Abstract
Background
Crucial roles of hematologic and immunologic responses in progression of coronavirus disease 2019 (COVID-19) remain largely unclear.
Objective
We sought to address the dynamic changes in hematologic and immunologic biomarkers and their associations with severity and outcomes of COVID-19.
Methods
A retrospective study including 548 patients with COVID-19 with clarified outcome (discharged or deceased) from a national cohort in China was performed. Cross-sectional and longitudinal variations were compared and the associations with different severity and outcomes were analyzed.
Results
On admission, the counts of lymphocytes, T-cell subsets, eosinophils, and platelets decreased markedly, especially in severe/critical and fatal patients. Increased neutrophil count and neutrophils-to-lymphocytes ratio were predominant in severe/critical cases or nonsurvivors. During hospitalization, eosinophils, lymphocytes, and platelets showed an increasing trend in survivors, but maintained lower levels or dropped significantly afterwards in nonsurvivors. Nonsurvivors kept a high level or showed an upward trend for neutrophils, IL-6, procalcitonin, D-dimer, amyloid A protein, and C-reactive protein, which were kept stable or showed a downward trend in survivors. Positive correlation between CD8+ T-cell and lymphocytes count was found in survivors but not in nonsurvivors. A multivariate Cox regression model suggested that restored levels of lymphocytes, eosinophils, and platelets could serve as predictors for recovery, whereas progressive increases in neutrophils, basophils, and IL-6 were associated with fatal outcome.
Conclusions
Hematologic and immunologic impairment showed a significantly different profile between survivors and nonsurvivors in patients with COVID-19 with different severity. The longitudinal variations in these biomarkers could serve to predict recovery or fatal outcome.
Key words: Hematologic indices, immunologic responses, COVID-19, severity, outcome
Abbreviations used: COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; HR, Hazard ratio; MERS, Middle East respiratory syndrome; NLR, Neutrophils-to-lymphocytes ratio; PCT, Procalcitonin; PLR, Platelets-to-lymphocytes ratio; SAA, Amyloid A protein; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Graphical abstract
On December 31, 2019, a cluster of pneumonia cases of unknown origins in Wuhan linked to exposure to a seafood market was reported.1 The causative agent was determined to be a novel β-coronavirus, which is currently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 The disease has been named coronavirus disease 2019 (COVID-19) by the World Health Organization. Until April 21, 2020, the outbreak of COVID-19 was reported in more than 2.3 million confirmed cases globally.3 Despite a reportedly lower fatality rate compared with the 10% fatality rate of severe acute respiratory syndrome (SARS)4 and 37% of Middle East respiratory syndrome (MERS),5 COVID-19 has killed 4642 people in China and 158,314 people outside China (by April 21, 2020).3
Studies have revealed some changes in hematologic and immunologic indices in patients with COVID-19.6, 7, 8 The acute phase of the disease was found to be marked lymphopenia, involving a dramatic loss of CD4+ T and CD8+ T cells.9 , 10 Several studies on SARS-CoV,11 MERS-CoV,12 or SARS-CoV-213 infections have investigated the correlation between abnormalities on laboratory indices including leukocytes, lymphocytes, and eosinophils counts, serum inflammatory cytokine levels, and the severity or mortality of the diseases. MERS-CoV infection induced T cells’ apoptosis by a combination of extrinsic and intrinsic apoptosis pathways, which might contribute to virus spread and the severe immunopathologic dysregulations.14 However, little is known about how different lymphocyte subsets and the kinetic changes in immuno-related biomarkers differ during different prognosis and outcomes of COVID-19. More importantly, whether these changes can help to predict the outcome of the disease remain largely unclear.
In this retrospective study, we aimed to analyze clinical manifestations and outcomes, hematologic, immunologic, and lymphocyte subsets, and infection-related bioindices in laboratory-confirmed cases based on a national cohort. With multivariate Cox regression analysis, we attempted to investigate the potential risk factors associated with the outcome of the disease.
Methods
Led by the China National Health Commission, a retrospective cohort to study the laboratory-confirmed COVID-19 admitted cases from 575 hospitals throughout China was established.6 The diagnosis of COVID-19 was made on the basis of World Health Organization interim guidance.15 By March 22, 2020, a total of 548 cases with the clarified outcome (discharged or deceased) from the cohort were included and patients with incomplete medical records or still in the hospitals were excluded. The severity of the disease was assessed according to the Seventh Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance from the National Health Commission of China.16 According to the severity, patients were categorized into mild/moderate, severe, or critical group upon admission. The study was approved by the National Health Commission and the Ethics Commission of the First Affiliated Hospital of Guangzhou Medical University. The requirement for informed consent was waived by the Ethics Commission because of the emerging infectious disease, which had been described previously.6
Data extraction
All medical records were copied and sent to the data processing center in Guangzhou, under the coordination of the National Health Commission. A team of experienced respiratory clinicians reviewed and abstracted the data. Data were entered into a computerized database and cross-checked. The clinical data (including demographic data, clinical symptoms and signs, comorbidities, laboratory findings, treatments during hospitalization, and clinical outcome) were extracted from electronic medical records. Laboratory assessments consisted of complete blood cell counts, T-cell subsets, inflammatory indices, coagulation function, and so forth. The differences in the clinical characteristics and laboratory findings in patients with different outcome or severity would be addressed. Longitudinal tracing of the laboratory indices during the hospitalization were performed. Furthermore, independent predicting factors associated with the fatal outcome would also be investigated.
Statistical analysis
Continuous variables with normal distribution were described using mean ± SD; otherwise, using median and interquartile range. Categorical variables were described as number (percentage). We conducted t test and Mann-Whitney test to identify differences between survivors and nonsurvivors for continuous variables. ANOVA or Kruskal-Wallis test was performed to compare the difference among 3 groups of patients with different severity, and then Tukey or Dunn test was used for multiple comparisons as appropriate. Chi-square test and Fisher exact test were applied to categorical variables, with false-discovery rate correction for multiple comparisons. We calculated the pairwise Spearman correlations (r) between indexes for different time or survivor/nonsurvivor outcome. We selected correlation strength by an absolute correlation |r| > 0.2. The selected connections were plotted as an undirected network graph, with the width of the edge proportional to correlation strength. A principal-component analysis of variables on admission or at end-hospitalization was conducted using R package FactoMineR (https://cran.r-project.org/web/packages/FactoMineR/, v1.42, R foundation, Vienna, Austria). Biplot was used to signify the proportions of the individual variable on principal components.
Differences in indexes between end-hospitalization and on admission were calculated and presented as Δ (Δindex(a) = index(a) at end-hospitalization minus index(a) on admission). Survival curves were depicted using the Kaplan-Meier method and compared using the log-rank test. The prognostic effect of each variable including Δindex was clarified by Cox proportional hazards regression model. A final model selection was performed by a backward selection of all factors with the Akaike information criterion. Estimates of adjusted hazard ratios (HRs), 95% CI, and P value are displayed. Analyses were conducted using R software, version 3.6.1 (http://CRAN.R-project.org, R Foundation, Vienna, Austria). All P values were based on 2-sided tests and were considered statistically significant at P < .05.
Results
Demographic and baseline clinical characteristics
A total of 548 patients with laboratory-confirmed diagnosis of COVID-19 were included, with 345 patients categorized into mild/moderate, 155 severe, and 48 critical degree on admission. The fatality rate was 60% in critical cases, followed by 34% in severe and 6% in mild/moderate patients (P < .001). The mean age for all patients was 56 years, with significantly older age for nonsurvivors than for survivors (66.9 vs 53.5 years; P < .001). Among all nonsurvivors, 67% were males, reaching 83% in critical patients. Symptoms of cough, sputum, and dyspnea were found significantly more common in severe or critical patients compared with mild/moderate counterparts (all P < .05), and in nonsurvivors as compared with survivors (all P < .05). Two hundred forty-one (44%) patients had at least 1 underlying comorbidity, of which 27% had hypertension, 11% had diabetes, and 3% had cerebrovascular disease, which was significantly higher in nonsurvivors than in survivors (all P < .001). Only 2 (0.4%) patients reported having asthma and 18 (3%) reported other allergic diseases, mostly drug hypersensitivity (Table I ).
Table I.
