Abstract
Objective
To describe the clinical profiles and risk factors for critical illness in hospitalized children and adolescents with coronavirus disease 2019 (COVID-19).
Study design
Children 1 month to 21 years of age with COVID-19 from a single tertiary care children's hospital between March 15 and April 13, 2020 were included. Demographic and clinical data were collected.
Results
In total, 67 children tested positive for COVID-19; 21 (31.3%) were managed as outpatients. Of 46 admitted patients, 33 (72%) were admitted to the general pediatric medical unit and 13 (28%) to the pediatric intensive care unit (PICU). Obesity and asthma were highly prevalent but not significantly associated with PICU admission (P = .99). Admission to the PICU was significantly associated with higher C-reactive protein, procalcitonin, and pro-B type natriuretic peptide levels and platelet counts (P < .05 for all). Patients in the PICU were more likely to require high-flow nasal cannula (P = .0001) and were more likely to have received Remdesivir through compassionate release (P < .05). Severe sepsis and septic shock syndromes were observed in 7 (53.8%) patients in the PICU. Acute respiratory distress syndrome was observed in 10 (77%) PICU patients, 6 of whom (46.2%) required invasive mechanical ventilation for a median of 9 days. Of the 13 patients in the PICU, 8 (61.5%) were discharged home, and 4 (30.7%) patients remain hospitalized on ventilatory support at day 14. One patient died after withdrawal of life-sustaining therapy because of metastatic cancer.
Conclusions
We describe a higher than previously recognized rate of severe disease requiring PICU admission in pediatric patients admitted to the hospital with COVID-19.
Keywords: COVID-19, critical care, children, SARS CoV-2
Abbreviations: ARDS, Acute respiratory distress syndrome; COVID-19, Coronavirus disease 2019; ED, Emergency department; ICU, Intensive care unit; PICU, Pediatric intensive care unit; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
See related article, p 199
The first reports of novel coronavirus disease 2019 (COVID-19) noted the infrequency of disease in children with one of the earliest studies including only 9 children under 14 years of age among 1011 total patients (0.89%).1 , 2 Since then, multiple reports have described children affected by COVID-19 with varying degrees of severity.3, 4, 5
Epidemiologic studies have consistently demonstrated that children are at lower risk of developing severe symptoms or critical illness compared with adults.5 , 6 In a study of 2143 pediatric patients in China with confirmed (n = 731) or suspected (n = 1412) COVID-19, over one-half had only mild illness, and <1% had severe or critical illness.5 In another study from China describing 36 children, no severe or critically ill case was observed.6 The only study to describe children requiring admission to a pediatric intensive care unit (PICU) was a study from Spain of 365 children tested for COVID-19.7 The authors found that 41 (11%) children tested had virus detected; 25 of 41 (61%) required hospitalization, and 4 of 41 (16%) were admitted to the PICU. Details of clinical characteristics were not described.
Overall, the incidence of critical illness in children with COVID-19 is not well known, with limited data on possible associated risk factors. The objectives of this study were (1) to describe the clinical profile of critically ill children with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection admitted to our tertiary care facility and (2) to study the risk factors associated with critical illness.
Methods
The Albert Einstein College of Medicine Institutional Review Board reviewed and approved this retrospective review of all children age 1 month to 21 years admitted at the Children's Hospital at Montefiore between March 15 and April 13, 2020 with a laboratory confirmed SARS-CoV-2 infection. Infection was confirmed by real-time reverse transcription polymerase chain reaction testing a specimen using nasopharyngeal swab on one of several different platforms adopted by the Clinical Microbiology Laboratory in an effort to increase testing availability (Abbott laboratories, Abbott Park, Illinois; Luminex Aries (Luminex Corporation, Austin, Texas); Cepheid Xpert Xpress, Sunnyvale, California and Hologic Panther Fusion, San Diego, California).
Demographic data, clinical signs and symptoms at presentation, laboratory and radiologic results, treatments, and outcomes on all pediatric patients admitted to the hospital during the study period were obtained from the electronic medical record (EPIC, Verona, Wisconsin). The decision to admit to the pediatric floor or intensive care unit (ICU) was at the discretion of treating physicians.
