Abstract
Background:
Prediabetes is strongly associated with high blood pressure; however, a little is known about prediabetes and high blood pressure comorbidity in the high-risk individuals. This is the first study in the world to assess the long-term effects of risk factors associated with high blood pressure and prediabetes comorbidity in the first-degree relatives (FDRs) of type 2 diabetes mellitus (T2DM) patients.
Materials and Methods:
The longitudinal data obtained from 1388 nondiabetic FDRs of T2DM patients with at least two visits between 2003 and 2011. We used univariate and bivariate mixed-effects logistic regressions with a Bayesian approach to identify longitudinal predictors of high blood pressure and prediabetes separately and simultaneously.
Results:
The baseline prevalence of high blood pressure, prediabetes, and the coexistence of both was 27.4%, 19.1%, and 29.8%, respectively. The risks of high blood pressure and prediabetes were increased by one-unit raise in the age (odds ratio [OR] of high blood pressure: 1.419 (95% credible intervals [CI], 1.077–1.877), prediabetes: 1.055 (95% CI: 1.040–1.068)) and one-unit raise in remnant-cholesterol (OR of high blood pressure: 1.093 (95%CI, 1.067–1.121), and prediabetes: 1.086 (95% CI, 1.043–1.119)). Obese participants were more likely to have high blood pressure (OR: 2.443 [95% CI, 1.978–3.031]) and prediabetes (OR: 1.399 [95% CI, 1.129–1.730]) than other participants.
Conclusion:
We have introduced remnant-cholesterol, along with obesity and age, as a significant predictor of prediabetes, high blood pressure, and the coexistence of both in the FDRs of diabetic patients. Obesity index and remnant-cholesterol showed the stronger effects on high blood pressure and prediabetes comorbidity than on each condition separately.
Keywords: Comorbidity, family history, hypertension, prediabetes, prehypertension, risk factor
INTRODUCTION
High blood pressure (prehypertension or hypertension), pre-type 2 diabetes mellitus (prediabetes), and the coexistence of both are risk factors for cardiovascular disease (CVD).[1,2,3] Similarly, type 2 diabetes mellitus, hypertension and often their coexistence are risk factor for CVD.[4,5] Majority of the patients with prediabetes develop type 2 diabetes mellitus (T2DM) over time. Recent studies have shown that the prevalence of ischemic heart disease is approximately the same among patients with prediabetes and those with T2DM.[6,7] On the other hand, increased within-visit blood pressure variability is particularly associated with the prevalence of prediabetes.[8] Globally, 1 in 3 apparently healthy adults have prehypertension, that is the state before hypertension; 1 in 4 has prediabetes and 1 in 10 has both prehypertension and prediabetes.[3]
Lately, the prevalence of prediabetes has been increasing at a higher rate than T2DM, specifically in developing countries.[7] However, most individuals with prediabetes remain undiagnosed. Overall, 16.8% of the Iranian adults population have prediabetes (impaired fasting glucose).[9] Moreover, more than half of the Iranian apparently normal adults, aged 25–65 years, have prehypertension, and 23.4% of them have hypertension.[10]
The first-degree relatives (FDRs) of patients with T2DM are at three times higher risk of developing diabetes than general population according to family-based studies.[11] Therefore, the family history of T2DM is an important risk factor for prediabetes.[12] Overall, 12.3% of the FDRs (siblings and children) of Iranian patients with T2DM had prediabetes, and these individuals with prediabetes were 23% more likely to develop hypertension, independent of known risk factors for hypertension.[13]
Previous cross-sectional studies have presented the prevalence and risk factors of only prediabetes in the FDRs of Iranian patients with T2DM.[7,14] Recently, a strong association of hypertension and prediabetes was reported in the FDRs of T2DM patients.[1,13,15] Coexistence of prehypertension and prediabetes has also been investigated in healthy Asian and United States population in the cross-sectional studies, however, the effect of risk factors have been left unmeasured.[1,3] There is no information available in the literature regarding the risk factors associated with high blood pressure and prediabetes comorbidity in the high-risk individuals such as FDRs of T2DM patients. The present longitudinal study with focus on high-risk FDRs of type 2 diabetic patients identified the causal-effect relationship of risk factors with prediabetes and hypertension and the coexistence of both conditions.
