Abstract
Background
Meningiomas are the most common primary intracranial tumors in adults. The relationship between meningiomas and exogenous sex hormones such as cyproterone acetate (CPA) is well documented, yet the underlying mechanisms remain unknown. Defining the histomolecular status of meningiomas developed on CPA would help us to better understand the oncogenesis of these tumors.
Methods
We identified 30 patients operated for a meningioma after long-term high-dose CPA therapy and with a history of CPA discontinuation before establishing the indication for surgical intervention. We used array-comparative genomic hybridization (to characterize copy number changes in those 30 meningiomas and subsequently performed next-generation sequencing with the National Institute of Cancer (INCa) solid tumor panel, which is a targeted panel of clinically actionable genes. We also examined grade, type, and clinical features.
Results
We identified AKT1 mutations or PIK3CA mutations in 33.3% of CPA meningiomas. AKT1 and PIK3CA mutations were mutually exclusive. Enrichment in oncogenic PIK3CA mutations in the CPA cohort was detected. CPA meningiomas showed chromosomal stability and were located mainly in the skull base. Ninety percent of CPA meningiomas were low-grade meningiomas and 63.4% were meningotheliomas. Half of our CPA cohort had microcystic components.
Conclusion
Our study shows that low-grade meningothelial meningiomas of the skull base are predominant in CPA meningiomas. We identified PIK3CA/AKT1 pathway as a hypothetical actor in onco-pharmacological interaction between meningiomas and CPA. This signaling pathway could be an interesting target for precision medicine trials in meningioma patients who have been subjected to CPA. Our results could invite the scientific community to review the current classification of meningiomas and to evolve toward more specific histomolecular classification.
Keywords: AKT1, cyproterone acetate, meningioma, molecular pathology, PIK3CA
Key Points.
1. Meningiomas developed on cyproterone acetate are mutated frequently on PI3K pathway.
2. Meningiomas developed on cyproterone acetate showed chromosomal stability.
Importance of the Study.
Our study aims to define the histomolecular characteristics of adult intracranial meningiomas arising on antiandrogen treatment. So far, there is little data available in the literature on this topic. Our cohort of 30 meningiomas developed on high-dose cyproterone acetate (CPA) constitutes the largest series reported in the literature. The identification of genetic and histologic abnormalities involved in meningiomas developed on CPA would enable better understanding of the meningiomagenesis. To reach our objectives, we used 2 different molecular technologies, array comparative genomic hybridization and next-generation sequencing. Our results represent a major step toward an integrated histomolecular classification. And our study suggests that PIK3CA/AKT1 enrichment in this particular population could place targeting therapy in a central position for the management of patients with meningioma.
Meningiomas are the most frequent brain tumors in adults.1 The prevalence is higher in females with a sex ratio of 2:1.2 From a histopathologic perspective, 80% of meningiomas are grade I according to the World Health Organization (WHO) classification.1 Among those, several architectural patterns have been identified, the most frequent being meningothelial, fibroblastic, and transitional meningiomas.3 The standard of care is surgical resection. Some meningiomas have a high risk of recurrence and are challenging for surveillance, raising the indication for adjuvant therapy.4 Although pharmacotherapies may offer some benefits to selected patients in terms of survival, their efficacy has not been demonstrated in prospective studies.5
Several studies have suggested an association between female sex hormones and the pathophysiology of meningiomas.6,7 The role of different sex hormones in the development of meningiomas has been evaluated using various approaches. Numerous reports have dealt with the presence or absence of hormonal receptors in the tumors8; almost all of those detected being high progesterone receptors; however, it remains difficult to determine whether their function is activating or inhibiting.9
Cyproterone acetate (CPA) has been associated with a risk of meningioma occurrence.10,11 CPA is a synthetic steroid derived from 17-hydroxyprogesterone with antiandrogenic and progestogenic properties. Long-term use of high-dose CPA seems to be a major risk factor for developing meningioma. Many studies have shown the regression of multiple meningiomas after discontinuation of progesterone agonist treatment.12,13 Although the association between CPA administration and multiple intracranial meningioma development is well documented, the underlying mechanisms remain unknown.
Even though molecular classification of meningiomas is still limited, it appears that there are 2 distinct pathways according to NF2 mutation status.14 Typical non-NF2-mutant meningiomas do not present major chromosomal copy number anomalies, whereas NF2-mutant meningiomas, or those with chromosome 22 monosomy, present a higher chromosomal instability.15 Recent genetic analysis showed that TRAF7, KLF4, AKT1, PIK3CA, and SMO genes were frequently mutated in non-NF2-mutant meningiomas.14 Although these mutations may be involved in tumor progression, their role in meningioma genesis has yet to be elucidated.16PIK3CA is mutated in 4.7% of all meningiomas and in 7% of non-NF2-mutant meningiomas.17,18 In addition to gene mutations, recurrent chromosomal copy number changes have been reported in meningioma, differing according to the grade.18 However, the histologic and genetic profiles of adult intracranial meningiomas developed on Androcur® use have barely been explored.
Here, we have assessed clinical cases of progression of meningiomas on progesterone agonist treatment by array-comparative genomic hybridization (array-CGH) and next-generation sequencing (NGS) and highlighted typical histologic and genetic features.
Materials and Methods
This is an observational study, descriptive and comparative, conducted at the University Hospital Center of Poitiers (UHCP), in collaboration with the Adolphe de Rothschild Foundation Hospital (RFH) in Paris. The study was validated by the Committee of Biologic Resources of UHCP (identification number BB0033-00068).
Patients and Specimens
We retrospectively selected patients operated for a meningioma with informed history of prolonged exposure to high-dose CPA therapy and documented meningioma evolution after CPA discontinuation. This selection was made regardless of grade, age, and sex to study a general CPA cohort. The tumor samples were obtained from patients who had undergone surgery between 2005 and 2017. None had received radiotherapy before the surgery. The specimens consisted of formalin-fixed, paraffin-embedded (FFPE) tissue sections, with at least 30% of tumor cell. All meningioma samples were reviewed by a neuropathologist (S.M.) and classified according to the 2016 WHO classification.19 Tumor samples of meningiomas developed on CPA cohort were collected from the tumor banks (5 from the tumor bank of UHCP and 25 from RFH).
