Abstract
Reduction of intraocular pressure (IOP) is the only reliable treatment for glaucoma that maintains the patient’s visual function throughout life, and IOP-lowering eyedrops are the mainstay of therapy. Ripasudil hydrochloride hydrate (brand name: Glanatec ophthalmic solution 0.4%; Kowa Company, Ltd., Japan) is a Rho-associated coiled-coil-containing protein kinase (ROCK) inhibitor that lowers IOP by increasing conventional aqueous outflow. Since the approval of ripasudil eyedrops in 2014, a large store of clinical data has been amassed in Japan. With regard to safety, conjunctival hyperemia is the most common adverse drug reaction (ADR) and is usually transient and mild. Blepharitis and allergic conjunctivitis are other major local ADRs. Systemic ADRs are rare, but we should be wary of allergic reactions. With regard to efficacy, ripasudil is expected to lower IOP in almost all glaucoma subtypes (including primary open-angle glaucoma, secondary glaucoma, and primary angle-closure glaucoma) and in all patterns of treatment initiation (monotherapy, combination therapy, switching therapy, and add-on therapy). However, the status of the trabecular meshwork may affect the IOP-lowering effect of ripasudil. In patient selection, current evidence-based information on the safety and efficacy of ripasudil should be fully considered. As irreversible damage to the trabecular meshwork would considerably affect efficacy, it may be better to start ripasudil treatment during an early stage of glaucoma.
Keywords: ripasudil, glaucoma, intraocular pressure, efficacy, safety
Introduction
Glaucoma, a chronic optic neuropathy, is a major cause of irreversible blindness around the world.1,2 Although the pathogenesis of glaucoma remains unclear, a consensus exists that progressive degeneration in the retinal ganglion cells (RGCs) is a key cellular event that leads to characteristic glaucomatous optic disc changes with associated visual field loss.3 Current treatment strategies stem from the concept of lowering intraocular pressure (IOP), the primary modifiable risk factor associated with disease progression,1,2 based on robust evidence from animal experiments showing the close relationship between IOP level and RCG death4 and major randomized controlled trials showing the protective effect of IOP lowering on visual field progression in glaucoma eyes.2
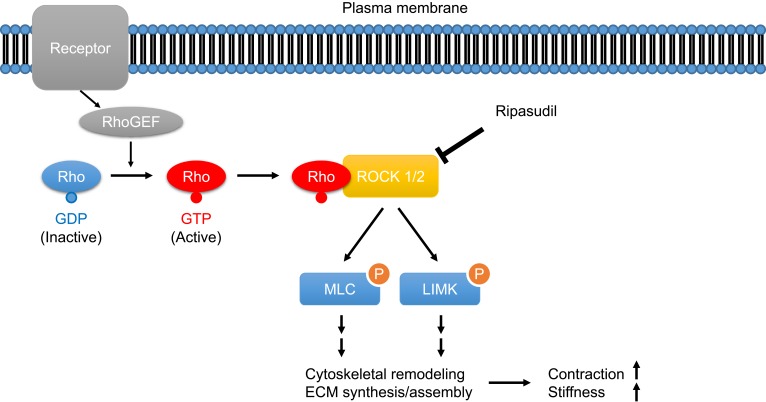
Ripasudil hydrochloride hydrate (brand name: Glanatec ophthalmic solution 0.4%; Kowa Company, Ltd., Japan; hereafter referred to as ripasudil) is the world’s first Rho-associated coiled-coil-containing protein kinase (ROCK) inhibitor eyedrop that lowers IOP by increasing conventional aqueous outflow through the trabecular meshwork and Schlemm’s canal.5 Rho is a Ras homologue of small GTPase that regulates various fundamental cell behaviors via Rho/ROCK signaling. In trabecular meshwork cells, Rho guanine nucleotide exchange factor (RhoGEF) activates Rho by catalyzing GDP–GTP exchange following binding of agonist to its receptor (e.g., G-protein coupled receptor). GTP-bound Rho then binds to the downstream effector proteins ROCK1 and ROCK2 and activates them. Activated ROCK1/2 phosphorylates myosin light chain (MLC) and Lin-1/Isl-1/Mec-3 kinase (LIMK) and enhances cytoskeletal remodeling and synthesis and assembly of extracellular matrix, resulting in increased tissue contraction and stiffness (Figure 1).6–10 The effects of ROCK inhibitors on cell components responsible for the trabecular outflow pathway have been well documented in preclinical studies. In cultured trabecular meshwork cells, addition of ROCK inhibitors caused morphological changes accompanied by disruption of actin bundles and disassembly of focal adhesion.11–15 ROCK inhibitors also affect cultured Schlemm’s canal endothelial (SCE) cells and increase their permeability.13,14,16 Many animal experiments support the efficacy of ROCK inhibitors in IOP reduction,11,12,17-21 and several ex vivo and in vivo experiments demonstrated conventional outflow tissue responses to ROCK inhibitors: increased aqueous humor outflow,11,12,17,20 expanded outflow route,22 and suppression of the fibrogenic response.21
Figure 1.