Demographic and baseline characteristics of patients with COVID-19 with different severity and outcome
| Characteristic | All patients |
Mild/moderate |
Severe |
Critical |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 548) | Survivors (n = 445) | Nonsurvivors (n = 103) | Total (n = 345) | Survivors (n = 324) | Nonsurvivors (n = 21) | Total (n = 155) | Survivors (n = 102) | Nonsurvivors (n = 53) | Total (n = 48) | Survivors (n = 19) | Nonsurvivors (n = 29) | |
| Demographic | ||||||||||||
| Survival or death rate (%) | 81.2∗ | 18.8 | 93.9∗ | 6.1 | 65.8∗ | 34.2 | 39.6 | 60.4 | ||||
| Days from symptom onset to admission | 13 (9-18) | 13 (10-18)∗ | 9 (6-17) | 13 (9-16) | 13 (9-16) | 14 (7-18) | 15 (8-21)† | 17 (11-24)∗ | 9 (6-18) | 9 (6-23) | 23 (12-54)∗ | 7 (3-9) |
| Days from symptom onset to end-hospitalization | 22 (17-29) | 23 (18-29)∗ | 20 (14-30) | 22 (18-27) | 22 (18-26) | 22 (19-30) | 25 (17-32)† | 27 (19-34)∗ | 19 (14-30) | 22 (18-39)† | 40 (27-61)∗ | 19 (12-22) |
| Sex, n (%) | ||||||||||||
| Male | 313 (57.1) | 244 (54.8)‡ | 69 (67.0) | 182 (52.8) | 170 (52.5) | 12 (57.1) | 93 (60.0) | 60 (58.8) | 33 (62.3) | 38 (79.2)†§ | 14 (73.7) | 24 (82.8) |
| Age (y) | ||||||||||||
| Mean ± SD | 56.0 ± 14.5 | 53.5 ± 13.9∗ | 66.9 ± 12.1 | 67.3 ± 12.1 | 67.5 ± 12.2 | 64.5 ± 9.3 | 60.9 ± 13.8† | 58.1 ± 13∗ | 64 ± 14.1 | 61.4 ± 13.6† | 56.3 ± 16 | 64.8 ± 10.9 |
| >60, n (%) | 223 (40.7) | 144 (32.4)∗ | 79 (76.7) | 106 (30.7) | 92 (28.4)∗ | 14 (66.7) | 85 (54.8)† | 42 (41.2)∗ | 43 (81.1) | 32 (66.7)† | 10 (52.6) | 22 (75.9) |
| Weight (kg) | ||||||||||||
| Mean ± SD | 68.3 ± 13.6 | 68.6 ± 14 | 66.4 ± 10.1 | 67.3 ± 12.1 | 67.5 ± 12.2 | 64.5 ± 9.3 | 70.3 ± 16.8 | 67.5 ± 11.5∗ | 76.6 ± 24 | 71.3 ± 14.2 | 75.8 ± 15.4 | 64.9 ± 9.9 |
| Ever smoke, n (%) | 32 (5.8) | 20 (4.5) | 12 (11.7) | 16 (4.6) | 14 (4.3) | 2 (9.5) | 10 (6.5) | 4 (3.9) | 6 (11.3) | 6 (12.5) | 2 (10.5) | 4 (13.8) |
| Clinical manifestations, n (%) | ||||||||||||
| Fever | 513 (93.6) | 413 (92.8) | 100 (97.1) | 317 (91.9) | 297 (91.7) | 20 (95.2) | 150 (96.8) | 98 (96.1) | 52 (98.1) | 46 (95.8) | 18 (94.7) | 28 (96.6) |
| Highest temperature (°C), median (IQR) | 38.6 (38.0-39.0) | 38.6 (38.0-39.0) | 38.5 (38.0-39.0) | 38.6 (38.1-39.0) | 38.7 (38.1-39.0) | 38.5 (38.1-39.0) | 38.5 (38.0-39.0) | 38.5 (38.0-39.0) | 38.8 (38.0-39.0) | 38.3 (38.0-39.0) | 38.1 (37.8-39.0) | 38.5 (38.0-39.0) |
| Rhinobyon | 14 (2.6) | 9 (2.0) | 5 (4.9) | 7 (2.0) | 6 (1.9) | 1 (4.8) | 5 (3.2) | 3 (2.9) | 2 (3.8) | 2 (4.2) | 0 (0.0) | 2 (6.9) |
| Headache | 36 (6.6) | 29 (6.5) | 7 (6.8) | 27 (7.8) | 25 (7.7) | 2 (9.5) | 5 (3.2) | 3 (2.9) | 2 (3.8) | 4 (8.3) | 1 (5.3) | 3 (10.3) |
| Cough | 416 (75.9) | 329 (73.9)‡ | 87 (84.5) | 243 (70.4) | 226 (69.8) | 17 (81.0) | 133 (85.8)† | 87 (85.3) | 46 (86.8) | 40 (83.3) | 16 (84.2) | 24 (82.8) |
| Sore throat | 18 (3.3) | 12 (2.7) | 6 (5.8) | 12 (3.5) | 10 (3.1) | 2 (9.5) | 5 (3.2) | 2 (2.0) | 3 (5.7) | 1 (2.1) | 0 (0.0) | 1 (3.4) |
| Sputum | 170 (31.0) | 126 (28.3)∗ | 44 (42.7) | 96 (27.8) | 85 (26.2)‡ | 11 (52.4) | 51 (32.9) | 32 (31.4) | 19 (35.8) | 23 (47.9)‖ | 9 (47.4) | 14 (48.3) |
| Fatigue | 186 (33.9) | 155 (34.8) | 31 (30.1) | 122 (35.4) | 114 (35.2) | 8 (38.1) | 46 (29.7) | 36 (35.3) | 10 (18.9) | 18 (37.5) | 5 (26.3) | 13 (44.8) |
| Hemoptysis | 9 (1.6) | 5 (1.1) | 4 (3.9) | 4 (1.2) | 3 (0.9) | 1 (4.8) | 4 (2.6) | 1 (1.0) | 3 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 242 (44.2) | 170 (38.2)∗ | 72 (69.9) | 114 (33.0) | 105 (32.4) | 9 (42.9) | 94 (60.6)† | 53 (52.0)∗ | 41 (77.4) | 34 (70.8)† | 12 (63.2) | 22 (75.9) |
| Vomiting | 18 (3.3) | 13 (2.9) | 5 (4.9) | 8 (2.3) | 8 (2.5) | 0 (0.0) | 7 (4.5) | 4 (3.9) | 3 (5.7) | 3 (6.3) | 1 (5.3) | 2 (6.9) |
| Diarrhea | 14 (2.6) | 13 (2.9) | 1 (1.0) | 11 (3.2) | 11 (3.4) | 0 (0.0) | 3 (1.9) | 2 (2.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myalgia | 67 (12.2) | 56 (12.6) | 11 (10.7) | 44 (12.8) | 42 (13.0) | 2 (9.5) | 20 (12.9) | 13 (12.7) | 7 (13.2) | 3 (6.3) | 1 (5.3) | 2 (6.9) |
| Shiver | 48 (8.8) | 39 (8.8) | 9 (8.7) | 30 (8.7) | 29 (9.0) | 1 (4.8) | 12 (7.7) | 7 (6.9) | 5 (9.4) | 6 (12.5) | 3 (15.8) | 3 (10.3) |
| Comorbidities, n (%) | ||||||||||||
| Asthma | 2 (0.4) | 1 (0.2) | 1 (1.0) | 1 (0.3) | 1 (0.3) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other allergic diseases | 18 (3.3) | 15 (3.4) | 3 (2.9) | 12 (3.5) | 12 (3.7) | 0 (0.0) | 5 (3.2) | 2 (2.0) | 3 (5.7) | 1 (2.1) | 1 (5.3) | 0 (0.0) |
| COPD | 7 (1.3) | 2 (0.4)∗ | 5 (4.9) | 2 (0.6) | 0 (0.0)∗ | 2 (9.5) | 3 (1.9) | 1 (1.0) | 2 (3.8) | 2 (4.2) | 1 (5.3) | 1 (3.4) |
| Diabetes | 61 (11.1) | 41 (9.2)∗ | 20 (19.4) | 33 (9.6) | 31 (9.6) | 2 (9.5) | 23 (14.8) | 7 (6.9)∗ | 16 (30.2) | 5 (10.4) | 3 (15.8) | 2 (6.9) |
| Hypertension | 148 (27.0) | 103 (23.1)∗ | 45 (43.7) | 73 (21.2) | 64 (19.8)‡ | 9 (42.9) | 52 (33.5)† | 33 (32.4) | 19 (35.8) | 23 (47.9)† | 6 (31.6) | 17 (58.6) |
| Coronary heart disease | 35 (6.4) | 24 (5.4) | 11 (10.7) | 14 (4.1) | 13 (4.0) | 1 (4.8) | 14 (9.0) | 10 (9.8) | 4 (7.5) | 7 (14.6)‖ | 1 (5.3) | 6 (20.7) |
| Cerebrovascular disease | 16 (2.9) | 8 (1.8)∗ | 8 (7.8) | 5 (1.4) | 2 (0.6)∗ | 3 (14.3) | 9 (5.8)‖ | 4 (3.9) | 5 (9.4) | 2 (4.2) | 2 (10.5) | 0 (0.0) |
| Hepatitis | 11 (2.0) | 9 (2.0) | 2 (1.9) | 6 (1.7) | 5 (1.5) | 1 (4.8) | 3 (1.9) | 3 (2.9) | 0 (0.0) | 2 (4.2) | 1 (5.3) | 1 (3.4) |
| Cancer | 17 (3.1) | 14 (3.1) | 3 (2.9) | 11 (3.2) | 10 (3.1) | 1 (4.8) | 5 (3.2) | 4 (3.9) | 1 (1.9) | 1 (2.1) | 0 (0.0) | 1 (3.4) |
| Renal diseases | 13 (2.4) | 11 (2.5) | 2 (1.9) | 6 (1.7) | 6 (1.9) | 0 (0.0) | 6 (3.9) | 4 (3.9) | 2 (3.8) | 1 (2.1) | 1 (5.3) | 0 (0.0) |
Data are presented as mean ± SD, medians (IQR), and n/N (%).