Our results were compared with a series of 150 adult meningiomas without informed history of Androcur® described by Abedalthagafi et al.17 This cohort was used as a “control cohort” in our article.
DNA Extraction and Quantification
6 × 10 µm FFPE sections per sample were subject to a deparaffinization protocol using Tween® 20 detergent, before DNA extraction with the Maxwell® 16 FFPE Tissue LEV Purification kit. DNA was quantified by Quantus® Fluorometer (Promega).
Array Genomic Comparative Hybridization
The minimum DNA input required to perform the hybridization was 2 µg. Tumoral and reference DNAs (Promega) were labeled using the ULS Labeling Kit (Agilent Technologies) and hybridized onto SurePrint G3 Human CGH Microarray Chip 4 × 180K (Agilent® Technologies) for 24 hours at 67°C in a rotating oven at 20 rpm. After washing, the microarrays were scanned on the Agilent SureScan Microarray Scanner. Agilent CytoGenomics® was used to feature extract tiff. images generated by the scanner and to analyze the extracted data. Somatic copy number changes of 5 consecutive probes with mean log2 ratios 0.25 for gains and –0.25 for losses were called using the ADM2 algorithm. A second analysis strategy detecting aberrations with mean log2 ratios of 0.07 (absolute value) was adopted for samples with low tumoral cell percentage (<50%) to report low-level copy number aberrations. Genomic coordinates were reported according to the Hg19.
To evaluate the copy number alterations (CNAs) of each patient, the number of detected CNA was calculated. One chromosome could carry several CNAs. We defined 3 chromosomal profiles: flat profiles with no chromosomal aberrations (0 CNA), simple profiles with 1–2 CNAs, and complex profiles with 3 or more CNAs. In addition, we calculated the cytogenetic abnormality score (CAS), as defined by Aizer et al.20 and previously used in the “control cohort” (Abedalthagafi et al.17).
Targeted NGS
An in-house actionable gene panel was designed as per the recommendations of the French National Institute of Cancer for the detection of theranostic mutations. The panel included a total of 212 primers, covering hotspot exons of 20 genes (Supplementary Table1). Double libraries were prepared using the Fluidigm Access Array system: after a pre-amplification step, multiplex polymerase chain reaction using target-specific primers was performed and samples were dual-barcoded during the target enrichment process. The amplicons were purified using Ampure XP beads and librairies were quantified and pooled with equimolar concentration. Sequencing was performed on a MiSeq platform using 300-cycle MiSeq Reagent Kit v2 (Illumina). Generated data (FASTQ) were processed according to an in-house developed bioinformatic pipeline. The workflow comprised 4 distinct steps: filtering data using fastQC and prinseq programs, aligning sequences according to the reference genome (hg19); using bowtie2 and BWA, pre-processing alignment files (.bam files); and using SAMtools, picardtools, GATK and FATBAM and FreeBayes. Finally, variants were annotated using ANNOVAR and homemade tools. All NGS analyses were performed in duplicate. For quality assessment, a positive control and 4 blanks were performed in the same run and analyzed with the same bioinformatic pipeline as the samples. All alterations were manually inspected by Integrated Genome Viewer (http://www.broadinstitute.org/igv). Only variants present in both duplicates with an allelic frequency ≥5% were considered. Clinical impact of variants was determined using Alamut® software and classified as per The Association of Molecular Pathology (AMP) guidelines..21 Briefly, variants with strong or potential clinical significance (Tier I and II) were called “pathogenic,” variants of unknown clinical significance were called “VOUS” (Tier III) and benign or likely benign variants were not reported.
Statistical Analysis
We used R3.3.2. software for the statistical analysis. The relationship between the variables was assessed with the chi-square independence test.
Results
Clinical and Histopathological Data
Our CPA cohort consisted of 29 female patients and only 1 male patient. The mean age was 50 years. The control cohort comprised 95 females and 55 males, with mean age of 58 years. The sex ratio was unbalanced in both cohorts with a strong prevalence of females in our cohort as they are the patients in whom Androcur® therapy is indicated, in a majority of cases. All women in the CPA cohort had received 25 mg/day Andocur® for the management of hirsutism. One male patient of our cohort had used Andocur® at a dose of 100 mg/day for the treatment of prostate cancer. Of the 30 patients who had been exposed to Andocur®, 26 (86.7%) had prolonged exposure >10 years and 21 (70%) showed clinical improvement and/or tumor regression on discontinuation of the treatment (Tables 1 and 2) but remained symptomatic. Twenty-six (86.7%) meningiomas were localized in the skull base and only 4 (13.3%) in the convexities (Figure 1B), whereas in the control cohort, 73 (48.7%) were localized in the skull base (Table 2). A positive relationship was found between the skull base localization of meningiomas and use of Andocur® (P = .0003; Table 2).
Table 1.
