Abstract
COVID-19 is a coronavirus responsible for a global pandemic that started in China in December 2019 and has quickly spread to almost all countries. Approximately 2% of cases are diagnosed in children. There is increasing evidence for transmission by asymptomatic or presymptomatic adults and children. The clinical features do not differ from those of other respiratory viral infections, although rare cases manifest an unusual rash involving the digits. Disease is generally mild in children but deaths have been reported. Risk groups for severe disease in children are yet to be delineated. All treatments remain experimental.
Keywords: Coronavirus, COVID-19, SARS-CoV-2
Introduction
COVID-19 is a respiratory infection caused by SARS-CoV-2, a single stranded RNA betacoronavirus first detected in Wuhan, China in December, 2019[1]. Like all human coronaviruses, this virus almost certainly originated in bats and spread to humans via an animal, in this case possibly the pangalon[2] at a live animal market. Four other coronavirus (229E, OC43, NL63, and HKU1) circulate worldwide year-round but with increased case numbers in winter and spring in temperate climates [1]. These four viruses typically cause mild respiratory tract infections in children and adults. Severity is somewhat comparable to rhinovirus infection; paediatric hospitalization is far less common than with influenza or respiratory syncytial virus (RSV) infection. SARS-CoV-1, presumed to spread to humans from civet cats, circulated in 2003 only with 9% mortality [3]. Middle East Respiratory syndrome (MERS), thought to spread to humans from camels, has circulated primarily in Saudi Arabia since 2012 with a startling 34% mortality rate [3]. The limited circulation of the latter two viruses is presumed to be because asymptomatic or mild infection is rare. Infected people are too ill even early in the course of infection to go out in public so generally infect only household contacts or health care workers (HCWs). Conversely, with SARS-CoV-2, many infected people are asymptomatic or presymptomatic yet have a viral load in the upper respiratory tract comparable to that of symptomatic patients [4], [5]. This renders contact tracing problematic, enabling the virus to spread to at least 187 of the 195 countries on the planet by May 11, 2020. It is difficult to determine the effectiveness of school closures, bans on public gatherings, universal use of masks and other public health policies on spread of the virus due to variations in testing algorithms, the date on which SARS-CoV-2 became endemic in a country, the proportion of people already infected when policies were put in place and the level of compliance with these policies.
Transmission of SARS-CoV-2
Transmission of SARS-CoV-2 is thought to occur only by contact with either fomites or large droplets. Each infected person infects an estimated 5.7 others ((the R0 (pronounced R naught) of the virus)) [6]. In an experiment with RSV, infection occurred with inoculation of the eyes or nose but not the mouth of volunteers [7]. These two routes of infection likely account for all cases of COVID-19 although it remains possible that entry of SARS-CoV-2 via the mouth leads to infection.
A vital measure to prevent spread by contact is to ensure that one’s hands are clean before touching one’s face after touching a person (since anyone can have asymptomatic COVID-19) or when in an environment where there could be SARS-CoV-2 on fomites (such as doorknobs or handrails). Although SARS-CoV-2 can be detected for days on fomites [8], it is not known for how long this is viable or transmittable virus. Virus can sometimes be detected in stool even after the infected person has negative respiratory specimens [9] but it is not known whether infection ever occurs from contact with feces or aerosolization from toilets.
With regard to spread by large droplets, when an infected symptomatic or asymptomatic person coughs, sneezes, spits, breaths, or talks, droplets will be produced and immediately fall to the ground. Spread of droplets is usually up to 1 meter but can be further with an unprotected sneeze. A surgical mask (also known as medical or procedure mask) worn properly by either the infected person or by their contacts is highly likely to prevent transmission. Nonetheless, the risk with a universal mask policy is that people are unlikely to consistently comply with the recommendation to wash their hands immediately before donning a mask and after doffing it and may touch their face frequently to scratch under or to adjust their mask. Cloth masks appear to be less effective than surgical masks but this could in part be related to wearing used or wet cloth masks [10].
As mentioned, the viral load in the upper respiratory tract is similar in asymptomatic versus symptomatic cases [4], [5]. Therefore, it is not surprising that spread from asymptomatic or presymptomatic cases is well documented in household settings or with other close contact [11]. It appears that adults are infectious for up to 3 days prior to symptom onset [12]. The relative contribution of presymptomatic and asymptomatic cases to spread remains controversial [13]. It seems unlikely that spread will occur by simply walking past an infected asymptomatic person.