Schematic illustration of the Rho/ROCK signaling pathway in trabecular meshwork cells.
Abbreviations: RhoGEF, Rho guanine nucleotide exchange factor; ROCK, Rho-associated coiled-coil-containing protein kinase; MLC, myosin light chain; LIMK, Lin-1/Isl-1/Mec-3 kinase; ECM, extracellular matrix.
Since the approval of ripasudil eyedrops in September 2014 for patients with glaucoma or ocular hypertension (OHT) that was uncontrolled by other IOP–lowering eyedrops, a large store of clinical data has been amassed in Japan. In this review, we will provide current evidence-based information on the safety and efficacy of ripasudil and discuss the selection of patients to maximize the benefits of this newly developed glaucoma eyedrop.
Safety of Ripasudil
The safety profile of ripasudil seems to be superior to that of prostaglandin analogues (PGAs) and other IOP-lowering agents. As with PGAs, the majority of ripasudil-related adverse reactions occur locally, and systemic adverse events rarely happen. The most frequent adverse drug reaction (ADR) in eyes treated with ripasudil is conjunctival hyperemia, probably due to relaxation of smooth muscle of the blood vessels resulting from inhibition of Rho kinase.5 In a Phase 2 randomized clinical study of K-115 monotherapy to identify the optimal dose for primary open-angle glaucoma (POAG) or OHT, conjunctival hyperemia was found in 32 (65.3%) of 49 patients in the 0.4% K-115 (ripasudil) group and in 7 (13.0%) of 54 patients in the placebo group.23 The incidence of conjunctival hyperemia was approximately the same as that in two Phase 3 randomized clinical trials that evaluated the additive IOP-lowering effects and the safety of ripasudil combined with timolol 0.5% (Ripasudil–Timolol Study) or latanoprost 0.005% (Ripasudil–Latanoprost Study). After the addition of ripasudil to first-line drugs, conjunctival hyperemia was noted as an adverse event in 68 (65.4%) of 104 patients in the Ripasudil–Timolol Study and in 57 (55.9%) of 102 patients in the Ripasudil–Latanoprost Study.24 Although its incidence is extremely high, conjunctival hyperemia induced by ripasudil instillation is generally mild and transient.23,25 In a multicenter prospective study investigating the kinetic change in ripasudil-induced conjunctival hyperemia, the median offset time of hyperemia was 90 min, and hyperemia disappeared within 60 min in approximately half of the patients.26 Accordingly, the frequency of conjunctival hyperemia would be underestimated in a real-world clinical setting. In fact, in the interim analysis of a 2-year prospective observational postmarketing surveillance study (ROCK-J study) of 3459 patients including all glaucoma subtypes, 244 (8.0%) of 3058 patients had ADRs after ripasudil administration, and the incidence rate of the most common ADR, conjunctival hyperemia, was reported to be 4.0%.27 Therefore, in daily clinical practice, it is important to pre-inform patients of mild and transient conjunctival hyperemia before ripasudil treatment.