Clinical Information on Patients in the Cyproterone Acetate (CPA) Cohort. CPA Cohort Consisted of 29 Female Patients and Only 1 Male Patient
| Age (years) | Sex | CPA indication | CPA daily dose (mg/d) | CPA exposure (years) | Symptoms appeared under CPA | Radio-clinical evolution after CPA discontinuation | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 63 | M | Prostate cancer | 100 | <10 | Left visual loss, memory disorders | Improvement |
| Patient 2 | 33 | F | Hirsutism | 25 | >10 | Right vestibular syndrom, right hypoacusis | Stable |
| Patient 3 | 49 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 4 | 48 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 5 | 52 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 6 | 29 | F | Hirsutism | 25 | <10 | Right V1, V2 and V3 deficit, right vestibular syndrom | Stable |
| Patient 7 | 52 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 8 | 29 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 9 | 64 | F | Hirsutism | 25 | >10 | Left visual loss | Stable |
| Patient 10 | 49 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 11 | 50 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 12 | 53 | F | Hirsutism | 25 | >10 | Left visual loss | Stable |
| Patient 13 | 38 | F | Hirsutism | 25 | >10 | Bilateral visual loss | Stable |
| Patient 14 | 62 | F | Hirsutism | 25 | <10 | Right visual loss | Improvement |
| Patient 15 | 68 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 16 | 47 | F | Hirsutism | 25 | >10 | Left exophtalmia, left visual loss | Improvement |
| Patient 17 | 49 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 18 | 51 | F | Hirsutism | 25 | >10 | Frontal swelling | Improvement |
| Patient 19 | 33 | F | Hirsutism | 25 | >10 | Frontal swelling | Improvement |
| Patient 20 | 47 | F | Hirsutism | 25 | >10 | Right visual loss | Stable |
| Patient 21 | 43 | F | Hirsutism | 25 | >10 | Right visual loss | Stable |
| Patient 22 | 37 | F | Hirsutism | 25 | >10 | Left visual loss | Stable |
| Patient 23 | 68 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 24 | 50 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 25 | 67 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 26 | 56 | F | Hirsutism | 25 | <10 | Frontal syndrom, phasic disorders | Improvement |
| Patient 27 | 47 | F | Hirsutism | 25 | >10 | Left visual loss | Improvement |
| Patient 28 | 49 | F | Hirsutism | 25 | >10 | Left visual loss | Stable |
| Patient 29 | 50 | F | Hirsutism | 25 | >10 | Right visual loss | Improvement |
| Patient 30 | 58 | F | Hirsutism | 25 | >10 | Bilateral ophtalmoplegia | Improvement |
Table 2.
Demographic and Pathological Data of the Cyproterone acetate (CPA) Cohort Compared With the Control Cohort
| CPA cohort | Control cohort | Chi2 | Degree of freedom | P | |
|---|---|---|---|---|---|
| Demographics data | |||||
| Total cases | 30 | 150 | |||
| Age, mean (range), y | 50 (29–68) | 58 (22–90) | |||
| Sex (F:M) | 29:1 | 95:55 | |||
| Localization | |||||
| Skull base | 26 (86.7%) | 73 (48.7%) | 13.10 | 1 | <.001 |
| Convexity | 4 (13.3%) | 77 (51.3%) | |||
| AKT1 status | |||||
| Mutated | 5 (16.7%) | 9 (6%) | 2.62 | 1 | .10 |
| Wild-type | 25 (83.3%) | 141 (94%) | |||
| PIK3CA status | |||||
| Mutated | 5 (16.7%) | 7 (4.7%) | 4.02 | 1 | .045 |
| Wild-type | 25 (83.3%) | 143 (95.3%) | |||
| PIK3CA/AKT1 pathway status | |||||
| Mutated | 10 (33.3%) | 16 (10.7%) | 8.64 | 1 | .003 |
| Wild-type | 20 (66.7%) | 134 (89.3%) | |||
| CAS | |||||
| 0 | 10 (40%) | 55 (36.7%) | 10.35 | 5 | .06 |
| 1–2 | 14 (56%) | 56 (37.3%) | |||
| 3–4 | 0 | 15 (10%) | |||
| 5–6 | 0 | 17 (11.3%) | |||
| 7–8 | 0 | 6 (4%) | |||
| 9 | 1 (4%) | 1 (0.7%) | |||
| WHO grade | |||||
| Grade I | 27 (90%) | 104 (69.3%) | 5.54 | 2 | .06 |
| Grade II | 3 (10%) | 41 (27.3%) | |||
| Grade III | 0 | 5 (3.4%) | |||
| Histopathology | |||||
| Chordoid | 1 (3.3%) | 0 | 23.74 | 5 | <.001 |
| Meningothelial | 19 (63.4%) | 38 (42.7%) | |||
| Metaplastic | 1 (3.3%) | 0 | |||
| Microcystic | 3 (10%) | 0 | |||
| Transitional | 6 (20%) | 45 (50.6%) | |||
| Fibroblastic | 0 | 6 (6.7%) |
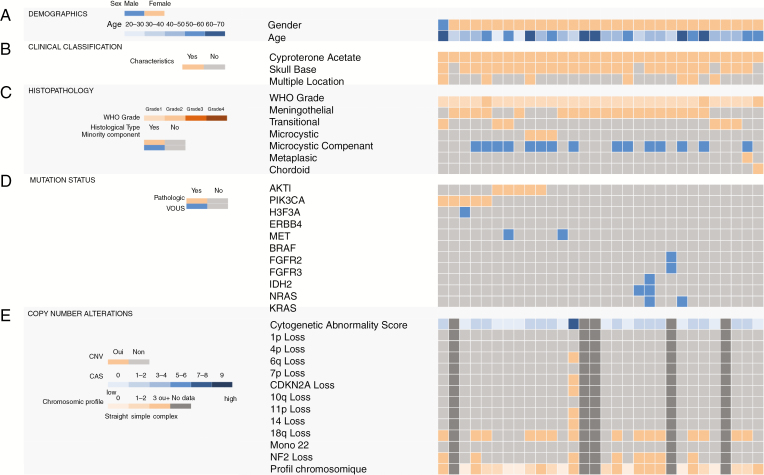
Fig. 1.
Mutation and copy number profiling of the clinical cohort of meningiomas developed on cyproterone acetate (CPA). Data are shown for all samples for which array-comparative genomic hybridization (array-CGH) and next-generation sequencing (NGS) were performed. (A) Patient demographic data. (B) Clinical classification. (C) Histopathological findings. (D) Mutation status. (E) Copy number alterations. The cytogenetic abnormality score (CAS) is plotted in the upper row. Unavailable data are represented in dark gray. The gene panel used for sequencing is available in Supplementary Table 1.
Twenty-seven (90%) meningiomas of the CPA cohort were grade I, and 3 (10%) were grade II (classified grade II based on focal invasion of the cerebral parenchyma n = 2 or due to the presence of a major chordoid component n = 1). There was no grade III meningioma in our series. The control cohort presented 104 (69.3%) grade I, 41 (27.3%) grade II, and 5 grade III (3.4%) meningiomas. Low-grade meningioma and exposure to Androcur® tended to be associated, although not significantly (P = .06; Figure 1 and Table 2).