Protection of health care workers
A meta-analysis of 4 studies reported no difference in the transmission of respiratory viruses to HCWs who wore surgical masks versus N95 masks (these filter minimum 95% of airborne particles) [14]. For coronaviruses, the authors report “When seasonal coronavirus (OC43, HKU1, 229E, NL63) was tested for by PCR in a non-cluster randomized trial of medical masks vsN95 respirators, 4.3% (9/212) of nurses in the medical mask group had RT-PCR confirmed coronavirus infection compared to 5.7% (12/210) in the N95 respirator group (p = 0.49)” [14]. N95 masks are recommended for HCWs when patients infected with respiratory viruses are undergoing medical procedures that produce abundant aerosols (droplets that are smaller than those produced spontaneously by a patient so they travel further and may be able to penetrate a surgical mask). Common examples of such procedures are tracheal intubation or extubation, positive pressure ventilation, delivery of medications via nebulisation and high-flow oxygen delivery [15]. The need for N95 masks with these procedures has not been formally studied but tracheal intubation was associated with an increased risk of transmission of SARS-CoV-1 to HCWs [15]. However, in an early unrecognized case of COVID-19 in Singapore, nosocomial transmission did not occur to 35 HCWs exposed to medical aerosol generating procedures for minimum 10 min while wearing only surgical masks [16]. Three of 94 HCWs with medium or high risk exposures to an unrecognized case in the United States (US) became infected but the infected HCWs did not consistently wear even surgical masks [17].
Clinical features of COVID-19 in children
As with all respiratory viruses, the spectrum of COVID-19 ranges from asymptomatic infection to severe pneumonia with respiratory failure. In a 2019 study of 214 adults and children, asymptomatic infection occurred in 70 to 85 % of RSV, parainfluenza, adenovirus, human rhinovirus and coronavirus infections compared to approximately 40% of influenza and 30% of human metapneumovirus infections [18]. To accurately determine the incidence of asymptomatic COVID-19 in children, viral detection and collection of data on symptoms needs to be performed on a large random group of children at the peak of an outbreak. Ideally, there would be follow-up data collection to determine how many were presymptomatic.
Recent estimates of the mean incubation period (time from exposure to symptoms) in patients of all ages from two different studies were 4.2 [6] and 5.1 [19] days with 2.5% being symptomatic before day 2.2 or after day 11.5 [19].
The only three studies to date to report clinical features on a substantial number of children included 171 inpatients in one hospital in China [20] 291 outpatients and inpatients throughout the US [21] and 100 children assessed in 17 emergency departments in Italy (reference = Children with Covid-19 in Pediatric Emergency Departments in Italy [58]. Cough was reported in 44% to 54%, fever in 42% to 56%, diarrhea in 9% to 13 % and nausea/vomiting in 10% to 11%.
These features do not differ from what one might expect with any respiratory virus so it is important to look for distinguishing features of COVID-19. The only feature recognized to date was first reported in April 2020 [22] and is distal vascular involvement of the toes, fingers or both (See Fig. 1, Fig. 2 for an example in a 2 year old child from Ecuador). A report from Italy characterizes these as chilblain-like lesions and describes 63 cases, primarily in adolescents [23]. Although only 2 of 11 tested had COVID-19 detected, lesions started a median of 10 days after the onset of respiratory symptoms so virus may have cleared by the time of testing. Another report from Italy describes 14 cases (11 in children) seen during the COVID-19 outbreak; 3 NP and 2 rectal swabs were negative for SARS-CoV-2 [24]. There is increasing evidence that thrombosis may play a role in the pathophysiology of COVID-19 which could explain this unusual manifestation in digits [25]. In terms of other cutaneous features, active surveillance in 88 patients in Italy (presumably all adults) with no recent drug exposure found that14 had a generalized erythematous viral rash, 3 had urticaria and one had vesicles [26]. In another report from Italy, 2 of 130 inpatients had vesicles [27]. Five of 103 inpatient adults in France had cutaneous manifestations (2 erythematous rashes, 2 urticarial rashes and herpes stomatitis) [28]. In areas where SARS-CoV 2 co-circulates with dengue, a petechial rash from COVID-19 has led to diagnostic confusion [29].
Fig. 1.
