Abstract
Trends in influenza and pneumococcal vaccine coverage in Thai patients with type 2 diabetes mellitus 2010–2018: Experience from a tertiary diabetes center in Bangkok.
Background
Routine vaccination is an important part of preventive services in treating patients with type 2 diabetes (T2DM). There are no available data in temporal trends of vaccination coverage rates in both influenza and pneumococcal vaccines among Thai patients with T2DM.
Aim
This study aimed to elucidate influenza and pneumococcal vaccination trends and to identify factors that affect vaccination rates in those patients.
Method
A retrospective study of randomly medical records stratified by 13 diabetologists was conducted in patients with T2DM from 2010 to 2018 at Theptarin Hospital, a private multi-disciplinary diabetes center in Bangkok. Adherence to influenza and pneumococcal vaccinations according to current guidance on adult immunization in Thailand had been studied. The rate of both vaccinations from each diabetologist had also been recorded.
Results
A total of 2114 medical records (female 51.7%, mean age 65.2 ± 12.8 years, BMI 26.5 ± 4.6 kg/m2, A1C 7.1 ± 1.3%, median duration of diabetes 13 years) were retrospectively reviewed covering a 9-year period. We audited 3554 selected outpatient visits for influenza and pneumococcal vaccinations rates as key performance index in each year. The overall vaccination rate was 39.6% for influenza, 17.4% for the pneumococcal vaccine, and only 13.7%, for both vaccines. The trends of influenza vaccination rates increased from 32.9% in 2010 to 52.2% in 2018 but the trends of pneumococcal vaccination rates were relatively stable at less than 20%. The rate of both vaccinations varied considerably from 0 to 44% among our diabetologists. Age ≥ 65 years, duration of DM ≥ 15 years, the presence of chronic respiratory disease, and moderate to severe Charlson Comorbidity Index (CCI) score were positively associated with both received vaccinations.
Conclusions
The completeness and timeliness of influenza and pneumococcal vaccinations were unsatisfactory in Thai patients with T2DM. More efforts are needed to increase both influenza and pneumococcal vaccination rates.
Keywords: Influenza vaccine, Pneumococcal vaccine, Type 2 Diabetes Mellitus, Coverage rate, Bangkok
Introduction
Vaccination is one of the most effective interventions to control transmission, decrease morbidity and mortality of seasonal influenza [1]. Patients with type 2 diabetes mellitus (T2DM) are a key target of routine annual influenza vaccination and periodically pneumococcal vaccination as epidemiologic studies suggested that these patients are at high risk for complications, hospitalization, and death from influenza and pneumococcal disease [2]. An increased incidence of pneumococcal infection followed by influenza infection had been observed [3]. As a result, both annual influenza vaccination and adherence to pneumococcal vaccination are an important part of preventive services in treating patients with T2DM from various organizations [4], [5], [6].
Although it has been recommended that persons with chronic diseases and those at increased risk of influenza complications should be vaccinated yearly, only a small proportion of high-risk individuals all over the world actually receive influenza vaccination especially in Asian countries [7]. The World Health Organization (WHO) proposed in 2005 that the influenza vaccination rate should attain coverage rate of 75% among the high-risk groups [8]. However, the current median influenza coverage rate is merely 50.3% among European countries [9]. When compared with Western countries, influenza vaccination coverage rate remains considerably low in most Asian countries [10], [11].
While influenza vaccination has been focused as key to prevent influenza pandemic in the future, the importance of pneumococcal vaccination should also receive attention in the immunocompromized patients including people with diabetes. Invasive pneumococcal diseases (such as meningitis and bacteremia) and pneumonia from Streptococcus pneumoniae are associated with increased mortality among individuals with diabetes [12]. Therefore, the Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination in individuals with diabetes [13]. There are currently two types of pneumococcal vaccines - Pneumococcal Conjugate Vaccine (PCV13) and Pneumococcal Polysaccharide Vaccine (PPV23). Guidelines for pneumococcal immunization across the world are complex and vary greatly between countries in terms of age groups and risk groups recommended for vaccination, as well as which vaccine should be administered. In Thailand, our current national guidance on adult immunization still reflects the previous version of ACIP recommendation which suggested routine PCV13 for adults ages 65 years and older and followed with PPV23 one year later [14]. For people with diabetes younger than 65 years, only PPV23 is suggested to be administered and repeated every 5 years.
Despite various campaigns and free influenza vaccination for high-risk groups from the Thai government, annual influenza vaccination uptake in the general Thai population remained suboptimal at the rate of lower than 30% [15]. There are no available data in temporal trends of vaccination coverage rates in both influenza and pneumococcal vaccines among Thai patients with T2DM. Based on the available data among patients with T2DM in East Asian countries [16], [17], [18], the annual influenza vaccination rate was only 30–60% and pneumococcal vaccination rate was less than 20%. In an effort to further improve both vaccines coverage rates, it is necessary to understand the current trends and associated factors of vaccination coverage rates. The primary objective of this study is to determine the trends of influenza and pneumococcal vaccine uptakes in individuals with T2DM who attended a routine outpatient diabetes clinic at our hospital. The secondary objective is to identify factors that affect vaccination rates in those patients.