Other ocular ADRs include allergic conjunctivitis, blepharitis, punctate keratitis, and others. In a retrospective review of 92 cases of all glaucoma subtypes, 6 patients (6.5%) had blepharitis, 5 (5.4%) had a topical allergy, and 2 (2.2%) complained of a burning sensation.28 Saito et al performed a data analysis of 103 consecutive ripasudil-treated glaucoma patients and reported the incidence of blepharitis (25.2%) and its risk factor: a past history of allergic reaction to other glaucoma medication.29 Tanihara et al conducted a multicenter, prospective, open-label study of 388 patients with POAG, OHT, or exfoliation glaucoma (XFG) and reported that the incidence of blepharitis and allergic conjunctivitis was 20.6% and 17.2%, respectively.30 In the interim analysis of the ROCK-J study, conjunctivitis (1.4%), blepharitis (0.8%), and eye pruritus (0.5%) were observed as ADRs.27 From these results, the incidence of ocular ADRs other than conjunctival hyperemia seems to be not very high, but care should be taken because these ADRs were often among the major reasons for discontinuation of ripasudil treatment.27,29-32 Nonocular ADRs of ripasudil are rare and generally not severe: constipation (0.6%), headache (0.1%), dizziness (0.1%), nausea (0.1%), and others.27,30 However, Inoue et al reported a significant decrease in blood pressure 1 month after ripasudil administration in a retrospective study of 51 POAG cases,33 and we observed one patient with uveitic glaucoma (UG) who had a severe backlash after ripasudil treatment.34 Therefore, severe nonocular ADRs, although rare, should also be borne in mind.
Efficacy of Ripasudil
The IOP-lowering efficacy of ripasudil has been well investigated in eyes with POAG or OHT. Concerning the efficacy of ripasudil as monotherapy, a multicenter, prospective, randomized, Latin-square crossover study observed a statistically significant reduction in IOP compared with placebo for at least 7 hours after ripasudil instillation in patients with POAG or OHT.35 Tanihara et al conducted a phase 2 clinical trial that aimed to identify the optimal dose of K-115 in 210 patients with POAG or OHT. The trial found that 0.4% K-115 (ripasudil) twice daily lowered mean IOP by 3.5 mmHg at trough (before instillation) and by 4.5 mmHg at peak (2 hours after instillation) 8 weeks after treatment.23 A report from the K-115 Clinical Study Group in Japan, which performed a prospective study to investigate the IOP-lowering effects of 0.4% K-115 (ripasudil) in 173 patients with POAG, OHT, or XFG, showed that ripasudil monotherapy reduced mean IOP from baseline to week 52 by 2.6 mmHg at trough and 3.7 mmHg at peak.30
Since current first-line medical therapy for POAG or OHT is topical PGAs or β-blockers, the effects of ripasudil in combination with these drugs were tested. The results from a pair of phase 3 trials (the Ripasudil–Timolol Study and Ripasudil–Latanoprost Study) comprising 413 patients with POAG or OHT showed that the mean reduction in IOP at 8 weeks from baseline was 2.4 mmHg at trough and 2.9 mmHg at peak after the addition of ripasudil to timolol, and 2.2 mmHg at trough and 3.2 mmHg at peak after the addition of ripasudil to latanoprost.24 In a small prospective study that attempted to assess the effect of the addition of ripasudil to latanoprost on IOP in 26 patients with POAG or normal tension glaucoma (NTG), mean IOP significantly changed from 16.7 mmHg at baseline to 15.5 mmHg at 1 month and 14.9 mmHg at 3 months (by intention-to-treat analysis).33 The efficacy of adjunctive use of ripasudil has been examined in other clinical settings. Inoue et al retrospectively analyzed 119 eyes with POAG, OHT, or NTG treated with an average of 3.8 anti-glaucoma medications and reported that the addition of ripasudil significantly lowered mean IOP from 19.8 mmHg at baseline to 17.5 mmHg at 1 month and 16.8 mmHg at 3 months.36 Inagazi et al conducted a prospective interventional study in patients with POAG treated with maximum medical therapy and demonstrated that the addition of ripasudil led to reductions in mean IOP from baseline of 2.8 mmHg at 3 months and 2.6 mmHg at 12 months.37,38 The K-115 Clinical Study Group carried out a prospective study in patients with POAG, OHT, or XFG and reported that ripasudil additive therapy reduced mean IOP at trough and at peak from baseline to week 52 by 1.4 and 2.4 mmHg (combined with PGAs), 2.2 and 3.0 mmHg (combined with β-blockers), and 1.7 and 1.7 mmHg (combined with PGAs/β-blockers).35
