nuclei with irregular contours, increased mitosis and, in some rare cases, necrosis.97 Mice with these tumors die within 18 weeks of age. Tumor cells from these mice can be serially transplanted for malignant glioma therapeutic studies.98,99 Moreover, the addition of PTEN mutation, mimicking the PI3K pathway activation seen in human NF1-GBM, results in a more aggressive malignancy, with a shorter latency to tumor development and attenuated survival.100 In addition, complementary modeling approaches using the MADM (Mosaic Analysis with Double Markers),101,102 viral transduction methods (RCAS/tVA system,103 CRISPR/Cas editing,104 or in utero CRISPR/Cas9 induced Nf1 and Trp53 loss also result in high-grade glioma formation.102,104
Insights from Nf1 Genetically Engineered Mice
Using these Nf1 genetically-engineered mouse glioma models, significant insights have been derived regarding the tumor cell of origin, the role of the tumor microenvironment, and the contributions of risk factors (sex, germline genetics, and genomic alterations) to brain tumor disease pathogenesis.
Cell of Origin
Brain tumors can arise from different progenitor cell populations that reside in distinct germinal (ventricular) zones. Nf1 optic glioma mice have been instructive for discovering that this type of low-grade glioma originates from specific progenitor cells that line the ventricular surface of the third ventricle (TVZ), rather than from the lateral ventricular subventricular zone (lv-SVZ). First, Nf1 mutation results in increased proliferation and glial differentiation in stem cells lining the TVZ, rather than the lv-SVZ.105 Second, in both human and mouse brain sections, proliferation in the TVZ disappears early in postnatal development.106 Third, only GFAP+, BLBP+, and CD133+ neural progenitor cells serve as the cells of origin for Nf1 optic glioma.84,105,107,108 No optic glioma formation is observed in Nf1+/− mice with somatic Nf1 inactivation in astrocytes or NG2+ glia.105,109 Fourth, Nf1 optic gliomagenesis requires somatic Nf1 loss to occur during late embryonic development, as postnatal loss in the same neuroglial progenitors does not result in tumor development.105,108 Lastly, there is a second progenitor cell population that can also give rise to optic gliomas in Nf1 genetically engineered mice.108 These Olig2+ oligodendrocyte progenitor cells serve as cells of origin for optic gliomas; however, the latency to tumor formation is nearly twice as long (6 months of age), suggesting that the specific cell of origin partially dictates glioma biology.96,110
In striking contrast, high-grade gliomas appear to originate from stem cell populations within the lv-SVZ of adult mice.111 In addition to serving as the cell of origin for these malignant tumors, depletion of these stem cells in established tumors also reduces tumor size and extends mouse survival.112 Similar to Nf1 optic gliomas, pathologically identical, but molecularly distinct tumors are generated when these molecular changes occur in adult neural stem cells compared to oligodendrocyte progenitors.113,114 However, using the MADM experimental platform, simultaneous Nf1 and Trp53 inactivation in neural stem cells or OPCs resulted in the formation of gliomas, which, in both cases, reflected a common cell of origin (oligodendrocyte progenitor cells).102
Tumor Microenvironment
Studies in Nf1 optic glioma mice have revealed an essential role for non-neoplastic (stromal) cells in tumor development and progression. One of the most important of these non-neoplastic cells, microglia, comprise 30–50% of the cellular content in both sporadic and NF1-associated low-grade gliomas,115 and are present at higher densities in murine Nf1 optic gliomas than in the optic nerves of normal mice.92,93,115,116 In Nf1 optic glioma-bearing mice, the microglia harbor a germline mutation in the Nf1 gene, which results in increased proliferation and migration, as well as increased production of key growth factors.117 In this regard, genetic reduction of the key receptor critical for directed migration of microglia (Cx3cr1), reduces microglia infiltration in Nf1-OPG mice and leads to a delay in glioma formation.118 Similarly, genetic or pharmacologic inhibition of microglia function attenuates Nf1 optic glioma growth.115,117,119 To identify how microglia control Nf1 optic glioma growth, Nf1+/− microglia were isolated from Nf1 optic glioma-bearing mice and compared to those from nontumor-bearing mice by RNA sequencing, revealing that tumor-associated microglia secrete CC-chemokine ligand 5 (Ccl5).120 Support for the importance of microglia Ccl5 in tumor maintenance was subsequently provided by demonstrating that the treatment of Nf1 optic glioma mice with Ccl5 neutralizing antibodies120 and the injection of optic glioma stem cells into Ccl5-deficient mice,121 resulted in reduced tumor growth and an absence of glioma formation, respectfully. It is worth noting that high-grade gliomas produce their own Ccl5, thus reducing their dependence on stromal cells for this growth factor.98 Other chemokines, like Cxcl12, may also be important for dictating the formation and growth of these tumors.122 While less well explored, it is likely that microglia in the tumor microenvironment also govern the growth and spread of NF1-malignant glioma.123–125
Microglia are not the only important nonneoplastic cells in the glioma microenvironment. Recently, T cells were found in both mouse and human NF1-LGGs.15,121 In murine Nf1-OPGs, these lymphocytes prime microglia to produce Ccl5 through the elaboration of paracrine factors (Figure 3). Additionally, Ccl5 levels in these mouse tumors correlate with the abundance of T cells and microglia.121 Importantly, glioma stem cells from murine Nf1 optic gliomas do not form low-grade gliomas following injection into the brains of mice lacking T cells, where the microglia fail to produce Ccl5.121 Taken together, these findings support a model in which a supportive neuroimmune axis is established to foster Nf1 glioma development and progression; however, the factors that attract and activate T cells in the setting of glioma remain to be fully elucidated.
