Abstract
Background
Erdheim–Chester disease (ECD), a rare inflammatory myeloid neoplasm, is known to be fundamentally reliant on the constitutive activation of the MAPK signaling pathway in the majority of patients. Consequently, inhibition of the V600E-mutant BRAF kinase has proven to be a safe and efficacious long-term therapeutic strategy for BRAF-mutant ECD patients. Nevertheless, in a subset of patients with CNS disease, the efficacy of long-term treatment may diminish, facilitating suboptimal responses or disease progression.
Methods
We retrospectively describe 3 BRAF-mutant ECD patients whose treatment with Vemurafenib was upgraded to Vemurafenib/Cobimetinib due to either disease progression, insufficient response, or unacceptable toxicity. CNS response to therapy was evaluated using magnetic resonance imaging (MRI) and extra-cranial disease was monitored using 18F-fludeoxyglucose positron emission tomography/computed tomography (PET/CT).
Results
Three patients with a mean age of 52.6 years were treated with Vemurafenib for a mean duration of 26.6 months (range: 6–52). Monotherapies were upgraded to Vemurafenib/Cobimetinib dual therapy. The combination therapy was administered for a mean duration of 21 months (range: 19–23). All patients exhibited clinical and neurological improvement. Regression of lesions on MRI was noted in 2 patients. Both patients characterized by a PET-avid disease responded to the biological treatment regimen with complete metabolic remissions.
Conclusion
Dual inhibition of BRAF and downstream MEK may be a safe and effective therapeutic strategy for BRAF-mutant ECD patients for whom BRAF inhibitor therapy proved insufficient and as such appropriate for the long-term management of CNS disease in ECD.
Keywords: BRAF, Cobimetinib, Erdheim–Chester disease, histiocytosis, Vemurafenib
Key Point.
Dual BRAF/MEK blockade induces CNS responses in Erdheim–Chester disease patients who previously failed on BRAF inhibitor monotherapy.
Importance of the Study.
Rare neoplastic diseases such as the Erdheim–Chester disease (ECD) render it impossible to execute large-scale randomized controlled trials and obtain robust evidence-based data. Therefore, precision medicine approaches are employed and treatment strategies rely on our understanding of the molecular mechanisms that underlie the pathogenesis of these diseases. Herein, the authors report the observation that dual inhibition of BRAF and MEK may be an effective therapeutic strategy for BRAF-mutant ECD patients who previously failed on BRAF inhibitor monotherapy. This combination therapy successfully treats the central nervous system involvements of ECD and is well tolerated for long-term use.
In the past two decades, marked progress had been made in enriching and refining the therapeutic armamentarium for Erdheim–Chester disease (ECD), a rare inflammatory myeloid neoplasm.1 In that time period, therapeutic strategies matured from rudimentary oncological approaches to molecular tailored precision medicine. In 1996, the absolute majority of patients described by Veyssier-Belot et al.2 were treated with corticosteroids, chemotherapy, radiotherapy, and surgery. Interferon-α based therapy was introduced in 20013 and thereafter became the first historically recognized line of therapy.4–6 Anti-cytokine therapy7,8 emerged following the understanding that ECD is associated with a systemic immune Th1-oriented perturbation.9 Nevertheless, the most substantial leap in the field was the recognition that ECD is a monoclonal neoplastic entity that is markedly reliant on the consecutive activation of the mitogen-activated protein kinase (MAPK) signal transduction pathway. Multiple genomic alterations were identified in ECD10 with the predominant V600E BRAF mutation detected in as many as half of the patients,11 causing a paradigm shift in the management of ECD. Since inhibition of similar alterations in other malignancies such as melanoma proved efficacious,12–14 these data were instrumental for employing molecularly tailored targeted therapies for ECD as well. Evidently, exceptional efficacy was documented with respect to the usage of BRAF inhibitors such as Vemurafenib or Dabrafenib in BRAF-mutant patients.15–18 As a result, in November 2017, Vemurafenib was approved by the FDA for the treatment of ECD patients harboring a V600 mutation.19 Moreover, recent evidence published in the medical literature demonstrated the efficacy of MEK inhibition in ECD patients.12,20 Ultimately, in October 2019, the FDA granted a breakthrough therapy designation to Cobimetinib for MEK inhibition in histiocytic neoplasms.
