Abstract
Background:
Cognitive impairment is a common complication of patients with temporal lobe epilepsy (TLE). Therefore, the aim of this study was to compare the effects of donepezil and memantine on improving the cognitive function of patients with TLE.
Materials and Methods:
In a clinical trial study, 70 patients with TLE were divided into two groups of 35 each: 10 mg doses of donepezil (first group) and memantine (second group) were applied for 16 weeks. The level of cognitive function of patients in both groups before and after treatment was determined using Montreal Cognitive Assessment (MoCA) test.
Results:
The mean score of MoCA before and after intervention was 23.55 ± 3.67 and 26.09 ± 2.5, respectively, in the group treated with memantine, and the mean score of intervention was significantly improved (P < 0.001). In the group treated with donepezil, the score before and after the operation was 23.87 ± 3.18 and 24.35 ± 2.17, respectively, and no significant difference was observed in this group (P = 0.38).
Conclusion:
Hence, memantine was better than donepezil in the improvement of cognitive impairment in patients with TLE.
Keywords: Cognitive impairment, donepezil, memantine, temporal lobe epilepsy
INTRODUCTION
Epilepsy is one of the most common neurological disorders, which from the neurophysiology point of view is equivalent to abnormal electrical charge and discharge of the brain. Epilepsy is reported in various forms and is categorized in different ways in terms of etiology and clinical symptoms, and its temporal type occurs with a series of mental, behavioral, cognitive, and motor disorders.[1] Loss of consciousness, amnesia, cognitive impairment, and emotional changes are common symptoms of temporal lobe epilepsy (TLE),[2] and based on the results of the recent researches, the most common neuropsychological effect of epilepsy is memory impairment, which can be associated with other disorders such as vocabulary and recognition.[3,4]
Cognitive impairment is one of the common problems of patients with TLE, as approximately 70% of patients with TLE having known problems with memory function.[4] Because the temporal lobe plays a role in the formation of memory, TLE communication with memory impairment is also acceptable, and this has been proven in various studies.[5] Lack of memory performance in the form of TLE is related to the memory-related brain structure, such as the hippocampus, which is directly involved in seizure activity. TLE may also interfere with neurophysiological memory tests, which measures the ability to recall new information within 30 min.[6,7] Studies have shown that patients with temporal lobe seizures are at high risk for cognitive problems.[8,9] Other factors associated with cognitive impairment include the duration of epilepsy, the onset of the disease, the frequency of seizures, and the history of recent head injury and the use of anticonvulsants.[10,11] According to some studies, the two drugs, donepezil and memantine, are effective in improving daily function, behavioral function, cognitive function, and overall clinical conditions.[12,13]
Memantine is a proprietary and noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor with a mild-to-moderate tendency. Memantine is more abundant in the regions of the hippocampus and the cerebral cortex, which are involved in recognition, learning, and memory.[14] Glutamate or glutamic acid is resulted in long-term strength of NMDA receptor. Increasing levels of glutamate are associated with the progression of neurotoxicity, suggesting the beneficial effect of memantine on blocking the negative effects of increasing glutamate levels. Memantine is well tolerated and its side effects are very rare and include dizziness, confusion, drowsiness, hallucinations, and nausea that can be eliminated after a dose reduction or drug discontinuation.[15]
Donepezil is a cholinesterase inhibitor that reduces the breakdown of synaptic acetylcholine, enhances its ability to stimulate postsynaptic receptors, and enhances the normal pattern of acetylcholine release in the brain.[16] Donepezil is prescribed in the countries of Europe and Japan during the moderate stages of Alzheimer's disease and in the United States at all stages.[17] Studies have shown that donepezil improves cognitive and neuropsychiatric performance and reduces the economic burden of nursing need. In patients with Alzheimer's disease, the use of this drug in comparison with placebo has improved general and cognitive function.[18,19] Many studies have shown the efficacious results of the use of memantine and donepezil to improve memory in Alzheimer's disease,[20,21] but studies on the effects of this drug in patients with epilepsy are limited. Therefore, due to the high prevalence of cognitive impairment in patients with epilepsy and the necessity of its treatment, this study was conducted to compare the effects of memantine and donepezil on improvement of cognitive function in patients with TLE.