Two retrospective studies evaluated the efficacy of ripasudil treatment in eyes with UG. Yasuda et al reviewed the medical records of 36 uveitic eyes with IOP elevation >21 mmHg (divided into inflammation-induced OHT and corticosteroid-induced OHT) and analyzed changes in IOP and anterior aqueous flare values (photon counts per millisecond [pc/ms]) measured by an aqueous flare meter (Kowa FM-700 Aqueous, Kowa Medicals, Japan) after ripasudil treatment. After a mean duration of 5.2 months, significant decreases in mean IOP and aqueous flare were seen in 20 eyes with inflammation-induced OHT from 26.4 mmHg and 28.1 pc/ms to 17.9 mmHg and 17.1 pc/ms, respectively. In the 16 eyes with corticosteroid-induced OHT, a significant reduction in mean IOP from 26.7 to 18.6 mmHg was observed, but the aqueous flare value remained unchanged from 18.7 to 22.6 pc/ms. Multiple linear regression analysis extracted higher IOP before treatment as a factor for predicting a greater decrease in IOP after treatment. In addition, although a significant IOP reduction after ripasudil treatment is clearly shown, the scatter plots indicate the presence of responders and nonresponders to ripasudil treatment.39 We investigated 21 eyes with open-angle UG, 19 of which were treated with topical, periocular, and/or systemic steroid therapy before ripasudil treatment. The median (minimum, maximum) IOP significantly decreased from 23 (13, 46) mmHg at baseline to 18 (12, 44) mmHg at 12 months. Changes in IOP varied widely between individuals in our cases: only 60% of eyes attained IOP reduction ≥2 mmHg at 1 month after ripasudil treatment. In the responder analysis (defined as a reduction ≥3 mmHg at 1 month), 52% were classified as ripasudil responders, and the median (minimum, maximum) IOP change from baseline to 1 month was – 0.5 (– 2, +8) mmHg in nonresponders and – 6 (– 36, –3) mmHg in responders.34 Thus, according to the results of these two studies, the magnitude of IOP reduction after ripasudil treatment was high in eyes with UG among ripasudil responders.
To obtain insight into ripasudil use in a real-world clinical setting, three retrospective studies were conducted and one prospective study is ongoing in Japan that targets all glaucoma subtypes. Sato et al reviewed 92 patients who received ripasudil as an additive glaucoma treatment, comprising 43 patients with POAG, 28 with NTG, 10 with secondary glaucoma (SG), 7 with XFG, and 4 with developmental glaucoma (DG), and reported that mean IOP decreased from 18.9 mmHg at baseline (n = 92) to 15.8 mmHg at 6 months (n = 55).28 Kawara et al (our group) examined 116 consecutive ripasudil-treated patients (76 POAG, 31 SG, and 9 DG patients) and showed that the median (interquartile range) IOP was significantly lowered from 19.0 (17.0~22.5) mmHg at baseline to 16.0 (15.0~20.0) mmHg at 6 months.31 Komizo et al analyzed the data from 58 patients who were treated with adjunctive ripasudil therapy (38 POAG, 6 XFG, and 14 SG patients) and found that the mean IOP value measured in the morning was significantly lower than that measured in the afternoon by 1.3 mmHg.40 In the interim analysis of a postmarketing surveillance prospective study (ROCK-J study) comprising 124 patients with OHT, 1389 with POAG, 1128 with NTG, 277 with SG, 64 with primary angle-closure glaucoma (PACG), 3 with childhood glaucoma, and 73 with other types of glaucoma or OHT, mean IOP was significantly decreased 3 months after ripasudil treatment in patients with POAG (– 2.9 mmHg), PACG (– 3.9 mmHg), OHT (– 3.8 mmHg), XFG (– 3.0 mmHg), UG (– 4.7 mmHg), and steroid-induced glaucoma (– 5.5 mmHg). However, the mean IOP change did not reach statistical significance in patients with neovascular glaucoma (– 2.8 mmHg; P = 0.669). Moreover, mean IOP was significantly reduced for all treatment initiation patterns: –2.8 mmHg in the “Initial monotherapy” group, –6.7 mmHg in the “Initial combination therapy” group, –2.7 mmHg in the “Switch from prior treatment” group, –2.4 mmHg in the “Add-on (only)” group, and – 3.1 mmHg in the “Add-on (with other glaucoma drug)” group.27
The accumulated evidence so far suggests that ripasudil is a promising anti-glaucoma drug that can lower IOP irrespective of glaucoma subtype and the pattern of treatment initiation.