Materials and methods
The current study is a retrospective analysis of randomly sampled paper-based medical records, stratified by 13 diabetologists, each with annual medical records of 350–500 samples. It was conducted in patients with T2DM from 2010 to 2018 at Theptarin Hospital, a private multi-disciplinary diabetes center in Bangkok, Thailand. This study was a part of our annual quality improvement program which has been carried out since 2010 in order to improve various aspects of diabetes care and benchmark results in each diabetologists. Over 2,000 registered T2DM patients follow-up regularly at our diabetes center. Patients with age <15 years, patients with type 1 diabetes mellitus (T1DM) and patients with other types of diabetes were excluded. The data on patient characteristics, smoking status, glycemic control, pattern of diabetes treatments, adherence to influenza and pneumococcal vaccinations according to current guidance on adult immunization in Thailand were collected [14]. We used medical records as a source to assess the receipt of influenza and pneumococcal vaccinations. The vaccination venue could be either inside our hospital or other healthcare services. Because influenza vaccination is provided for free in people with diabetes in the government healthcare programs since 2008, some patients might receive free influenza vaccination elsewhere. The presence of comorbidities was determined by the Charlson Comorbidity Index (CCI) [19] which composed of 19 medical conditions. Each comorbidity category has an associated weight (from 1 to 6) and the sum of all the weights results in a single comorbidity score for each patient. A score of zero indicates that no comorbidities was found. The higher the score, the more likely the predicted outcome will result in higher mortality. Patients were divided into three groups: mild, with CCI scores of 1–2; moderate, with CCI scores of 3–4; and severe, with CCI scores ≥5 [20]. The rate of both influenza and pneumococcal vaccinations from each diabetologist over the study period was also collected. This study was approved by the Institutional Review Board (IRB) committee of Theptarin Hospital (EC No.6-2018)
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range -IQR), as appropriate and categorical variables were presented as proportions. Variables analyzed in the univariate analysis included age ≥ 65 years, sex, body mass index (BMI) ≥ 30 kg/m2, duration of diabetes mellitus (DM) ≥ 15 years, current smoking status, the presence of chronic pulmonary disease, the presence of cardiovascular disease, glycated hemoglobin (A1C) ≥ 9.0%, insulin usage, and moderate to severe CCI (CCI ≥ 3) based on previous literatures for associated factor in vaccine uptake. Factors achieving a P-value < 0.1 were included in the multivariate models to determine associated factors with influenza and pneumococcal vaccinations. P-value ≤ 0.05 was considered statistically significant. All statistical analyses were conducted using the Statistical Package for the Social Sciences (version 22.0; SPSS, Armonk, NY, USA).
Results
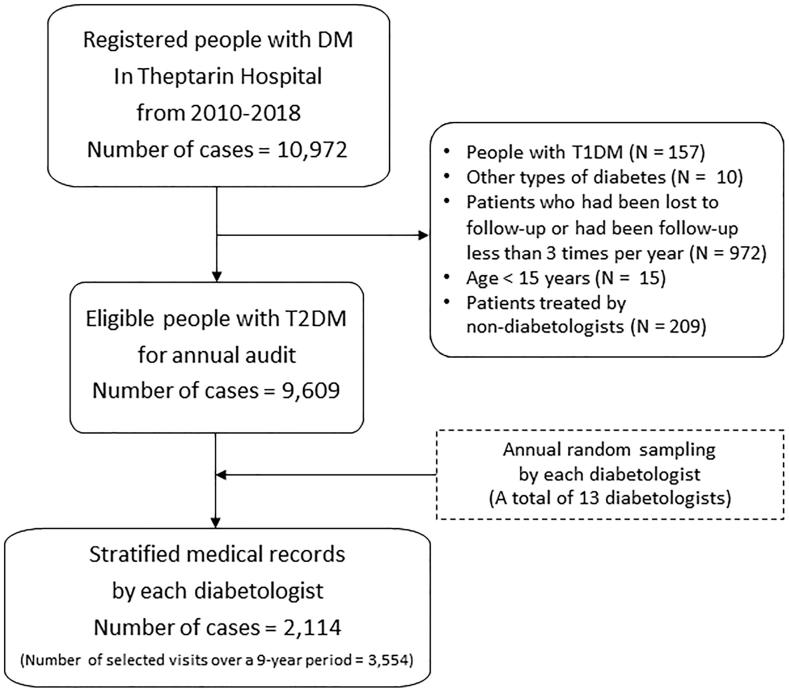
A total of 2114 medical records (female 51.7%, mean age 65.2 ± 12.8 years, BMI 26.5 ± 4.6 kg/m2, A1C 7.1 ± 1.3%, median duration of diabetes 13 years) with 3554 selected outpatient visits were selected based on results from random sampling method as shown in Fig. 1. This number of medical records represented 20% of all visits during the study period. In this retrospective study, 40% of all selected medical records had been audited for more than once (ranging from 2 to 7 times) during the 9-year period. Insulin usage rate was 23.9% and moderate to severe CCI (CCI ≥ 3) were presented in 78.8% of all patients. The details of audited patients were presented in Table 1. The overall vaccination rate was 39.6% for influenza, 17.4% for the pneumococcal vaccine, and only 13.7% for both vaccines. When stratified by each diabetologist, the rate of both influenza and pneumococcal vaccinations varied considerably from 0 to 44% during the study period.