In this longitudinal study, the repeated measurements of potential risk factors were considered as time-varying covariates. The repeated measurements of multiple outcome variables took into account the participants' changes, during the long follow-up period. We applied the mixed model regression to construct models in which repeated measurements of the risk factors were included to predict repeated measurements of the outcome variables. The main objective of the present study was to identify and to compare the risk factors of high blood pressure (prehypertension or hypertension), prediabetes, and the coexistence of both conditions in the nondiabetic FDRs of patients with T2DM, based on a 9-year cohort study.
METHODS
Study participants and data collection
The present study was conducted within the framework of the Isfahan Diabetes Prevention Cohort Study (IDPCS), an open cohort in central Iran to determine the various potential risk factors for the type 2 diabetes in the FDRs of patients with T2DM. The recruitment procedures and examination methods of IDPCS have been described elsewhere.[14] 3140 (837 men and 2303 women) FDRs of patients with T2DM attended the Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences in Iran. According to the inclusion criteria [Supplementary Figure 1 (184.8KB, tif) ], participants with T2DM (412 out of 3140 FDRs of T2DM patients) were excluded at the baseline because they were treated by glucose-lowering agents. The American Diabetes Association (ADA) criteria were adopted to diagnose diabetes (fasting plasma glucose [FPG] concentration ≥126 mg/dL and/or 2-h postload plasma glucose levels ≥200 mg/dL during an oral glucose tolerance test (OGTT) and/or hemoglobin A1C (HbA1C) level ≥6.5%),[16] as shown in [Supplementary Figure 1 (184.8KB, tif) ] for recruitment details. Therefore, the total number of 2728 participants were included in the baseline assessment, 1340 participants were also excluded from the longitudinal analysis because they did not attend any follow-up examination. We retrieved the information of 1388 participants with a mean (standard error [SE]) age of 43.0 (6.4) years old (range 30–73 years old) in the longitudinal assessments [Supplementary Figure 1 (184.8KB, tif) ], all of whom had at least two visits during the follow-up period (mean of 3 and range of 2–8 years). The study was conducted between 2003 and 2011. There was no statistically significant difference between the baseline characteristics of attendees at the follow-up visits and nonattendees. These characteristics include age, height, weight, waist circumference, hip circumference, waist-to-hip ratio (WHR), body mass index (BMI), systolic blood pressure (SBP) and diastolic blood pressure (DBP), physical activity, HbA1C level, FPG, 2-h postload plasma glucose levels, triglyceride, low density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol, and remnant-cholesterol concentration.
A full family history was obtained, and physical examination was performed on all participants. The standardized blood pressure was measured; various potential related risk factors were recorded for prediabetes, diabetes, and high blood pressure. The fasting serum lipid profile was measured and a standard 75 g 2-h OGTT was performed. All participants received follow-up tests according to the Standard of Medical Care in Diabetes[16] to update information on lifestyle, anthropometric, and demographic characteristics and to collect data on the diagnosis of prehypertension, hypertension, prediabetes, and diabetes. The normal glucose tolerance (NGT) was defined by FPG of <100 mg/dL, 2-h postload plasma glucose levels during 75-g OGTT of <140 mg/dL and HbA1C at baseline of <5.7%, respectively;[17] the repeat test for participants with NGT was carried out at least at 3-year intervals. Otherwise, tests were usually repeated annually. Tenets of the Declaration of Helsinki were followed in this study, and each participant signed a copy of informed consent form.