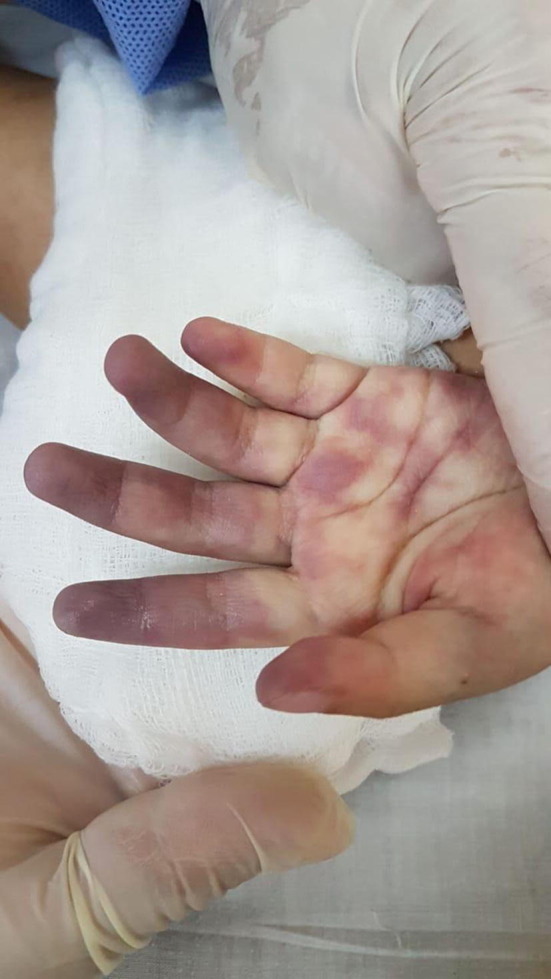
Appearance of hands in a child with proven COVID-19 on day 10 of respiratory symptoms.
Fig. 2.
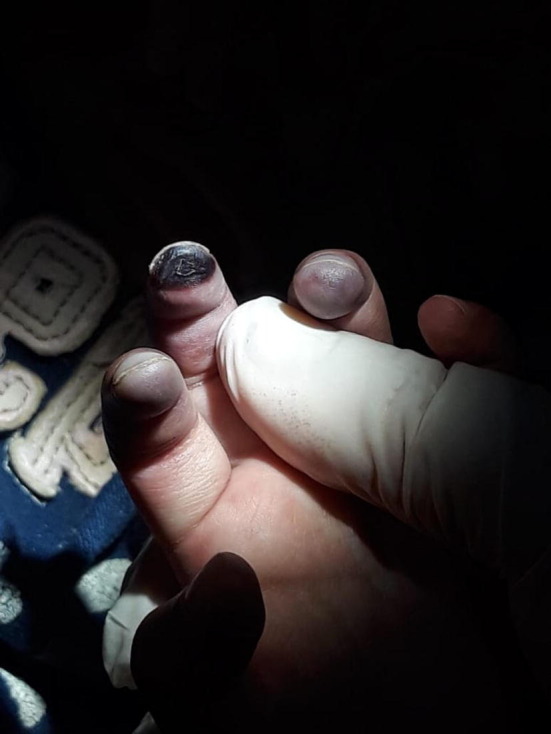
Follow-up photo 7 days later (Unlike previously reported cases, this child has a necrotic digit.).
A multisystem inflammatory syndrome sometimes leading to shock was first described in children in countries with extensive SARS-CoV-2 circulation in May 2020 (reference is: Hyperinflammatory shock in children during COVID-19 pandemic [57].) but the virus is often not detectable. Virus may have cleared by the time of presentation. Causation by SARS-CoV-2 seems likely but remains unproven.
Neurologic manifestations including dizziness (17%), headache (13%) and impairment of taste (6%) and smell (5%) were reported in 214 adult inpatients in China. Five had cerebral infarcts and one had an intracranial bleed [30]. A case of encephalitis with detection of SARS-CoV-2 in cerebral spinal fluid has been reported in an adult [31]. Pediatric neurologic manifestations reported to date are limited to a 6 week-old infant with a possible seizure [32]. Myocarditis has been reported in adults [33]. In the recent reports of the pediatric multisystem inflammatory syndrome, myocardial dysfunction was reported in 7 of 8 cases and dilated coronary arteries in one case.
Adults with severe pneumonia who progress to respiratory failure most commonly manifest pneumonia with Acute Respiratory Distress Syndrome (ARDS). The time between symptom onset and hospitalization in adults is longer than one might predict for a viral pneumonia (7–12 days [34], [35]). Some manifest a cytokine release syndrome, suggesting that the immune response to the virus may be the primary problem. There are no reports yet of the clinical features of children with respiratory failure.