Fig. 1.
Flow selection of audited medical records from 2010 to 2018.
Table 1.
Demographic data in studied patients during 2010–2018 (3554 selected outpatient visits from 2114 medical records).
| Clinical characteristics | Results |
|---|---|
| Age (years) | 65.2 ± 12.8 |
|
2.8% |
|
43.5% |
|
27.9% |
|
20.8% |
|
5.0% |
| Female (%) | 51.7% |
| BMI (kg/m2) | 26.5 ± 4.6 |
|
1.6% |
|
20.5% |
|
17.9% |
|
40.1% |
|
19.9% |
| Smoking status | |
|
76.7% |
|
14.7% |
|
8.6% |
| Duration of DM (years) | 14.1 ± 9.6 |
| A1C (% NGSP) | 7.1 ± 1.3% |
| Pattern of DM treatments | |
|
3.8% |
|
72.3% |
|
5.6% |
|
18.3% |
| Comorbidities | |
|
10.9% |
|
8.6% |
|
7.5% |
|
1.5% |
| Charlson Comorbidity Index (CCI) | |
|
21.2% |
|
31.2% |
|
47.6% |
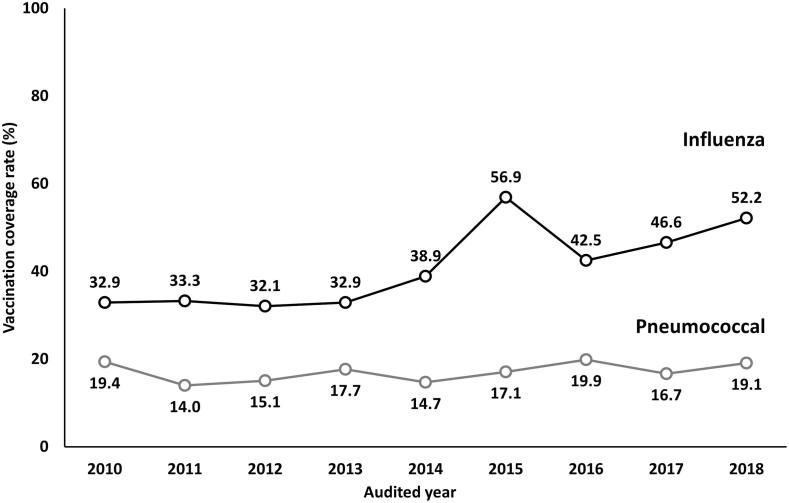
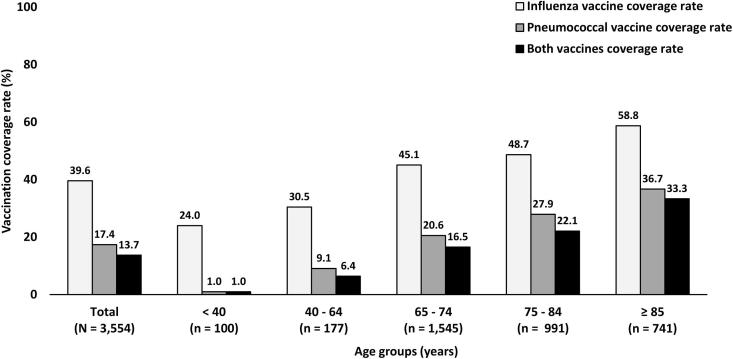
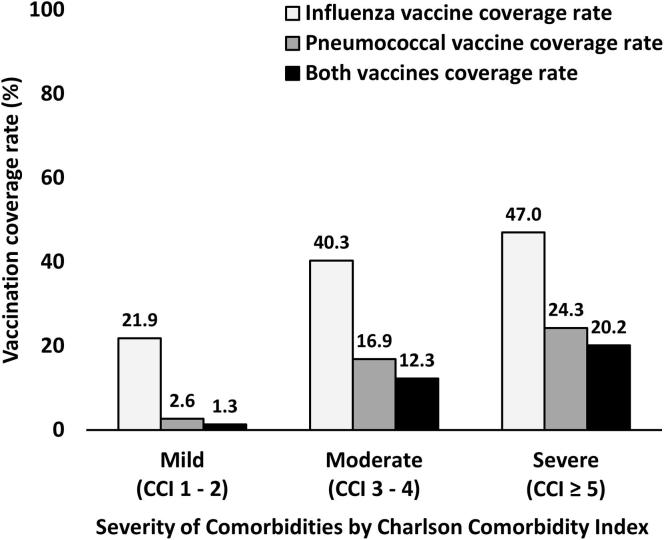
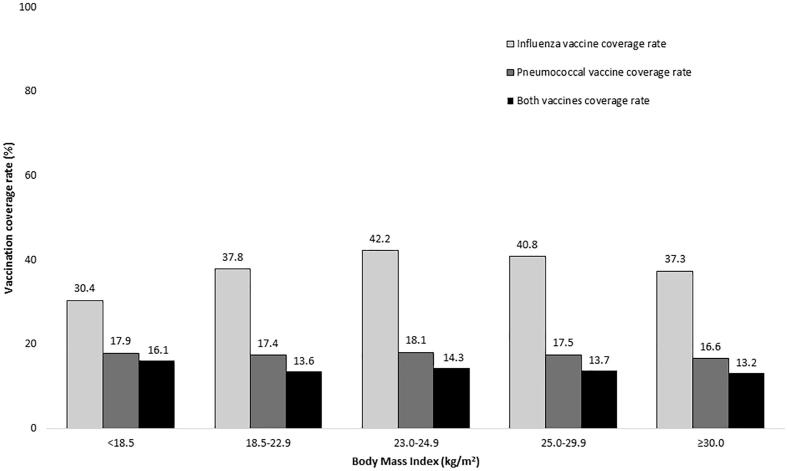
As shown in Fig. 2, influenza vaccination rates show an increasing trend going from 32.9% in 2010 to 52.2% in 2018 but pneumococcal vaccination rates remain relatively stable at less than 20%. When the vaccination coverage rates were stratified by age groups, younger patients consistently received influenza and pneumococcal vaccinations less than elderly patients as revealed in Fig. 3. However, the rate of both influenza and pneumococcal vaccinations remained suboptimal in T2DM patients aged ≥ 85 years with only one-third of these patients having received both vaccines. In T2DM patients with severe comorbidities (CCI ≥ 5), only 47.0% of these