Patient Selection
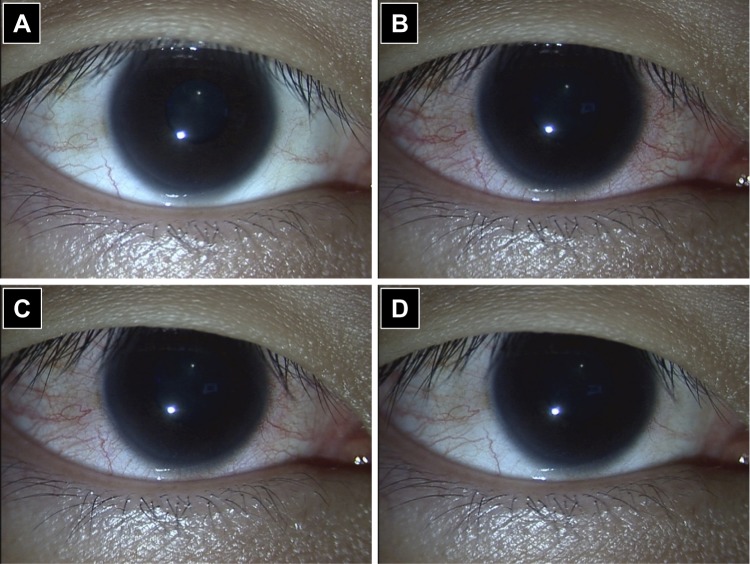
In clinical practice, PGA eyedrops remain the major medical treatment option for patients with glaucoma or OHT due to their well-balanced profile between safety and efficacy. Ocular ADRs, such as conjunctiva hyperemia, increased iris pigmentation, and eyelash changes, frequently occur in eyes treated with PGAs. Moreover, prolonged use of topical PGAs increases the frequency of periocular changes referred to as prostaglandin-associated periorbitopathy (PAP), including deepening of the upper eyelid sulcus (DUES), upper eyelid ptosis/retraction, loss of inferior orbital fat pads and enophthalmos, eyelid pigmentation, and eyelash changes.41 Notwithstanding these local ADRs, a favorable systemic side-effect profile compared with β-adrenergic antagonists is one of the key reasons for the designation of PGAs as first-line medical therapy. Ripasudil’s systemic side-effect profile is equal or superior to that of PGAs because the reported systemic adverse effects are minimal except for allergic reactions, and most of the local adverse events are transient and/or mild in patients treated with ripasudil compared with those in patients treated with PGAs.23–25,27,30 Cosmetic problems associated with long-term use of PGAs could be serious for young patients (especially women) or patients with unilateral glaucoma. The presence of DUES makes it difficult to measure IOP by Goldmann applanation tonometry, and Miki et al reported that preoperative use of bimatoprost might be related to recurrent IOP elevation after trabeculectomy.42 To the best of our knowledge, ripasudil-associated DUES has not been reported. However, blepharitis and conjunctivitis sometimes occur during ripasudil treatment and are among the major reasons for treatment discontinuation.27,29-32 Corticosteroid ointment may help manage ripasudil-related blepharitis and thereby allow continued ripasudil treatment, but to date, there is no consensus recommendation for the management of ripasudil-related blepharitis. Transient ocular hyperemia is inevitable, as discussed above (Figure 2). The safety profile of ripasudil compared with that of other anti-glaucoma agents is one of the key factors in patient selection.
Figure 2.
Transient conjunctival hyperemia after ripasudil instillation. (A) Before instillation; (B) 15 minutes after instillation; (C) 30 minutes after instillation; (D) 2 hours after instillation.