MATERIALS AND METHODS
This was a randomized clinical trial performed in Al-Zahra and Kashani Hospitals of Isfahan University of Medical Sciences, Isfahan, Iran, during 2017–2018. Based on the observation, electroencephalogram (EEG) findings and neuroimaging patients with TLE referring to the outpatient clinic were enrolled in the study. The study was approved by the Ethics Committee of the Isfahan Medical Sciences and was approved in the Iranian Registry of Clinical Trial (IRCT20180113038344N1).
The inclusion criteria included patients with TLE that was diagnosed by a neurologist, absence of other neurological diseases other than epilepsy, with age more than 18 years and <55 years old, the history of failing treatment of improving cognitive function, and willingness to participate in the study. Furthermore, patients with mental retardation and progressive neurological disease did not meet inclusion criteria. Furthermore, allergy to memantine or donepezil drugs, the onset of any side effects associated with the use of these drugs, and the lack of satisfaction to continue the study at any time of the study were considered as exclusion criteria.
TLE was diagnosed by a history of characteristic partial seizure symptoms. The diagnosis was confirmed by the capture of a typical episode during EEG or video-EEG, with epileptiform activity over one or both temporal regions.
The sampling method was convenience–nonrandom, and the patients were admitted to the clinic if they were eligible. All patients had informed consent for entering into the study.
All patients were examined by a neurological assistant, and a biography of the patient was obtained including demographic information (name, age, sex, education, and marital status) and information about the disease (when the disease began and drugs). All of this information was recorded in a special form to the patient.
Patients were randomly allocated into two groups using random allocation software; the first group was treated with donepezil. The drug was started at a dose of 5 mg, and after 4 weeks, if the patient tolerated, it was increased to 10 mg daily and the total dose of the drug was administered to patients for 16 weeks.
In the second group, memantine was started at a dose of 5 mg daily, and in case of patient tolerance, it increased to 10 mg after a week, and in this group, the duration of treatment was 16 weeks.
The data collection tool was a memory and cognitive function questionnaire of Montreal Cognitive Assessment (MoCA) that all patients were asked to complete the questionnaire before intervention. This questionnaire is in fact a tool for evaluating mild cognitive impairment with a maximum score of 30. The questionnaire consists of eight parts of the visuospatial/executive, memory, attention, naming, abstraction, language, delayed recall, and orientation, giving each section a separate score. The short-term memory recall section is included 5 points about learning and recall words, visuospatial abilities are included 3 points about clock-drawing task and one point about cube copy, executive function is included 4 points about alternation task adapted, attention, concentration and working memory is included one point about target detection, 3 points about subtraction task, and one point about digits forward and backward, language is included three item about naming task and repeating of two complex sentence (2 points) and a fluency task and finally orientation to time and place is included 6 points. Validity and reliability of this questionnaire have been confirmed in various studies previously.[22] The cognitive impairment was considered MoCA score below 26.
During the 1st and 2nd months of intervention, the patient was contacted by regular telephone conversation and asked for the occurrence of possible complications. In case of complications, the patient was treated with a medical treatment.
After completing the drug (16 weeks), all patients were called out to the outpatient clinic of Al-Zahra Hospital and the questionnaire for measuring memory and cognitive function of MoCA was completed again by the patients.
Statistical analysis
The sample size of the study was 35 individuals in each group according to the sample size formula for the comparison of the meanings with a 95% confidence level, 80% test power, the standard deviation of cognitive impairment score which was found to be about 1.17 in other studies and the least significant difference between the two groups which was considered as 0.8. All patients' information was entered into SPSS version 25 (IBM, New York, United States) and analyzed by Chi-square test (to compare frequency values of variables between groups), t-test (to compare mean values of variables between groups), and paired samples test (to compare mean values of variable before and after intervention) and a significant level of P < 0.05 was considered.