COPD, Chronic obstructive pulmonary disease; IQR, interquartile range.
P < .01, survivors compared with nonsurvivors.
P < .01, compared with mild/moderate.
P < .05, survivors compared with nonsurvivors.
P < .05, compared with severe.
P < .05, compared with mild/moderate.
Initial laboratory findings among hospitalized patients with different outcome and severity
On admission, the hematologic profile varied among patients with different outcome and severity (Table II ). Compared with the survivors, eosinopenia, lymphopenia, and decreased platelets were much common in nonsurvivors (all P < .05), which accounted for 80.5%, 96.6%, and 34.5%, respectively. The trend of the gradual decreasing of eosinophils and lymphocytes among patients with varying degree of severity was identical. In contrast, nonsurvivors showed significantly elevated level of leukocytes, neutrophils, the neutrophils-to-lymphocytes ratio (NLR), and the platelets-to-lymphocytes ratio (PLR) (all P < .05). Moreover, compared with mild/moderate, severe and critical patients showed a higher incidence of abnormal leukocytes, neutrophils, NLR, and PLR.
Table II.
Initial laboratory findings among hospitalized patients with different outcome and severity
| Laboratory findings | All patients |
Patients with different outcome |
Patients with different severity |
|||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 548) | Survivors (n = 445) | Nonsurvivors (n = 103) | P value | Mild/moderate (n = 345) | Severe (n = 155) | Critical (n = 48) | P value | |
| Hematologic | ||||||||
| Leukocyte (×109/L) | 6.09 (4.23-8.83) | 5.70 (4.05-7.98) | 9.12 (6.02-12.92) | <.001 | 5.36 (3.95-7.47) | 7.55 (4.67-10.81)∗ | 10.22 (8.06-15.13)∗† | <.001 |
| <3.5 or >9.5 | 189/516 (36.63) | 146/429 (34.03) | 43/87 (49.43) | .0095 | 98/334 (29.34) | 70/147 (47.62)∗ | 21/35 (60.00)∗ | <.001 |
| Neutrophils (×109/L) | 4.53 (2.80-7.58) | 3.96 (2.51-6.33) | 8.18 (5.21-11.65) | <.001 | 3.74 (2.37-5.80) | 6.35 (3.38-9.69)∗ | 9.56 (7.03-13.79)∗† | <.001 |
| <1.8 or >6.3 | 212/506 (41.90) | 151/419 (36.04) | 61/87 (70.11) | <.001 | 103/329 (31.31) | 81/143 (56.64)∗ | 28/34 (82.35)∗‡ | <.001 |
| Lymphocytes (×109/L) | 0.90 (0.61-1.25) | 0.98 (0.70-1.32) | 0.57 (0.46-0.74) | <.001 | 1.04 (0.73-1.38) | 0.69 (0.51-0.97)∗ | 0.59 (0.43-0.78)∗ | <.001 |
| <1.1 | 339/513 (66.08) | 255/426 (59.86) | 84/87 (96.55) | <.001 | 183/334 (54.79) | 125/145 (86.21)∗ | 31/34 (91.18)∗ | <.001 |
| Monocytes (×109/L) | 0.34 (0.23-0.47) | 0.34 (0.23-0.47) | 0.29 (0.21-0.48) | .25 | 0.34 (0.23-0.47) | 0.29 (0.22-0.50) | 0.39 (0.28-0.48) | .3 |
| <0.1 | 17/502 (3.39) | 11/415 (2.65) | 6/87 (6.90) | .096 | 9/326 (2.76) | 6/142 (4.23) | 2/34 (5.88) | .51 |
| Eosinophils (×109/L) | 0.01 (0-0.04) | 0.01 (0-0.04) | 0 (0-0.02) | .0081 | 0.01 (0-0.04) | 0 (0-0.02)§ | 0 (0-0.01) | <.001 |
| <0.03 | 336/499 (67.33) | 266/412 (64.56) | 70/87 (80.46) | .006 | 198/324 (61.11) | 110/142 (77.46)∗ | 28/33 (84.85)‡ | <.001 |
| Basophils (×109/L) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | 0.01 (0.01-0.03) | .11 | 0.01 (0.01-0.02) | 0.01 (0-0.02) | 0.02 (0.02-0.04)∗† | <.001 |
| <0.02 | 299/498 (60.04) | 254/411 (61.80) | 45/87 (51.72) | .1 | 211/323 (65.33) | 80/142 (56.34) | 8/33 (24.24)∗† | <.001 |
| Platelets (×109/L) | 200.50 (145.25-263.75) | 204.00 (156.00-273.00) | 159.00 (108.00-219.50) | <.001 | 203.00 (155.00-265.00) | 194.00 (124.50-263.00) | 209.00 (171.00-245.50) | .094 |
| <125 | 75/510 (14.71) | 45/423 (10.64) | 30/87 (34.48) | <.001 | 33/329 (10.03) | 37/146 (25.34)∗ | 5/35 (14.29) | <.001 |
| Hemoglobin (g/L) | 126.00 (115.00-137.00) | 126.00 (115.00-137.00) | 121.00 (112.00-137.50) | .36 | 127.00 (115.00-137.00) | 123.00 (115.00-135.00) | 128.00 (111.00-141.00) | .31 |
| <130 | 300/509 (58.94) | 247/422 (58.53) | 53/87 (60.92) | .77 | 189/329 (57.45) | 93/145 (64.14) | 18/35 (51.43) | .25 |
| NLR | 4.91 (2.48-10.27) | 3.71 (2.27-7.54) | 13.45 (9.33-23.60) | <.001 | 3.37 (2.05-6.65) | 8.96 (4.62-17.04)∗ | 16.06 (11.26-26.35)∗† | <.001 |
| >5 | 243/504 (48.21) | 163/417 (39.09) | 80/87 (91.95) | <.001 | 110/328 (33.54) | 100/142 (70.42)∗ | 33/34 (97.06)∗† | <.001 |
| PLR | 220.59 (149.53-325.14) | 211.36 (146.10-310.88) | 282.61 (182.95-384.62) | <.001 | 199.62 (142.86-282.76) | 270.09∗ (183.36-382.49) | 323.52§ (231.76-490.39) | <.001 |
| >300 | 153/506 (30.24) | 115/419 (27.45) | 38/87 (43.68) | .0041 | 72/328 (21.95) | 61/144 (42.36)∗ | 20/34 (58.82)∗ | <.001 |
| Lymphocyte subsets‖ | ||||||||
| CD4+ T cell (/μL) | 343.00 (191.00-562.00) | 361.00 (232.50-576.50) | 182.00 (136.50-230.00) | <.001 | 386.00 (287.50-591.00) | 226.50∗ (144.25-362.25) | 182.00 (129.00-442.00) | <.001 |
| CD8+ T cell (/μL) | 222.00 (122.00-321.00) | 240.50 (131.25-358.00) | 112.00 (52.00-141.00) | <.001 | 252.50 (168.75-371.75) | 126.50∗ (61.25-164.50) | 121.00 (63.00-277.00) | <.001 |
| CD3+ T cell (/μL) | 577.00 (354.00-884.75) | 628.00 (413.00-947.00) | 296.00 (201.00-397.50) | <.001 | 662.00 (452.00-950.00) | 367.50∗ (210.00-556.00) | 310.00 (222.00-908.00) | <.001 |