Pediatric data obtained from the websites of countries with more than 1000 total adult and pediatric cases on April 13, 2020 showed that 1.9% of cases were in children and that death occurred in 5 of 5501 infected children (0.09%) (unpublished data). This figure is difficult to interpret given the inconsistency in the indications for testing children. Understandably, early in the epidemic most children with CVOID-19 were admitted to hospital given the severity of disease in adults. However, by the time that the virus became endemic in the US in March 2020, it was apparent that most children could be safely cared for at home and only 147 of the first 745 US pediatric cases with known hospitalization status were admitted (20%) of which 15 (2% of all cases and 10% of those admitted) went to the intensive care unit [21] and 3 died (0.4% of all cases and 2% of those admitted). In preliminary data from New York, all 46 admitted children were alive (14 were still in hospital), 7 (15%) required ICU care of which 4 required mechanical ventilation and none required renal replacement therapy [36].
As with other coronaviruses, vertical transmission is yet to be proven [37]. SARS-CoV-2 has been detected from neonates but this could represent colonization rather than infection. All neonates with virus detected have done well. If the mother is infected, she can breastfeed while wearing a mask [37].
The risk factors for severe COVID-19 in children are yet to be established. Preliminary US data suggest that comorbidities increase the risk of admission [21] but they may also lower the threshold for admission. There are no data yet on risk factors for ICU admission or death although all 6 children admitted to US ICUs with available data had comorbidities [21]. It seems likely that children with pulmonary or cardiac disease or immunosuppression are at increased risk for severe disease but were this risk substantial, one might expect there to have been more deaths.
It is not clear yet whether asthma increases the risk of severe COVID-19 in adults or children [38]. There were 40 children with asthma amongst the first 345 children diagnosed in the US with data on comorbidities available but the severity of COVID-19 in these children is not reported [21]. Infection with viruses other than rhinovirus increased the chance of treatment failure in asthmatic children [39] so it seems likely that COVID-19 will do the same. There are theoretical concerns that obese children and those with cystic fibrosis will be at increased risk of severe COVID-19 [40].
Diagnosis
Viral detection
It is impressive that molecular testing for SARS-CoV-2 became available in many countries within one month of the virus being identified. A nasopharyngeal (NP) swab is commonly obtained for testing. The yield from NP specimens for respiratory viruses appears to be higher than from throat swabs or saliva [41], but this is yet to be confirmed for SARS-CoV-2. The yield appears to be higher from bronchoalveolar lavage specimens than from upper respiratory samples [42], but lower tract specimens are only available from intubated patients. It seems plausible that the viral load is higher in the upper respiratory tract early in the course of disease, but with pneumonia will eventually be higher in the lower tract. It is impossible to establish the true sensitivity of the many available molecular assays as there is no reference standard and NP specimens may be obtained incorrectly. The accuracy of diagnosis would be even more important were there an effective treatment. Virus was shed for a median of 14 days in one study [43] but it is not known whether patients with small amounts of detectable virus remain infectious.
Serology
There is a race to develop accurate serologic assays. They could prove useful for diagnosis in cases where COVID-19 seems likely but virus cannot be detected. Assuming that reinfection is rare or results in mild disease, those with antibodies could be allowed to resume their previous work and activities. Again though, there is no reference standard and it seems possible that antibodies (especially IgM) could cross-react with other viruses or that reinfection could occur despite the presence of IgG.
Chest imaging
Findings on plain radiographs are not distinctive from findings with any other viral respiratory tract infection. Typical findings on computed tomography (CT) chest in adults are “ground-glass opacities, multifocal patchy consolidation, and/or interstitial changes with a peripheral distribution” [44]. An early case series described CT chest findings in 20 infected children. Four had normal imaging, 6 had unilateral disease and 10 had bilateral disease. All with abnormal findings had subpleural disease, 10 had consolidation with surrounding halos and 12 had ground glass appearance [45]. From 1014 patients in Wuhan (only 7 were children), the sensitivity of CT in patients with COVID-19 detected was 97% (95%CI, 95–98%, 580/601 patients) while specificity was only 25% (95% CI 22–30%, 105/413 patients) [44]. This suggests that CT chest should not be used to diagnose COVID-19.