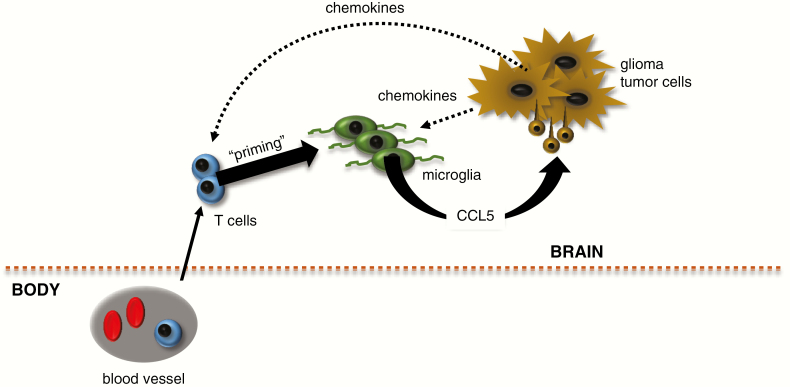
Figure 3.
Tumor microenvironment is involved in glioma progression. Glioma cells produce chemokines that attract both T lymphocytes (from blood vessels) and microglia. In response to paracrine factors released by T cells (“priming”), microglia produce growth factors, like Ccl5, which increase tumor cell proliferation or survival.
Risk Factors
Another use for Nf1 optic glioma mice is an opportunity to define the etiologies for risk factors operative in patients with NF1 (Figure 4), including sex, germline genetics, the presence of additional genomic alterations, and background genomic variation.
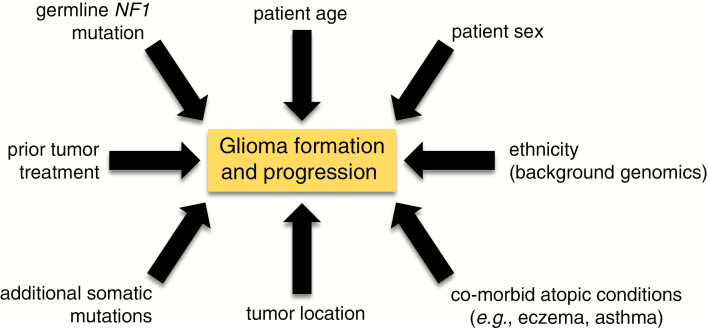
Figure 4.
Risk factors for glioma formation. Research over the past 20 years has revealed numerous factors that can alter the risk of NF1-associated glioma formation and progression. As discussed in the text, these include the germline NF1 gene mutation, patient age, patient sex, background genomics (ethnicity/race), co-existing atopic conditions (eczema, asthma), tumor location within the neuroaxis, the presence of additional somatic mutations in the tumor, and prior tumor treatment (radiation therapy).
Sex
As mentioned above, girls with NF1 more frequently lose vision from their OPG than boys.24,43,126 Using Nf1-OPG mice, this sexual dimorphism reflects in part differences in gonadal sex hormones. In this regard, only female Nf1 optic glioma mice exhibit increased RGC death and RNFL thinning sufficient to result in reduced visual acuity.43 In mice, this is due to estrogen receptor-beta (ERβ) activation in microglia, which leads to the production of neurotoxins that induce axonal injury in female Nf1 optic glioma mice. Pharmacologic inhibition of the estrogen receptor (ERβ) or estrogen depletion by surgical or chemical ovariectomy reverses the RNFL thinning and RGC loss.126 Future work will be required to define the mechanisms underlying estrogen reprogramming of microglia and the paracrine factors responsible for microglia-mediated axonal damage. In addition, there could be sex chromosome effects that mediate sexually dimorphic differences in glioma penetrance or progression.127–129
Germline Genetics
Several lines of evidence have raised the intriguing possibility that not all germline NF1 gene mutations are functionally equivalent. First, population-based studies have revealed that a subgroup of patients with specific mutations (eg, Arg1809 missense mutations) lack the signature nerve sheath tumors that characterize NF1 (neurofibromas).130–133 Second, human induced pluripotent stem cells with different NF1 gene mutations exhibit different levels of neurofibromin and dopamine, supporting the notion of mutational specificity.134 Third, using Nf1 mutant mice in which the germline Nf1 knockout allele is replaced with actual NF1 patient mutations, the identical somatic Nf1 loss has differential effects on the ability of these mice with different germline Nf1 gene mutations to develop optic gliomas.96,135 For example, mice with an Arg1276Pro mutation develop optic gliomas similar to those with an artificial knockout germline Nf1 allele, but with fewer infiltrating microglia, whereas those with an Arg681X germline Nf1 gene mutation form optic gliomas with larger volumes and proliferative rates.96 In addition, mice with a Gly848Arg germline Nf1 gene mutation fail to form optic gliomas,135 while those harboring a Cys383X germline Nf1 mutation develop optic gliomas with reduced penetrance. Moreover, the glioma stem cells from mouse optic gliomas with different germline Nf1 mutations exhibit different levels of microglia and T cell infiltration owing to mutation-specific differences in chemokine production.96
Secondary Genomic Alterations
While most NF1-OPG harbor no additional genomic alterations, some patient tumors harbor co-existing mutations, including heterozygous PTEN deletion or KIAA1549:BRAF duplication.87 When modeled in mice, the differential effects of these alterations were confirmed: While coexpression of the KIAA1549:BRAF fusion gene did not further increase Nf1 optic glioma growth in mice, heterozygous Pten inactivation dramatically increased tumor volume, proliferation and microglia infiltration.136 Similarly, in the context of Nf1/Trp53-driven high grade gliomagenesis, the addition of somatic heterozygous Pten loss in NPcis mice leads to the development of more aggressive gliomas with near complete penetrance.100 As additional genetic alterations are identified through large-scale sequencing efforts,15 opportunities may arise to understand how these co-existing mutations influence glioma biology.