| CD45+ (/μL) | 910.00 (561.25-1291.00) | 964.00 (657.50-1356.00) | 452.00 (358.50-574.50) | <.001 | 1047.00 (725.50-1396.00) | 574.50∗ (456.25-856.25) | 624.00 (371.00-1275.00) | <.001 |
| CD4/CD8 ratio | 1.67 (1.24-2.49) | 1.55 (1.17-2.41) | 1.98 (1.65-2.65) | .035 | 1.52 (1.17-2.36) | 1.94 (1.55-2.58) | 1.63 (1.37-2.82) | .13 |
| CD4+ T-cell % | 39.28 (32.25-44.75) | 38.94 (32.01-44.90) | 40.90 (36.62-44.13) | .41 | 39.80 (33.65-44.75) | 38.39 (30.51-43.56) | 39.41 (27.84-48.16) | .74 |
| CD8+ T-cell % | 22.95 (16.58-28.95) | 23.13 (17.20-30.02) | 19.53 (13.89-24.61) | .077 | 24.20 (18.87-30.31) | 18.52 (15.57-23.29)∗ | 19.53 (13.87-30.12) | .0099 |
| CD3+ Lymphocyte % | 67.08 (57.23-71.69) | 67.03 (57.44-72.04) | 67.13 (54.76-70.93) | .67 | 68.43 (59.97-72.61) | 61.12 (48.60-71.03) | 66.10 (52.21-70.63) | .065 |
| Other indices | ||||||||
| IL-6 (pg/mL) | 7.54 (5.82-10.15) | 7.24 (5.58-9.78) | 9.74 (7.53-13.22) | <.001 | 7.40 (5.66-9.81) | 8.43 (6.18-10.52)‡ | 8.33 (5.46-12.63) | .037 |
| >7 | 242/418 (57.89) | 177/337 (52.52) | 65/81 (80.25) | <.001 | 139/256 (54.30) | 82/129 (63.57) | 21/33 (63.64) | .17 |
| SAA (mg/L) | 182.80 (73.40-249.60) | 173.70 (61.20-249.70) | 198.25 (161.45-245.25) | .0041 | 166.65 (43.93-250.30) | 198.80∗ (153.20-241.80) | 191.25 (162.50-255.80) | .0078 |
| >10 | 437/483 (90.48) | 351/397 (88.41) | 86/86 (100.00) | .0018 | 265/308 (86.04) | 139/141 (98.58)∗ | 33/34 (97.06) | <.001 |
| CRP (mg/L) | 38.30 (9.65-94.35) | 27.60 (6.33-72.85) | 108.90 (55.70-160.00) | <.001 | 20.70 (5.30-60.90) | 78.00∗ (39.85-143.00) | 73.80∗ (26.25-151.65) | <.001 |
| >5 | 414/499 (82.97) | 325/410 (79.27) | 89/89 (100.00) | <.001 | 239/317 (75.39) | 140/147 (95.24)∗ | 35/35 (100.00)∗ | <.001 |
| PCT (ng/mL) | 0.05 (0.05-0.09) | 0.05 (0.05-0.07) | 0.14 (0.08-0.33) | <.001 | 0.05 (0.05-0.05) | 0.07 (0.05-0.17)∗ | 0.13 (0.07-0.24)∗† | <.001 |
| >0.5 | 22/477 (4.61) | 8/393 (2.04) | 14/84 (16.67) | <.001 | 7/304 (2.30) | 10/139 (7.19)§ | 5/34 (14.71)∗ | .0011 |
| Ferritin (ng/mL) | 616.06 (342.21-1249.44) | 557.96 (300.78-968.50) | 1274.80 (739.57-2000.00) | <.001 | 493.76 (274.10-892.38) | 927.22∗ (493.80-1938.43) | 1029.75∗ (760.51-1853.34) | <.001 |
| >275 | 367/452 (81.19) | 287/370 (77.57) | 80/82 (97.56) | <.001 | 207/279 (74.19) | 130/143 (90.91)∗ | 30/30 (100.00)∗ | <.001 |
| D-dimer (μg/mL) | 0.84 (0.45-1.97) | 0.69 (0.41-1.49) | 2.70 (1.04-16.92) | <.001 | 0.61 (0.39-1.23) | 1.17 (0.64-4.40)∗ | 4.68 (1.25-18.02)∗ | <.001 |
| >0.5 | 320/459 (69.72) | 242/376 (64.36) | 78/83 (93.98) | <.001 | 178/292 (60.96) | 113/133 (84.96)∗ | 29/34 (85.29)§ | <.001 |
| APTT (s) | 26.70 (23.00-30.88) | 26.70 (23.00-30.75) | 27.20 (23.00-32.00) | .73 | 27.00 (23.00-31.15) | 26.70 (23.00-29.08) | 23.70 (22.00-32.00) | .16 |
| >40 | 17/474 (3.59) | 13/391 (3.32) | 4/83 (4.82) | .73 | 12/303 (3.96) | 3/138 (2.17) | 2/33 (6.06) | .47 |
| PT (s) | 11.20 (10.50-12.00) | 11.10 (10.40-12.00) | 12.00 (11.00-13.10) | <.001 | 11.00 (10.20-11.80) | 11.80 (11.00, 12.50)∗ | 12.65 (11.63-13.55)∗‡ | <.001 |
Data are presented as medians (IQR) and n/N (%). N is the total number of patients with available data.
APTT, Activated partial thromboplastin time; IQR, interquartile range; PT, prothrombin time.
P < .01, compared with mild/moderate.
P < .01, compared with severe.
P < .05, compared with severe.
P < .05, compared with mild/moderate.
Lymphocyte subsets by flow cytometry were detected in a total of 141 subjects (19 with fatal outcome), with different severity (94 mild/moderate cases, 34 severe cases, and 13 critical cases).
T-cell subsets were further analyzed in 141 patients on admission (demography presented in Table E1 in this article’s Online Repository at www.jacionline.org). In patients with different outcomes, nonsurvivors showed a significant decrease in the total number of CD3+ T cells, CD4+ T cells, and CD8+ T cells in peripheral blood, compared with survivors (all P < .001). A decreased tendency of CD8+ T-cell counts was detected in nonsurvivors (P = .077). In the comparison among patients with different severity, a significant decrease in total counts of CD3+ T, CD4+ T, and CD8+ T cells was found in more severe patients. The percentage of CD8+ T cells in severe/critical patients was significantly lower compared with mild/moderate patients (P < .01).
Compared with survivors, nonsurvivors showed significantly increased levels of IL-6, C-reactive protein (CRP), procalcitonin (PCT), amyloid A protein (SAA), ferritin, and D-dimer (all P < .01). CRP, PCT, and ferritin also showed a significant increasing trend with severer condition of the disease. Regarding the coagulation indicators, the indexes of D-dimer and prothrombin time increased significantly in nonsurvivors, or the patients with severer condition.
Dynamic changes in hematologic and immunologic biomarkers
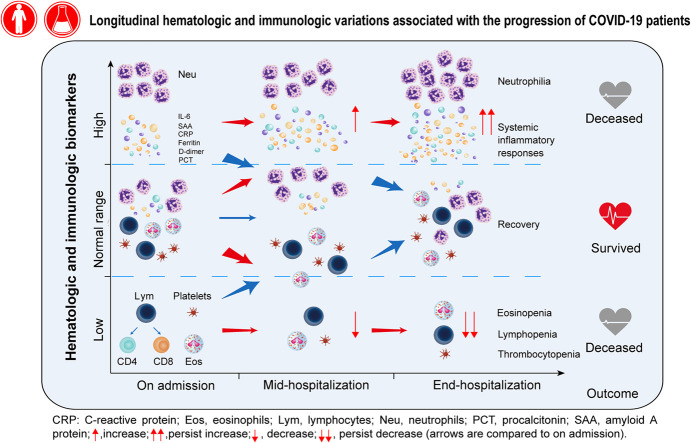
Fig 1 illustrates a schematic diagram of dynamic changes in hematologic and inflammatory biomarkers in survivors and nonsurvivors during progression of COVID-19 on admission, midhospitalization, and end-hospitalization.
Fig 1.