To date, no head-to-head clinical trials have been conducted allowing direct comparison of the efficacy of ripasudil vs. another anti-glaucoma drug. Further, ripasudil is approved in Japan as a second-line treatment for glaucoma or OHT with inadequate response to PGAs and/or β-blockers or in cases where these drugs are contraindicated. As a result, ripasudil treatment is generally initiated in chronic cases. However, considering that ripasudil acts by increasing conventional aqueous humor outflow by changing the status of the trabecular meshwork and endothelial cells of Schlemm’s canal,5 ripasudil treatment should be started during an early stage of glaucoma, before irreversible damage to the components of the trabecular meshwork occurs. Based on this notion, patients with steroid-induced OHT or UG may be good candidates for ripasudil treatment, because their eyes often show a sudden-onset rise in IOP associated with corticosteroid use or deterioration of inflammation, indicating that the drainage function of the trabecular meshwork is likely sufficiently preserved to maintain normal IOP. This idea is endorsed by the presence of ripasudil responders among patients with steroid-induced OHT or UG who show a considerable reduction in IOP soon after ripasudil treatment.34,39 The advantage of early intervention with ripasudil should be evaluated in future studies.
Recently, pleiotropic effects of ripasudil have been reported, including neuroprotection, increased ocular blood flow, anti-inflammation, anti-scarring, decreased macular thickness in eyes with diabetic macular edema, and changes in corneal endothelial cells.43–46 However, these auxiliary effects should not be used for patient selection, because no human clinical trials have proven the true efficacy and/or safety of ripasudil in patients with such ocular conditions.
Conclusions
Ripasudil is well tolerated and effective against almost all subtypes of glaucoma. With regard to safety, conjunctival hyperemia is the most common ADR, but it is unlikely to be a reason for discontinuation if the patient is pre-informed, as it is usually transient and mild. On the other hand, blepharitis and allergic conjunctivitis are ADRs to be carefully monitored because they may be causes for ripasudil discontinuation. The systemic adverse effects of ripasudil are minimal, except for allergic reactions. With regard to efficacy, ripasudil is expected to lower IOP in almost all glaucoma subtypes, regardless of the treatment initiation pattern. However, the status of the trabecular meshwork may affect the IOP-lowering effect of ripasudil and hereby determine whether an eye is a responder or a non-responder. In patient selection, both the safety and the efficacy profiles of ripasudil should be considered. Because irreversible damage to the trabecular meshwork would considerably affect the drug’s efficacy, it may be better to start ripasudil treatment during an early stage of glaucoma.
ROCK inhibitors are a new class of topical IOP-lowering drugs. More data from clinical trials and postmarketing studies will be required to establish best practices for the treatment of patients with glaucoma or OHT using ROCK inhibitor ophthalmic solution. Several ROCK inhibitors other than ripasudil are currently being studied or used clinically. Among these, netarsudil (Rhopressa, Aerie Pharmaceuticals) was approved by the US Food and Drug Administration in 2017 for treatment of open-angle glaucoma and OHT, and its safety and efficacy are now being evaluated in clinical practice.43 The set of clinical data on ripasudil introduced in this review would be a benchmark for evaluation of the safety and efficacy of netarsudil and other future topical ROCK inhibitors.
Disclosure
The authors report no conflicts of interest in this review.
References
- 1.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7 [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenwick EK, Man RE, Aung T, Ramulu P, Lamoureux EL. Beyond intraocular pressure: optimizing patient-reported outcomes in glaucoma. Prog Retin Eye Res. 2019;100801. doi: 10.1016/j.preteyeres.2019.100801 [DOI] [PubMed] [Google Scholar]
- 4.Nuschke AC, Farrell SR, Levesque JM, Chauhan BC. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: axon transport, injury and soma loss. Exp Eye Res. 2015;141:111–124. doi: 10.1016/j.exer.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 5.Honjo M, Tanihara H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn J Ophthalmol. 2018;62(2):109–126. doi: 10.1007/s10384-018-0566-9 [DOI] [PubMed] [Google Scholar]