RESULTS
Seventy patients with TLE were studied in the two groups of 35 each receiving donepezil and memantine. During the study, six patients were excluded due to lack of referring, two from the memantine group and four from the doping group, and the data were analyzed on 33 patients receiving memantine and 31 patients receiving donepezil [Figure 1].
Figure 1.
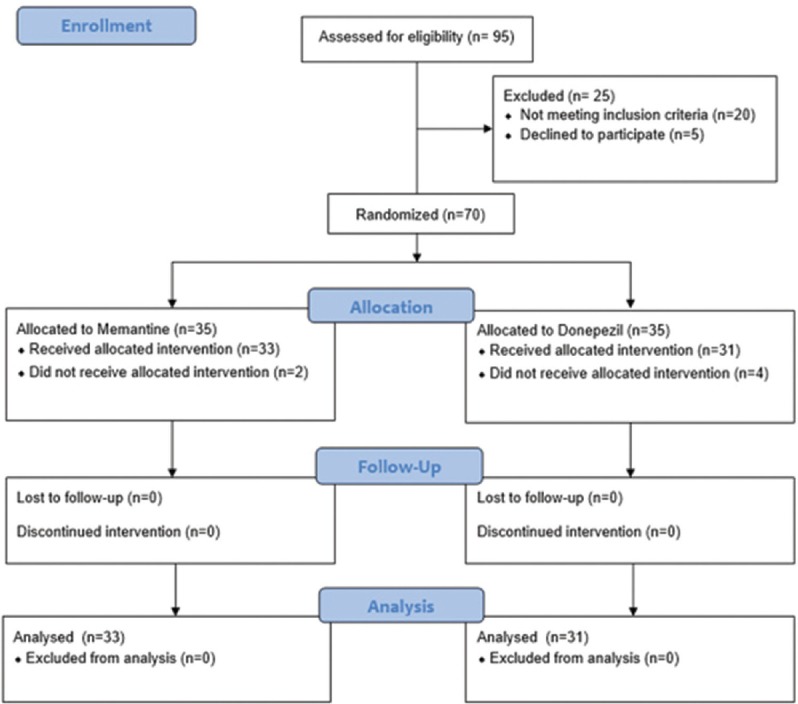
Consort diagram of patients in each step of the study
There were no significant differences between the two groups in terms of demographic and baseline variables including age and sex distribution and education level [Table 1].
Table 1.
Distribution of the age, sex, and level of education of the two groups
| Variables | Groups | P | |
|---|---|---|---|
| Memantine | Donepezil | ||
| Average of age (years) | 39.1±9.8 | 38.1±11.9 | 0.72* |
| Gender (%) | |||
| Male | 16 (48.5) | 15 (48.4) | 0.9** |
| Female | 17 (51.5) | 16 (51.6) | |
| Education | |||
| Under the diploma | 3 (9.1) | 4 (12.9) | 0.86** |
| Diploma | 11 (33.3) | 9 (29) | |
| University | 19 (57.6) | 18 (58.1) | |
*Independent t-test, **Chi-square
The mean total scores of MoCA before and after intervention in the memantine group were 23.55 ± 3.67 and 26.09 ± 2.5, respectively. The mean score of MoCA in the memantine group was significantly improved (P < 0.001). In the donepezil group, the total scores before and after intervention were 23.87 ± 3.18 and 24.35 ± 2.17, respectively, and the changing of total score in the donepezil group was not statistically significant (P = 0.38). There was no significant difference between the two groups based on total score before intervention (P = 0.71), but the mean total score after intervention in the memantine group was significantly higher than the donepezil group (P = 0.004) and the difference of changing the score in the memantine group was significantly higher than the donepezil group (P = 0.001).
Compared to the results of the study before intervention, of 64 patients, 51 (79.7%) had cognitive impairment (score <26), of which 27 were from the memantine group and 24 from the donepezil group (81.8% vs. 77.4%), but the difference between the two groups was not statistically significant (P = 0.66). In the interventional part, the number of patients with cognitive impairment was 32 (50%), of which 11 were from the memantine group and 21 from the donepezil group (33.3% vs. 67.7%), and the difference between the two groups was statistically significant (P = 0.006).