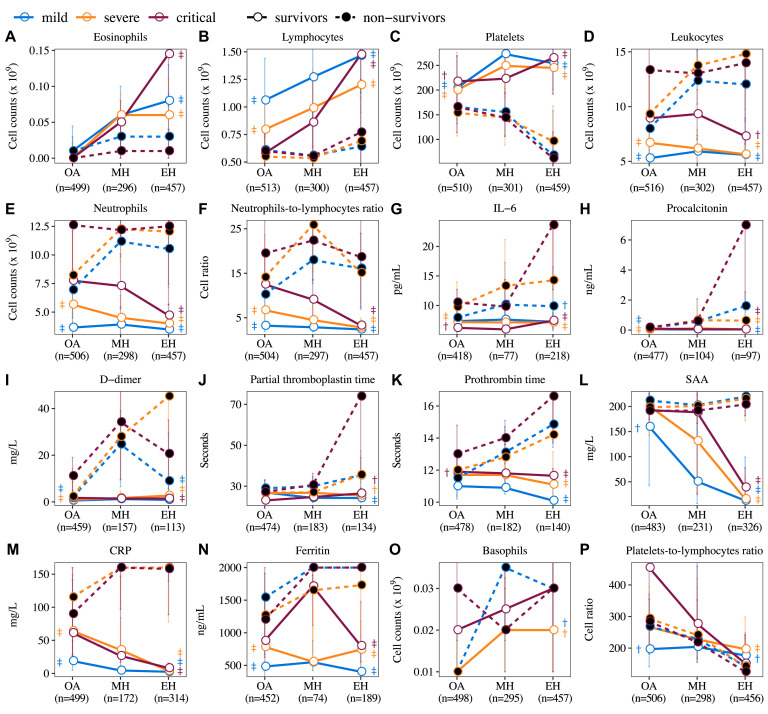
Dynamic changes in hematologic and immunologic biomarkers in patients with COVID-19 across 3 time periods, including on admission (OA), midhospitalization (MH), and end-hospitalization (EH). Medium value of 3 time periods is shown in patients with mild, severe, or critical severity on admission for both survivors and nonsurvivors. The significant difference between survivors and nonsurvivors in each of 3 severity groups was compared using Kruskal-Wallis test and indicated as †P ≤ .05 and ‡P ≤ .01.
To further understand their dynamic changes, we classified these biomarkers into 3 distinct patterns. Three hematologic cells in pattern 1, including eosinophils, lymphocytes, and platelets, showed a significant continuous upward trend in survivors, but a downward trend or kept low in nonsurvivors (Fig 1, A-C). On admission, regardless of the severity or the outcome, most patients presented an identical eosinopenia. Level of lymphocytes was inversely proportional to disease severity, with the highest in mild survivors and the lowest in critical survivors. During hospitalization, survivors showed an increasing trend for both eosinophils (6-fold in midterm, and 8-fold at the end of hospitalization, P < .001) and lymphocytes (1.2-fold in midterm, and 1.4-fold at the end of hospitalization, P < .001); however, nonsurvivors remained at low level without a noteworthy increase. For platelets, survivors showed a significantly higher level on admission and an increasing trend during hospitalization (1.25-fold in midterm and 1.24-fold at the end of hospitalization, P < .001), whereas nonsurvivors started with a low level and the levels dropped significantly afterwards (1.07-fold and 2.13-fold, P < .001).
Biomarkers in pattern 2 including leukocytes, neutrophils, NLR, IL-6, PCT, D-dimer, prothrombin time, SAA, CRP, and ferritin were maintained at significantly lower levels or showed a slightly downward trend in survivors. In contrast, these biomarkers showed an upward trend or maintained higher levels in nonsurvivors (Fig 1, D-N). Take an example of IL-6; in severe or critical patients, significantly higher levels were found in nonsurvivors (severe: 9.7 pg/mL, critical: 10.5 pg/mL) than in survivors (severe: 7.2 pg/mL, critical: 6.2 pg/mL) on admission (P < .001). During hospitalization, survivors had comparable levels of IL-6 across 3 time points, whereas at end-hospitalization, a 1.2-, 1.5-, and 2.2-fold increase compared with on admission was found in mild/moderate, severe, or critical cases with fatal outcome, respectively (Fig 1, L). Furthermore, there was no clear pattern of basophils and PLR (Fig 1, O and P).
Correlation networks and principal-component analysis for immunologic cells and biomarkers
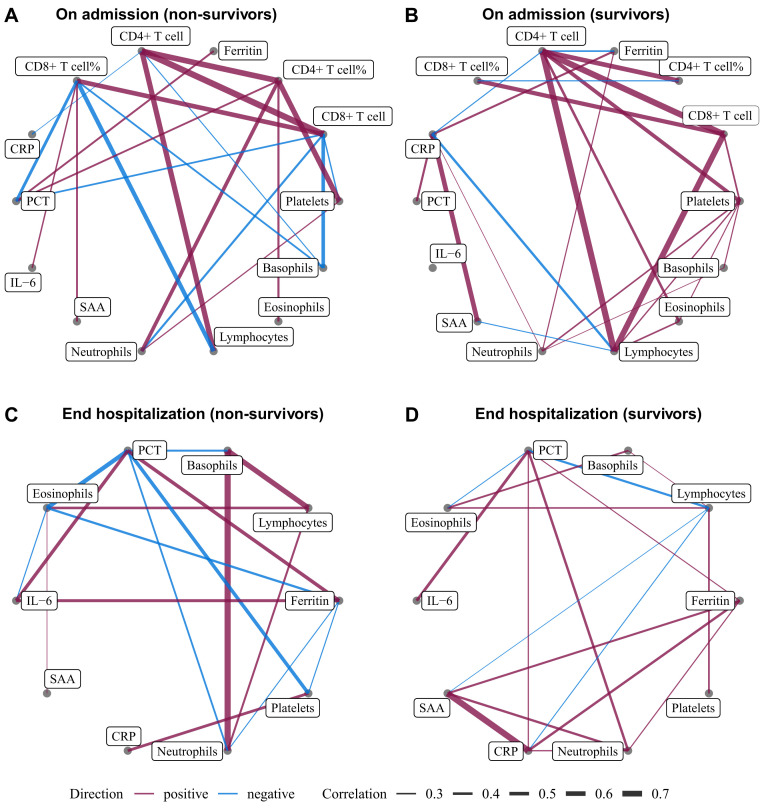
On admission (Fig 2 ), both nonsurvivors and survivors showed a strong positive correlation between CD4+ T-cell and CD8+ T-cell count (r = 0.69 and r = 0.71, respectively), and CD4+ T versus lymphocyte count (r = 0.62 and r = 0.72, respectively). Similar positive correlations were observed between CD4+ T-cell count and CD4+ T-cell% (r = 0.65 and r = 0.61) and CD8+ T-cell count and CD8+ T-cell% in all patients (r = 0.58 and r = 0.57). We observed a positive correlation between CD8+ T cells and lymphocyte count in survivors (r = 0.69), but not in nonsurvivors. In contrast, nonsurvivors showed a negative correlation between lymphocyte count and CD8+ T-cell% (r = −0.56). CD8+ T-cell% and CD8+ cell counts were 2 highly connected hub nodes in the nonsurvivor biomarker network (6 edges, Fig 2, A).
Fig 2.
Correlation networks for immunologic cells and biomarkers. Networks showed different profiles of correlations in COVID-19 nonsurvivors (A and C) and survivors (B and D), on admission (Fig 2, A and B) and end hospitalization (Fig 2, C and D). The width of the edge showing stronger or weaker interactions is proportional to the absolute value of biomarker-biomarker correlation (|r|). Edges were shown only when |r| > 0.2. A purple edge indicates a positive correlation, and a blue edge indicates a negative correlation. PCT, Procalcitonin.
When at end-hospitalization, strong correlations between basophil and neutrophil (r = 0.53), and between basophil and lymphocyte counts (r = 0.47), were observed in nonsurvivors but not in survivors. Furthermore, nonsurvivors showed stronger negative correlations between PCT and eosinophils (r = −0.42 in nonsurvivors and r = −0.23 in survivors), PCT versus platelets (r = −0.37), and CRP and platelets (r = 0.33) than survivors. Collectively, strong correlations between immunologic cells and infection biomarkers were still found in nonsurvivors, but not in survivors.