Treatment
There was minimal investment in finding treatments or vaccines for coronaviruses until 2020 as disease due to 229E, OC43, NL63, and HKU1 is usually mild and disease due to MERS is limited to the Middle East. The anti-viral agent thought to hold the most promise is remdesivir [46], an RNA polymerase inhibitor with in-vitro activity against MERS [47]. This drug has been studied for Ebola virus disease but appears of COVID-19 to be less promising than other options [48]. The largest study to date described a 68% response rate but lacked a control group so is impossible to interpret [49]. Lopinavir-ritonavir is a protease inhibitor with extensive use as an antiretroviral agent, but did not suppress viral replication in the initial randomized controlled trial [50]. However, treatment was started on median day 13 of symptoms which may be too late to procure benefit so further trials are ongoing. Hydroxychloroquine and chloroquine have been used for decades for malaria and for rheumatologic conditions. They inhibit SARS-CoV-2 entry by an unknown mechanism so could be effective for prophylaxis or for treatment. Trials to date are not definitive as they are small with a high risk of bias, sometimes using monotherapy and sometimes adding azithromycin (for unclear reasons) [51]. Because end-stage COVID-19 is reminiscent of a hyper-inflammatory state with cytokine storm, the IL-6 inhibitor tocilizumab is being studied. A detailed summary of medications for treatment of SARS-Cov-2 compiled by pharmacists and paediatric infectious diseases physicians in North America was published April 2020 [46]. In keeping with the Infectious Diseases Society of America recommendations for adults [52], they recommended that none of these medications be used outside of clinical trials. Given the absence of effective medications, convalescent plasma warrants study [53].
Based on experience with previous coronoaviruses, oral corticosteroids were considered potentially detrimental for treatment of COVID-19 [38] although this has not been formally studied. This led to great concern as to whether asthma exacerbations should be managed with corticosteroids when SARS-CoV-2 is circulating in the community. The consensus to date is that there is insufficient evidence to avoid corticosteroids for management of asthma [38].
The management of respiratory failure due to COVID-19 is controversial. The initial mortality rate was 25% in adults who required mechanical ventilation in New York with many survivors still ventilated when the data were published [36]. It has been observed that some patients with respiratory failure with COVID-19 have high pulmonary compliance so perhaps do not fit the typical pattern of ARDS. This should be taken into account when determining whether to use non-invasive ventilation, high PEEP and prone positioning [54]. For the first 32 adults in the US managed with ECMO, 10 had died, 17 remained on ECMO and 5 were successfully decannulated in the initial report [55].
Predicting the future
The future evolution of this pandemic is of interest to every person alive today. The following all seem possible in any given country:
-
1.
There is only ever one wave of COVID-19. Based on the R0, this would require that an estimated 82% of people are infected during the first wave [6], leading to herd immunity such that spread is minimal if the virus is reintroduced. However, perhaps this estimate will prove to be incorrect and most countries will only ever see one wave despite achieving a lower level of herd immunity. A single wave is reminiscent of what happened when Zika virus reached the Americas in 2015, differences being that an estimated 80% of Zika virus infections are asymptomatic and Zika virus requires a mosquito to spread person-to-person.
-
2.
There are repeated waves of COVID-19 until herd immunity develops.
-
3.
We learn that reinfection with SARS-CoV-2 or a mutated version is common so COVID-19 becomes endemic (like 229E, OC43, NL63, and HKU1 have done). It is difficult to predict whether infection will occur year round (but more commonly in the winter and spring in temperate climates) as with these other coronaviruses or seasonally (as with RSV and influenza). The severity of infection with RSV and influenza is higher in infants and in the elderly. One might predict the same pattern with COVID-19 but it is striking to what a degree infants have been spared from severe disease in the first wave.
-
4.
There are repeated waves of COVID19 until an effective vaccine is employed. Although experts suggest that there may be a vaccine during 2021, this seems optimistic given that the first study of an RSV vaccine ended in 1966 [56], yet there is still no licensed vaccine. After decades of development, influenza vaccines have only about 50% efficacy in recent years. However, we would readily adopt a well-tolerated SARS-CoV-2 vaccine with 50% efficacy given the havoc the virus is wreaking worldwide.
Conclusion
The course of history has been changed by SARS-CoV-2. The general public has become aware of the basic principles of infection control. We will eventually emerge from the havoc wreaked by this virus, hopefully with better tools to prevent and manage future pandemic threats.
Educational aims
-
•
Realize how little we know about the spectrum of disease of COVID-19 in children.
-
•
Understand modes of SARS-CoV-2 transmission and the resulting infection control polices.
-
•
Know when to suspect COVID-19 in a child and how to confirm the diagnosis.
-
•
Be able to provide parents with an accurate assessment of the prognosis and treatment options if their child has COVID-19 infection.
-
•
Be aware of possible scenarios for how this pandemic may evolve.
Directions for future research
-
•
Surveillance studies and development of a validated serologic assay to determine the incidence of asymptomatic infections in all age groups.
-
•
Incidence and severity of reinfection in all age groups.
-
•
Randomized controlled trials of remdesivir, lopinavir-ritonavir, hydroxychloroquine, tocilizumab and convalescent plasma for treatment of COVID-19.