Before intervention, there was no significant difference between groups based on the scores of visuospatial/executive, language, naming, attention, abstraction, delayed recall, and orientation to time and place (P > 0.05).
The study of MoCA test showed that in the memantine group, scores of language, visuospatial/executive, and orientation to time and place significantly increased (P < 0.05) and the scores of attention and delayed recall in the memantine group significantly decreased after intervention (P < 0.05). However, the changing scores of abstraction and naming in the memantine group after intervention was not statistically significant (P > 0.05). In the donepezil group, the score of abstraction was significantly increased after intervention (P = 0.03), but the changing scores of visuospatial/executive, language, naming, attention, delayed recall, and orientation to time and place were not statistically significant (P > 0.05). After intervention, the mean score of orientation to time and place in the memantine group was significantly higher than the donepezil group (P = 0.023), but there was no significant difference between groups based on visuospatial/executive, language, naming, attention, abstraction, and delayed recall (P > 0.05) [Table 2].
Table 2.
Mean and standard deviation of Montreal Cognitive Test score in both groups before and after intervention
| Variables | Time | Groups | P* | |
|---|---|---|---|---|
| Memantine | Donepezil | |||
| Visuospatial/executive | Before | 3.58±1.58 | 3.77±1.33 | 0.59* |
| After | 3.97±1.13 | 3.84±1.01 | 0.91* | |
| Difference | 0.39±0.45 | 0.07±0.33 | 0.08* | |
| P | 0.017** | 0.78** | ||
| Naming | Before | 2.97±0.17 | 2.84±0.37 | 0.07* |
| After | 2.79±0.6 | 2.71±0.46 | 0.083* | |
| Difference | −0.18±0.43 | −0.13±0.09 | 0.10* | |
| P | 0.06** | 0.21** | ||
| Attention | Before | 5.42±0.75 | 5.13±0.81 | 0.6* |
| After | 4.64±1.19 | 4.97±0.87 | 0.28* | |
| Difference | −0.78±0.44 | −0.16±0.06 | 0.09* | |
| P | <0.001** | 0.39** | ||
| Language | Before | 2.55±0.56 | 2.68±0.48 | 0.32* |
| After | 2.85±0.36 | 2.65±0.61 | 0.11* | |
| Difference | 0.3±0.2 | −0.03±0.13 | 0.04* | |
| P | 0.002** | 0.77** | ||
| Abstraction | Before | 1.48±0.51 | 1.32±0.6 | 0.25* |
| After | 1.73±0.52 | 1.61±0.56 | 0.71* | |
| Difference | 0.25±0.01 | 0.29±0.04 | 0.31* | |
| P | 0.073** | 0.037** | ||
| Delayed recall | Before | 3.52±1.2 | 3.45±0.99 | 0.68* |
| After | 2.79±1.11 | 3.13±0.99 | 0.82* | |
| Difference | −0.73±0.09 | −0.32 | 0.12* | |
| P | <0.001** | 0.12** | ||
| Orientation to time and place | Before | 5.55±0.62 | 5.42±0.85 | 0.5* |
| After | 5.82±0.46 | 5.45±0.77 | 0.023* | |
| Difference | 0.27±0.16 | 0.03±0.08 | 0.04* | |
| P | 0.018** | 0.28** | ||
| Total score | Before | 23.55±3.67 | 23.87±3.18 | 0.71* |
| After | 26.09±2.5 | 24.35±2.17 | 0.004* | |
| Difference | 2.54±1.17 | 0.48±1.01 | 0.001* | |
| P | <0.001** | 0.38** | ||
*Significant level of difference between the two groups before and after intervention by t-test, **Significant level of difference before and after intervention in each group in terms of t-paired test
DISCUSSION
Studies and experiments have shown that epileptic seizures have an effect on memory and cognitive function. Patients with epilepsy have complained of cognitive and memory problems. According to the results of our study, the prevalence of cognitive impairment was 79.7% in the preinterventional study, which is higher than Olshansky's finding (54%).[7]
The results of our study showed that memantine was effective on improving cognitive impairment in patients with TLE. With 16 weeks of treatment with memantine, the prevalence of memory impairment decreased from 81.8% to 33.3%, whereas in the treatment group with donepezil, the prevalence of cognitive impairment decreased from 77.4% to 67.7%, which was less than the memantine group. The results of a study by Leeman-Markowski et al. have shown that the use of memantine leads to memory impairment in patients with epilepsy.[12]