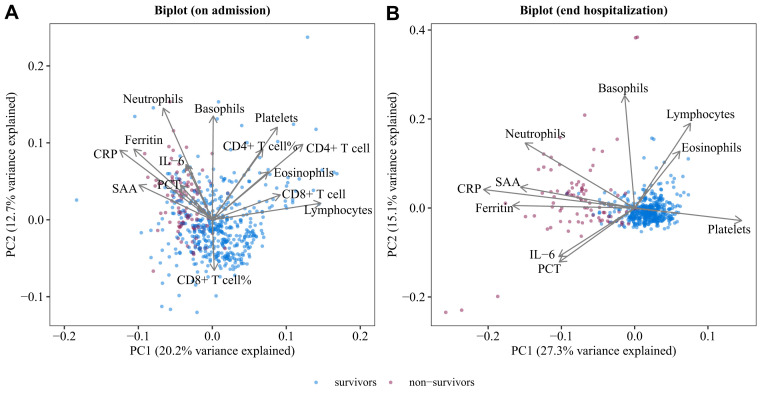
A biplot via principal-component analysis showed the configuration of biomarkers on admission and at end-hospitalization, which is shown in Fig 3 . On admission, the first principal component (PC1) separated nonsurvivors from survivors. Twelve biomarkers significantly associated positively or negatively with PC1, with lymphocytes (20.1%), CD4+ T cell (13.9%), and CD8+ T cell (7.9%) having the biggest positive contribution. Notably, the spectrum of lymphocytes and their subsets in the principal-component analysis is consistent with what is shown in the biomarker correlation networks (Fig 2). At end-hospitalization, nonsurvivors and survivors showed more marked separation along PC1. Platelets (12.6%), lymphocytes (3.4%), and eosinophils (2.3%) were positively associated with PC1, whereas CRP (25.1%), ferritin (16.4%), and SAA (14.3%) showed negative contribution on PC1.
Fig 3.
PCA biplot of biomarkers on admission (A) or at end hospitalization (B). Samples are shown as dots and colored by outcomes (survivors and nonsurvivors). Biomarkers shown as lines with arrows. The configuration of biomarkers on biplot represented the relationship between variables and principal components. PC, Principal component; PCA, Principal-component analysis; PCT, procalcitonin.
Predicted factors for the fatal outcome
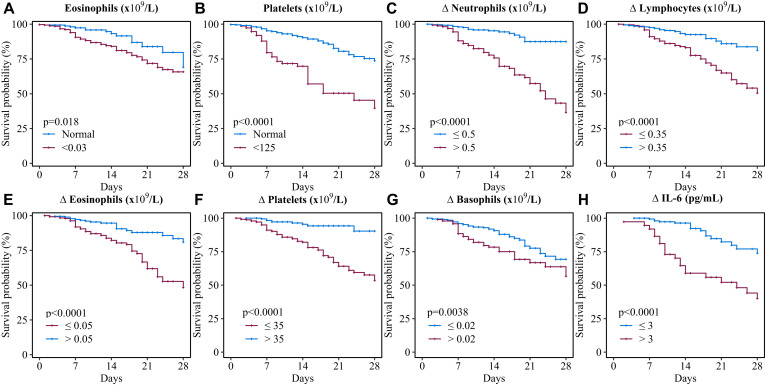
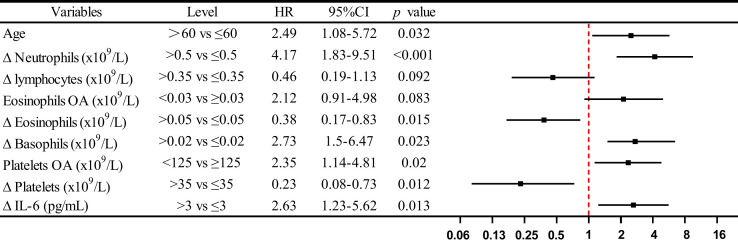
The Kaplan-Meier and log-rank test showed a significant difference in survival curve in patients with COVID-19 categorized by the level of eosinophils, platelets on admission, and the level of changes in neutrophils, lymphocytes, eosinophils, platelets, basophils, and IL-6 from on admission to end-hospitalization, respectively (Fig 4 ). Furthermore, on the basis of multivariate Cox regression model, we found age more than 60 years (HR, 2.49; 95% CI, 1.08-5.72), Δneutrophils greater than 0.5 × 109/L (HR, 4.17; 95% CI, 1.83-9.51), eosinophils on admission less than 0.03 × 109/L (HR, 2.12; 95% CI, 0.91-4.98), Δbasophils greater than 0.02 × 109/L (HR, 2.73; 95% CI, 1.5-6.47), platelets on admission less than 125 × 109/L (HR, 2.35; 95% CI, 1.14-4.81), and ΔIL-6 greater than 3 pg/mL (HR, 2.63; 95% CI, 1.23-5.62) were the risk factors, whereas Δlymphocytes greater than 0.35 × 109/L (HR, 0.46; 95% CI, 0.19-1.13), Δeosinophils greater than 0.05 × 109/L (HR, 0.38; 95% CI, 0.17-0.83), Δplatelets greater than 35 × 109/L (HR, 0.23; 95% CI, 0.08-0.73) were the protective factors for fatal outcome (Fig 5 ).
Fig 4.
Kaplan-Meier survival plots for different prognostic factors. Kaplan-Meier survival plots according to (A) eosinophil OA, (B) platelets OA, (C) Δ neutrophils, (D) Δ lymphocytes, (E) Δ eosinophils, (F) Δ basophils, (G) Δ platelets, and (H) Δ IL-6. Δ index(a) = index(a) end hospitalization − index(a) OA. OA, On admission.
Fig 5.
Risk factors of fatal outcome in the multivariate Cox proportional hazards regression model. Shown in the figure are the HR and the 95% CI associated with the end point. Δ index(a) = index(a) end hospitalization − index(a) OA. OA, On admission.
Discussion
In this study, longitudinal variations in hematologic and immunologic biomarkers associated with the severity and progression of COVID-19 were investigated. Some novel findings were found. First, not only the lymphocytes and their subsets but also the eosinophils decreased at the early stage, associating with disease severity and clinical outcome. Altered immunologic interactions among T-cell subsets and hematologic indices in nonsurvivors indicated impaired immune responses in COVID-19. Second, during the hospitalization, dynamic changes in hematologic and immunologic indices varied in survivors and nonsurvivors. Finally, multivariate Cox regression model suggested that restored levels of lymphocytes, eosinophils, and platelets could serve as the predictors of recovery, whereas progressive increases in neutrophils, basophils, and IL-6 were risk factors for fatal outcomes of COVID-19.
Coronavirus infections (SARS and MERS) are confirmed to activate both innate and adaptive immune responses. With the progression of the disease, uncontrolled inflammatory responses may lead to subsequently local and systematic tissue injury. A marked decrease in lymphocyte count has been reported to be associated with the disease severity of SARS and COVID-19.17, 18, 19 We confirmed observations of a significantly lower lymphocyte count and higher lymphopenia percentage on admission in severe/critical patients with COVID-19 than in mild/moderate ones. Furthermore, we extend the observation by comparing lymphocyte levels between survivors and nonsurvivors, showing that the initial levels of absolute lymphocyte count were significantly lower and lymphopenia percentage significantly higher in nonsurvivors, regardless of the initial disease severity. Our data also revealed that the decreased lymphocyte count was recovered to the normal level in survivors, whereas it was sustained at low level in nonsurvivors, suggesting that restored lymphocyte numbers are associated with the curative outcome of COVID-19.
We further analyzed T-lymphocyte subsets and confirmed the observation of significant reduction in CD3+, CD4+, and CD8+ T-cell subsets in severe/critical patients than in mild/moderate ones.10 , 20 , 21 Unfortunately, we could not obtain the kinetic changes in T-cell subsets, because samples only on admission were taken with flow cytometry in our patients. However, given the notification of marked decreased initial counts of CD3+, CD4+, and CD8+ T-cell subsets in nonsurvivors than in survivors, we could speculate that marked impairment of T lymphocytes is associated with fatal outcome in patients with COVID-19. Decline in T lymphocytes via T-cell apoptosis in patients with SARS and MERS is implicated to diminish the T-cell response, leading to further exuberant inflammatory responses.22 , 23 A recent report by Wang et al’s group24 proved that SARS-CoV-2 could directly infect T cells through receptor-dependent spike protein–mediated membrane fusion, despite a very low expression level of angiotensin-converting enzyme 2 receptor in T cells. Further research is needed to investigate whether SARS-CoV-2 infection could induce T-cell apoptosis.