patients received influenza vaccination and only 24.3% of these patients received pneumococcal vaccination as shown in Fig. 4. When stratifying the rate of vaccinations by BMI category, the influenza vaccine coverage rate was lowest in patients with BMI < 18.5 kg/m2 when compared with other BMI categories but the pneumococcal vaccine coverage rate was lowest in patients with BMI ≥ 30 kg/m2 as shown in Fig. 5.
Fig. 2.
Trends of influenza and pneumococcal vaccination rates from 2010 to 2018 in Thai patients with T2DM.
Fig. 3.
Influenza and pneumococcal vaccination rates stratified by age groups.
Fig. 4.
Influenza and pneumococcal vaccination rates stratified by severity of comorbidities.
Fig. 5.
Influenza and pneumococcal vaccination rates stratified by BMI category.
In the univariate analysis, factors associated with influenza vaccination were older age, female, duration of DM ≥ 15 years, current smoking, the presence of chronic pulmonary disease, insulin usage, and moderate to severe CCI. However, only older age, long-duration of DM ≥ 15 years, the presence of chronic pulmonary disease, and moderate to severe CCI remained significant in the multivariate analysis as shown in Table 2. These four factors also remained significant in the multivariate analysis of associated factors in pneumococcal vaccinations as shown in Table 3. Based on multivariate analysis, T2DM patients with CCI ≥ 3 were more likely to receive influenza vaccine (OR = 1.86; CI 95% 1.49–2.33) and pneumococcal vaccine (OR = 4.97; CI 95% 3.06–8.09).
Table 2.