Various studies on the use of memantine have shown that treatment with memantine improves the overall cognitive and functional relationships and has beneficial effects on agitation and aggressive behaviors.[13]
Based on the results of our study, memantine therapy relatively improved cognitive impairment in terms of attention, visuospatial/executive, language, delayed recall, and orientation to time and place, whereas treatment with donepezil only improves the abstraction, and in other areas, no significant difference was observed. On the other hand, cognitive impairment is commonly found in Alzheimer's disease. The most studies have been conducted on the effects of the two drugs in Alzheimer's patients, few studies have been done on the effect of these two drugs on the treatment of cognitive impairment in patients with TLE according to these limited studies, it has been shown that memantine is effective on improving daily function, behavioral function, cognitive function and clinical conditions of patients. Memantine is a noncompetitive NMDA antagonist and reduces some of the behavioral symptoms of Alzheimer's disease and has a significant impact on general, functional, and cognitive conditions of patients.[21] The use of donepezil and memantine combination for moderate-to-severe Alzheimer's has recently been investigated. A group of studies have reported that it reduces the cognitive and functional decline and delays the need for a nurse at home.[20] Some studies have shown the effective results in the use of memantine in improving memory in Alzheimer's disease.[21] The mechanism and effectiveness of antidementive drugs in other patient groups (dementia, Parkinson's, and multiple sclerosis) may be similar to patients with epilepsy.[23]
In a study performed in 2016, Marimuthu et al. reported that once-daily memantine (10 mg) treatment significantly improved cognition, memory, and quality of life in epileptic patients with mild-to-moderate cognitive impairment and was found to have a favorable safety profile.[24] In our study, the result was in line with this study because memantine was effective on improvement of memory and cognitive. The mechanism and effectiveness of antidementive drugs in other patient groups (dementia, Parkinson's, and multiple sclerosis) may be similar to patients with epilepsy. There are limited studies that have looked at these drugs in patients with epilepsy, and these studies have not provided enough evidence to generalize the entire population of the disease.
Study limitations
This is a clinical trial study on a small number of cases which may lead to imprecise estimation of findings. Furthermore, lack of comparison group pushed us just to describe findings. Furthermore, lack of blindness was considered a limitation in this study.
CONCLUSION
According to the results of this study, evidence of the efficacy of memantine in improving cognitive impairment was found in MoCA tests in patients with TLE. However, despite subjective changes in the improvement of cognitive impairment, there was no evidence that donepezil was effective in improving cognitive impairment in MoCA test. At the same time, further studies are recommended in this area.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences. This design was registered in Research Faculty of Medical School of Isfahan by code 395112.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The present article is derived partly from the results of thesis on specialty in the field of neurology, sponsored by the Research Deputy of the Faculty of Medicine of Isfahan University of Medical Sciences. Therefore, the authors of the article praise and appreciate their efforts.