Genomic Modifiers
Since individuals with NF1 within the same family can exhibit different clinical features and phenotypic severity, it is likely that other factors influence glioma penetrance, including genomic modifiers.137 While challenging to study in people, NPcis mice have been used to identify potential modifier genes. For example, susceptibility to astrocytoma has been linked to genes on mouse chromosome 11, such that mice that inherit the NPcis mutation from their mothers have an increased risk for astrocytoma.138 Additionally sexually dimorphic differences in glioma susceptibility are linked to loci on mouse chromosome 19,139 with the Arlm1 gene representing one potential modifier of astrocytoma resistance in males.140 Finally, the Scram1 locus on mouse chromosome 5 affects the incidence and latency of spinal cord astrocytoma development, but does not affect the overall latency of astrocytomas.141
Preclinical Drug Discovery and Evaluation
In addition to risk assessment, one of the most widely exploited applications for mouse models has been in drug discovery and preclinical testing. For example, Nf1 optic glioma mice have been used as preclinical platforms to evaluate the efficacy of RAS pathway inhibitors. As such, successful trials using MEK (PD0325901), PI3K (NPV-BKM120), and mTOR (rapamycin) inhibitors have demonstrated decreased tumor cell proliferation and tumor size.81,142 Similarly, increasing cAMP levels in Nf1-OPG using an inhibitor of the enzyme responsible for cAMP degradation (phosphodiesterase-4 inhibitor; Rolipram) reduces tumor proliferation and volume.143 Unfortunately, some of these therapies in mice require drug doses that are not easily tolerated in children, and tumor growth suppression with these pathway-targeted therapies requires continual drug exposure.142,144 In addition, the cancer stem cells from Nf1-OPG acquire adaptive responses that promote resistance to mTOR and MEK inhibition95 which may further limit their efficacy. For this reason, additional molecularly targeted or combinatorial therapies are needed.
While tumor-directed therapies have been the mainstay of treatment, therapies aimed at reducing stromal cell support of tumor growth represent another opportunity. In this regard, suppressing microglia function with minocycline or inhibiting the JNK pathway activated in microglia have resulted in reduced tumor growth.115,117,119 Future strategies that aim to disrupt this immune axis may offer new avenues for pursuit.
In addition to tumor- and stroma-directed therapies, recent studies have also focused on identifying therapies that block further visual decline or result in improved visual acuity. Using Nf1-OPG mice, treatment with Lovastatin, a HMG CoA reductase inhibitor that blocks RAS activity, resulted in preservation of RGC numbers two months after the cessation of therapy.93 Interestingly, tumor proliferation returned to pretreatment levels, suggesting neuroprotective and neurorestorative strategies might emerge as future adjuvant approaches for NF1-OPG.
Future Directions
With the insights provided by basic and preclinical translational research, several clinical trials have been designed to identify more effective therapies for NF1-OPG (Table 1). Most of these studies use chemotherapeutic agents to halt tumor progression. However, emerging therapies are being considered that target cells and signals in the tumor microenvironment, as well as focused on restoring vision loss. Other approaches, including gene editing (NCT02465060), are in early stages of development.145
Table 1.