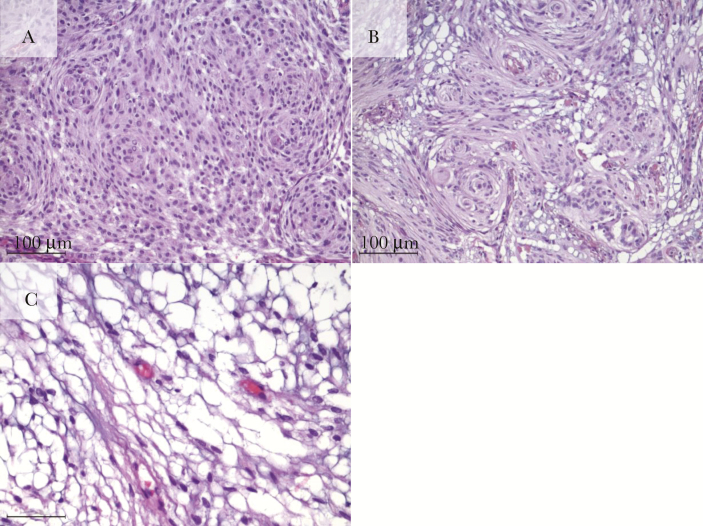
Our CPA cohort included 19 (63.4%) meningothelial meningiomas, 6 (20%) transitional meningiomas, 3 (10%) microcystic, 1 (3.3%) metaplastic, and 1 (3.3%) chordoid (Figure 1 and Table 2). Fifteen (50%) meningiomas developed on Androcur® treatment had microcystic components. In addition, there were 38 (42.7%) meningothelial meningiomas in the control cohort, 45 (50.6%) transitional meningiomas, 6 (6.7%) were fibroblastic meningiomas, and none were of the microcystic, metaplastic, or chordoid subtypes (Figure 3). A positive association was shown between meningothelial and microcystic histology and Androcur® use (P = .0002), whereas a negative correlation seemed to exist between transitional subtype and having been exposed to Androcur® (P = .0002; Figure 1 and Table 2).
Fig. 3.
Hematoxylin and eosin stain sections of the 3 main subtypes of meningiomas developed on cyproterone acetate (CPA): (A) meningothelial, characterized by sheets, whorls or syncytia of neoplastic cells which have round or oval centrally located nuclei and dispersed chromatin, smooth nuclear profiles and small indistinct nucleoli. (B) Transitional defined by mixed histology, typically containing meningothelial, and fibrous components and (C) microcystic with extracellular spaces, scattered throughout the meningioma substrate. (Scale length: 100 µm).
Meningiomas Developed on Cyproterone Acetate Are Mutated Frequently on PIK3CA/AKT1 Pathway
Five (16.7%) meningiomas of the CPA cohort were AKT1 mutated; all 5 had the same E17K nonsense pathogenic mutation in exon 3. These 5 AKT1 mutant meningiomas were all located in the skull base. One AKT1-mutated meningioma carried an additional mutation in the MET gene, G1108S. This mutation was classified as VOUS according to AMP recommendations. The control cohort presented fewer meningiomas with E17K mutation in AKT1 (6%) than our cohort, but the difference was not statistically significant (P = .10; Figure 1 and Table 2).
Five (16.7%) meningiomas of the CPA cohort had a mutation in the PIK3CA gene. All PIK3CA mutations were missense mutations known to be pathogenic: E542G and E542K in exon 9; H1047L, H1047R, and G1049R in exon 20. One of these meningiomas carried 2 concomitant PIK3CA mutations, E542K and H1047R at different allele frequencies (58% and 7% respectively). The H1047L PIK3CA mutant meningioma also had 1 VOUS: A22V in H3F3A gene. Four of these 5 PIK3CA mutated meningiomas were located in the skull base. In Abedalthagafi et al. cohort, only 4.7% of the meningiomas carried oncogenic PIK3CA variants, 4 in exons 9 and 20 (E545K and 3 cases had H1047R mutations) and the rest in exons 1, 5, and 8, respectively (E110del, N345K, E453K mutations). PIK3CA mutational state was associated with Androcur® exposure with a significant P-value (P = .045; Figure 1 and Table 2).
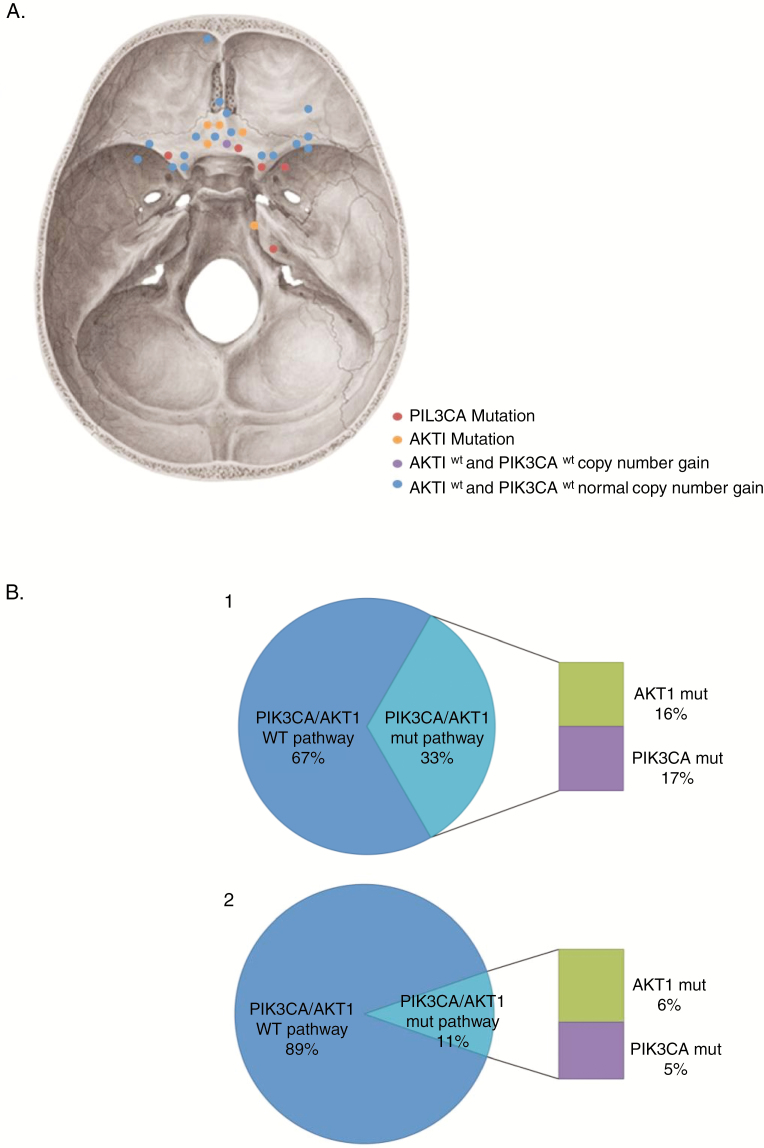
AKT1 and PIK3CA mutations in our CPA cohort were mutually exclusive. All in all, 33.3% meningiomas harbored at least 1 pathogenic mutation of the PIK3CA/AKT1 pathway. Compared with the control cohort, whereby only 10.7% had oncogenic mutations in the PIK3CA/AKT1 pathway, a positive association was established between AKT1 or PIK3CA mutational status and Androcur® exposure (P = .003; Figures 1 and 2 and Table 2).