REFERENCES
- 1.Mehvari J, Zare M, Andami R, Ghadimi K, Tabrizi N. Ictal and interictal electroencephalography of mesial and lateral temporal lobe epilepsy: A comparative study. Caspian J Neurol Sci. 2017;4:222–30. [Google Scholar]
- 2.Rafiee Zadeh A, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int J Physiol Pathophysiol Pharmacol. 2019;11:95–104. [PMC free article] [PubMed] [Google Scholar]
- 3.Rafiee Zadeh A, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11:105–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–72. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 5.Helmstaedter C. Effects of chronic temporal lobe epilepsy on memory functions. In: Arzimanoglou A, Aldenkamp A, Cross H, Lassonde M, editors. Cognitive Dysfunction in Children with Temporal Lobe Epilepsy: Progress in Epileptic Disorders Series. London: John Libbey; 2005. pp. 13–30. [Google Scholar]
- 6.Eskin SB, Hermanson S. Nutrition labeling at fast-food and other chain restaurants. Issue Brief (Public Policy Inst (Am Assoc Retired Pers)) 2004;Jul;(IB71):1–6. [PubMed] [Google Scholar]
- 7.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 8.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 9.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lay C, Doré J, Rigottier-Gois L. Separation of bacteria of the Clostridium leptum subgroup from the human colonic microbiota by fluorescence-activated cell sorting or group-specific PCR using 16S rRNA gene oligonucleotides. FEMS Microbiol Ecol. 2007;60:513–20. doi: 10.1111/j.1574-6941.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 11.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeman-Markowski BA, Meador KJ, Moo LR, Cole AJ, Hoch DB, Garcia E, et al. Does memantine improve memory in subjects with focal-onset epilepsy and memory dysfunction. A randomized, double-blind, placebo-controlled trial? Epilepsy Behav. 2018;88:315–24. doi: 10.1016/j.yebeh.2018.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher RS, Bortz JJ, Blum DE, Duncan B, Burke H. A pilot study of donepezil for memory problems in epilepsy. Epilepsy Behav. 2001;2:330–4. doi: 10.1006/ebeh.2001.0221. [DOI] [PubMed] [Google Scholar]
- 14.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 15.Cabuk B, Etus V, Bozkurt SU, Sav A, Ceylan S. Neuroprotective effect of memantine on hippocampal neurons in infantile rat hydrocephalus. Turk Neurosurg. 2011;21:352–8. doi: 10.5137/1019-5149.JTN.4119-11.1. [DOI] [PubMed] [Google Scholar]
- 16.Cummings JL. Cholinesterase inhibitors: A new class of psychotropic compounds. Am J Psychiatry. 2000;157:4–15. doi: 10.1176/ajp.157.1.4. [DOI] [PubMed] [Google Scholar]
- 17.Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): Randomised double-blind trial. Lancet. 2004;363:2105–15. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson's disease: A randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72:708–12. doi: 10.1136/jnnp.72.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 20.Koola MM, Nikiforuk A, Pillai A, Parsaik AK. Galantamine-memantine combination superior to donepezil-memantine combination in Alzheimer's disease: Critical dissection with an emphasis on kynurenic acid and mismatch negativity. J Geriatr Care Res. 2018;5:57–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Ridha BH, Crutch S, Cutler D, Frost C, Knight W, Barker S, et al. A double-blind placebo-controlled cross-over clinical trial of DONepezil in posterior cortical atrophy due to underlying Alzheimer's Disease: DONIPAD study. Alzheimers Res Ther. 2018;10:44. doi: 10.1186/s13195-018-0363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong A, Xiong YY, Kwan PW, Chan AY, Lam WW, Wang K, et al. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord. 2009;28:81–7. doi: 10.1159/000232589. [DOI] [PubMed] [Google Scholar]
- 23.Durães F, Pinto M, Sousa E. Old drugs as new treatments for neurodegenerative diseases Pharmaceuticals (Basel) 2018;11 doi: 10.3390/ph11020044. pii: E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marimuthu P, Varadarajan S, Krishnan M, Shanmugam S, Kunjuraman G, Ravinder JR, et al. Evaluating the efficacy of memantine on improving cognitive functions in epileptic patients receiving anti-epileptic drugs: A double-blind placebo-controlled clinical trial (Phase IIIb pilot study) Ann Indian Acad Neurol. 2016;19:344–50. doi: 10.4103/0972-2327.179971. [DOI] [PMC free article] [PubMed] [Google Scholar]