- 6.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80(2):197–206. doi: 10.1016/j.exer.2004.08.029 [DOI] [PubMed] [Google Scholar]
- 7.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298(3):C749–C763. doi: 10.1152/ajpcell.00317.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Liu X, Zhong Y. Rho/Rho-associated kinase pathway in glaucoma (review). Int J Oncol. 2013;43(5):1357–1367. doi: 10.3892/ijo.2013.2100 [DOI] [PubMed] [Google Scholar]
- 9.Wecker T, Han H, Borner J, Grehn F, Schlunck G. Effects of TGF-beta2 on cadherins and beta-catenin in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013;54(10):6456–6462. doi: 10.1167/iovs.13-12669 [DOI] [PubMed] [Google Scholar]
- 10.Torrejon KY, Papke EL, Halman JR, et al. TGFbeta2-induced outflow alterations in a bioengineered trabecular meshwork are offset by a rho-associated kinase inhibitor. Sci Rep. 2016;6(1):38319. doi: 10.1038/srep38319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honjo M, Inatani M, Kido N, et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001;119(8):1171–1178. doi: 10.1001/archopht.119.8.1171 [DOI] [PubMed] [Google Scholar]
- 12.Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42(1):137–144. [PubMed] [Google Scholar]
- 13.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 14.Kaneko Y, Ohta M, Inoue T, et al. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci Rep. 2016;6(1):19640. doi: 10.1038/srep19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1–2):40–51. doi: 10.1089/jop.2017.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm’s canal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53(6):3092–3103. doi: 10.1167/iovs.11-8018 [DOI] [PubMed] [Google Scholar]
- 17.Tokushige H, Inatani M, Nemoto S, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48(7):3216–3222. doi: 10.1167/iovs.05-1617 [DOI] [PubMed] [Google Scholar]
- 18.Nishio M, Fukunaga T, Sugimoto M, et al. The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes. Curr Eye Res. 2009;34(4):282–286. doi: 10.1080/02713680902783763 [DOI] [PubMed] [Google Scholar]
- 19.Van de Velde S, Van Bergen T, Sijnave D, et al. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Invest Ophthalmol Vis Sci. 2014;55(2):1006–1016. doi: 10.1167/iovs.13-13157 [DOI] [PubMed] [Google Scholar]
- 20.Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015;24(1):51–54. doi: 10.1097/IJG.0b013e3182952213 [DOI] [PubMed] [Google Scholar]
- 21.Pattabiraman PP, Rinkoski T, Poeschla E, Proia A, Challa P, Rao PV. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. Am J Pathol. 2015;185(2):496–512. doi: 10.1016/j.ajpath.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Mukherjee D, Navarro I, et al. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur J Pharmacol. 2016;787:20–31. doi: 10.1016/j.ejphar.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanihara H, Inoue T, Yamamoto T, et al. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156(4):731–736. doi: 10.1016/j.ajo.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 24.Tanihara H, Inoue T, Yamamoto T, et al. Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133(7):755–761. doi: 10.1001/jamaophthalmol.2015.0525 [DOI] [PubMed] [Google Scholar]
- 25.Tanihara H, Inoue T, Yamamoto T, et al. Phase 1 clinical trials of a selective Rho kinase inhibitor, K-115. JAMA Ophthalmol. 2013;131(10):1288–1295. doi: 10.1001/jamaophthalmol.2013.323 [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto E, Ishida W, Sumi T, et al. Evaluation of offset of conjunctival hyperemia induced by a Rho-kinase inhibitor; 0.4% Ripasudil ophthalmic solution clinical trial. Sci Rep. 2019;9(1):3755. doi: 10.1038/s41598-019-40255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanihara H, Kakuda T, Sano T, et al. Safety and efficacy of ripasudil in japanese patients with glaucoma or ocular hypertension: 3-month interim analysis of ROCK-J, a post-marketing surveillance study. Adv Ther. 2019;36(2):333–343. doi: 10.1007/s12325-018-0863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato S, Hirooka K, Nitta E, Ukegawa K, Tsujikawa A. Additive intraocular pressure lowering effects of the rho kinase inhibitor, ripasudil in glaucoma patients not able to obtain adequate control after other maximal tolerated medical therapy. Adv Ther. 2016;33(9):1628–1634. doi: 10.1007/s12325-016-0389-3 [DOI] [PubMed] [Google Scholar]