Nevertheless, achieving longstanding disease control in the case of ECD seems to necessitate chronic treatment, as the majority of BRAF-mutant patients relapse following interruption of BRAF inhibitor therapy.21 On the other hand, chronic BRAF inhibition embodies its own inherent challenges: long-term treatment is often difficult to tolerate.22–24 In some patients, a variety of treatment-associated adverse events may require dosage modification or cessation of therapy. Chronic low-dose therapy may be insufficient for central nervous system (CNS) involvement due to limited drug penetrance to the brain,25,26 thus culminating in inadequate treatment efficacy and plateauing of clinical improvement. Finally, the potential emergence of resistance also remains a concern. In such cases and in patients with CNS disease in particular, the addition of an MEK inhibitor may be a reasonable therapeutic strategy. Herein, we report the efficacy of Vemurafenib–Cobimetinib combination therapy in 3 BRAF-mutant ECD patients with extensive CNS disease, for whom Vemurafenib monotherapy proved insufficient.
Patients and Methods
In this IRB-approved retrospective study, 3 ECD patients harboring the BRAF V600E mutation were evaluated. Written informed consent was obtained from all the patients. The descriptive characteristics of these patients are recorded in Table 1. All 3 patients had histologically proven ECD with associated classical radiological findings as previously described in the literature.27 Confirmation of the BRAF V600E mutation was performed using pyrosequencing-based methods and by detection of the BRAF V600E mutant DNA in the patients’ urine. Treatment efficacy was monitored by routine clinical and neurological evaluations, measurement of C-reactive protein (CRP), whole-body 18F-fludeoxyglucose positron emission tomography/computed tomography (PET/CT), and brain-directed magnetic resonance imaging (MRI). Since all 3 patients exhibited prominent CNS involvement characterized primarily by cerebellar dysfunction, neurological deterioration was the prime determinant incorporated into the decision to upgrade the therapeutic regimen. Each biological agent was administered according to the standard manufacturer’s recommendations.
Table 1.
The clinical and radiological chronicles of three ECD patients
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Sex, age (years) | Female, 51 | Male, 64 | Male, 43 |
| Intracranial ECD involvement sites, per MRI | (1)nSOL—pons, lt. middle cerebellar peduncle, lt. cerebellar peri-dentate region (2)Pituitary (DI) | SOL—involving the midbrain, lt. superior and middle cerebellar peduncles | (1)nSOL—lt. cerebellar peri-dentate region, cerebellar atrophy (2)Pituitary (DI) |
| Extracranial ECD involvement sites, per multiple modalitiesa | Bone marrow, peri-renal infiltration, peri-orbital xanthelasma, lungs, retro-orbital masses involving orbital muscles, retinal infiltration | Bone marrow, aortic coating, peri-renal infiltration | None |
| Previous treatments | Steroids, IFNa, IFNa+vinblastin, anakinra, cladribine | None | Steroids, cyclophosphamide, rituximab, plasmapheresis, cladribine |
| Time points for the emergence of first documented ECD symptoms and diagnosis | Symptoms: 2005 Diagnosis: 2007 | Symptoms: 2007 Diagnosis: 2015 | Symptoms: 2005 Diagnosis: 2014 |
| Neurological baseline prior to targeted therapy | Rapid deterioration, severe cerebellar syndrome—dysarthria, ataxia, patient bedridden ECOG: 4 | Rapid deterioration, severe cerebellar syndrome, dysarthria, loss of gag reflex necessitating PEG insertion, loss of functional motor capacity of the left arm, patient confined to a wheelchair ECOG: 4 | Indolent disease. Severe cerebellar atrophy, dysarthria, dysmetria, patient confined to a wheelchair ECOG: 4 |
| Monotherapy type, duration (months), and dosage | Vemurafenib 52 months (April 2013– July 2017) 960 mg/day | Vemurafenib 14 months (December 2015– February 2017) 1920 mg/day | Vemurafenib monotherapy 6 months (March 2016–August 2016) 960 mg/day Cobimetinib monotherapy 2 months (March 2017–May 2017) 60 mg/day |
| Monotherapy clinical gain | Rapid neurological deterioration halted, marked neurological improvement: improved dysarthria with partial regaining of speech fluency, regaining of locomotion up to distances of 500 m ECOG: 2 | Rapid neurological deterioration halted, mild improvement in cerebellar function ECOG: 3 | For both monotherapies independently: Incremental clinical improvement in alertness, upper body strength, in the capacity to lift the arms above the shoulders and maintain an erect posture ECOG: 4 |
| Time to initial response | 3 weeks | 1 month | Vemurafenib monotherapy: 1 month Cobimetinib monotherapy: 2 months |
| Time to maximal response | 3 months | 4 months | Vemurafenib monotherapy: 1 month Cobimetinib monotherapy: 2 months |
| Monotherapy imaging effect, per MRI | Normalization of the pontocerebellar abnormalities | Decrease in the sizes of the SOLs, decrease in the compression of the fourth ventricle | No significant change in MRI studies |