Associated factors for received influenza vaccination.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| Age ≥ 65 yrs | 2.13 | 1.85–2.44 | <0.001 | 1.39 | 1.17–1.64 | <0.001 |
| Female | 1.17 | 1.03–1.34 | 0.021 | 1.06 | 0.92–1.23 | 0.407 |
| BMI ≥ 30 kg/m2 | 0.89 | 0.75–1.05 | 0.166 | |||
| Duration of DM ≥ 15 yrs | 2.01 | 1.75–2.31 | <0.001 | 1.60 | 1.37–1.87 | <0.001 |
| Current smoking | 0.78 | 0.61–1.00 | 0.049 | 0.93 | 0.72–1.22 | 0.608 |
| Chronic Pulmonary diseases | 1.92 | 1.50–2.47 | <0.001 | 1.73 | 1.34–2.25 | <0.001 |
| Cardiovascular disease | 1.17 | 0.94–1.45 | 0.154 | |||
| A1C ≥ 9.0% | 1.03 | 0.80–1.32 | 0.823 | |||
| Insulin-treated regimen | 1.20 | 1.03–1.40 | 0.022 | 1.14 | 0.96–1.35 | 0.139 |
| Moderate to Severe Comorbidities (CCS ≥ 3) | 2.85 | 2.36–3.44 | <0.001 | 1.86 | 1.49–2.33 | <0.001 |
Note: Factors achieving a P-value < 0.1 from the univariate analysis were included in the multivariate models to determine associated positively factors with influenza vaccinations.
Table 3.
Associated factors for received pneumococcal vaccination.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| Age ≥ 65 yrs | 3.52 | 2.88–4.30 | <0.001 | 1.91 | 1.53–2.38 | <0.001 |
| Female | 1.15 | 0.97–1.37 | 0.120 | |||
| BMI ≥ 30 kg/m2 | 0.93 | 0.75–1.16 | 0.535 | |||
| Duration of DM ≥ 15 yrs | 2.42 | 2.03–2.90 | <0.001 | 1.55 | 1.29–1.88 | <0.001 |
| Current smoking | 0.83 | 0.60–1.15 | 0.256 | |||
| Chronic Pulmonary diseases | 3.05 | 2.34–3.98 | <0.001 | 2.64 | 2.00–3.48 | <0.001 |
| Cardiovascular disease | 1.24 | 0.95–1.62 | 0.108 | |||
| A1C ≥ 9.0% | 0.85 | 0.61–1.19 | 0.351 | |||
| Insulin-treated regimen | 1.09 | 0.89–1.33 | 0.395 | |||
| Moderate to Severe Comorbidities (CCS ≥ 3) | 9.99 | 6.35–15.71 | <0.001 | 4.97 | 3.06–8.09 | <0.001 |
Note: Factors achieving a P-value < 0.1 from the univariate analysis were included in the multivariate models to determine associated positively factors with pneumococcal vaccinations.
Discussions
Our main findings from this study were that the trends of influenza vaccination rates increased over the 9-year study period but remained suboptimal as half of T2DM patients did not receive influenza vaccination. For pneumococcal vaccination, the rate of vaccination was relatively stable at less than 20% throughout the study period. The rate of both influenza and pneumococcal vaccinations varied considerably from 0 to 44% among our diabetologists. The strongest predictive factors to receive both influenza and pneumococcal vaccinations were the presence of moderate to severe co-morbiditis. Our results implied that T2DM had largely been ignored as an indicator for influenza and pneumococcal vaccines unless the patients have complex comorbidities. These results highlight the need for pro-active vaccination service to younger patients with T2DM.
In Thailand, the Ministry of Public Health has been providing seasonal influenza vaccine to people with chronic illness free of charge since 2008. However, eligible patients must receive influenza vaccine from the public sector or private providers who participate in the National Health Insurance Plan [21]. Despite having this program in place for over a decade, the uptake of influenza vaccination among Thai people remained suboptimal. For pneumococcal vaccine, the patients must cover the cost of this vaccine themselves. Therefore, the pneumococcal vaccine coverage rate is much lower than in other countries [22], [23]. Beside socio-economic status, attitudes and beliefs of eligible patients toward vaccination play a major role in the decision-making process [24], [25]. Adult vaccination is often undervalued and there is a lack of assertiveness from healthcare professionals when compared with childhood vaccination. Despite more opportunities to get vaccinated in patients with T2DM when visiting routine outpatient clinics, vaccination rates for these populations have been suboptimal all over the world. Vaccination coverage has been set as one of the key measures for successful diabetes program and also a key public health action to prevent the spread of infectious diseases [4]. Physicians and related healthcare professionals should take a role to review immunization histories and to provide vaccinations for their patients. Our present results suggest that active hospital-based vaccination in adult patients with T2DM needs improvement and more effective reminder systems for attending diabetologists should be implemented.
Based on our study, the different vaccine coverage rates by different age groups imply that different targeted interventions should be employed. Younger T2DM patients should receive more information and be more aware of the importance of vaccines as one of the core components in diabetes care.
However, healthcare professionals rarely offer information or discuss routine vaccinations with people with diabetes in busy clinic settings. Insufficient information to patients from healthcare professionals, and/or lack of assertiveness by treating physicians had been cited as one of the main barriers to increase vaccination rates [25]. Attitudes towards vaccination have changed over time especially in vaccine-hesitant individuals [26]. Some patients who refused vaccines in the past might change their minds to actively receive vaccines during pandemic period or if their physician insists on the importance of vaccine as a part of their diabetes care. Vaccine hesitancy exists across all socioeconomic strata of the population. Patient-oriented and trust-worthy information should be offered to these people.