Fig. 2.
Diagrams summarizing the mutation analysis of the PIK3CA/AKT1 pathway in meningiomas developed on cyproterone acetate (CPA). (A) Schematic diagram depicting the approximate location of CPA meningiomas in the skull base. PIK3CA mutant, AKT1 mutant, AKT1 wild-type and PIK3CA wild-type with copy number gain and PIK3CA wild-type and AKT1 wild-type with normal copy number are distinguished by different colored spots. (B) Diagram showing the distribution of pathogenic mutations of AKT1 and PIK3CA genes in the CPA cohort (1) compared to the control cohort (2).
Other point mutations were identified in the CPA cohort. One meningioma had a KRAS mutation R149K. Two meningiomas carried mutations in the NRAS gene: C118Y and D119N. The meningioma with the KRAS mutation (R149K), NRAS mutation (D119N) also carried a mutation in the IDH2 gene, R149W. FGFR2 was mutated in 1 meningioma A284V and further mutated in the FGFR3 gene, F384L. All of these mutations were categorized as VOUS.
Among the 5 AKT1 mutated meningiomas of the CPA cohort, 2 were transitional meningiomas, 2 had microcystic subtype, and the remaining 1 was a meningothelial meningioma. All were WHO grade I. Four of the 5 PIK3CA-mutant meningiomas in the CPA series presented a meningothelial pattern and 1 was a transitional meningioma. The PIK3CA-mutated meningiomas were classified grade I, except for the meningioma that carried double PIK3CA mutations, classified as grade II meningioma because of a focal invasion of the parenchyma (Figure 1).
Chromosome Instability of Meningiomas Developed on Cyproterone Acetate
In 5 meningiomas, array-CGH could not be carried out due to insufficient DNA quantity. Among the 25 meningiomas analyzed, 24 (96%) had a low CAS score and none presented focal amplification. They included the 5 AKT1-mutated, the 5 PIK3CA-mutated, and the 3 microcystic meningiomas. The only meningioma within the CPA cohort with a high CAS score was a meningothelial meningioma grade I, located in the skull base, AKT1, and PIK3CA wild-type. A majority of low CAS meningiomas (n = 126, 84%) were likewise found in the cohort without Androcur® exposure. No significant correlation between the CAS and Androcur® exposure was revealed, though a trend was noted (P = .06; Figure 1 and Table 2).
Seven (28%) meningiomas of the CPA cohort had a flat genomic profile on array-CGH, 8 (32%) had a simple profile, and 10 (40%) presented a complex profile (Figure 1 and Supplementary Table 2). The most frequently detected cytogenetic aberrations involved chromosomes 19 and 22. Chromosome 19 CNA was found in 16 (53.3%) meningiomas, whereas CNA of chromosome 22 was revealed in 9 (30%) meningiomas. Eight (26.67%) meningiomas presented 22q loss, a chromosomic region containing NF2 gene (band 22q12). Two meningiomas with NF2 gene deletion were PIK3CA mutant. The only meningioma with a high CAS score also presented a complex genomic profile with a gain of 3q and 14q regions, comprising the genes PIK3CA (band 3q26) and AKT1 (band 14q32; Table 3). One complex case with CAS zero presented an atypical chromosomal profile with multiple whole-chromosome gains: chromosomes 4, 5, 8, 10, 11, 12, 13, 14, 17, 19, 20, and 21 (Table 3). Monosomy of chr22 was also highlighted in the control cohort without Androcur® exposure, but the frequency of chr19 CNA appeared less frequent. Complete information on detected CNA is available in Supplementary Table 2.
Table 3.