Clinical trials for NF1-OPG
| Trial | Study number | Status | Sponsor | Phase | Ages | Disease status | Primary outcome |
|---|---|---|---|---|---|---|---|
| Selumetinib | NCT03871257 | Not yet recruiting | NCI | III | 2–21 y | Progressive | Event-free survival and visual acuity |
| Selumetinib | NCT03326388 | Not yet recruiting | Great Ormond Street Hospital for Children NHS Foundation Trust | I/II | 3–18 y | Progressive, relapsed | Maximum tolerated dose, objective response rate |
| Trametinib | NCT03363217 | Recruiting | St. Justine’s Hospital | I/II | 1 month to 25 y | Refractory | Objective response rate |
| MEK162 | NCT02285439 | Recruiting | Children’s Hospital Los Angeles | I/II | 1–18 y | Recurrent, refractory, progressive | Objective response rate |
| Vinblastine +/− Bevacizumab | NCT02840409 | Recruiting | The Hospital for Sick Children | II | 6 months to 18 y | Unresectable or progressive | Response rate |
| Selumetinib | NCT01089101 | Recruiting | NCI | II | 3–21 y | Recurrent, refractory | Response rate |
| Trametinib | NCT02465060 | Recruiting | NCI | II | >18 y | Advanced refractory | Response rate |
| RAD0001 (everolimus) | NCT01158651 | Active, not recruiting | University of Alabama at Birmingham | II | 1–21 y | Chemotherapy refractory, radiologically progressive | Objective response rate |
| Lenalidomide | NCT01553149 | Active, not recruiting | NCI | II | <21 y | Recurrent, refractory, or progressive | Objective response rate |
| TAK-580 | NCT03429803 | Active, not recruiting | Karen D. Wright, MD | I/II | <18 y | Refractory | Dose limiting toxicity and progression free survival |
| Pomalidomide | NCT02415153 | Active, not recruiting | NCI | I | 3–20 y | Recurrent, progressive, or refractory | Maximum tolerated dose |
| Sorafenib | NCT01338857 | Completed | NYU Langone Health | II | >2 y | Recurrent or progressive | Sorafenib ineffective for the treatment of recurrent or progressive PLGA |
NCI = National Cancer Institute; NYU = New York University.
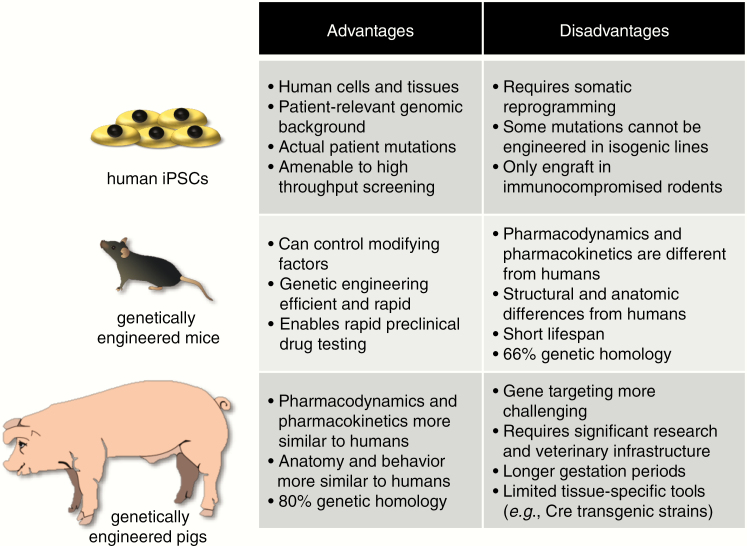
While mouse models have proven to be extremely useful tools for studying NF1-glioma, successful preclinical experiments in rodents do not always translate well into effective treatments for patients. For this reason, additional models are currently being developed (Figure 5). Genetically engineered minipigs have recently been generated that better recapitulate the full spectrum of NF1-associated features, including café au lait macules, neurofibromas, and OPG.146 Given the greater similarities between swine and people with respect to brain structure and function, as well as pharmacokinetic and pharmacodynamics profiles, these animals may be better suited for preclinical drug testing than mice. In addition to these animal models, researchers are also working to establish human induced pluripotent stem cell models of NF1-tumors.147 These studies are still in their early stages, but may yield tractable platforms as have been recently reported for high-grade glioma.148,149 Taken together, the implementation of these complementary model systems are likely provide unprecedented insights into the pathobiology of these tumors and result in the development of more effective treatments for patients with NF1-associated glioma.
Figure 5.
Preclinical models of NF1-glioma. Several preclinical models have been developed for NF1-associated low-grade gliomas, including human induced pluripotent stem cells (hiPSCs), genetically engineered mice, and genetically engineered swine. Each of these platforms has limitations and advantages.
Funding
D.H.G. is funded by a Research Program Award grant from the National Institutes of Health (1-R35-NS07211-01).
Acknowledgments
The authors would like to thank the members of the Gutmann Laboratory for their helpful comments and suggestions. D.H.G. is supported by a Research Program Award grant from the National Institutes of Health (1-R35-NS07211-01).
Conflict of interest statement
The authors have no relevant conflicts to disclose.
Authorship statement
A.C. wrote the initial draft of the manuscript and designed some of the figures. D.H.G. edited the manuscript and finalized the figures.