Acute respiratory distress syndrome (ARDS) was defined using Berlin criteria8 (PaO2/FiO2 ratio 200-300 as mild ARDS, 100-200 as moderate ARDS, and <100 as severe ARDS). Oxygenation index was calculated using the formula (fraction inspired oxygen × mean airway pressure) × 100/partial pressure of arterial oxygen. Management of ARDS was based on ARDSNet guideline of low tidal volume and limiting peak/plateau pressure under 30 cm of water.9 Sepsis, severe sepsis, and septic shock were defined as per the pediatric surviving sepsis guidelines.10 Acute kidney injury was defined using the Kidney Disease: Improving Global Outcomes classification based upon the change in serum level of creatinine and creatinine clearance.11 Virus-associated sepsis was defined as presence of ≥2 systemic inflammatory syndrome criteria, severe sepsis as sepsis with organ dysfunction or tissue hypoperfusion, and septic shock as severe sepsis with volume resistant hypotension.10 Obesity was defined as body mass index >30 kg/m2, and asthma was recorded if it was documented in the electronic medical record by a physician.
Statistical Analyses
Clinical data were presented as counts and percentage, mean and SD, and median and IQR. Comparison of means and medians was performed using the 2-sample Student t test or Wilcoxon rank-sum test, respectively. Categorical data were compared using Pearson χ2 or Fisher exact test if any expected cell size numbered <5. All tests were 2-tailed with a level of significance of P < .05. Statistical analyses were performed using STATA v 13.1 (StataCorp, College Station, Texas).
Results
From March 15 to April 13, a total 1747 children and adolescents visited the emergency department (ED); 194 were tested for SARS-CoV-2 infection. Test results were positive in 67 (34.5%) patients with 46 subsequent admissions to the hospital, with 13 (28.3%) patients requiring PICU care. In 21 patients, SARS-CoV-2 infection was confirmed but did not require hospitalization. As the number of patients screened for COVID-19 was restricted during the first weeks of the outbreak because of limited testing availability, the number of mildly symptomatic patients is not known, and, therefore, these 21 patients are not included in the analysis.
The median age of the hospitalized cohort was 13.1 (IQR 0.4, 19.3) years with a preponderance of male sex (31, 67.4%). The majority of patients were Hispanic/Latino (78.8%, P = .001), reflecting the demographics of the Bronx community. Median body mass index was 22.8 kg/m2 (IQR 17.6, 32.9). Eighty-four percent of patients admitted to the PICU were 11 years of age or older.
The most common symptoms at admission were cough (63%) and fever (60.9%). Patients reported a median duration of symptoms of 3 (IQR 1, 5) days prior to admission. No patient in this cohort reported travel to areas heavily affected by COVID-19 prior to symptom onset, but 20 (43.5%) reported a COVID-confirmed contact. Demographic and clinical comparison of the 46 patients admitted to the medical unit and PICU is presented in Table I . The only clinical symptom found to be significantly associated with PICU admission was shortness of breath (92.3% vs 30.3%, P < .001).
Table I.
Demographics and baseline characteristics of pediatric patients with COVID-19
| Clinical characteristics | Admitted to medical unit (n = 33) | Admitted to PICU (n = 13) | P value∗ | |