Acknowledgments
Acknowledgements
The authors are grateful to Dr Nelly Chavez who provided the patient photos and obtained parental consent.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial disclosure
Neither author has a financial interest related to this topic to disclose.
Conflict of interest
Neither author has a conflict of interest related to this manuscript.
References
- 1.Ogimi C., Kim Y.J., Martin E.T., Huh H.J., Chiu C.H., Englund J.A. What's new with the old coronaviruses? J Pediatric Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa037. Epub 2020/04/22. doi: 10.1093/jpids/piaa037. PubMed PMID: 32314790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol: CB. 2020;30(8):1578. doi: 10.1016/j.cub.2020.03.063. Epub 2020/04/22. doi: 10.1016/j.cub.2020.03.063. PubMed PMID: 32315626; PubMed Central PMCID: PMCPMC7169893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. Epub 2020/04/03. doi: 10.1016/j.cmi.2020.03.026. PubMed PMID: 32234451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New Engl J Med. 2020 doi: 10.1056/NEJMoa2008457. Epub 2020/04/25. doi: 10.1056/NEJMoa2008457. PubMed PMID: 32329971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. Epub 2020/02/20. doi: 10.1056/NEJMc2001737. PubMed PMID: 32074444; PubMed Central PMCID: PMCPMC7121626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200282. Epub 2020/04/08. doi: 10.3201/eid2607.200282. PubMed PMID: 32255761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall C.B., Douglas R.G., Jr., Schnabel K.C., Geiman J.M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immunity. 1981;33(3):779–783. doi: 10.1128/iai.33.3.779-783.1981. Epub 1981/09/01. PubMed PMID: 7287181; PubMed Central PMCID: PMCPMC350778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020 doi: 10.1001/jama.2020.3227. Epub 2020/03/05. doi: 10.1001/jama.2020.3227. PubMed PMID: 32129805; PubMed Central PMCID: PMCPMC7057172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect = Wei mian yu gan ran za zhi. 2020 doi: 10.1016/j.jmii.2020.03.021. Epub 2020/04/12. doi: 10.1016/j.jmii.2020.03.021. PubMed PMID: 32276848; PubMed Central PMCID: PMCPMC7141453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006577. Epub 2015/04/24. doi: 10.1136/bmjopen-2014-006577. PubMed PMID: 25903751; PubMed Central PMCID: PMCPMC4420971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2 – Singapore, January 23-March 16, 2020. MMWR Morbidity Mortality Weekly Rep. 2020;69(14):411–415. doi: 10.15585/mmwr.mm6914e1. Epub 2020/04/10. doi: 10.15585/mmwr.mm6914e1. PubMed PMID: 32271722; PubMed Central PMCID: PMCPMC7147908 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0869-5. Epub 2020/04/17. doi: 10.1038/s41591-020-0869-5. PubMed PMID: 32296168. [DOI] [PubMed] [Google Scholar]
- 13.He D., Zhao S., Lin Q., Zhuang Z., Cao P., Wang M.H. The relative transmissibility of asymptomatic cases among close contacts. Int J Infect Dis: IJID. 2020 doi: 10.1016/j.ijid.2020.04.034. Epub 2020/04/22. doi: 10.1016/j.ijid.2020.04.034. PubMed PMID: 32315808; PubMed Central PMCID: PMCPMC7166025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12745. Epub 2020/04/05. doi: 10.1111/irv.12745. PubMed PMID: 32246890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS one. 2012;7(4) doi: 10.1371/journal.pone.0035797. Epub 2012/05/09. doi: 10.1371/journal.pone.0035797. PubMed PMID: 22563403; PubMed Central PMCID: PMCPMC3338532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng K., Poon B.H., Kiat Puar T.H., Shan Quah J.L., Loh W.J., Wong Y.J. COVID-19 and the risk to health care workers: a case report. Ann Internal Med. 2020 doi: 10.7326/L20-0175. Epub 2020/03/17. doi: 10.7326/l20-0175. PubMed PMID: 32176257; PubMed Central PMCID: PMCPMC7081171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzerling A., Stuckey M.J., Scheuer T., Xu K., Perkins K.M., Resseger H. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morbidity Mortality Weekly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. Epub 2020/04/17. doi: 10.15585/mmwr.mm6915e5. PubMed PMID: 32298249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanti M., Birger R., Ud-Dean M., Filip I., Morita H., Comito D. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol Infect. 2019;147:e176. doi: 10.1017/S0950268819000505. Epub 2019/05/08. doi: 10.1017/s0950268819000505. PubMed PMID: 31063096; PubMed Central PMCID: PMCPMC6518513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Internal Med. 2020 doi: 10.7326/M20-0504. Epub 2020/03/10. doi: 10.7326/m20-0504. PubMed PMID: 32150748; PubMed Central PMCID: PMCPMC7081172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. New Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. Epub 2020/03/19. doi: 10.1056/NEJMc2005073. PubMed PMID: 32187458; PubMed Central PMCID: PMCPMC7121177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morbidity and mortality weekly report. 2020;69(14):422-6. .Epub 2020/04/10. doi: 10.15585/mmwr.mm6914e4. PubMed PMID: 32271728; PubMed Central PMCID: PMCPMC7147903 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed]