Our findings are also in keeping with a recent study from France that falling vaccine coverage rate could be observed after a flu pandemic [27]. As the trend of influenza vaccination rate in our cohort continuously increased until the peak rate at almost 60% in 2015 and then the trend dropped the year after. There was a peak period of seasonal flu activity in Thailand during 2015–2016. This phenomenon is explained by the possibility of vaccine hesitancy effect regarding its effectiveness against the pandemic [28]. Another possible explanation is our hospital policy to start collecting influenza vaccination as a key quality improvement program since 2015. Therefore, it could affect the surge of vaccination rate in the first year after implementation. However, these observations underscore the need for sustainable interventions to increase vaccination uptake. Even among European Union countries, none could achieve the target for influenza vaccination of 75% in people living with chronic disease (the median uptake rate was only 50%) [9]. Global efforts to increase seasonal influenza vaccine coverage rate must involve more proactive strategies from healthcare providers around the world to prevent the future influenza pandemic.
During influenza outbreaks, pneumococcal vaccines may help prevent secondary pneumococcal infections [29]. The rate of pneumococcal vaccination uptakes in patients with T2DM are variable and difficult to benchmark when compared with influenza vaccine coverage rate. In contrast to some higher income countries such as South Korea which the coverage rate of PPV dramatically increased after the national policy to give free vaccine in all elderly Koreans aged ≥ 65 years [17], pneumococcal vaccine has to be paid out-of-pocket in Thailand. Although pediatricians generally recommend PCV in infants, it has not been included in the National Immunization Program and the vaccine coverage is still low in children [30]. PCV13 vaccine has been approved for adults in Thailand in immunocompromized conditions and people with diabetes age ≥ 65 years old since 2011 but lack data to establish cost-effectiveness in general people. As a result, less than 5–10% of Thai people with T2DM received pneumococcal vaccination from a previous study in Thailand [31]. As shown in this study, the coverage rate of our patients with severe comorbidities was almost 10 times greater than patients with mild comorbidities. This data implies that the importance of pneumococcal vaccination among the general T2DM people has been overlooked and continuing efforts to increase pneumococcal vaccination should be emphasized in all people with diabetes, not only people with more complex comorbidities. In light of the ongoing of an outbreak of coronavirus disease 2019 (COVID-19) caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), routine vaccination of available vaccines and rules of sick day managements became increasingly important to keep people with diabetes from preventable illnesses [32]. Because symptoms and signs of seasonal flu and flu-like illness infections could be similar to COVID-19, counseling regarding routine influenza and pneumococcal vaccination should be provided to all patients and the role of telehealth or home vaccination by healthcare personnel might be applied in order to get all unvaccinated patients to receive both vaccines.
It is important to note that our study has several limitations. First, this study was conducted only in a private hospital in Bangkok. The findings could not be generalized to other patients with T2DM in Thailand. The socio-economic factor influences the decision to receive both vaccines in many patients. In private settings, the patients have to pay the cost of influenza and pneumococcal vaccine for themselves. Therefore, this study may have underestimated the rate of influenza vaccination if patients did not inform the treating physicians that they were already received influenza vaccination from other places. For the pneumococcal vaccination, our vaccine coverage rate may be overestimated from the patients with higher socio-economic status in private settings. Similarly, the health beliefs and knowledge of influenza among various diabetologists in our hospital could affect the overall rate of vaccination in our study. But our study could serve as a reference for future studies on vaccinations among Thai people with diabetes. Second, data on vaccination in some patients were documented by treating physicians as self-report data from their patients. The recall bias from these patients could affect the results. However, an earlier study reported that self-reporting may be the only effective and feasible way to gather data from diverse population [33]. Additionally, this study was conducted based on medical audits over the 9-year period with a uniform format. It would not affect greatly the trend of vaccination coverage rates. Third, some factors such as education level, economic status, previous history of vaccine-preventable hospitalizations could not be retrieved from our medical records which might affect the associated factors in receiving both vaccines [34]. Finally, the sample size of audited medical records was not planed as this study was a part of quality improvement program since 2010. The stratification by characteristics that may impact the results improves the sample quality but a convenience sample may not reliably infer to a population.