Definition and assessment of outcomes and covariates
The blood pressure was measured twice for patients in a seated position after 5 min rest using a standard mercury sphygmomanometer (ALPK2, Japan). Cases with high blood pressure were identified according to the Seventh Report of Joint National Committee criteria on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) criteria. The prehypertension blood pressure was defined by 120 mmHg< SBP <139 mmHg and/or 80 mmHg< DBP <89 mmHg. Hypertension blood pressure was defined by SBP ≥140 mmHg and/or DBP ≥90 or if the patient was using antihypertensive medication.[18] The blood pressure as an ordinal response variable was classified in three groups of normal blood pressure, prehypertension, and hypertension.
The ADA criteria were used to diagnose prediabetes. Prediabetes is characterized by an FPG concentration between 100 mg/dL and 125 mg/dL and/or from a 75-g OGTT, which is defined by 2-h postload plasma glucose levels of ≥140 and ≤199 mg/dL and/or HbA1C level of 5.7%–6.4%.[17] Therefore, we considered plasma glucose levels as a binary response variable, normal plasma glucose, and prediabetes. The morning 10- h fasting blood samples were collected for biochemical investigations. Fasting plasma glucose was assessed by the glucose oxidase method (Clinical Chemistry Analyzer Liasys, Roma, Italy). A 75-g OGTT was performed, and plasma glucose concentrations were measured before and 2-h after taking glucose (75-g OGTT).
The HbA1c measurement was performed by ion-exchange chromatography. Total cholesterol and HDL-C were measured, and LDL-C and remnant-cholesterol calculated by the Friedewald's equation[19] and subtracting HDL-C and LDL-C from the total cholesterol, respectively. All biochemical parameters were measured in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using the enzyme-linked method.
Anthropometric assessments, including weight, height, waist and hip circumference, were conducted while participants were lightly clothed without footwear. Body weight was measured to the nearest 0.1 kg using the Seca (Hamburg, Germany) scale. Height was measured with individuals in a standing position with their shoulders in the normal position using the Seca stadiometer. The BMI (kg/m2) was calculated as the weight (kg) divided by height (m) squared.
Physical activity was expressed as total metabolic equivalents (TMETs) in hours per day. To calculate the TMETs for each person, we reproduced the times (h/day) reported for each physical activity by its related TMETs coefficient using standard tables.[20]
Information on anthropometric indices, family and personal medical history, blood pressure, physical activity, total cholesterol, HDL-C, LDL-C, triglyceride, and plasma glucose was collected at baseline and through follow-ups. All clinical and laboratory measurements at the baseline and follow-ups were made using the same standardized protocol.[13]
Statistical analysis
Descriptive data were reported as mean ± SE for the quantitative variables and percentage (%) for the categorical variables. At baseline, the mean age of five groups (normal people, participants with the high blood pressure or prediabetes, both prediabetes and prehypertension, and both prediabetes and hypertension) compared using one-way analysis of variance. Where the mean difference of age was statistically significant, Tukey's test was used to compare the mean difference of age between two groups. One-way analysis of covariance was used to determine differences of continuous variables, other than age, between multiple groups and was adjusted by age; post hoc analysis, using Bonferroni correction, was adopted to perform multiple comparisons between two groups. The Chi-square test was used for the comparing categorical variables across different groups at baseline. The number of patients with high blood pressure, prediabetes, and the coexistence of both were changing during 9 years of follow-up, therefore, we presented the prevalence of these conditions, annually, in Supplementary Table 1, and the mean of prevalence (overall prevalence) was used over the duration of the study.
Supplementary Table 1.