- 22.Alramthan A., Aldaraji W. A case of COVID-19 presenting in clinical picture resembling chilblains disease. First report from the Middle East. Clin Exp Dermatol. 2020 doi: 10.1111/ced.14243. Epub 2020/04/18. doi: 10.1111/ced.14243. PubMed PMID: 32302422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo V., Neri I., Filippeschi C., Oranges T., Argenziano G., Battarra V.C. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16526. Epub 2020/04/25. doi: 10.1111/jdv.16526. PubMed PMID: 32330334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recalcati S., Barbagallo T., Frasin L.A., Prestinari F., Cogliardi A., Provero M.C. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16533. Epub 2020/04/25. doi: 10.1111/jdv.16533. PubMed PMID: 32330324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P. COVID-19 coagulopathy in Caucasian patients. British J Haematol. 2020 doi: 10.1111/bjh.16749. Epub 2020/04/25. doi: 10.1111/bjh.16749. PubMed PMID: 32330308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16387. Epub 2020/03/28. doi: 10.1111/jdv.16387. PubMed PMID: 32215952. [DOI] [PubMed] [Google Scholar]
- 27.Tammaro A., Adebanjo G.A.R., Parisella F.R., Pezzuto A., Rello J. Cutaneous manifestations in COVID- 19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16530. Epub 2020/04/25. doi: 10.1111/jdv.16530. PubMed PMID: 32330340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedou M., Carsuzaa F., Chary E., Hainaut E., Cazenave-Roblot F., Masson Regnault M. Comment on “Cutaneous manifestations in COVID-19: a first perspective ” by Recalcati S. J Eur Acad Dermatol Venereol: JEADV. 2020 doi: 10.1111/jdv.16519. Epub 2020/04/22. doi: 10.1111/jdv.16519. PubMed PMID: 32314436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. Epub 2020/03/28. doi: 10.1016/j.jaad.2020.03.036. PubMed PMID: 32213305; PubMed Central PMCID: PMCPMC7156802. [DOI] [PMC free article] [PubMed]
- 30.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. Epub 2020/04/11. doi: 10.1001/jamaneurol.2020.1127. PubMed PMID: 32275288; PubMed Central PMCID: PMCPMC7149362. [DOI] [PMC free article] [PubMed]
- 31.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-8. Epub 2020/04/07. doi: 10.1016/j.ijid.2020.03.062. PubMed PMID: 32251791. [DOI] [PMC free article] [PubMed]
- 32.Dugue R, Cay-Martinez KC, Thakur K, Garcia JA, Chauhan LV, Williams SH, et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020. Epub 2020/04/25. doi: 10.1212/wnl.0000000000009653. PubMed PMID: 32327489. [DOI] [PMC free article] [PubMed]
- 33.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet (London, England). 2020. Epub 2020/04/27. doi: 10.1016/s0140-6736(20)30912-0. PubMed PMID: 32334650. [DOI] [PMC free article] [PubMed]
- 34.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020. Epub 2020/02/08. doi: 10.1001/jama.2020.1585. PubMed PMID: 32031570; PubMed Central PMCID: PMCPMC7042881. [DOI] [PMC free article] [PubMed]
- 35.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. Jama. 2020 doi: 10.1001/jama.2020.6775. Epub 2020/04/23. doi: 10.1001/jama.2020.6775. PubMed PMID: 32320003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muldoon K.M., Fowler K.B., Pesch M.H., Schleiss M.R. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104372. Epub 2020/04/27. doi: 10.1016/j.jcv.2020.104372. PubMed PMID: 32335336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams E.M., Szefler S.J. Managing asthma during COVID-19: an example for other chronic conditions in children and adolescents. J Pediatrics. 2020 doi: 10.1016/j.jpeds.2020.04.049. Epub 2020/04/25. doi: 10.1016/j.jpeds.2020.04.049. PubMed PMID: 32330469; PubMed Central PMCID: PMCPMC7172836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merckx J., Ducharme F.M., Martineau C., Zemek R., Gravel J., Chalut D. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics. 2018;142(1) doi: 10.1542/peds.2017-4105. Epub 2018/06/06. doi: 10.1542/peds.2017-4105. PubMed PMID: 29866794. [DOI] [PubMed] [Google Scholar]