| Monotherapy- associated adverse events | Emergence of multiple keratoacanthomas (excised) | None | Vemurafenib monotherapy Grade 3 skin toxicity: deterioration of existing pressure ulcers → cessation of therapy Cobimetinib monotherapy Grade 3 skin toxicity: macular-pustular rash necessitating hospitalization → therapy restarted at a decreased dosage following complete resolution of the skin-related adverse event |
| Basis for conversion to Vemurafenib/ Cobimetinib dual therapy | Slow neurological deterioration including slowing of speech, compromised fine motor skills, gait disturbances necessitating the usage of ambulatory aids, re-emergence of pontocerebellar abnormalities on MRI | Worsening of dysarthria, horizontal nystagmus, marked gait disturbances, recurrent hospitalizations due to gag reflex insufficiency associated aspiration pneumonia | Worsening dysarthria, loss of the ability to balance the head, intolerable skin toxicity at effective doses |
| Vemurafenib/ Cobimetinib dual therapy duration (months) and dosage | 19 months (July 2017– February 2019) 960 mg/day (VEM), 60 mg/day (COBI) | 23 months (March 2017– February 2019) 1440 mg/day (VEM), 40–60 mg/day (COBI) | 21 months (May 2017–February 2019) 480 mg/day (VEM), 20–60 mg/day (COBI)b |
| Time to initial response | 1 month | 1 week | 1 month |
| Time to maximal response | 3 months | Maximal response not yet achieved | 6 months |
| Dual therapy clinical gain | Improvement in speech fluency and fine motor skills, stabilization of gait disturbances ECOG: 2 | Marked neurological improvement: regaining of the ability to ambulate with aids for short distances, improvement in speech fluency, gradual improvement in swallowing, reconstitution of the strength of the left arm ECOG: 3 | Further improvement in the ability to maintain an erect posture, executes functional motions with both arms, moves lower limbs, a further increase in appetite and body weight ECOG: 4 |
| Dual therapy imaging effect, per MRI | Normalization of pontocerebellar abnormalities on MRI | Complete regression of the SOLs on MRI | No significant change in MRI studies |
| Dual therapy- associated adverse events | None | Grade 1 diarrhea, grade 1 maculopapular rash | Grade 1 macular rash |
| Overall multifocal PET/CT response to treatmentc | Complete metabolic response | Complete metabolic response | Disease not PET avid |
COBI, cobimetinib; ECOG, eastern cooperative oncology group performance score; DI, diabetes insipidus; IFNa, interferon alpha; lt., left; PEG, Percutaneous endoscopic gastrostomy; SOL, space-occupying lesions; nSOL, non-space-occupying lesions; VEM, vemurafenib.
aBone scintigraphy, CT, PET/CT, MRI, confocal scanning laser ophthalmoscopy, and dermoscopy.
bDue to former cutaneous adverse events, the dosage was moderately escalated from 20 to 60 mg/day over a period of 2 months.
cPET/CT performed prior to monotherapy and following dual therapy.
Results
Three patients (2 males, 1 female) with a mean age of 52.6 years were described in this study (Table 1). All 3 patients suffered from ECD-related symptoms for over a decade. The various disease involvement sites included the CNS (n = 3), pituitary gland (n = 2), skeleton (n = 2), retroperitoneum and peri-renal fat (n = 2), aortic coating (n = 1), and orbits (n = 1). Profound disease-associated morbidity was specifically related to prominent CNS-associated involvements in all patients. These included non-space-occupying lesions (n = 2) and space-occupying lesions (n = 1) of the pontocerebellar region as well as cerebellar atrophy (n = 1).
Following molecular confirmation of a V600 BRAF mutation, all patients received Vemurafenib monotherapy. The mean duration of therapy was 26.6 months (range: 6–52). Treatment was administered at a full dosage of 1920 mg/day for 2 weeks and then adjusted per patient depending on their basal performance, extent of disease, efficacy, and adverse events as described in Table 1. All 3 patients benefited from this treatment regimen with patient 1 exhibiting marked improvement as well as normalization of her MRI studies (Figure 1) and patient 2 presenting with stabilization of his neurological deterioration and partial regression of his CNS space-occupying lesions (Figure 2). Patient 3 gained only incremental clinical benefit with no radiological response and suffered from Vemurafenib-associated skin toxicity at doses higher than 480 mg/day. Treatment with Vemurafenib was ceased and 7 months later a trial of Cobimetinib was attempted at an initial dose of 60 mg/day for 21 consecutive days per cycle. This attempt, albeit producing a positive clinical outcome, also yielded significant cutaneous toxicity as well, which necessitated restarting the treatment from 20 mg/day and gradually escalating to 60 mg/day over a period of 3 months. Once a full dose was achieved again, no significant skin toxicity was noted.