Two Meningiomas Developed on Cyproterone Acetate (CPA) With Atypical and Complex Chromosome Profile
| Loss | Cytobands | Genes with copy number loss | Gain | Cytobands | Genes with copy number gain | |
|---|---|---|---|---|---|---|
| Patient 13 | 2q | q36,1-q37,3 | 1p | p31,1-p11,2 | NOTCH2 | |
| 6q | q16,3-q22,1 | 1q | Whole | |||
| 6q | q22,33-q25,2 | 2p | p23,3-p11,1 | |||
| 8 | whole | 3q | q26,1-q29 | PIK3CA | ||
| 9p | p24,02-p21,1 | CDKN2A, CDKN2B | 6q | q22,32-q22,33 | ||
| 10p | p13-p12,31 | 7 | Whole | EGFR | ||
| 10p | p12,1-p11,1 | 9q | Whole | KLF4 | ||
| 10q | q21,1 | 10q | q11,22-q11,23 | |||
| 10q | q23,1-q23,31 | 11q | q14,3-q25 | |||
| 10q | q25,2-q26,2 | 12p | Whole | |||
| 13q | q13,3-q31,1 | 13q | q12,11-q13,3 | |||
| 14q | q23,1-q32,33 | 13q | q31,1-q34 | |||
| 17q | q11,2 | 14q | q11,2-q23,1 | AKT1 | ||
| 18p | Whole | 15q | q22,2-q26,3 | |||
| 18q | q22,1-q23 | 17p | p13,1-p11,1 | |||
| 19q | q12-q13,11 | 19p | p13,3-p13,2 | |||
| 19q | q13,2-q13,43 | 19p | p13,11-p12 | |||
| 20p | p12,3-p12,2 | 20q | q11,23-q13,33 | |||
| 20p | p11,23-p11,1 | 22q | q13,1-q13,33 | |||
| 20q | q11,21-q11,22 | |||||
| 21q | q11,2-q22,13 | |||||
| 22q | q11,21-q13,1 | NF2, SMARCB1, CHEK2 | ||||
| Patient 30 | — | — | — | 4 | Whole | |
| — | — | — | 5 | Whole | PIK3R1, hTERT | |
| — | — | — | 8 | Whole | ||
| — | — | — | 10 | Whole | SUFU | |
| — | — | — | 11 | Whole | ||
| — | — | — | 12 | Whole | ||
| — | — | — | 13 | Whole | ||
| — | — | — | 14 | Whole | AKT1 | |
| — | — | — | 17 | Whole | RPS6KB1, POLR2A, BRIP1, SMARCE1 | |
| — | — | — | 19 | Whole | ||
| — | — | — | 20 | Whole | ||
| — | — | — | 21 | Whole |
Discussion
From a molecular standpoint, our results suggest that meningiomas arising on Androcur® are significantly enriched in pathogenic PIK3CA mutations. The gene PIK3CA encodes the catalytic subunit of the phosphatidylinositol 3-kinase enzyme involved in cellular proliferation through the PIK3CA/AKT1/mTOR signaling. Pathogenic mutations in PIK3CA lead to overactivation of the PIK3 pathway.22 The most frequently identified mutation in our cohort was H1047R in exon 20, in accordance with PIK3CA-mutated meningiomas described in the literature.23 The detected mutations in the CPA series (E542K and E542G in exon 9, H1047L, H1047R, and G1049R in exon 20) were hotspot mutations, underlining a role in meningioma development.24 Among them, E542G mutation has previously been reported in other tumors but not yet documented in meningiomas.25PIK3CA and AKT1 mutations are known to be mutually exclusive17,18 as was the case in our CPA cohort. We identified 2 PIK3CA mutations in the same meningioma tumor, which is in accordance with the intratumoral heterogeneity described in the literature.18 Our results indicate a higher rate of PIK3CA/AKT1 pathway mutations in meningiomas developing on Androcur® treatment than in sporadic meningiomas. Peyre et al.26 recently studied a series of progestin-associated meningiomas and also reported a higher frequency of PIK3CA mutations in comparison with a control population. Incidence of PIK3CA mutation was higher compared with our study (35% vs 16.7%) but they did not observe any AKT1 mutation enrichment. It is worth noting that Peyre et al. included patients with different progestin treatment (eg, egestrol acetate and chlormadinone acetate), whereas in our study, only patients with history of high-dose CPA treatment were included. Finally, only exons 9 and 20, which are known to contain most of the relevant oncogenic mutations, were analyzed in our study, whereas Peyre et al. analyzed all the exons of the gene.
Prior studies have shown that PIK3CA and AKT1 mutations were more frequent in skull base meningiomas17,27 and the results of our CPA cohort point toward preferential skull base localization for meningiomas arising on Androcur® use. Although correlating mutational status to tumor location, we can hypothesize that a mutation in the PIK3CA/AKT1 signaling constitutes a favorable environment to onco-pharmacologic interaction between meningioma and Androcur®. In further investigation, it would be interesting to compare a CPA cohort, similar to ours, to a matched control for skull base location.
From a cytogenetic perspective, PIK3CA- or AKT1-mutated meningiomas have a stable chromosome profile.16,17 That much said, our data did not disclose a statistically significant association regarding the cytogenetic abnormality score and whether or not Androcur® treatment had occurred. We can, therefore, extrapolate this stable genomic profile, characteristic of meningiomas harboring a PIK3CA or AKT1 mutation, to meningiomas arising on Androcur® treatment. Interestingly, 2 wild-type meningiomas AKT1 and PIK3CA in our cohort presented copy number gains involving these genes: 1 meningioma had AKT1 gain and the other had both AKT1 and PIK3CA gains. These 2 tumors might have an activation of the PIK3CA/AKT1 signaling path via different mechanisms than classic point mutations, further supporting the hypothesis that this molecular pathway could be a major actor of meningiomas developed on Androcur®.
Because PIK3CA and AKT1 are therapeutic targets,28,29 the molecular characterization of meningiomas developing on Androcur® impels a reevaluation of the diagnostic and therapeutic strategy of these tumors. The surgical management of patients affected by meningiomas at the skull base can be quite problematic, depending on proximal neurovascular structures. Tumoral growth and surgical resection could lead to significant morbidity.30 Several clinical trials are ongoing for molecules targeting PIK3CA/AKT1 signaling in several cancer types (eg, NCT01226316, NCT03006172, NCT02822482) and a recent case report described the response of an AKT1-mutant meningioma to AKT1 inhibition.31 Targeting oncogenes would hinder cellular proliferation in patients not showing a suitable clinical and/or radiological response after Androcur® discontinuation. Acknowledging molecular profile of meningioma may help in deferring the surgical indication in cases of contra-indication to surgery or high-risk procedures, which are often the case in skull base meningiomas; or patients in whom stopping Androcur® treatment is unsought in light of the clinical context. The outcome of our report highlights the importance of PIK3CA/AKT1 inhibition and suggests a need for further phase II and III prospective studies.