Number (percent) and status of first-degree relatives of type 2 diabetic patients that newly entered into the present open cohort in each year
| Participants’status | Years | Overall | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First year | Second year | Third year | Forth year | Fifth year | Sixth year | Seventh year | Eighth year | Ninth year | ||
| Without pre-T2DM and/or HBP | 64 (18.9) | 188 (29.7) | 105 (25.3) | 119 (29.5) | 52 (21.1) | 52 (19.0) | 19 (11.8) | 25 (15.4) | 23 (22.7) | 647 (23.7) |
| Only pre-T2DM | 56 (16.6) | 116 (18.3) | 109 (26.2) | 83 (20.6) | 41 (16.9) | 47 (17.2) | 23 (14.5) | 23 (14.1) | 23 (22.7) | 521 (19.1) |
| Only HBP (pre-HTN or HTN) | 105 (31.3) | 190 (30.0) | 69 (16.7) | 99 (24.7) | 69 (28.7) | 70 (25.7) | 60 (37.5) | 68 (41.0) | 17 (17.0) | 747 (27.4) |
| Pre-T2DM and pre-HTN* | 60 (17.6) | 106 (16.8) | 97 (23.5) | 61 (15.3) | 49 (20.3) | 73 (26.5) | 34 (21.1) | 30 (17.9) | 22 (21.6) | 532 (19.5) |
| Pre-T2DM and HTN** | 52 (15.6) | 33 (5.3) | 34 (8.3) | 40 (9.9) | 32 (13.1) | 31 (11.6) | 24 (15.1) | 19 (11.5) | 16 (15.9) | 281 (10.3) |
| Total number | 337 (100) | 633 (100) | 414 (100) | 402 (100) | 243 (100) | 273 (100) | 160 (100) | 165 (100) | 101 (100) | 2728 (100) |
*Coexisting Pre-HTN and Pre-T2DM, **Coexisting HTN and Pre-T2DM. Pre-T2DM=Pretype 2 diabetes mellitus; HBP=High blood pressure; HTN=Hypertension; Pre-HTN=Prehypertension
In the longitudinal assessment, separate univariate logistic and cumulative logistic regressions were applied to evaluate the longitudinal associations between risk factors and fasting plasma glucose (as a binary outcome variable) and blood pressure variables (as an ordinal outcome variable). We proposed the bivariate longitudinal ordinal-binary logistic regression to consider the coexistence between two outcomes. The unbalanced longitudinal data (dropouts) are shown in Supplementary Figure 2 (359.8KB, tif) . In order to handle the dropout data, longitudinal mixed-effects (with random subject effects) logistic regressions were performed in both univariate and bivariate settings, accordingly. We adopted the Bayesian approach as a novel statistical method to estimate parameters while the missing data were handled on covariates in the abovementioned models by OpenBUGS version 3.2.3.[21] The description of missing covariates is reported in Supplementary Table 2. The results of these models were demonstrated as odds ratios (ORs) and related 95% credible intervals (CI).
Supplementary Table 2.
Description of the missing data in the covariates during 9 years
| Name | Description | Type | Missingness (%) |
|---|---|---|---|
| Obesity | Obese/normal case | Binary | 10.4 |
| Remnant cholesterol | Total-C−(HDL-C + LDL-C); mg/dl | Continuous | 12.5 |
| Physical activity | TMET h/day | Continuous | 17 |
HDL-C=High-density lipoprotein cholesterol; LDL-C=Low-density lipoprotein cholesterol; Total-C=Total cholesterol; TMET=Total metabolic equivalent
The bivariate model of longitudinal ordinal-binary responses with random subject effect is shown in Equations 1 and 2.
Where i = 1, 2,…, 1388 is the number of subject, t = 1,2,3…, 9 is the time point of subject i and π1it, c is its corresponding probability belong to the category c, and c = 1, 2 and κc, β01, β02, β11, β21 are fixed effect terms; (ui1, ui2) represent subject-specific random intercepts for i-th subject.
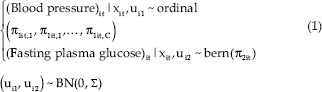
The cumulative probabilities is 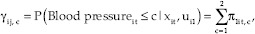 where
where
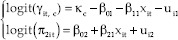
The covariance matrix Σ reflects the inherent association between prediabetes and high blood pressure as the longitudinal ordinal and binary outcomes, respectively.