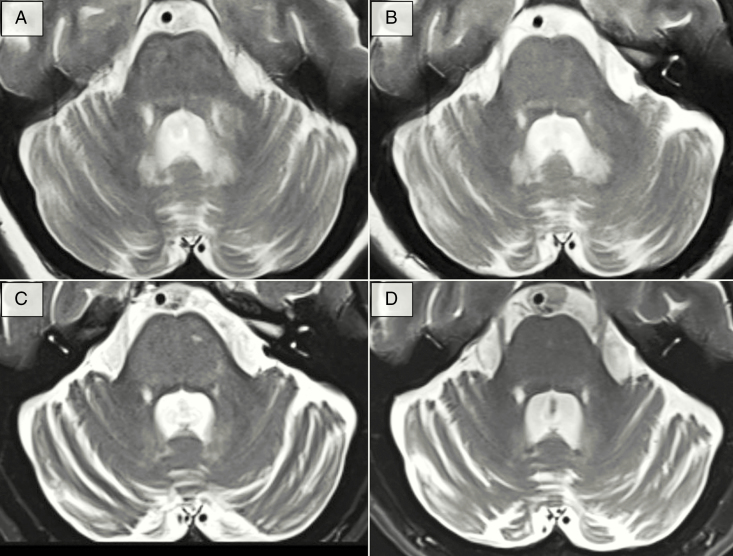
Figure 1.
Cerebellar MRI findings of patient 1. Axial T2-weighted MR images of patient 1 at the level of the cerebellum, middle cerebellar peduncles, and pons. (A) Baseline MR study prior to biological therapy exhibits a diffuse, heterogeneous hyperintense signal involving mainly the pons and left middle cerebellar peduncle. (B) Fourteen months following initiation of Vemurafenib monotherapy, these loci of abnormal signal attenuate. The state of near normalization persists for 2 years before new minute hyperintensities involving the left region of the pons emerge (C). These loci intensify over the course of 11 months and 2 consecutive scans. (D) Normalization of the hyperintense signals occurs 9 months following initiation of Vemurafenib/Cobimetinib dual therapy. Nevertheless, over the course of her disease, the patient exhibits loss of cerebellar mass regardless of any therapeutic interventions.
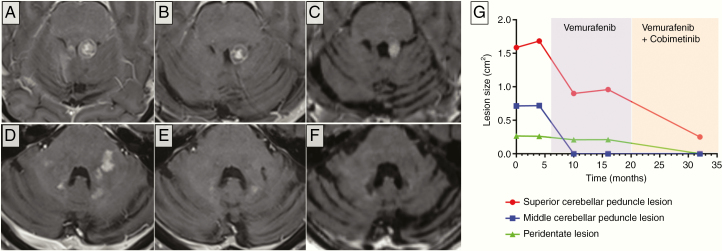
Figure 2.
Cerebellar MRI findings of patient 2. Contrast-enhanced axial T1-weighted MR images of patient 2 at the levels of the superior cerebellar peduncles (A–C) and middle cerebellar peduncles (D–F). (A) Baseline MR study prior to biological therapy reveals a contrast-enhancing space-occupying lesion involving the left superior cerebellar peduncle, compressing the fourth ventricle. Following 4 months of Vemurafenib monotherapy, partial regression of the lesion is noted. (B) The lesion then stabilizes and maintains its dimensions, evident 10 months following Vemurafenib monotherapy. (C) Fifteen months pursuant initiation of Vemurafenib/Cobimetinib dual therapy, marked regression of the lesion is noted. (D–F) Similar dynamics appear in respect to the lesions involving the middle cerebellar peduncles. (D) Multiple contrast-enhancing lesions appear at a baseline involving mainly the left middle cerebellar peduncle as well as in proximity to the fourth ventricle. (E) Marked regression of these lesions occurs in response to Vemurafenib. (F) Complete regression appears following Vemurafenib/Cobimetinib dual therapy. Note that despite treatment, a marked loss of cerebellar mass occurs over the natural history of the disease. (G) Quantification of lesions’ dimensions between June 2015 to January 2018.