In conclusion, the completeness and timeliness of influenza and pneumococcal vaccinations were unsatisfactory in Thai patients with T2DM as reported from previous studies around the world. To the best of our knowledge, this study provides an insight into preventive care services for Thai patients with T2DM. Even though the positive trend had been observed for influenza vaccination, the pneumococcal vaccination rates especially in younger patients remained severely suboptimal. Our findings suggest that younger T2DM patients are overlooked when compared with elderly patients. An increase in vaccination coverage rates should be encouraged as a key quality improvement initiative [35]. Use of health information technology for identification of unvaccinated patients, promoting a positive attitude toward vaccination to patients, and periodic assessment with giving feedback to treating physicians should be employed to help achieve higher vaccination rates.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
CRediT authorship contribution statement
Yotsapon Thewjitcharoen: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Siriwan Butadej: Investigation, Resources. Malidaeng Areeya: Investigation, Resources. Nalin Yenseung: Investigation, Resources. Soontaree Nakasatien: Conceptualization, Methodology, Formal analysis, Writing - review & editing. Nampetch Lekpittaya: Investigation, Resources. Worawit Kittipoom: Investigation, Resources. Sirinate Krittiyawong: Investigation, Resources. Thep Himathongkam: Supervision.
Declaration of Competing Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Acknowledgments
The authors wish to thank Dr.Tinapa Himathongkam for excellent language editing and Dr. Krittadhee Karndumri for assisting in statistical analysis. Parts of this manuscript had previously been presented as a poster in International Diabetes Federation (IDF) meeting 2019, Busan, South Korea.
Funding
No source of funding was applied in this retrospective study.
Author contributions
TY, BS, MA, YN, and NS collected data, performed the statistical analyses, interpreted the data and drafted the manuscript. LN, KW, KS, and HT contributed to interpretation of the data and revised the manuscript critically before submission. KS and HT made substantial contributions to the discussion of results. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Ethical approval and consent to participant
This retrospective study is approved by the Ethics board committee of Theptarin Hospital (No.06/2018). No inform consent to participant was required as a retrospective study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100227.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Remschmidt C., Wichmann O., Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta-analysis. BMC Med. 2015;13:53. doi: 10.1186/s12916-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith S.A., Poland G.A. Use of influenza and Pneumococcal vaccines in people with diabetes. Diabetes Care. 2000;23:95–108. doi: 10.2337/diacare.23.1.95. [DOI] [PubMed] [Google Scholar]
- 3.Song J.Y., Nahm M.H., CheongKim H.J. WJ. Impact of preceding flu-like illness on the serotype distribution of pneumococcal pneumonia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43:S37-47. [DOI] [PubMed]
- 5.Diabetes Canada Clinical Practice Guidelines Expert Committee. Influenza, Pneumococcal, Hepatitis B and Herpes zoster vaccinations. Can J Diabetes 2018;42:S142-4. [DOI] [PubMed]
- 6.Haneda M., Noda M., Origasa H., Noto H., Yabe D., Fujita Y. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9:657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldenkar A., Lim F., Yung C.F., Lwin M.O. Acceptance and uptake of influenza vaccines in Asia: a systematic review. Vaccine. 2019;37:4896–4905. doi: 10.1016/j.vaccine.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Influenza vaccines: WHO position paper [J]. Wkly Epidemiol Rec 2005;80:27-8. [PubMed]
- 9.Jorgensen P., Mereckiene J., Cotter S., Johansen K., Tsolova S., Brown C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36:442–452. doi: 10.1016/j.vaccine.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palache A., Oriol-Mathieu V., Abelin A., Music T. Influenza Vaccine Supply task force (IFPMA IVS). Seasonal influenza vaccine dose distribution in 157 countries (2004–2011) Vaccine. 2014;32:6369–6376. doi: 10.1016/j.vaccine.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert J.A. Seasonal and pandemic influenza: global fatigue versus global preparedness. Lancet Respir Med. 2018;6:94–95. doi: 10.1016/S2213-2600(17)30466-6. [DOI] [PubMed] [Google Scholar]
- 12.Kyaw M.H., Rose C.E., Jr, Fry A.M., Singleton J.A., Moore Z., Zell E.R. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi: 10.1086/431521. [DOI] [PubMed] [Google Scholar]
- 13.CDC. Use of PCV13 and PPSV23 vaccine for adults with immunocompromising conditions. MMWR 2012;61:816-9. [PubMed]
- 14.Infectious Disease Society of Thailand: Recommended adult and elderly immunization schedule 2018. Available from: https://ddc.moph.go.th/uploads/ckeditor/c20ad4d76fe97759aa27a0c99bff6710/files/adult.pdf [Accessed 25 April 2020].