References
- 1. Sørensen SA, Mulvihill JJ, Nielsen A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med. 1986;314(16):1010–1015. [DOI] [PubMed] [Google Scholar]
- 2. Bader JL. Neurofibromatosis and cancer. Ann N Y Acad Sci. 1986;486:57–65. [DOI] [PubMed] [Google Scholar]
- 3. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [DOI] [PubMed] [Google Scholar]
- 5. Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glombova M, Petrak B, Lisy J, Zamecnik J, Sumerauer D, Liby P. Brain gliomas, hydrocephalus and idiopathic aqueduct stenosis in children with neurofibromatosis type 1. Brain Dev. 2019;41(8):678–690. [DOI] [PubMed] [Google Scholar]
- 7. Guillamo JS, Creange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1). A retrospective study of 104 patients. Brain. 2002;126(1):152–160. [DOI] [PubMed] [Google Scholar]
- 8. Byrne S, Connor S, Lascelles K, Siddiqui A, Hargrave D, Ferner RE. Clinical presentation and prognostic indicators in 100 adults and children with neurofibromatosis 1 associated non-optic pathway brain gliomas. J Neurooncol. 2017;133(3):609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(Pt 3):473–481. [DOI] [PubMed] [Google Scholar]
- 10. Gutmann DH, Rasmussen SA, Wolkenstein P, et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology. 2002;59(5):759–761. [DOI] [PubMed] [Google Scholar]
- 11. Vinchon M, Soto-Ares G, Ruchoux MM, Dhellemmes P. Cerebellar gliomas in children with NF1: pathology and surgery. Childs Nerv Syst. 2000;16(7):417–420. [DOI] [PubMed] [Google Scholar]
- 12. Huttner AJ, Kieran MW, Yao X, et al. Clinicopathologic study of glioblastoma in children with neurofibromatosis type 1. Pediatr Blood Cancer. 2010;54(7):890–896. [DOI] [PubMed] [Google Scholar]
- 13. Sellmer L, Farschtschi S, Marangoni M, et al. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet J Rare Dis. 2017;12(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenfeld A, Listernick R, Charrow J, Goldman S. Neurofibromatosis type 1 and high-grade tumors of the central nervous system. Childs Nerv Syst. 2010;26(5):663–667. [DOI] [PubMed] [Google Scholar]
- 15. D’Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med. 2019;25(1):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blanchard G, Lafforgue MP, Lion-François L, et al. NF France network. Systematic MRI in NF1 children under six years of age for the diagnosis of optic pathway gliomas. Study and outcome of a French cohort. Eur J Paediatr Neurol. 2016;20(2):275–281. [DOI] [PubMed] [Google Scholar]
- 17. Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001;22(10):1963–1969. [PMC free article] [PubMed] [Google Scholar]
- 18. Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. [DOI] [PubMed] [Google Scholar]
- 19. Listernick R, Darling C, Greenwald M, Strauss L, Charrow J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J Pediatr. 1995;127(5):718–722. [DOI] [PubMed] [Google Scholar]
- 20. Segal L, Darvish-Zargar M, Dilenge ME, Ortenberg J, Polomeno RC. Optic pathway gliomas in patients with neurofibromatosis type 1: follow-up of 44 patients. J AAPOS. 2010;14(2):155–158. [DOI] [PubMed] [Google Scholar]
- 21. Sellmer L, Farschtschi S, Marangoni M, et al. Serial MRIs provide novel insight into natural history of optic pathway gliomas in patients with neurofibromatosis 1. Orphanet J Rare Dis. 2018;13(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111(3):568–577. [DOI] [PubMed] [Google Scholar]
- 23. Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalin‐Hajdu E, Décarie JC, Marzouki M, Carret AS, Ospina LH. Visual acuity of children treated with chemotherapy for optic pathway gliomas. Pediatric Blood Cancer. 2014;61(2):223–227. [DOI] [PubMed] [Google Scholar]
- 26. King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A(2):95–99. [DOI] [PubMed] [Google Scholar]
- 27. Habiby R, Silverman B, Listernick R, Charrow J. Precocious puberty in children with neurofibromatosis type 1. J Pediatr. 1995;126(3):364–367. [DOI] [PubMed] [Google Scholar]
- 28. Sani I, Albanese A. Endocrine long-term follow-up of children with neurofibromatosis type 1 and optic pathway glioma. Horm Res Paediatr. 2017;87(3):179–188. [DOI] [PubMed] [Google Scholar]
- 29. Deliganis AV, Geyer JR, Berger MS. Prognostic significance of type 1 neurofibromatosis (von Recklinghausen disease) in childhood optic glioma. Neurosurgery. 1996;38(6):1114–1118; discussion 1118. [DOI] [PubMed] [Google Scholar]
- 30. Grill J, Laithier V, Rodriguez D, Raquin MA, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159(9):692–696. [DOI] [PubMed] [Google Scholar]
- 31. Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. [DOI] [PubMed] [Google Scholar]
- 33. Banc A, Stan C, Florian IS. Optical coherence tomography as a marker of vision in children with optic pathway gliomas. Childs Nerv Syst. 2018;34(1):51–60. [DOI] [PubMed] [Google Scholar]
- 34. Anastasaki C, Morris SM, Gao F, Gutmann DH. Children with 5’-end NF1 gene mutations are more likely to have glioma. Neurol Genet. 2017;3(5):e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolcekova A, Nemethova M, Zatkova A, et al. Clusterring of mutations in the 5’tertile of the NF1 gene in Slovak patients with optic pathway glioma. Neoplasma. 2013;60:6. [DOI] [PubMed] [Google Scholar]
- 36. Sharif S, Upadhyaya M, Ferner R, et al. A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype–phenotype correlations. J Med Genet. 2011;48(4):256–260. [DOI] [PubMed] [Google Scholar]