Ultimately, the monotherapies were upgraded to Vemurafenib/Cobimetinib dual therapy due to signs of disease progression (patient 1), suboptimal response (patient 2), or inability to withstand treatment-related toxicities at effective doses (patient 3). Dual therapy was administered for a mean duration of 21 months (range: 19–23) and doses were adjusted per patient. While Vemurafenib was administered readily, Cobimetinib was administered in 28 day cycles consisting of 21 days on therapy and 7 days off therapy. All 3 patients obtained a meaningful clinical benefit from this regimen, characterized by satisfactory rehabilitation following partial recuperation of multiple neurological faculties. Furthermore, 2 patients exhibited improvement in their MRI studies. Patient 1 presented with normalization of her pontocerebellar disease-related abnormalities (Figure 1) and patient 2 demonstrated significant regression of all CNS-associated space-occupying lesions (Figure 2). All patients presented with normalization/near normalization of their CRP levels following biological therapy and 2 out of the 3 exhibited gradual resolution of their ECD-associated microcytic anemia. Finally, both patients who have had a PET-avid disease responded to the biological treatment regimen with complete metabolic remissions (Figure 3).
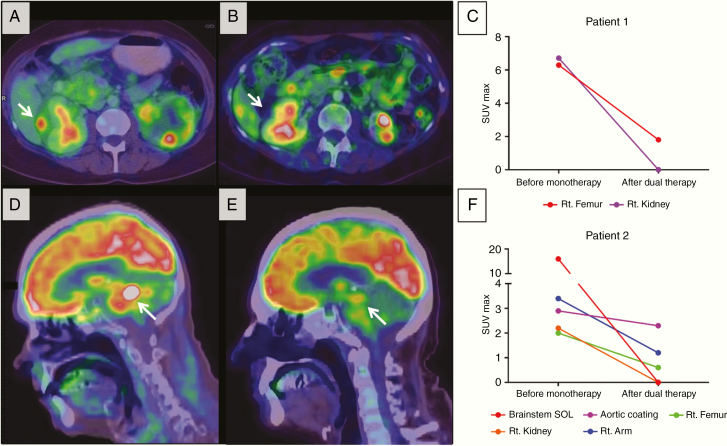
Figure 3.
PET/CT findings of patients 1 and 2. (A and B) Axial PET/CT fusion images at the level of the kidneys of patient 1 prior to initiation of monotherapy (A) and following dual therapy (B). Note the complete regression of the PET-avid space-occupying lesion compressing the right kidney (white arrow). (C) Graphic representation of the SUVmax values measured in different lesions from patient 1. (D–F) Sagittal PET/CT fusion images at the level of the brainstem of patient 2 prior to initiation of monotherapy (D) and following dual therapy (E). A significant decrease in FDG uptake is noted in the brainstem lesion (white arrow). (F) Graphic representation of the SUVmax values measured in different lesions from patient 2.
All 3 patients were monitored for the emergence of treatment-associated adverse events. These included a monthly dermatologist evaluation and a cardiac echocardiogram and ophthalmological evaluation every 3 monthly cycles of Cobimetinib. The treatment-associated adverse events documented at the time of follow-up are recorded in Table 1.
Discussion
Herein we describe the advantageous qualities of dual BRAF/MEK blockade in BRAF-mutant ECD patients in the settings of disease progression, suboptimal response, and treatment-related toxicity on BRAF inhibitor therapy. The described response dynamics underlie the importance of properly understanding the role of the MAPK axis in histiocytic neoplasms.