- 40.Sinha I.P., Harwood R., Semple M.G., Hawcutt D.B., Thursfield R., Narayan O. COVID-19 infection in children. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30152-1. Epub 2020/04/01. doi: 10.1016/s2213-2600(20)30152-1. PubMed PMID: 32224304; PubMed Central PMCID: PMCPMC7154504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson J.L., Lee B.E., Kothapalli S., Craig W.R., Fox J.D. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clinical infectious diseases. 2008;46(7):e61–e64. doi: 10.1086/529386. Epub 2008/05/01. doi: 10.1086/529386. PubMed PMID: 18444806; PubMed Central PMCID: PMCPMC7107872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in Different types of clinical specimens. Jama. 2020 doi: 10.1001/jama.2020.3786. Epub 2020/03/12. doi: 10.1001/jama.2020.3786. PubMed PMID: 32159775; PubMed Central PMCID: PMCPMC7066521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X., Xing Y., Jia J., Ni W., Liang J., Zhao D. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi: 10.1016/j.scitotenv.2020.138812. Epub 2020/04/27. doi: 10.1016/j.scitotenv.2020.138812. PubMed PMID: 32335406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. Epub 2020/02/27. doi: 10.1148/radiol.2020200642. PubMed PMID: 32101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia W., Shao J., Guo Y., Peng X., Li Z., Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatric Pulmonol. 2020;55(5):1169–1174. doi: 10.1002/ppul.24718. Epub 2020/03/07. doi: 10.1002/ppul.24718. PubMed PMID: 32134205; PubMed Central PMCID: PMCPMC7168071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiotos K., Hayes M., Kimberlin D.W., Jones S.B., James S.H., Pinninti S.G. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc. 2020 doi: 10.1093/jpids/piaa045. Epub 2020/04/23. doi: 10.1093/jpids/piaa045. PubMed PMID: 32318706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. Epub 2020/02/26. doi: 10.1074/jbc.AC120.013056. PubMed PMID: 32094225; PubMed Central PMCID: PMCPMC7152756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of Ebola virus disease therapeutics. New Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. Epub 2019/11/28. doi: 10.1056/NEJMoa1910993. PubMed PMID: 31774950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. New Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. Epub 2020/04/11. doi: 10.1056/NEJMoa2007016. PubMed PMID: 32275812; PubMed Central PMCID: PMCPMC7169476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. New Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. Epub 2020/03/19. doi: 10.1056/NEJMoa2001282. PubMed PMID: 32187464; PubMed Central PMCID: PMCPMC7121492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander P.E., Debono V.B., Mammen M.J., Iorio A., Aryal K., Deng D. COVID-19 research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020 doi: 10.1016/j.jclinepi.2020.04.016. Epub 2020/04/25. doi: 10.1016/j.jclinepi.2020.04.016. PubMed PMID: 32330521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.America IDSo. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. [DOI] [PMC free article] [PubMed]
- 53.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. Epub 2020/03/03. doi: 10.1016/s1473-3099(20)30141-9. PubMed PMID: 32113510; PubMed Central PMCID: PMCPMC7128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. Jama. 2020 doi: 10.1001/jama.2020.6825. Epub 2020/04/25. doi: 10.1001/jama.2020.6825. PubMed PMID: 32329799. [DOI] [PubMed] [Google Scholar]
- 55.Jacobs J.P., Stammers A.H., St Louis J., Hayanga J.W.A., Firstenberg M.S., Mongero L.B. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: experience with 32 patients. ASAIO J (American Society for Artificial Internal Organs: 1992) 2020 doi: 10.1097/MAT.0000000000001185. Epub 2020/04/23. doi: 10.1097/mat.0000000000001185. PubMed PMID: 32317557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. Epub 1969/04/01. 10.1093/oxfordjournals.aje.a120955 PubMed PMID: 4305198. [DOI] [PubMed] [Google Scholar]
- 57.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Lancet Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 May 7:31094–31111. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parri N, Lenge M, Buonsenso D; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group.N Engl J Med. 2020 May 1. 10.1056/NEJMc2007617. [DOI]