- 37. Xu M, Xiong H, Han Y, et al. Identification of mutation regions on NF1 responsible for high- and low-risk development of optic pathway glioma in neurofibromatosis type I. Front Genet. 2018;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abadin SS, Zoellner NL, Schaeffer M, Porcelli B, Gutmann DH, Johnson KJ. Racial/ethnic differences in pediatric brain tumor diagnoses in patients with neurofibromatosis type 1. J Pediatr. 2015;167(3):613–620. e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warrington NM, Sun T, Luo J, et al. The cyclic AMP pathway is a sex-specific modifier of glioma risk in type I neurofibromatosis patients. Cancer Res. 2015;75(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Porcelli B, Zoellner NL, Abadin SS, Gutmann DH, Johnson KJ. Associations between allergic conditions and pediatric brain tumors in Neurofibromatosis type 1. Fam Cancer. 2015;15(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu GT, Brodsky MC, Phillips PC, et al. Optic radiation involvement in optic pathway gliomas in neurofibromatosis. Am J Ophthalmol. 2004;137(3):407–414. [DOI] [PubMed] [Google Scholar]
- 42. Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–445. [DOI] [PubMed] [Google Scholar]
- 43. Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014;75(2):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fisher MJ, Loguidice M, Gutmann DH, et al. Gender as a disease modifier in neurofibromatosis type 1 optic pathway glioma. Ann Neurol. 2014;75(5):799–800. [DOI] [PubMed] [Google Scholar]
- 45. Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110(1):1–7. [DOI] [PubMed] [Google Scholar]
- 46. Demaerel P, de Ruyter N, Casteels I, Renard M, Uyttebroeck A, van Gool S. Visual pathway glioma in children treated with chemotherapy. Eur J Paediatr Neurol. 2002;6(4):207–212. [DOI] [PubMed] [Google Scholar]
- 47. Massimi L, Tufo T, Di Rocco C. Management of optic-hypothalamic gliomas in children: still a challenging problem. Expert Rev Anticancer Ther. 2007;7(11):1591–1610. [DOI] [PubMed] [Google Scholar]
- 48. Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–114. [DOI] [PubMed] [Google Scholar]
- 49. Okada K, Yamasaki K, Tanaka C, Fujisaki H, Osugi Y, Hara J. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn J Clin Oncol. 2013;43(11):1073–1079. [DOI] [PubMed] [Google Scholar]
- 50. Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. [DOI] [PubMed] [Google Scholar]
- 51. Molloy PT, Bilaniuk LT, Vaughan SN, et al. Brainstem tumors in patients with neurofibromatosis type 1: a distinct clinical entity. Neurology. 1995;45(10):1897–1902. [DOI] [PubMed] [Google Scholar]
- 52. Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46(6):1652–1660. [DOI] [PubMed] [Google Scholar]
- 53. Mahdi J, Shah AC, Sato A, et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology. 2017;88(16):1584–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leonard JR, Perry A, Rubin JB, King AA, Chicoine MR, Gutmann DH. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology. 2006;67(8):1509–1512. [DOI] [PubMed] [Google Scholar]
- 55. Sievers P, Appay R, Schrimpf D, et al. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019;138(3):497–504. [DOI] [PubMed] [Google Scholar]
- 56. Alturkustani M, Ang LC. Rosette-forming glioneuronal tumour of the 4th ventricle in a NF1 patient. Can J Neurol Sci. 2012;39(1):95–96. [DOI] [PubMed] [Google Scholar]
- 57. Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reinhardt A, Stichel D, Schrimpf D, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–291. [DOI] [PubMed] [Google Scholar]
- 59. Rodriguez FJ, Brosnan-Cashman JA, Allen SJ, et al. Alternative lengthening of telomeres, ATRX loss and H3-K27M mutations in histologically defined pilocytic astrocytoma with anaplasia. Brain Pathol. 2019;29(1):126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salloum R, McConechy MK, Mikael LG, et al. Characterizing temporal genomic heterogeneity in pediatric high-grade gliomas. Acta Neuropathol Commun. 2017;5(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu GF, O’Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62(3):599–608. [DOI] [PubMed] [Google Scholar]
- 62. Ballester R, Marchuk D, Boguski M, et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63(4):851–859. [DOI] [PubMed] [Google Scholar]
- 63. Martin GA, Viskochil D, Bollag G, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. [DOI] [PubMed] [Google Scholar]
- 64. Upadhyaya M. NF1 gene structure and NF1 genotype/phenotype correlations. In: Neurofibromatoses, Vol. 16 Karger Publishers; 2008:46–62. [Google Scholar]
- 65. Scheffzek K, Welti S. Neurofibromin: protein domains and functional characteristics. In: Neurofibromatosis Type 1. Springer; 2012:305–326. [Google Scholar]
- 66. Fahsold R, Hoffmeyer S, Mischung C, et al. Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am J Hum Genet. 2000;66(3):790–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colman SD, Williams CA, Wallace MR. Benign neurofibromas in type 1 neurofibromatosis (NF1) show somatic deletions of the NF1 gene. Nat Genet. 1995;11(1):90–92. [DOI] [PubMed] [Google Scholar]
- 68. Serra E, Puig S, Otero D, et al. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997;61(3):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kluwe L, Friedrich RE, Mautner VF. Allelic loss of the NF1 gene in NF1-associated plexiform neurofibromas. Cancer Genet Cytogenet. 1999;113(1):65–69. [DOI] [PubMed] [Google Scholar]
- 70. Gutmann DH, Donahoe J, Brown T, James CD, Perry A. Loss of neurofibromatosis 1 (NF1) gene expression in NF1-associated pilocytic astrocytomas. Neuropathol Appl Neurobiol. 2000;26(4):361–367. [DOI] [PubMed] [Google Scholar]
- 71. Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275(39):30740–30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. [DOI] [PubMed] [Google Scholar]
- 73. DeClue JE, Papageorge AG, Fletcher JA, et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69(2):265–273. [DOI] [PubMed] [Google Scholar]