- 29.Saito H, Kagami S, Mishima K, Mataki N, Fukushima A, Araie M. Long-term side effects including blepharitis leading to discontinuation of ripasudil. J Glaucoma. 2019;28(4):289–293. doi: 10.1097/IJG.0000000000001203 [DOI] [PubMed] [Google Scholar]
- 30.Tanihara H, Inoue T, Yamamoto T, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94(1):e26–e34. doi: 10.1111/aos.12829 [DOI] [PubMed] [Google Scholar]
- 31.Kawara K, Kanamori A, Mori S, et al. Intraocular pressure-lowering effect of ripasudil hydrochloride hydrate and reasons for discontinuation of use in clinical practice. Int J Ophthalmol CliniRes. 2018;5(1):083. [Google Scholar]
- 32.Kusakabe M, Imai Y, Natsuaki M, Yamanishi K. Allergic contact dermatitis due to ripasudil hydrochloride hydrate in eye-drops: a case report. Acta Derm Venereol. 2018;98(2):278–279. doi: 10.2340/00015555-2832 [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, Ishida K, Tomita G. Effectiveness and safety of switching from prostaglandin analog monotherapy to prostaglandin/timolol fixed combination therapy or adding ripasudil. Jpn J Ophthalmol. 2018;62(4):508–516. doi: 10.1007/s10384-018-0599-0 [DOI] [PubMed] [Google Scholar]
- 34.Kusuhara S, Katsuyama A, Matsumiya W, Nakamura M. Efficacy and safety of ripasudil, a Rho-associated kinase inhibitor, in eyes with uveitic glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256(4):809–814. doi: 10.1007/s00417-018-3933-9 [DOI] [PubMed] [Google Scholar]
- 35.Tanihara H, Inoue T, Yamamoto T, et al. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a randomized, open-label, crossover study. Acta Ophthalmol. 2015;93(4):e254–e260. doi: 10.1111/aos.12599 [DOI] [PubMed] [Google Scholar]
- 36.Inoue K, Okayama R, Shiokawa M, Ishida K, Tomita G. Efficacy and safety of adding ripasudil to existing treatment regimens for reducing intraocular pressure. Int Ophthalmol. 2018;38(1):93–98. doi: 10.1007/s10792-016-0427-9 [DOI] [PubMed] [Google Scholar]
- 37.Inazaki H, Kobayashi S, Anzai Y, et al. Efficacy of the additional use of ripasudil, a rho-kinase inhibitor, in patients with glaucoma inadequately controlled under maximum medical therapy. J Glaucoma. 2017;26(2):96–100. doi: 10.1097/IJG.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 38.Inazaki H, Kobayashi S, Anzai Y, et al. One-year efficacy of adjunctive use of Ripasudil, a rho-kinase inhibitor, in patients with glaucoma inadequately controlled with maximum medical therapy. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):2009–2015. doi: 10.1007/s00417-017-3727-5 [DOI] [PubMed] [Google Scholar]
- 39.Yasuda M, Takayama K, Kanda T, Taguchi M, Someya H, Takeuchi M. Comparison of intraocular pressure-lowering effects of ripasudil hydrochloride hydrate for inflammatory and corticosteroid-induced ocular hypertension. PLoS One. 2017;12(10):e0185305. doi: 10.1371/journal.pone.0185305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komizo T, Ono T, Yagi A, Miyata K, Aihara M. Additive intraocular pressure-lowering effects of the Rho kinase inhibitor ripasudil in Japanese patients with various subtypes of glaucoma. Jpn J Ophthalmol. 2019;63(1):40–45. doi: 10.1007/s10384-018-0635-0 [DOI] [PubMed] [Google Scholar]
- 41.Tan P, Malhotra R. Oculoplastic considerations in patients with glaucoma. Surv Ophthalmol. 2016;61(6):718–725. doi: 10.1016/j.survophthal.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 42.Miki T, Naito T, Fujiwara M, et al. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS One. 2017;12(7):e0181550. doi: 10.1371/journal.pone.0181550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanna AP, Johnson M. Rho kinase inhibitors as a novel treatment for glaucoma and ocular hypertension. Ophthalmology. 2018;125(11):1741–1756. doi: 10.1016/j.ophtha.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami Y, Song YS, Ishibazawa A, et al. Effect of ripasudil on diabetic macular edema. Sci Rep. 2019;9(1):3703. doi: 10.1038/s41598-019-40194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wada Y, Higashide T, Nagata A, Sugiyama K. Effects of ripasudil, a rho kinase inhibitor, on blood flow in the optic nerve head of normal rats. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):303–311. doi: 10.1007/s00417-018-4191-6 [DOI] [PubMed] [Google Scholar]
- 46.Matsumura R, Inoue K, Shiokawa M, et al. Changes in corneal endothelial cell shape after treatment with one drop of ROCK inhibitor. Int Ophthalmol. 2020;40(2):411–417. doi: 10.1007/s10792-019-01198-2 [DOI] [PubMed] [Google Scholar]