On a histopathologic level, it appeared that meningiomas arising on Androcur® exposure were essentially low-grade meningothelial meningiomas. Once again, if we associate this outcome with tumor location, it appears that meningiomas developed on Androcur® treatment were mainly meningothelial meningiomas of the skull base. Lee et al. showed that most of the meningiomas occurring in the skull base have meningothelial histology.3 Our results are consequently consistent with data from the literature. Our CPA cohort contained only 3 malignant meningiomas, and all the remaining ones were benign. We did not find a statistically significant association between the use of Androcur® and WHO grade. These findings are in line with epidemiological studies that report a vast majority of grade I among meningiomas.1,2
Previous genomic studies revealed a direct link between quantitative chromosomal abnormalities and malignancy of meningiomas.24,25,32,33 All meningiomas in our CPA cohort had low CAS, except for one. This observation is consistent with the fact that almost all were grade I meningiomas. However, our study was not designed to determine whether CAS is associated with molecular grade of tumor or with drug exposure. Moreover, several authors had reported that PIK3CA- or AKT1-mutated meningiomas were grade I16,17 as was the case in our study. Three meningiomas of the CPA series were classified grade II according to histoprognostic criteria of the 2016 WHO classification. One had a dominant chordoid component and the 2 others presented on the initial histopathologic reports a focal invasion of the underlying parenchyma that was not seen on slide review. Although the malignancy of a chordoid meningioma is indisputable, focal invasion of the cerebral parenchyma is more controversial. Although it is well-known that parenchymal infiltration in a grade I meningioma confers relapse and mortality rates similar to that of a grade II meningioma, the previous WHO classification did not consider invasion of the cerebral parenchyma as a grading criteria.34 To avoid confusion, the current 2016 WHO classification henceforth recognizes the invasion of the underlying parenchyma as an atypical character and a sufficient argument to classify as grade II.1,19 Genetically speaking, the 2 meningiomas with focal invasion of the underlying parenchyma had low CAS, simple chromosomal profile, and 1 was mutated for PIK3CA, manifesting chromosomal stability as per Abedalthagafi et al.’s conclusions. From a molecular angle, they would have a slow tumoral progression comparable with that of a grade I meningioma. Further genomic tumor explorations are needed to identify additional molecular markers predictive of meningioma progression and to ultimately integrate the molecular findings into histological classifications, as with gliomas.35
Half of the meningiomas in the CPA cohort showed microcystic components: 3 had major components and 12 had minor microcystic components of variable importance. Knowing that microcystic meningiomas commonly represent only 1.6% of meningiomas,36 our findings imply an association between microcystic component and exposure to Androcur®. Nevertheless, meningiomas are polymorphous tumors and minor components have not been thoroughly described. The presence of microcystic histology might be an indirect marker of underlying genetic mechanisms that could play a role in the onco-pharmacologic meningioma-Androcur® interaction. Variation in tumor size post-discontinuation of the treatment could thereby be attributed to the variation in microcystic components ratio. Several hypotheses have been put forward on the pathogenesis of intra- and extracellular microcysts,37,38 but further implication has yet to be demonstrated first in sporadic meningiomas and later in meningiomas occurring on Androcur® exposure.
Posterior meninges develop from the mesoderm embryonic tissue, whereas meninges of the anterior floor and anterior cranial vault originate from the neural crest.3,39 Genomic studies have demonstrated that genetic aberrations mimicked this structure. Alterations of NF2 gene preferentially concerned meninges of the posterior cranial fossa and cerebral convexity, whereas alterations of TRAF7, SMO, KLF4, AKT1, and PIK3CA genes involved meninges of the anterior cranial fossa.17,40 Moreover, meningiomas of the anterior and middle cranial cavities are NF2 wild-type and almost all are benign with chromosomal stability. On the other hand, meningiomas arising from cerebral and cerebellar convexities harbor mutations in NF2 gene and/or chromosome 22 deletion and are more likely to be atypical tumors with chromosomal instability.40 In our study, we found that 2 PIK3CA mutant meningiomas had NF2 gene deletion, which is uncommon but has been reported.18 To the best of our knowledge, there have not been yet any previous publications on the molecular status of meningiomas arising on Androcur® treatment. Moreover, the strength of our study lies in its sample size: with 30 meningiomas developed on CPA intake, it constitutes the largest cohort described in the literature. As for the limitations, our gene panel only contained actionable genes with prospective therapeutic targets and the other genes described in the literature for meningioma, such as NF2, were not characterized. This strategy has the advantage of detecting fewer mutations with uncertain significance.
In addition, because the molecular characterization of tumor sample requires tumor material, our recruitment was built on operated meningiomas that did not sufficiently respond to withdrawal of Androcur® treatment. This might create a selection bias regarding underlying oncogenic mechanisms. Nonetheless, despite the need for surgical intervention, 70% of the patients showed clinical and/or radiological improvement after discontinuation of the treatment. It is, therefore, conceivable that the histomolecular characteristics observed in operated meningiomas are the same as in nonoperated meningiomas.
Finally, the sex ratio between our cohort and the control cohort was quite different and a subsequent study with age and sex-matched control would be of great interest to ascertain our results.
In conclusion, the advent of new genetic technologies over the past years has generated major progress in understanding the molecular and cytogenetic events involved in meningiomagenesis. Given the current state of evidence, several oncogenic mechanisms exist, each with a different molecular pathway. The refinement of our knowledge on molecular genetics of meningiomas could have a direct impact on the diagnosis and management of these tumors. Our publication is the first to analyze the histomolecular status of meningiomas developed on Androcur® treatment. The discovery of divergences between sporadic meningiomas and those arising on Androcur® intake facilitates understanding of the underlying oncogenesis.
Funding
Our study was financed by Alienor donation funds. This funding project was created by the University Hospital Center of Poitiers (UHCP) and dedicated to research and innovation in medical health.
Supplementary Material
Acknowledgments
The authors wish to thank A. Le Bechec, a clinical bioinformatician of the University Hospital of Strasbourg, for sharing the FatBam tool. We thank J. Arsham, an American translator, for having reviewed and revised the original English-language text. We thank M. Proquin, P. Rivet, and S. Martin for their technical help.
Conflict of interest statement. All authors declare that there is no conflict of interest regarding the publication of this article.