- 74. Lau N, Feldkamp MM, Roncari L, et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J Neuropathol Exp Neurol. 2000;59(9):759–767. [DOI] [PubMed] [Google Scholar]
- 75. Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005;65(1):236–245. [PubMed] [Google Scholar]
- 76. Largaespada DA, Brannan CI, Jenkins NA, Copeland NG. Nf1 deficiency causes Ras-mediated granulocyte/macrophage colony stimulating factor hypersensitivity and chronic myeloid leukaemia. Nat Genet. 1996;12(2):137–143. [DOI] [PubMed] [Google Scholar]
- 77. Bollag G, Clapp DW, Shih S, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12(2):144–148. [DOI] [PubMed] [Google Scholar]
- 78. Hiatt KK, Ingram DA, Zhang Y, Bollag G, Clapp DW. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1−/− cells. J Biol Chem. 2001;276(10):7240–7245. [DOI] [PubMed] [Google Scholar]
- 79. Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chang T, Krisman K, Theobald EH, et al. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest. 2013;123(1):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17(6):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–2760. [DOI] [PubMed] [Google Scholar]
- 83. Patmore DM, Welch S, Fulkerson PC, et al. In vivo regulation of TGF-β by R-Ras2 revealed through loss of the RasGAP protein NF1. Cancer Res. 2012;72(20):5317–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. [DOI] [PubMed] [Google Scholar]
- 85. Anastasaki C, Gutmann DH. Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum Mol Genet. 2014;23(25):6712–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kluwe L, Hagel C, Tatagiba M, et al. Loss of NF1 alleles distinguish sporadic from NF1-associated pilocytic astrocytomas. J Neuropathol Exp Neurol. 2001;60(9):917–920. [DOI] [PubMed] [Google Scholar]
- 87. Rodriguez FJ, Ligon AH, Horkayne-Szakaly I, et al. BRAF duplications and MAPK pathway activation are frequent in gliomas of the optic nerve proper. J Neuropathol Exp Neurol. 2012;71(9):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li J, Perry A, James CD, Gutmann DH. Cancer-related gene expression profiles in NF1-associated pilocytic astrocytomas. Neurology. 2001;56(7):885–890. [DOI] [PubMed] [Google Scholar]
- 89. Gutmann DH, McLellan MD, Hussain I, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gutmann DH, James CD, Poyhonen M, et al. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003;61(10):1397–1400. [DOI] [PubMed] [Google Scholar]
- 91. Bajenaru ML, Hernandez MR, Perry A, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–8577. [PubMed] [Google Scholar]
- 92. Bajenaru ML, Garbow JR, Perry A, Hernandez MR, Gutmann DH. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57(1):119–127. [DOI] [PubMed] [Google Scholar]
- 93. Toonen JA, Ma Y, Gutmann DH. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. 2017;19(6):808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hegedus B, Hughes FW, Garbow JR, et al. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. J Neuropathol Exp Neurol. 2009;68(5):542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen YH, McGowan LD, Cimino PJ, et al. Mouse low-grade gliomas contain cancer stem cells with unique molecular and functional properties. Cell Rep. 2015;10(11):1899–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro-Oncology. 2019: May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. [DOI] [PubMed] [Google Scholar]
- 98. Pan Y, Smithson LJ, Ma Y, Hambardzumyan D, Gutmann DH. Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival. Oncotarget. 2017;8(20):32977–32989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen YH, Cimino PJ, Luo J, Dahiya S, Gutmann DH. ABCG1 maintains high-grade glioma survival in vitro and in vivo. Oncotarget. 2016;7(17):23416–23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kwon CH, Zhao D, Chen J, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121(3):479–492. [DOI] [PubMed] [Google Scholar]
- 102. Liu C, Sage JC, Miller MR, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ozawa T, Riester M, Cheng YK, et al. Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer Cell. 2014;26(2):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, et al. Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun. 2015;6:7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee Da Y, Gianino Scott M, Gutmann David H. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dahiya S, Lee DY, Gutmann DH. Comparative characterization of the human and mouse third ventricle germinal zones. J Neuropathol Exp Neurol. 2011;70(7):622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24(20):2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Solga AC, Toonen JA, Pan Y, et al. The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget. 2017;8(29):47206–47215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Solga AC, Gianino SM, Gutmann DH. NG2-cells are not the cell of origin for murine neurofibromatosis-1 (Nf1) optic glioma. Oncogene. 2014;33(3):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pan Y, Bush EC, Toonen JA, et al. Whole tumor RNA-sequencing and deconvolution reveal a clinically-prognostic PTEN/PI3K-regulated glioma transcriptional signature. Oncotarget. 2017;8(32):52474–52487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alcantara Llaguno S, Chen J, Kwon CH, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alcantara Llaguno SR, Wang Z, Sun D, et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell. 2015;28(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Alcantara Llaguno S, Sun D, Pedraza AM, et al. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat Neurosci. 2019;22(4):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Simmons GW, Pong WW, Emnett RJ, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kim KY, Ju WK, Hegedus B, Gutmann DH, Ellisman MH. Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma. Neuroscience. 2010;170(1):178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. [DOI] [PubMed] [Google Scholar]