|---|---|---|---|---|
| Age (y) | 3.6 (0.1, 17.2) | 14.8 (11.6, 15.9) | .19 | |
| Sex | Male, n (%) | 23 (69.6) | 8 (61.5) | .73 |
| Female, n (%) | 10 (30.4) | 5 (38.5) | ||
| Race | White, n (%) | 1 (3) | 2 (15.4) | .001 |
| Black, n (%) | 3 (9.1) | 2 (15.4) | ||
| Latino, n (%) | 26 (78.8) | 3 (23.1) | ||
| Other, n (%) | 3 (9.1) | 6 (46.2) | ||
| Weight (kg) | 40 (5.3, 99.6) | 56.4 (41, 78.1) | .25 | |
| BMI (kg/m2) | 30 (15.1, 35.7) | 23.5 (19.5, 49.0) | .22 | |
| Comorbidities | Obesity, n (%) | 9 (27.3) | 3 (23.1) | .99 |
| BMI >35, n (%) | 8 (24.2) | 3 (23.1) | .99 | |
| BMI >40, n (%) | 5 (15.5) | 3 (23.1) | .67 | |
| BMI >50, n (%) | 3 (9.1) | 1 (7.6) | .99 | |
| Asthma, n (%) | 8 (24.2) | 3 (23.1) | .99 | |
| Immunosuppressed, n (%) | 1 (3) | 1 (7.6) | .47 | |
| Seizure disorder, n (%) | 1 (3) | 3 (23.1) | .06 | |
| Malignancy, n (%) | 0 (0) | 1 (7.6) | .27 | |
| Heart disease, n (%) | 0 (0) | 1 (7.6) | .27 | |
| Presenting symptoms/history | T-max by history (°C) | 38.7 (38, 38.9) | 38.9 (38.9, 38.9) | .4 |
| Cough, n (%) | 19 (57.6) | 9 (69.2) | .52 | |
| Shortness of breath, n (%) | 10 (30.3) | 12 (92.3) | <.001 | |
| Known sick contact, n (%) | 14 (42.4) | 6 (46.2) | .99 | |
| Travel history | 0 (0) | 0 (0) | – | |
| Symptom (d) median (IQR) | 2.5 (1, 5.5) | 3 (2, 5) | .44 | |
| History of ibuprofen use, n (%) | 5 (15.2) | 3 (23.1) | .67 | |
| Vital signs | Adm temp (°C) median (IQR) | 37.9 (37, 38.7) | 37.1 (36.9, 38.2) | .32 |
| T-max (°C) median (IQR) | 39 (37.9, 39.5) | 38.9 (37.9, 40.1) | .46 | |
| Hosp d of T-max median (IQR) | 1 (1, 2) | 2 (1, 2) | .32 | |
| Adm HR/min mean (SD) | 136 (36) | 117 (27.1) | .1 | |
| Adm SpO2 (%) median (IQR) | 98 (95,100) | 98 (97, 100) | .9 | |
| Adm SBP mm Hg mean (SD) | 113.8 (17.6) | 111.6 (17.4) | .71 | |
| Adm DBP mm Hg mean (SD) | 66 (12.6) | 68 (12.1) | .64 | |
Adm, admission; BMI, body mass index; DBP, diastolic blood pressure; HD, hospital day; SBP, systolic blood pressure; SpO2, arterial oxygen saturation; T-max, temperature maximum.
Data expressed in number (percentages), mean (SD), or median (IQR).
Comparison of patients admitted to medical unit vs patients admitted to PICU using χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
Obesity was present in 14 (30.4%) admitted patients and asthma in 11 (24.4%) but neither was significantly associated with the need for PICU admission (P < .99 for both). A higher proportion of patients in the PICU had a preexisting history of seizure disorder (3 patients, 25%) compared with just 1 patient (3%) admitted to the medical unit. There was no significant difference in the usage of ibuprofen prior to hospitalization among patients admitted to medical unit compared with those admitted to the PICU.
Laboratory Test Results
Patients admitted to the PICU had lower platelet counts on admission compared with patients admitted to the medical unit (P = .03) (Table II ). Conversely, levels of inflammatory markers including C-reactive protein, procalcitonin, and pro-brain natriuretic peptide were significantly elevated in patients admitted to PICU compared with those admitted to the medical unit (P < .05 for all). Patients admitted to the PICU also had higher blood urea nitrogen (13 [IQR 10, 16] vs 10 [IQR 7, 11], P = .03) and trended toward having higher creatinine levels (0.7 [IQR 0.4, 1.1] vs 0.5 [IQR 0.2, 0.8], P = .12) on admission.
Table II.