In case of SARS, the CD4+/CD8+ ratio did not change significantly among patients with diverse severity or at different time points.11 , 25 From current research, CD4+/CD8+ ratio was found with no obvious difference among patients with different severity, but with a significant increase in nonsurvivors than in survivors. These findings indicate that greater loss of CD8+ T cells might lead to fatal outcome in patients with COVID-19. CoV-specific T cells, especially CD4+ and CD8+ T cells, are crucial for virus clearance and limiting further damage to host. Compared with CD4+ T cells, which largely function via indirect mechanisms, virus-specific CD8+ T cells directly target infected cells.26 Greater loss of CD8+ T cells might correlate to impaired virus elimination in the early stage. Intriguingly, we found different correlations between CD8+ T cells and lymphocytes in nonsurvivors than in survivors, indicating that other mechanisms underlying CD8+ T-cell exhaustion might be involved. A pilot study including 13 severe cases showed that the CD8+ T cells were functionally exhausted in patients with COVID-19 through upregulation of an immune-checkpoint inhibitory receptor, NKG2A. NKG2A+ CD8+ T cells were found with increased percentage and displayed a reduced ability to produce CD107a, IFN-γ, IL-2, and granzyme B. The upregulated expression of NKG2A normalized in recovery subjects.27 Aside from reduction in cell numbers, functional exhaustion of CD8+ cells might contribute to disease progression of COVID-19.
In our study, most of the severe/critical and fatal patients demonstrated eosinopenia, showing much lower level than mild/moderate and survival subjects on admission. This result was consistent with those of other studies with acute infections caused by SARS-CoV,28 MERS-CoV,12 and SARS-CoV-2.29 , 30 The decline in eosinophils might be owing to the response to stress of acute SARS-CoV-2 infection.31 Patients with severe respiratory syncytial virus infection had significantly higher plasma cortisol levels and significantly lower eosinophil counts.32 Other studies also reported significantly decreased eosinophils in subjects with stress of heat33 and force swim.34 However, whether SARS-CoV-2 has a direct effect on eosinophils remain unknown. Recently, a study found that the toxin of Clostridium difficile transferase induces pathogenic host inflammation via a Toll-like receptor 2–dependent pathway, resulting in the suppression of a protective host eosinophilic response.35 In experimental human endotoxemia, circulatory eosinopenia after an innate immune challenge (intravenous challenge with endotoxin) is mediated by CD49d-mediated homing of eosinophils to the tissues.36 Similarly, with Liu et al’s report,29 we found that eosinophils continually increased and reached significantly higher levels in survivors as compared with nonsurvivors, and a greater increase in eosinophils was associated with better outcome in our Cox regression model. All the above findings suggest that eosinophils might contribute to antiviral immunity in the lungs, and levels of eosinophil counts at early stage and the kinetic changes may predict COVID-19 progression and recovery.
Our study indicated the platelets count as a clinical indicator of disease severity and risk of mortality during hospitalization. Thrombocytopenia was identified as a significant risk factor for disease severity and mortality in both patients infected with SARS-CoV37 , 38 and patients infected with MERS-CoV.39 Furthermore, platelet count was selected in 2 prognosis regression models by multivariate analysis.38 , 40 A recent meta-analysis with 1779 patients with COVID-19 revealed that the platelet count was significantly lower in severe patients and even lower in nonsurvivors.41 A hypothesis suggested that damage to the lung tissue and pulmonary endothelial cells by virus infection would result in (1) platelet activation, aggregation, and entrapment, and further increase the consumption of platelets/megakaryocytes, and (2) reducing the production of platelets in the lungs.37
Neutrophil dysfunction was previously reported as a distinct inflammatory signature involved in the pathogenesis of SARS and MERS.22 Severe outcomes, including acute lung injury, acute respiratory distress syndrome, and death, are associated with massive neutrophil infiltration in the lung and dramatically elevated neutrophil counts in the peripheral blood.11 , 42, 43, 44 Higher neutrophil count on admission was found in severe or critical than in mild/moderate patients in our cohort, consistent with previous reports.1 , 45 Notably, kinetic analysis further revealed that a significant increase in initial neutrophil count, which persisted at higher levels at end-hospitalization, was correlated with the fatal outcome. The NLR has been reported as an independent predictor of disease severity in patients with COVID-19.45 , 46 We confirmed observation of higher initial NLR in severe/critical patients, and further evaluated the potential of NLR as a prognostic factor. Compared with those in survivors with low initial and a progressively declined NLR, significantly higher NLR sustained from on admission to end-hospitalization in nonsurvivors. Our results indicate that NLR may serve as a potential factor for early identification of severity and to further predict outcomes in COVID-19.
Increased proinflammatory cytokine/chemokine responses–induced immunopathology, defined as cytokine storm, has been implicated in human coronavirus pathogenesis.22 It is speculated that SARS-CoV-2 first binds to alveolar epithelial cells, and then the virus activates the innate immune system and the adaptive immune system, resulting in the release of a large number of cytokines, including IL-6. Abnormally high levels of these cytokines/chemokine are considered to lead to tissue damage, accounting for respiratory failure or multiple organ failure. Among various cytokines and chemokines (IL-2, IL-8, IL-17, GCSF, IP-10, and TNF-α) identified, IL-6 is one of the key cytokines with increased plasma level in both SARS and MERS, as well as in COVID-19.21 , 47, 48, 49 In accord with previous studies,20 , 21 IL-6 was found at a higher level on admission in nonsurvivors, as well as a marked increase especially at the end of hospitalization compared with those in survivors. This indicates that a sustained high level of IL-6 is related to a fatal outcome of SARS-CoV-2 infection. Interfering IL-6 by tocilizumab has been reported to effectively improve clinical symptoms and repress the deterioration of severe and critical patients.50 , 51 Although not sufficient, our findings provide further evidence that, in addition to antiviral treatment of directly targeting or blocking viral entry, IL-6 may serve as a potential therapeutic target for severe/critical patients with COVID-19.
Other conventional makers related to inflammatory damage, such as CRP, were also significantly elevated in nonsurvivors at an early stage and showed an upward trend with progression, which is similar as reported in SARS.52 Ferritin and SAA also belong to acute-phase proteins and can be used as prognostic markers for tissue injury or acute infections.53 , 54 SAA demonstrated a positive correlation with the extent of pneumonia in SARS.55 In the current study, consistently high levels of serum ferritin and SAA were present in nonsurvivors during the hospitalization. Our study, with another study on COVID-19,9 out a dynamic increasing of ferritin in nonsurvivors since on admission. The longitudinal observation on these indexes indicated the progressive immune-mediated damage in deceased patients.
From biomarker correlation networks, we found a significant interaction among hematologic and immunologic cells in both survivors and nonsurvivors on admission, but with different patterns. It might indicate that the immunology profile associated with host antiviral defense varied in different status. However, comparing with subsiding of cell-to-cell interactions in survivors, strong correlations sustained till end-hospitalization in nonsurvivors, suggesting that higher virus load plus inflammatory turbulence might have contributed to mortality of COVID-19. Currently, Cao et al56 reported that no benefit was observed with lopinavir-ritonavir treatment beyond standard care in severe patients with COVID-19. It is reasonable that combining usage of anti-inflammatory and antiviral medications may be more effective than using either alone. Beside tocilizumab, novel immune-mediated strategies (eg, mesenchymal stem cells) are under trial to dampen inflammatory responses and attenuate the inflammatory injury in COVID-19.
Our study has several limitations. First, because of the design of a retrospective study, some indices were not detected in all patients. Lack of follow-up data of T-cell subsets resulted in failure to perform a longitudinal analysis to unravel the recovery trends. Second, except for the serum IL-6 and some clinical infection indexes, other proinflammatory cytokines or chemokines associated with lymphocytes were not detected. Further studies are necessary to characterize the role of T-cell immune response in COVID-19. Third, data on the virus shedding or viremia profile are not available, and further studies are needed to describe the association between the virus-loading kinetics and the dynamic immune responses.
Conclusions
Our study demonstrates hematologic and immunologic variations associated with the severity and progression of COVID-19. Since the early stage of the disease, not only the lymphocytes and T-cell subsets but also eosinophils present a marked decrease, associating with the disease severity and clinical outcome. Altered immunologic interactions among T-cell subsets and hematologic indices suggests the impaired immune response since the onset of the disease. Dynamic changes in hematologic and immunologic indices varied in survivors and nonsurvivors, and could serve as the predictors of recovery or fatal outcome of COVID-19.
Clinical implications.
Restored lymphocytes, eosinophils, and platelets could predict recovery, whereas progressive increases in neutrophils and IL-6 were associated with mortality. Altered interactions among immunologic and hematologic indices indicated impaired immune responses in COVID-19.