- 118. Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. [DOI] [PubMed] [Google Scholar]
- 120. Solga AC, Pong WW, Kim KY, et al. RNA sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for Neurofibromatosis-1 glioma growth. Neoplasia. 2015;17(10):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pan Y, Xiong M, Chen R, et al. Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes Dev. 2018;32(7–8):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Warrington NM, Woerner BM, Daginakatte GC, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67(18):8588–8595. [DOI] [PubMed] [Google Scholar]
- 123. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen Z, Feng X, Herting CJ, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77(9):2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017;214(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang W, Warrington NM, Taylor SJ, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl med. 2019;11(473) Epub Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sun T, Warrington NM, Luo J, et al. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest. 2014;124(9):4123–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ippolito JE, Yim AK-Y, Luo J, Chinnaiyan P, Rubin JB. Sexual dimorphism in glioma glycolysis underlies sex differences in survival. JCI Insight. 2017; 2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rojnueangnit K, Xie J, Gomes A, et al. High incidence of noonan syndrome features including short stature and pulmonic stenosis in patients carrying NF1 missense mutations affecting p.Arg1809: genotype–phenotype correlation. Hum Mutat. 2015;36(11):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Upadhyaya M, Huson SM, Davies M, et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype–phenotype correlation. Am J Hum Genet. 2007;80(1):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Trevisson E, Morbidoni V, Forzan M, et al. The Arg1038Gly missense variant in the NF1 gene causes a mild phenotype without neurofibromas. Mol Genet Genomic Med. 2019;7(5):e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pinna V, Lanari V, Daniele P, et al. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur J Hum Genet. 2015;23(8):1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Anastasaki C, Woo AS, Messiaen LM, Gutmann DH. Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning. Hum Mol Genet. 2015;24(12):3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Toonen JA, Anastasaki C, Smithson LJ, et al. NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum Mol Genet. 2016;25(9):1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kaul A, Toonen JA, Gianino SM, Gutmann DH. The impact of coexisting genetic mutations on murine optic glioma biology. Neuro Oncol. 2015;17(5):670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Easton DF, Ponder MA, Huson SM, Ponder BA. An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF1): evidence for modifying genes. Am J Hum Genet. 1993;53(2):305–313. [PMC free article] [PubMed] [Google Scholar]
- 138. Reilly KM, Tuskan RG, Christy E, et al. Susceptibility to astrocytoma in mice mutant for Nf1 and Trp53 is linked to chromosome 11 and subject to epigenetic effects. Proc Natl Acad Sci U S A. 2004;101(35):13008–13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Walrath JC, Fox K, Truffer E, Gregory Alvord W, Quiñones OA, Reilly KM. Chr 19(A/J) modifies tumor resistance in a sex- and parent-of-origin-specific manner. Mamm Genome. 2009;20(4):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Amlin-Van Schaick JC, Kim S, DiFabio C, Lee MH, Broman KW, Reilly KM. Arlm1 is a male-specific modifier of astrocytoma resistance on mouse Chr 12. Neuro Oncol. 2012;14(2):160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Amlin-Van Schaick J, Kim S, Broman KW, Reilly KM. Scram1 is a modifier of spinal cord resistance for astrocytoma on mouse Chr 5. Mamm Genome. 2012;23(3–4):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–1528. [DOI] [PubMed] [Google Scholar]
- 143. Warrington NM, Gianino SM, Jackson E, et al. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010;70(14):5717–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Banerjee S, Gianino SM, Gao F, Christians U, Gutmann DH. Interpreting mammalian target of rapamycin and cell growth inhibition in a genetically engineered mouse model of Nf1-deficient astrocytes. Mol Cancer Ther. 2011;10(2):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Walker JA, Upadhyaya M. Emerging therapeutic targets for neurofibromatosis type 1. Expert Opin Ther Targets. 2018;22(5):419–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Isakson SH, Rizzardi AE, Coutts AW, et al. Genetically engineered minipigs model the major clinical features of human neurofibromatosis type 1. Commun Biol. 2018;1:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wegscheid ML, Anastasaki C, Gutmann DH. Human stem cell modeling in neurofibromatosis type 1 (NF1). Exp Neurol. 2018;299(Pt B):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Linkous A, Balamatsias D, Snuderl M, et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26(12):3203–3211.e3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sancho-Martinez I, Nivet E, Xia Y, et al. Establishment of human iPSC-based models for the study and targeting of glioma initiating cells. Nat Commun. 2016;7:10743. [DOI] [PMC free article] [PubMed] [Google Scholar]