Admission laboratory test results and imaging studies in patients with COVID-19 admitted to medical unit and PICU
| Parameters | Admitted to medical unit (n = 33) | Admitted to ICU (n = 13) | P value∗ |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.2 (10.8, 15.5) | 12.4 (12, 15.2) | .62 |
| Platelets (k/uL) | 244 (195, 361) | 194 (138, 238) | .03 |
| WBC (k/uL) | 7.0 (5.4, 11.8) | 9.7 (6.9, 17.1) | .08 |
| ALC (cells/uL) | 1377 (536, 2232) | 1184 (880, 2534) | .92 |
| AST (U/L) | 75 (35, 112) | 36 (32, 40) | .02 |
| ALT (U/L) | 51.5 (25.5, 132.5) | 31.5 (11.5, 45) | .10 |
| Total bilirubin (mg/dL) | 0.4 (0.3, 0.8) | 0.5 (0.1, 0.6) | .053 |
| BUN (mg/dL) | 10 (7, 11) | 13 (10, 16) | .03 |
| Creatinine (mg/dL) | 0.5 (0.2, 0.8) | 0.7 (0.4, 1.1) | .12 |
| C-reactive protein (mg/dL) | 1.9 (0.5, 4.3) | 6.6 (2.0, 11.8) | .02 |
| Peak C-reactive protein (mg/dL) | 3.3 (0.5, 6.6) | 12.1 (2, 19.8) | .06 |
| Procalcitonin (ng/mL) | 0.1 (0.1, 0.2) | 11.5 (1.4, 21.5) | .03 |
| Pro-BNP (pg/mL) | 60 (60, 85) | 1112 (1051, 1734) | .01 |
| Troponin (ng/mL) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | .13 |
| CPK (U/L) | 183 (100, 379) | 199 (109, 302) | .95 |
| Lactate (mmol/L) | 1.9 (1.5, 2.1) | 1.5 (1.2, 1.5) | .36 |
| D-dimer (ug/mL FEU) | 0.8 (0.3, 1.1) | 0.8 (0.7, 2.3) | .36 |
| LDH (U/L) | 417 (402, 765) | 420 (378, 569) | .84 |
| +Blood culture admission | 1 (3.0) | 3 (23.1) | .10 |
| +Respiratory culture | 0 (0) | 3 (23.1) | .08 |
| +Urine culture | 2 (6.1) | 3 (23.1) | .27 |
| Chest radiograph performed | 19 (57.6) | 12 (92.3) | .04 |
| Normal CXR | 4 (21.1) | 2 (16.7) | <.99 |
| Bilateral opacities | 12 (63.2) | 8 (66.7) | <.99 |
| Unilateral opacity | 3 (15.8) | 2 (16.7) | <.99 |
| Pleural effusion | 0 (0) | 1 (8.3) | .39 |
| LV dysfunction by echocardiogram | 0/3 (0) | 2/4 (50) | .43 |
ALC, absolute lymphocyte count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CPK, creatine phosphokinase; CXR, chest radiograph; LDH, lactate dehydrogenase; LV, left ventricular; pro-BNP, pro-brain natriuretic peptide; WBC, white blood cell count.
Comparison of patients admitted to floor vs patients admitted to PICU using χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
Chest radiographs were performed more often in patients admitted to the PICU compared with those admitted to the medical unit (92.3% vs 57.6%, respectively, P = .04) and showed opacities in 10 of 13 of those admitted to the PICU and in 19 of 33 admitted to the medical unit. There was no difference in the finding of unilateral vs bilateral opacities among patients in the medical unit compared with the PICU (P < .99). One patient admitted to the PICU also had a pleural effusion on admission requiring drainage as he was requiring high ventilatory support.
Management and Clinical Outcomes
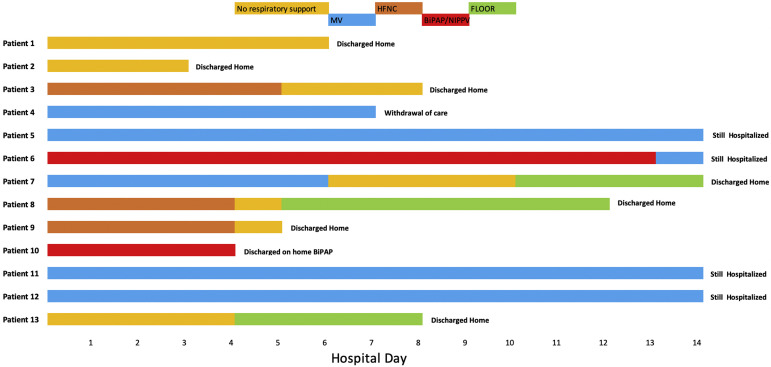
All 33 patients admitted to the medical unit except 1 were discharged home with a median length of stay of 3 (IQR 2, 4) days (Table III ). The one exception was a 15-year-old patient who was transferred to the PICU on day 1 of hospitalization for worsening hypoxemia. He required high-flow nasal cannula oxygen support for 4 days before being discharged home on day 11. Details of the 13 patients admitted to the PICU are shown in the Figure (available at www.jpeds.com) and Table IV (available at www.jpeds.com). The majority of patients initially admitted to the PICU (8 of 13, 61.5%) were discharged home with a median length of stay of 7 (IQR 6, 11) days. Four patients remain hospitalized in the PICU at day 14. In 1 patient with metastatic disease from underlying malignancy, the family chose to withdraw care (Table IV). All patients admitted to the PICUs had signs of systemic inflammatory syndrome, and 3 (23.1%) developed septic shock requiring vasopressor support and fluid resuscitation.