Authorship statement: Analysis of the data: Sylvain Portet, Rania Naoufal, Gaëlle Tachon, Anaïs Chalant, Amir Naar, Serge Milin, Lucie Karayan-Tapon. Conceived the experiments: Sylvain Portet, Rania Naoufal, Gaëlle Tachon, Amir Naar, Benoit Bataille, Lucie Karayan-Tapon. Experiments performance: Sylvain Portet, Rania Naoufal. Wrote the paper: Sylvain Portet, Rania Naoufal, Gaëlle Tachon, Lucie Karayan-Tapon. Data collection: Sylvain Portet, Adrien Simonneau, Benoit Bataille.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–1095; discussion 1088. [DOI] [PubMed] [Google Scholar]
- 3. Lee JH, Sade B, Choi E, Golubic M, Prayson R. Meningothelioma as the predominant histological subtype of midline skull base and spinal meningioma. J Neurosurg. 2006;105(1):60–64. [DOI] [PubMed] [Google Scholar]
- 4. Mathiesen T, Lindquist C, Kihlström L, Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39(1):2–7; discussion 8. [DOI] [PubMed] [Google Scholar]
- 5. Cossu G, Levivier M, Daniel RT, Messerer M. The role of mifepristone in meningiomas management: a systematic review of the literature. Biomed Res Int. 2015;2015:267831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller RE. Breast cancer and meningioma. J Surg Oncol. 1986;31(3):182–183. [DOI] [PubMed] [Google Scholar]
- 7. Bickerstaff ER, Small JM, Guest IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21(2):89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blaauw G, Blankenstein MA, Lamberts SW. Sex steroid receptors in human meningiomas. Acta Neurochir (Wien). 1986;79(1):42–47. [DOI] [PubMed] [Google Scholar]
- 9. Roser F, Nakamura M, Bellinzona M, Rosahl SK, Ostertag H, Samii M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004;57(10):1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernat AL, Oyama K, Hamdi S, et al. Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: a case series of 12 patients. Acta Neurochir (Wien). 2015;157(10):1741–1746. [DOI] [PubMed] [Google Scholar]
- 11. Gil M, Oliva B, Timoner J, Maciá MA, Bryant V, de Abajo FJ. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: evidence from a population-based cohort study. Br J Clin Pharmacol. 2011;72(6):965–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cebula H, Pham TQ, Boyer P, Froelich S. Regression of meningiomas after discontinuation of cyproterone acetate in a transsexual patient. Acta Neurochir (Wien). 2010;152(11):1955–1956. [DOI] [PubMed] [Google Scholar]
- 13. Vadivelu S, Sharer L, Schulder M. Regression of multiple intracranial meningiomas after cessation of long-term progesterone agonist therapy. J Neurosurg. 2010;112(5):920–924. [DOI] [PubMed] [Google Scholar]
- 14. Peyre M, Kalamarides M. Molecular genetics of meningiomas: building the roadmap towards personalized therapy. Neurochirurgie. 2018;64(1):22–28. [DOI] [PubMed] [Google Scholar]
- 15. Domingues P, González-Tablas M, Otero Á, et al. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6(13):10671–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zadeh G, Karimi S, Aldape KD. PIK3CA mutations in meningioma. Neuro Oncol. 2016;18(5):603–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bi WL, Zhang M, Wu WW, Mei Y, Dunn IF. Meningioma genomics: diagnostic, prognostic, and therapeutic applications. Front Surg. 2016;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banan R, Hartmann C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta Neurochir (Wien). 2017;159(3):403–418. [DOI] [PubMed] [Google Scholar]
- 20. Aizer AA, Abedalthagafi M, Bi WL, et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro Oncol. 2016;18(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the association for molecular pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El-Habr EA, Levidou G, Trigka EA, et al. Complex interactions between the components of the PI3K/AKT/mtor pathway, and with components of MAPK, JAK/STAT and notch-1 pathways, indicate their involvement in meningioma development. Virchows Arch. 2014;465(4):473–485. [DOI] [PubMed] [Google Scholar]
- 24. Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94(4):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu W, Tong GX, Manolidis S, Close LG, Assaad AM, Su GH. Novel mutant-enriched sequencing identified high frequency of PIK3CA mutations in pharyngeal cancer. Int J Cancer. 2008;122(5):1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peyre M, Gaillard S, de Marcellus C, et al. Progestin-associated shift of meningioma mutational landscape. Ann Oncol. 2018;29(3):681–686. [DOI] [PubMed] [Google Scholar]
- 27. Yesilöz Ü, Kirches E, Hartmann C, et al. Frequent AKT1E17K mutations in skull base meningiomas are associated with mtor and ERK1/2 activation and reduced time to tumor recurrence. Neuro Oncol. 2017;19(8):1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez S, Schwab CL, Cocco E, et al. Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014;135(2):312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu Y, Savage RE, Eathiraj S, et al. Targeting AKT1-E17K and the PI3K/AKT Pathway with an Allosteric AKT Inhibitor, ARQ 092. PLoS One. 2015;10(10): e0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33(4):260–266. [DOI] [PubMed] [Google Scholar]
- 31. Weller M, Roth P, Sahm F, et al. Durable control of metastatic AKT1-mutant WHO grade 1 meningothelial meningioma by the AKT inhibitor, AZD5363. J Natl Cancer Inst. 2017;109(3):1–4. [DOI] [PubMed] [Google Scholar]
- 32. Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–391. [DOI] [PubMed] [Google Scholar]
- 33. Tang M, Wei H, Han L, et al. Whole-genome sequencing identifies new genetic alterations in meningiomas. Oncotarget. 2017;5(0):17070–17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barresi V, Caffo M, Tuccari G. Classification of human meningiomas: lights, shadows, and future perspectives. J Neurosci Res. 2016;94(12):1604–1612. [DOI] [PubMed] [Google Scholar]
- 36. Matsushima N, Maeda M, Takamura M, Matsubara T, Taki W, Takeda K. MRI findings of atypical meningioma with microcystic changes. J Neurooncol. 2007;82(3):319–321. [DOI] [PubMed] [Google Scholar]
- 37. Michaud J, Gagné F. Microcystic meningioma. Clinicopathologic report of eight cases. Arch Pathol Lab Med. 1983;107(2):75–80. [PubMed] [Google Scholar]
- 38. Cuccurullo L, Parlato C, Luongo M, Accardo M. Ultrastructural profile of microcystic meningioma. Pathologica. 2009;101(3):115–118. [PubMed] [Google Scholar]
- 39. O’Rahilly R, Müller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45(5):588–608. [PubMed] [Google Scholar]
- 40. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.