- 15.Owusu J.T., Prapasiri P., Ditsungnoen D., Leetongin G., Yoocharoen P., Rattanayot J. Seasonal influenza vaccine coverage among high-risk populations in Thailand, 2010–2012. Vaccine. 2015;33:742–747. doi: 10.1016/j.vaccine.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M.C., Chou Y.L., Lee P.L., Yang Y.C., Chen K.T. Influenza vaccination coverage and factors affecting adherence to influenza vaccination among patients with diabetes in Taiwan. Hum VaccinImmunother. 2014;10:1028–1035. doi: 10.4161/hv.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T.U., Song J.Y., Noh J.Y., Cheong H.J., Kim W.J. Influenza and Pneumococcal vaccine coverage rates among patients admitted to a Teaching Hospital in South Korea. Infect Chemother. 2015;47:41–48. doi: 10.3947/ic.2015.47.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Yue N., Zheng M. Influenza vaccination coverage of population and the factors influencing influenza vaccination in mainland China: a meta-analysis. Vaccine. 2018;36:7262–7269. doi: 10.1016/j.vaccine.2018.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y.Q., Gou R., Diao Y.S., Yin Q.H., Fan W.X., Liang Y.P. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15:58–66. doi: 10.1631/jzus.B1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muangchana C., Thamapornpilas P., Karnkawinpong O. Immunization policy development in Thailand: the role of the Advisory Committee on Immunization Practice. Vaccine. 2010;28(Suppl 1):A104–A109. doi: 10.1016/j.vaccine.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Yang TU, Kim E, Park YJ, Kim D, Kwon YH, Shin JK, et al. Successful introduction of an underutilized elderly pneumococcal vaccine in a national immunization program by integrating the pre-existing public health infrastructure.Vaccine 201 18;34:1623-9. [DOI] [PubMed]
- 23.Naito T., Yokokawa H., Watanabe A. Impact of the national routine vaccination program on 23-valent pneumococcal polysaccharide vaccine vaccination rates in elderly persons in Japan. J Infect Chemother. 2018;24:496–498. doi: 10.1016/j.jiac.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Casalino E., Ghazali A., Bouzid D., Antoniol S., Pereira L., Kenway P. Patient’s behaviors and missed opportunities for vaccination against seasonal epidemic influenza and evaluation of their impact on patient’s influenza vaccine uptake. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayama M., Wetmore C.M., Mokdad A.H. Characteristics associated with the uptake of influenza vaccination among adults in the United States. Prev Med. 2012;54:358–362. doi: 10.1016/j.ypmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Lane S., MacDonald N.E., Marti M., Dumolard L. Vaccine hestiancy around the globe: analysis of three years of WHO/UNICEF Joint Reporting Form data 2015–2017. Vaccine. 2018;36:3861–3867. doi: 10.1016/j.vaccine.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verger P., Fressard L., Cortaredona S., Lévy-Bruhl D., Loulergue P., Galtier F. Trends in seasonal influenza vaccine coverage of target groups in France, 2006 to 2015: impact of recommendations and 2009 influenza a(H1N1) pandemic. Euro Surveill. 2018;23:1700801. doi: 10.2807/1560-7917.ES.2018.23.48.1700801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocquier A., Cortaredona S., Fressard L., Loulergue P., Raude J., Sultan A. Trajectories of seasonal influenza vaccine uptake among French people with diabetes: a nationwide retrospective cohort study, 2006–2015. BMC Public Health. 2019;19:918. doi: 10.1186/s12889-019-7209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.Y., Tang X.F., Du C.H., Wang B.B., Bi Z.W., Dong B.R. Comparison of dual influenza and pneumococcal polysaccharide vaccination with influenza vaccination alone for preventing pneumonia and reducing mortality amongthe elderly: a meta-analysis. Hum VaccinImmunother. 2016;12:3056–3064. doi: 10.1080/21645515.2016.1221552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulpeng W., Leelahavarong P., Rattanavipapong W., Sornsrivichai V., Baggett H.C., Meeyai A. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31:2839–2847. doi: 10.1016/j.vaccine.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalermsri C, Paisansudhi S, Kantachuvesiri P, Pramyothin P, Washirasaksri C, Srivanichakorn, et al. The effectiveness of holistic diabetic management between Siriraj Continuity of Care clinic and medical out-patient department. J Med Assoc Thai 2014;97 (Suppl 3):S197-205. [PubMed]
- 32.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopskin D, Birkenfeld AL, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020. Published Online April 23, 2020 Available from: DOI:10.1016/S2213-8587(20)30152-2 [Accessed 25 April 2020]. [DOI] [PMC free article] [PubMed]
- 33.Mac Donald R., Baken L., Nelson A., Nichol K.L. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med. 1999;16:173–177. doi: 10.1016/s0749-3797(98)00159-7. [DOI] [PubMed] [Google Scholar]
- 34.Fan J., Cong S., Wang N., Bao H., Wang B., Feng Y. Influenza vaccination rate and its association with chronic diseases in China: results of a national cross-sectional study. Vaccine. 2020;38:2503–2511. doi: 10.1016/j.vaccine.2020.01.093. [DOI] [PubMed] [Google Scholar]
- 35.Tan L.L.J. A review of the key factors to improve adult immunization coverage rates: what can the clinician do? Vaccine. 2018;36:5373–5378. doi: 10.1016/j.vaccine.2017.07.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.