Acknowledgments
We sincerely thank all the health care providers fighting against this public crisis and all the patients involved in the study. We thank the hospital staff for their efforts in collecting the information. We are indebted to the coordination of Drs Zong-jiu Zhang, Ya-hui Jiao, Bin Du, Xin-qiang Gao, and Tao Wei (National Health Commission), Yu-fei Duan and Zhi-ling Zhao (Health Commission of Guangdong Province), and Zi-jing Liang, Qing-hui Huang, Wen-xi Huang, and Ming Li (Guangzhou Institute of Respiratory Health), which greatly facilitated the collection of patients’ data. We thank Wei Xu (Department of Biostatistics, Princess Margaret Cancer Centre, Toronto, Ontario, Canada) for his suggestion on the statistical analysis. We also thank Li-qiang Wang, Wei-peng Cai, Zi-sheng Chen (the Sixth Affiliated Hospital of Guangzhou Medical University), Mu-lin Feng, Run-dong Qin, Ren-bin Huang, Si-yang Yao, Jing-wei Liu, Xin-liu Lin, Tian Luo, Li He, Yu-kai Liu, Chen Zhan, Li-man Fang, Shu-xin Zhong, Chang-xing Ou, Xiao-min Peng, Si-ni Cui, Yuan Wang, Mou Zeng, Xin Hao, Qi-hua He, Jing-pei Li, Xu-kai Li, Wei Wang, Li-min Ou, Ya-lei Zhang, Jing-wei Liu, Xin-guo Xiong, Wei-juna Shi, San-mei Yu, Run-dong Qin, Si-yang Yao, Bo-meng Zhang, Xiao-hong Xie, Zhan-hong Xie, Wan-di Wang, Xiao-xian Zhang, Hui-yin Xu, Zi-qing Zhou, Ying Jiang, Ni Liu, Jing-jing Yuan, Zheng Zhu, Jie-xia Zhang, Hong-hao Li, Wei-hua Huang, Lu-lin Wang, Jie-ying Li, Li-fen Gao, Jia-bo Gao, Cai-chen Li, Xue-wei Chen, Jia-bo Gao, Ming-shan Xue, Shou-xie Huang, Jia-man Tang, Wei-li Gu, and Jin-lin Wang (Guangzhou Institute of Respiratory Health) for their dedication to data entry and verification. We express sincere sympathies and deep condolences to the victims and bereaved families.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Coronavirus disease 2019 (COVID-19) situation report-92. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-covid-19.pdf?sfvrsn=38e6b06d_4 Available at: Accessed April 21, 2020.
- 4.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 5.Niu P., Zhang S., Zhou P., Huang B., Deng Y., Qin K. Ultrapotent human neutralizing antibody repertoires against Middle East respiratory syndrome coronavirus from a recovered patient. J Infect Dis. 2018;218:1249–1260. doi: 10.1093/infdis/jiy311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang S.M., Na B.J., Jung Y., Lim H.S., Seo J.E., Park S.A. Clinical and laboratory findings of Middle East respiratory syndrome coronavirus infection. Jpn J Infect Dis. 2019;72:160–167. doi: 10.7883/yoken.JJID.2018.187. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Clinical management of severe acute respiratory infection when COVID-19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at: Accessed March 18, 2020.
- 16.National Health Commission & State Administration of Traditional Chinese Medicine Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). March 3, 2020. https://www.who.int/docs/default-source/wpro---documents/countries/china/covid-19-briefing-nhc/1-clinical-protocols-for-the-diagnosis-and-treatment-of-covid-19-v7.pdf?sfvrsn=c6cbfba4_2 Available at: Accessed March 13, 2020.
- 17.Cui W., Fan Y., Wu W., Zhang F., Wang J.Y., Ni A.P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis. 2003;37:857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., Qiu Z., Han Y., Wang Z., Fan H., Lu W. Rapid loss of both CD4+ and CD8+ T lymphocyte subsets during the acute phase of severe acute respiratory syndrome. Chin Med J (Engl) 2003;116:985–987. [PubMed] [Google Scholar]
- 20.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online ahead of print March 12, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed]
- 21.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;30:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu H., Zhou J., Wong B.H., Li C., Chan J.F., Cheng Z.S. Middle East respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Xu W, Hu G, Xia S, Sun Z, Liu Z, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion [published online ahead of print April 7, 2020]. Cell Mol Immunol. https://doi.org/10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed]
- 25.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y.S., Chen C.H., Chao A., Chen E.S., Wei M.L., Chen L.K. Molecular signature of clinical severity in recovering patients with severe acute respiratory syndrome coronavirus (SARS-CoV) BMC Genomics. 2005;6:132. doi: 10.1186/1471-2164-6-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print February 19, 2020]. Allergy. https://doi.org/10.1111/all.14238. [DOI] [PubMed]
- 31.He L., Ding Y., Zhang Q., Che X., He Y., Shen H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto R.A., Arredondo S.M., Bono M.R., Gaggero A.A., Diaz P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117:e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 33.Ju X.H., Xu H.J., Yong Y.H., An L.L., Jiao P.R., Liao M. Heat stress upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: an in vivo and in vitro study. Animal. 2014;8:1462–1468. doi: 10.1017/S1751731114001268. [DOI] [PubMed] [Google Scholar]
- 34.Reis F.G., Marques R.H., Starling C.M., Almeida-Reis R., Vieira R.P., Cabido C.T. Stress amplifies lung tissue mechanics, inflammation and oxidative stress induced by chronic inflammation. Exp Lung Res. 2012;38:344–354. doi: 10.3109/01902148.2012.704484. [DOI] [PubMed] [Google Scholar]
- 35.Cowardin C.A., Buonomo E.L., Saleh M.M., Wilson M.G., Burgess S.L., Kuehne S.A. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol. 2016;1:16108. doi: 10.1038/nmicrobiol.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassani M, Leijte G, Bruse N, Kox M, Pickkers P, Vrisekoop N, et al. Differentiation and activation of eosinophils in the human bone marrow during experimental human endotoxemia [published online ahead of print January 10, 2020]. J Leukoc Biol. 10.1002/JLB.1AB1219-493R. [DOI] [PubMed]
- 37.Yang M., Ng M.H., Li C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review) Hematology. 2005;10:101–105. doi: 10.1080/10245330400026170. [DOI] [PubMed] [Google Scholar]
- 38.He W.Q., Chen S.B., Liu X.Q., Li Y.M., Xiao Z.L., Zhong N.S. Death risk factors of severe acute respiratory syndrome with acute respiratory distress syndrome [in Chinese] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15:336–337. [PubMed] [Google Scholar]
- 39.Arabi Y.M., Al-Omari A., Mandourah Y., Al-Hameed F., Sindi A.A., Alraddadi B. Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 40.Liu X.Q., Chen S.B., He G.Q., Li Y.M., He W.Q., Chen R.C. Management of critical severe acute respiratory syndrome and risk factors for death [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:329–333. [PubMed] [Google Scholar]
- 41.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y.H., Lin A.S., Chao T.Y., Lu S.N., Liu J.W., Chen S.S. A cluster of patients with severe acute respiratory syndrome in a chest ward in southern Taiwan. Intensive Care Med. 2004;30:1228–1231. doi: 10.1007/s00134-004-2311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohd H.A., Memish Z.A., Alfaraj S.H., McClish D., Altuwaijri T., Alanazi M.S. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14:464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng D.L., Al Hosani F., Keating M.K., Gerber S.I., Jones T.L., Metcalfe M.G. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Li SM, Liu J, Liang BY, Wang XB, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. MedRxiv preprint. 10.1101/2020.02.16.20023671. [DOI] [PMC free article] [PubMed]
- 46.Liu JY, Liu Y, Xiang P, Pu L, Xiong HF, Li CS, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel cronavirus in the early stage. medRxiv preprint. 10.1101/2020.02.10.20021584. [DOI] [PMC free article] [PubMed]
- 47.Chollet-Martin S., Jourdain B., Gibert C., Elbim C., Chastre J., Gougerot-Pocidalo M.A. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;154:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 48.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou YG, Fu BQ, Zheng XH, Wang DS, Zhao CC, Qi YJ. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. BioRxiv. 10.1101/2020.02.12.945576. [DOI]
- 51.Xu XL, Han MF, Li TT, Sun W, Wang DS, Fu BQ, et al. Effective treatment of severe COVID-19 patients with tocilizumab [published online ahead of print April 29, 2020]. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed]
- 52.Wang J.T., Sheng W.H., Fang C.T., Chen Y.C., Wang J.L., Yu C.J. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Buck M., Gouwy M., Wang J.M., Van Snick J., Opdenakker G., Struyf S. Structure and expression of different serum amyloid A (SAA) variants and their concentration-dependent functions during host insults. Curr Med Chem. 2016;23:1725–1755. doi: 10.2174/0929867323666160418114600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yip T.T., Chan J.W., Cho W.C., Yip T.T., Wang Z., Kwan T.L. Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid A protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin Chem. 2005;51:47–55. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.