Table III.
Clinical outcomes and therapies administered to patients with COVID-19
| Parameters no. (%) or median (IQR) | Admitted to floor (n = 33) | Admitted to PICU (n = 13) | P value∗ |
|---|---|---|---|
| ARDS | 0 (0) | 10 (76.9) | <.0001 |
| Mild ARDS | 0 (0) | 4 (30.8) | .004 |
| Moderate ARDS | 0 (0) | 5 (38.5) | .001 |
| Severe ARDS | 0 (0) | 1 (7.7) | .28 |
| Severe sepsis | 0 (0) | 4 (30.8) | .004 |
| Septic shock | 0 (0) | 3 (23.1) | .019 |
| Vasopressor | 0 (0) | 2 (15.4) | .11 |
| AKI | 0 (0) | 5 (38.5) | .001 |
| RRT | 1 (3)† | 1 (7.7) | .99 |
| Prone positioning | 0 (0) | 1 (7.7) | .33 |
| Medical therapy | |||
| Hydroxychloroquine | 6 (18.2) | 4 (30.8) | .44 |
| Remdesivir | 2 (7.1) | 6 (46.2) | .007 |
| Methylprednisolone | 5 (15.2) | 6 (46.2) | .051 |
| Antibiotics <48 h | 18 (58.1) | 7 (53.9) | .80 |
| Antibiotics >48 h | 10 (33.3) | 4 (30.8) | .99 |
| Respiratory support | |||
| NC | 9 (27.3) | 4 (30.8) | .99 |
| Duration of NC | 5.5 (2, 11) | 1.5 (1.0, 3.5) | .10 |
| HFNC | 1 (3.1) | 7 (53.9) | .0001 |
| Duration of HFNC | 0 (0, 0) | 4 (1, 5) | .82 |
| Non-IMV | 0 (0.0) | 2 (15.4) | .08 |
| Duration of Non-IMV | 0 (0, 0) | 8 (3, 13) | .005 |
| IMV | 0 (0.0) | 6 (46.2) | <.0001 |
| Duration of IMV | 0 (0, 0) | 9 (7, 14) | .004 |
| Outcomes | |||
| Hospital stay | 3 (2, 4) | 7 (6, 11) | .19 |
| PICU stay | – | 7 (5, 8) | – |
| Survivors | 33 (100) | 12 (92.3) | .32 |
AKI, acute kidney injury; HFNC, high flow nasal cannula; IMV, Invasive mechanical ventilation; NC, nasal cannula; RRT, renal replacement therapy.
Comparison of patients admitted to floor vs patients admitted to PICU using χ2 test, Fisher exact test, or Wilcoxon rank-sum test.
Chronic home RRT for end stage renal disease.
Figure.
Outcomes for individual patients in the PICU. Respiratory support modalities as a function of time (in hospitalization days) presented by patient. Light orange color denotes no respiratory support. Blue color denotes mechanical ventilation (MV). Dark orange color denotes high flow nasal cannula (HFNC). Red color denotes bilevel positive airway pressure/non-invasive positive pressure ventilation (BiPAP/NIPPV). Green color denotes transfer to the pediatric floor.