The MAPK signaling pathway is well known for its role in governing cellular proliferation, differentiation, and apoptosis.28 Particularly, aberrant signal transduction through the RAS–RAF–MEK–ERK axis has been repeatedly implicated in a multitude of malignancies, including melanoma, non-small cell lung carcinoma, and colorectal cancer. As such, small molecule mediated targeted inhibition of MAPK signaling elements is both actively investigated and clinically recognized as an acceptable therapeutic strategy in a number of cancers.12,13,17,29,30 Nonetheless, the responses generated by this strategy are often hindered by the swift emergence of resistance, such as in the case of melanoma.31,32 Resistance often stems from the inherent genomic instability of malignant tumors, which gives rise to drug-resistant clonal variants.33 However, in contrast to the majority of other MAPK-driven neoplasms, ECD progresses indolently and is a relatively genomically stable neoplasm. Previous data published in the literature advocate that the stable phenotype of the ECD histiocyte may be attributed to BRAF-associated oncogene-induced senescence.34 In the absence of rigorous mutagenesis and clonal divergence, resistance to targeted therapy in ECD appears to be an uncommon event, with BRAF V600 mutant patients exhibiting satisfactory and durable responses to long-term BRAF inhibition. Nevertheless, ECD histiocytes seem to be persistent, as the majority of BRAF-mutant patients relapse pursuant cessation of BRAF-targeted therapy.21
Constant, long-term inhibition of the MAPK axis is detrimental in achieving durable disease control. However, chronic BRAF inhibition is difficult to tolerate.22 In the long range, treatment-related toxicities and consequent dosage reductions may hinder the therapeutic efficacy of the drug, especially when CNS involvement is present. BRAF inhibitor monotherapy is also associated with the emergence of secondary malignancies.35,36 This phenomenon of paradoxical oncogenesis is attributed to the dimerization of wildtype RAF isoforms35,37 and the selection of RAS-mutant malignant cells on BRAF inhibitor therapy,36,38,39 resulting in over-activation of the MAPK axis. Nonetheless, evidence suggests that this might be circumvented by utilizing a double BRAF/MEK blockade.40
Herein, we demonstrate the rationale of utilizing the dual blockade approach as an advanced line of therapy in CNS involved ECD in the settings of BRAF inhibitor therapy insufficiency. Several plausible mechanisms may underlie the rationale for such an approach.
One possible explanation for disease progression on monotherapy is the emergence of resistance, which better fits in the case of patient 1—who initially responded and then progressed on Vemurafenib. Numerous biological mechanisms elucidating how resistance to BRAF inhibition emerges were described in the medical literature. Among them are upregulation of upstream elements,41 secondary mutations to the BRAF gene,42 emergence of alternatively spliced inhibitor-resistant BRAF isoforms,43 ERK re-activation in a BRAF-independent fashion,44–46 silencing of negative MAPK regulators,47 and signal transduction via a parallel cascade, such as the IGF-1R/AKT/PI3K/mTOR pathway.48 The mechanism by which BRAF-mutant ECD patients progress on BRAF inhibitor therapy remains unknown. However, given the genomic stability of ECD, it is plausible that such progression occurs due to the upregulation of the mutant BRAF on the protein level, rather than the emergence of a second alteration that confers resistance to BRAF inhibition. One possible mechanism fitting the ECD scenario was previously postulated and described by Poulikakos and Lito et al.40,49 The V600E-mutant BRAF functions as a RAS-independent monomer that is inhibitor sensitive. Continuous inhibition of this element decreases downstream ERK signaling, relieves the ERK-dependent feedback inhibition of RAS, thus promoting the formation of RAS-dependent, inhibitor-resistant V600E BRAF dimers. In agreement with this premise, treatment of BRAF wildtype ECD patients with a BRAF inhibitor would result in paradoxical activation of RAS and hyperactivation of ERK, thus promoting disease progression—a finding previously described by Cohen-Aubart et al.20 The case of patient 2 may reflect a scenario of suboptimal response to Vemurafenib monotherapy. Such response may be secondary to inadequate drug penetrance to the brain. Previous studies highlight the poor CNS penetration of Vemurafenib.25,26 Patient 2 presented with severe brainstem involvement with exceptionally high FDG uptake on PET/CT studies. As such full-dose Vemurafenib may have been insufficient to generate a complete response in all the lesions. Patient 3 exhibited monotherapy-related skin toxicities that necessitated dose reduction. These may have caused suboptimal CNS response and progression on therapy secondary to inadequate drug penetrance to the brain as well. In this particular case, monotherapy-related toxicities prevented reaching sufficient doses. In this scenario, the dual blockade approach provides a dual benefit: both increased efficacy and decreased toxicity.
Among the limitations of this descriptive study are its small cohort, retrospective design, and lack of control group. Additionally, PET/CT data were available in only 2 time points: prior to biological therapy and following dual therapy. As such, the marked effect of therapy as seen on PET/CT cannot be attributed to either the monotherapy or the dual therapy, but to the entire therapeutic scheme as a whole.
In conclusion, the dual pharmacological blockade of both BRAF and MEK may be superior to monotherapy for the chronic management of BRAF V600 mutant ECD patients who suffer from CNS disease. Dual therapy may be considered as a feasible advanced line of therapy in distinct settings such as disease progression on BRAF inhibition, suboptimal response to BRAF inhibition, or inability to receive effective doses of a BRAF inhibitor. Further studies exploring whether monotherapy inadequacy in ECD stems from poor drug penetrance to the brain, therapy-induced auto-adaptations of the MAPK axis, or alternatively from the emergence of secondary mutations in ECD are needed.