ARDS and Mechanical Ventilation
ARDS was documented in 10 of the 13 patients (76.9%) admitted to the PICU compared with none on the medical unit (Table IV). Seven (53.9%) were supported with high-flow nasal cannula, 4 of which required escalation to invasive mechanical ventilation. Two patients (15.4%) required noninvasive ventilatory support, of which one required escalation to invasive mechanical ventilation. A total of 6 patients (46.2%) required invasive ventilatory support, for a median duration of invasive mechanical ventilatory support of 9 (IQR 7, 14) days (Table III). These patients had moderate to severe hypoxemia diagnostic of ARDS, with a median PaO2/FiO2 ratio on day 1 of 178 which worsened to a PaO2/FiO2 ratio of 105 by day 3. Worsening hypoxemia and lung disease also was reflected in the escalation of ventilatory support by day 3 (Table V; available at www.jpeds.com). Lung protective strategies for mechanical ventilation in ARDS were insufficient in these patients by day 3 with a median PEEP requirement of 10 cm water, resulting in a median peak pressure of 35 cm water. One patient was successfully extubated after 6 days of ventilatory support, and 1 patient died after withdrawal of care. The remaining 4 patients remained on mechanical ventilatory support on day 14 of hospitalization.
Treatment
Hydroxychloroquine was administered to 30.8% of patients admitted to the PICU compared with 18.2% of those admitted to the medical unit (Table IV). Compassionate use of Remdesivir was administered more often to patients in the PICU compared with the medical unit (46% vs 7%, P = .007), and there was a trend toward increased use of methylprednisolone in patients admitted to the PICU (46% vs 15%, P = .051). There was no age-related difference in patients who received Remdesivir or hydroxychloroquine both in the medical unit and in the PICU. Empiric antibiotics were begun on 25 (54%) patients, with 14 (56%) patients continuing antibiotic therapy; there was no significant difference among patients cared for in the medical unit vs PICU in terms of antibiotic usage and duration. Acute kidney injury was diagnosed in 5 (38.4%) patients admitted to the PICU, of which 4 resolved with volume resuscitation. One patient (7.7%) required renal replacement therapy for severe fluid overload associated with septic shock and multiorgan failure.
Discussion
We describe 46 hospitalized children with SARS-CoV-2 infection diagnosed in the first weeks of the New York City pandemic to add to the limited data on pediatric SARS-CoV-2 infection. As expected, patients admitted to the PICU were noted to have more severe symptoms and markers of inflammatory response. However, as this cohort is from a unique, dense, urban setting, we note findings not previously observed in other hospitalized cohorts of children with COVID-19. Our patients had a higher rate of PICU admission per hospitalization (28.2%), which may be a reflection of a variety of social determinants that influence health outcomes. In previous studies,5, 6, 7 the ICU admission rate in children ranged from 1.7% to 16%.3 , 5 , 7 In adults, the ICU admission rates range from 5% to 32%.1 , 2 , 12, 13, 14, 15 Among PICU admissions, 84.6% were ≥11 years of age. The presence of comorbidities, including obesity, has been described to be one of the risk factors for critical illness with COVID-19 in children5; however, in our small sample, age and obesity were not associated with increased likelihood of PICU admission. The overall high prevalence of obesity could partially explain our higher PICU admission rate. However, obesity may also be a marker for other risk factors associated with an increased risk of critical illness, such as poverty.16 For example, Bronx County has the highest poverty rate of the New York City boroughs and is the least healthy county in New York State. Previous studies have demonstrated an association between social factors, such as poverty, and the increased risk of prolonged hospital stay as well as PICU admission for many different conditions.17, 18, 19, 20
Less than one-half of our patients had the history of a known sick contact, which is lower than previously reported.3 , 21 This high rate of community spread could be explained partially by the high population density in the Bronx, which is approximately 33 000 people per square mile, well above the national average of 90 people per square mile22 and approximately 10 times more dense than Wuhan, China. The lack of a known sick contact reported in our study may have implications for how healthcare providers identify and screen for potential cases.
Children are reported to have milder SARS-CoV-2 disease, but our findings suggest that a subset of pediatric patients develop severe disease requiring PICU admission. This subset had significantly higher markers of inflammation (CRP, pro-brain natriuretic peptide, procalcitonin) compared with patients in the medical unit. Inflammation likely contributed to the high rate of ARDS we observed, although serum levels of interleukin-6 and other cytokines linked to ARDS were not determined.
ARDS is reported in 3%-5.8% of all patients with COVID-19,2 , 5 in 17%-42% of patients with COVID-19 pneumonia,1 , 13 , 14 , 21 and in about 67% of patients requiring ICU care.12 Our rate of ARDS is higher than previously reported in children admitted to the hospital but is in line with that reported in critically ill adults at 67%.12 The need for mechanical ventilation was seen in 46% of our patients admitted to the PICU and 60% of patients with ARDS. These patients required high levels of ventilatory support because of worsening in oxygenation in the first few days of hospitalization. Our overall intubation rate (6 of 44, 13%) is higher than that reported by others: 1.7% to 10% in children3 , 7; and 2.3% to 12.5% in adults.1 , 2 , 13 , 14 , 21 Our PICU intubation rate of 46% (6 of 13 patients) is higher than reported ICU rates of 15%-47%1 , 13, 14, 15 but lower compared with rates reported in adults.23 , 24
Our study has several limitations. We present observational data from a single, unique, urban, academic medical center with a limited sample size, predisposing to type II error. However, as COVID-19 is a new disease with a dearth of literature in the pediatric population, our study sheds light by providing additional data from hospitalized children. Larger multicenter experience and mechanistic data are needed to identify predictors of severe disease.
Data Statement
Data sharing statement available at www.jpeds.com.
Acknowledgments
We thank Mark S. Teen, MD, Isabel F. Pesola, MD1, Roshan S. Patel, MD, Glenn E. Mann, MD, Agathe Streiff, MD, Valerie Ivanova, DO, Krupa Desai, MD, MSc, Lewis Diamond, MB, BCh, and Joanne Spaliaras, MD for data collection.
Footnotes
Supported by NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA (UL1TR001073) and the Departments of Anesthesiology and Pediatrics, Albert Einstein College of Medicine, Montefiore Medical Center, Children's Hospital at Montefiore. M.C. serves on the Editorial Board of The Journal of Pediatrics and is a member of the United States Preventive Services Task Force (USPSTF). This manuscript does not necessarily represent the views of the USPSTF. The other authors declare no conflicts of interest.
Appendix
Table IV.
Profile of 13 critically ill children in PICU with COVID-19
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 14 | 20 | 15 | 11 | 14 | 4 mo | 19 | 15 | 15 | 4 | 11 | 15 | 14 |
| Sex | F | M | F | M | M | F | M | M | M | M | M | F | F |
| Race | Black | Other | Other | Black | Other | Other | Other | Hispanic | Hispanic | White | White | Hispanic | Black |
| BMI (kg/m2) | 23.54 | 19.5 | 33.05 | 18.85 | 21.06 | 20 | 30.5 | 49.64 | 49.02 | 19.5 | 14.7 | 50.65 | 21 |
| Reason for PICU admission | DKA | DKA | PNA AHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | PNAAHRF | DKA |
| Comorbidities | None | T2DM | Obesity | Metastatic cancer | SeizuresAsthma | CHD | Obesity | T2DM | Obesity | SeizuresDCM | SeizuresQuadriparesis | HTNOSA | T2DM |
| WBC (k/uL) | 28.2 | 8.8 | 5.9 | 14.8 | 17.1 | 20.8 | 10 | 6.7 | 9.7 | 5.9 | 6.9 | 8.6 | 18.1 |
| ALC (k/uL) | 2.8 | 0.9 | 0.53 | 1.12 | 2.7 | 5.6 | 0.9 | 1.3 | 0.97 | 1.5 | 0.62 | 1.1 | 2.5 |
| CRP (mg/dL) | 2 | 1.2 | 3.4 | N | 11.8 | 3.3 | 8.1 | 5 | 14.4 | 9.2 | 1.5 | 33.3 | 1.1 |
| Procalcitonin (ng/mL) | N | N | N | N | 1.4 | 0.6 | N | N | 0.9 | N | N | 21.5 | 1.2 |
| Sepsis | N | N | N | N | Y | Y | N | N | N | Y | N | Y | N |
| AKI | Y | Y | N | N | Y | Y | N | N | N | N | Y | Y | Y |
| Respiratory support | None | None | HFNC | IMV | IMV | IMV | IMV | HFNC | HFNC | BIPAP | IMV | IMV | None |
| Remdesivir | N | N | N | N | Y | Y | N | Y | Y | N | Y | Y | N |
| HCQ | N | N | N | N | N | N | Y | Y | Y | Y | N | N | N |
| Steroids | N | N | N | N | Y | N | Y | Y | Y | N | Y | Y | N |
| Days resp support | 0 | 0 | 5 | 5 | 14 | 14 | 10 | 4 | 4 | 4 | 14 | 14 | 0 |
| PICU LOS | 2 | 3 | 5 | 5 | >14 | >14 | 10 | 5 | 4 | 4 | >14 | >14 | 5 |
| HLOS (d) | 3 | 4 | 7 | 5 | >14 | >14 | 14 | 12 | 5 | 4 | >14 | >14 | 7 |
| Outcome | Home | Home | Home | Death | Hosp | Hosp | Home | Home | Home | Home | Hosp | Hosp | Home |
Bolded numbers are for patients with Obesity (BMI>30 kg/m2).