Whether upfront dual BRAF/MEK inhibition should be considered as first-line therapy in selected cases of ECD remains a topic of debate. On the one hand, the profound impairment of proliferative signaling provided by the combination therapy might effectively hinder disease progression. As such, one could argue in favor of such a bold strategy in cases where swift control of an exceedingly aggressive disease is necessary. Additionally, the BRAF inhibitor-associated risk of paradoxical oncogenesis is lessened upon using combined inhibition.40 On the other hand, one should consider the fallbacks of such a strategy. First, in the majority of cases, a single agent proves sufficient in achieving disease control and as such, the addition of a second drug upfront seems redundant. Moreover, given the chronic-targeted treatment foreseen in ECD, adding a second drug would potentially expose the patient to additional adverse effects, as well as to a substantial financial burden. Recently, a phase II clinical trial designed to evaluate the efficacy and safety of the BRAF inhibitor Dabrafenib and the MEK inhibitor Trametinib as a combination therapy in patients with BRAF V600E positive ECD was announced at MD Anderson Cancer Center (NCT03794297).
No doubt, elucidating the mechanisms of disease progression on inhibitor therapy as well as the efficacy and suitability of BRAF/MEK inhibition in histiocytic neoplasms may provide the data required to tailor advanced line therapies for these patients.
Funding
This work (O.A.W,. and E.L.D.) supported by the National Institutes of Health/National Cancer Institute Core Grant [P30 CA008748].
Authorship Contributions
R.D.M. and O.S. oversaw the medical management of the patients and designed the research. R.D.M. wrote the paper. R.D.M. and R.W. were responsible for data acquisition and figure design. J.L. performed the MRI-based neuro-radiological assessment of the patients, L.D. and D.G. overviewed and quantified data from the PET/CT scans of the patients, and E.L.D, O.A.W, O.R.H., and S.S. provided expert consultation as well as critical revision of the manuscript for important intellectual content. Supervision: O.S.
Conflict of interest statement. Dr. Diamond discloses unpaid support from Third Rock Ventures, outside the submitted work.
References
- 1. Haroche J, Cohen-Aubart F, Charlotte F, et al. The histiocytosis Erdheim-Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol. 2015;11(9):1033–1042. [DOI] [PubMed] [Google Scholar]
- 2. Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, et al. Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore). 1996;75(3):157–169. [DOI] [PubMed] [Google Scholar]
- 3. Esmaeli B, Ahmadi A, Tang R, Schiffman J, Kurzrock R. Interferon therapy for orbital infiltration secondary to Erdheim-Chester disease. Am J Ophthalmol. 2001;132(6):945–947. [DOI] [PubMed] [Google Scholar]
- 4. Arnaud L, Hervier B, Néel A, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778–2782. [DOI] [PubMed] [Google Scholar]
- 5. Hervier B, Arnaud L, Charlotte F, et al. Treatment of Erdheim-Chester disease with long-term high-dose interferon-α. Semin Arthritis Rheum. 2012;41(6):907–913. [DOI] [PubMed] [Google Scholar]
- 6. Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen-Aubart F, Maksud P, Saadoun D, et al. Variability in the efficacy of the IL1 receptor antagonist anakinra for treating Erdheim-Chester disease. Blood. 2016;127(11):1509–1512. [DOI] [PubMed] [Google Scholar]
- 8. Dagna L, Corti A, Langheim S, et al. Tumor necrosis factor α as a master regulator of inflammation in Erdheim-Chester disease: rationale for the treatment of patients with infliximab. J Clin Oncol. 2012;30(28):e286–e290. [DOI] [PubMed] [Google Scholar]
- 9. Arnaud L, Gorochov G, Charlotte F, et al. Systemic perturbation of cytokine and chemokine networks in Erdheim-Chester disease: a single-center series of 37 patients. Blood. 2011;117(10):2783–2790. [DOI] [PubMed] [Google Scholar]
- 10. Diamond EL, Durham BH, Haroche J, et al. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. [DOI] [PubMed] [Google Scholar]
- 12. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 13. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 14. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 15. Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495–1500. [DOI] [PubMed] [Google Scholar]
- 16. Haroche J, Cohen-Aubart F, Emile JF, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAFV600E-mutated Erdheim-Chester disease. J Clin Oncol. 2015;33(5):411–418. [DOI] [PubMed] [Google Scholar]