AKI, acute kidney injury; ALC, absolute lymphocyte count; ASD, atrial septal defect; BiPAP, bilevel positive pressure ventilation; BMI, body mass index; CRP, C-reactive protein; DKA, diabetic ketoacidosis; DM, diabetes mellitus; F, female; HD, hospital day; HFNC, high flow nasal cannula; HLOS: hospital length of stay; Hosp, hospitalized; HTN, hypertension; IMV, invasive mechanical ventilation; LOS, length of stay; M, male; MSSA, methicillin-sensitive Staphylococcus aureus; N, no; NAFLD, nonalcoholic fatty liver disease; OSA, obstructive sleep apnea; PDA, patent ductus arteriosus; PNA, pneumonia; RRT, renal replacement therapy; T2DM, type 2 diabetes mellitus; UTI, urinary tract infection; WBC, white blood cell count; Y, yes.
Table V.
Oxygenation and mechanical ventilation parameters
| Parameters median (IQR) | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| PaO2/FiO2 ratio | 178 (130, 219) | 175 (130, 219) | 105 (105, 160) |
| Oxygenation index | 7.7 (6.7, 11.6) | 10 (8.8, 12) | 10.6 (9, 15) |
| Tidal volume (mL) | 336 (231, 424) | 297 (260, 350) | 264 (194, 365) |
| Tidal volume (mL/kg) | 7.8 (6.4, 8.3) | 6.6 (5.8, 7.5) | 6.4 (5.5, 7) |
| PEEP (cm of water) | 10 (7.75, 11.5) | 10 (8, 12) | 10 (8.5, 12) |
| MAP (cm of water) | 16 (15, 17) | 16 (15, 24) | 17 (17, 24) |
| Peak pressure (cm of water) | 29.5 (25.5, 40.25) | 30 (27, 37) | 35.5 (25.25, 37.5) |
| FiO2 | 1.0 (0.7, 1.0) | 0.45 (0.4, 0.5) | 0.57 (0.55, 0.75) |
FiO2, oxygen concentration; MAP, mean airway pressure; PaO2, partial pressure of oxygen; PEEP, positive end expiratory pressure.
Data expressed as median (IQR).
Supplementary Data
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W., Zhang Q., Chen J., Xiang R., Song H., Shu S. Detection of 2019-nCoV in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145:e20200702. [Google Scholar]
- 6.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagarro A., Epalza C., Santos M., Sanz-Santaeufemia F., Otheo E., Moraleda C. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020:e201346. doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Weiss S.L., Peters M.J., Alhazzani W., Agus MS D., Flori H R., Nadel S. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 11.Kellum J.A., Lameire N., KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H., Harris K.M., Gordon-Larsen P. Life course perspectives on the links between poverty and obesity during the transition to young adulthood. Popul Res Policy Rev. 2009;28:505–532. doi: 10.1007/s11113-008-9115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan H.T., Foster C.M., Bratton S.L. Social factors associated with prolonged hospitalization among diabetic children. Pediatrics. 2002;109:40–44. doi: 10.1542/peds.109.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Keet C.A., Matsui E.C., McCormack M.C., Peng R.D. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol. 2017;140:822–827. doi: 10.1016/j.jaci.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunwell J.R., Travers C., Fitzpatrick A.M. Inflammatory and comorbid features of children admitted to a PICU for status asthmaticus. Pediatr Crit Care Med. 2018;19:e585–e594. doi: 10.1097/PCC.0000000000001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrist E., Riley C.L., Brokamp C., Taylor S., Beck A.F. Neighborhood poverty and pediatric intensive care use. Pediatrics. 2019;144:e20190748. doi: 10.1542/peds.2019-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.census.gov/quickfacts/fact/table/bronxcountybronxboroughnewyork/IPE120218#IPE120218
- 22.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.