- 17. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatia A, Ulaner G, Rampal R, et al. Single-agent dabrafenib for BRAFV600E-mutated histiocytosis. Haematologica. 2018;103(4):e177–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oneal PA, Kwitkowski V, Luo L, et al. FDA approval summary: vemurafenib for the treatment of patients with Erdheim-Chester disease with the BRAFV600 mutation. Oncologist. 2018;23(12):1520–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohen Aubart F, Emile JF, Maksud P, et al. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180(1):150–153. [DOI] [PubMed] [Google Scholar]
- 21. Cohen Aubart F, Emile JF, Carrat F, et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood. 2017;130(11):1377–1380. [DOI] [PubMed] [Google Scholar]
- 22. Blank CU, Larkin J, Arance AM, et al. Open-label, multicentre safety study of vemurafenib in 3219 patients with BRAFV600 mutation-positive metastatic melanoma: 2-year follow-up data and long-term responders’ analysis. Eur J Cancer. 2017;79:176–184. [DOI] [PubMed] [Google Scholar]
- 23. Lloyd-Lavery A, Hodgson T, Coupe N, et al. Delayed oral toxicity from long-term vemurafenib therapy. Br J Dermatol. 2016;174(5):1159–1160. [DOI] [PubMed] [Google Scholar]
- 24. Sloot S, Fedorenko IV, Smalley KS, Gibney GT. Long-term effects of BRAF inhibitors in melanoma treatment: friend or foe? Expert Opin Pharmacother. 2014;15(5):589–592. [DOI] [PubMed] [Google Scholar]
- 25. Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaFV600E inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murrell J, Board R. The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: opportunities and unanswered questions. Cancer Treat Rev. 2013;39(8):833–838. [DOI] [PubMed] [Google Scholar]
- 27. Mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim-Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. [DOI] [PubMed] [Google Scholar]
- 29. Johanns TM, Ferguson CJ, Grierson PM, Dahiya S, Ansstas G. Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF-mutated high-grade glioma. J Natl Compr Canc Netw. 2018;16(1):4–10. [DOI] [PubMed] [Google Scholar]
- 30. Khunger A, Khunger M, Velcheti V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: clinical evidence and experience. Ther Adv Respir Dis. 2018;12:1753466618767611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solit DB, Rosen N. Towards a unified model of RAF inhibitor resistance. Cancer Discov. 2014;4(1):27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang GA, Polsky D. Mutational heterogeneity in melanoma: an inconvenient truth. J Invest Dermatol. 2015;135(12):2913–2918. [DOI] [PubMed] [Google Scholar]
- 34. Cangi MG, Biavasco R, Cavalli G, et al. BRAFV600E-mutation is invariably present and associated to oncogene-induced senescence in Erdheim-Chester disease. Ann Rheum Dis. 2015;74(8):1596–1602. [DOI] [PubMed] [Google Scholar]
- 35. Boussemart L, Girault I, Malka-Mahieu H, et al. Secondary tumors arising in patients undergoing braf inhibitor therapy exhibit increased BRAF-CRAF heterodimerization. Cancer Res. 2016;76(6):1476–1484. [DOI] [PubMed] [Google Scholar]
- 36. Callahan MK, Rampal R, Harding JJ, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med. 2012;367(24):2316–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gibney GT, Messina JL, Fedorenko IV, Sondak VK, Smalley KS. Paradoxical oncogenesis—the long-term effects of BRAF inhibition in melanoma. Nat Rev Clin Oncol. 2013;10(7):390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Danysh BP, Rieger EY, Sinha DK, et al. Long-term vemurafenib treatment drives inhibitor resistance through a spontaneous KRAS G12D mutation in a BRAF V600E papillary thyroid carcinoma model. Oncotarget. 2016;7(21):30907–30923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22(5):668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J, Yao Z, Jonsson P, et al. A secondary mutation in BRAF confers resistance to RAF inhibition in a BRAF. V600E-mutant brain tumor. Cancer Discov. 2018;8(9):1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480(7377):387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goetz EM, Ghandi M, Treacy DJ, Wagle N, Garraway LA. ERK mutations confer resistance to mitogen-activated protein kinase pathway inhibitors. Cancer Res. 2014;74(23):7079–7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen CH, Kim SH, Trousil S, et al. Loss of cohesin complex components STAG2 or STAG3 confers resistance to BRAF inhibition in melanoma. Nat Med. 2016;22(9):1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18(6):683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]