In our proposed model, the obesity index (according to 85th percentile value of waist circumference and/or WHR and/or BMI, separately for each gender), sex, remnant-cholesterol, physical activity, and the baseline age were considered as covariates. Multicollinearity was observed between anthropometric measurements including waist circumference, WHR and BMI [P < 0.01, Supplementary Table 3]. Therefore, we created a local definition for the obesity index that was coded as a binary variable. The values <85th percentile of waist circumference and/or WHR and/or BMI were coded 0, and the values higher than the 85th percentile were coded 1. The statistically significant multicollinearity was also found between triglyceride, total cholesterol, HDL-C, and LDL-C components of lipid profile using Pearson's coefficient [P < 0.01, Supplementary Table 3]. The remnant-cholesterol was introduced as representative of lipid profile because multicollinearity was detected between anthropometric indicators and lipid profile (P < 0.01). The statistical significance level of all tests was two-tailed and set to P < 0.05. Moreover, the 95% CIs do not include the one value for considering significant effects of risk factors in the Bayesian models.
Supplementary Table 3.
Correlation between anthropometric variables and lipid profile at baseline
| Waist (cm) | WHR | BMI (kg/m2) | TG (mg/dl) | Total-C (mg/dl) | LDL-C (mg/dl) | |
|---|---|---|---|---|---|---|
| WHR | r†=0.716** | |||||
| BMI (kg/m2) | r=0.739** | r=0.475** | ||||
| TG (mg/dl) | r=0.244** | r=0.188** | r=0.115** | |||
| Total-C (mg/dl) | r=0.135** | r=0.019 | r=0.111* | r=0.333** | ||
| LDL-C (mg/dl) | r=0.065** | r=0.017 | r=0.069** | r=0.125** | r=0.851** | |
| HDL-C (mg/dl) | r=−0.173** | r=−0.231** | r=−0.044* | r=−0.234** | r=−0.273** | r=−0.103** |
*Correlation is significant at the 0.05 level, **Correlation is significant at the 0.01 level, †Pearson correlation coefficients. BMI=Body mass index; WHR=Waist-to-hip ratio; TG=Triglyceride; Total-C=Total cholesterol; HDL-C=High-density lipoprotein cholesterol; LDL-C=Low-density lipoprotein cholesterol
RESULTS
Results of the baseline assessment
The baseline characteristics of the 412 (13.12%) excluded patients who had T2DM and 2728 (86.88%) participants without T2DM are shown in Supplementary Table 4. Those who had T2DM were older and had a higher age-adjusted mean of BMI, waist circumference, WHR, triglyceride, total cholesterol, remnant-cholesterol, and a higher proportion of obesity index, compared to those without T2DM.
Supplementary Table 4.
Baseline characteristics in participants who with/without diabetes
| Characteristic | Without T2DM | With T2DM | P |
|---|---|---|---|
| Age (year), mean (SE) | 42.96 (0.13) | 45.47 (0.34) | 0.000** |
| BMI (kg/m2), mean (SE) | 28.90 (0.08) | 29.67 (0.22) | 0.002* |
| Waist (cm), mean (SE) | 89.10 (0.19) | 92.88 (0.48) | 0.000** |
| WHR, mean (SE) | 0.68 (0.001) | 0.70 (0.004) | 0.001* |
| TG (mg/dl), mean (SE) | 164.86 (2.03) | 191.89 (5.39) | 0.000** |
| Total-C (mg/dl), mean (SE) | 196.97 (0.79) | 206.10 (2.01) | 0.002* |
| HDL-C (mg/dl), mean (SE) | 45.41 (0.24) | 45.16 (0.56) | 0.505 |
| LDL-C (mg/dl), mean (SE) | 118.76 (0.71) | 122.70 (1.73) | 0.288 |
| remnant cholesterol (mg/dl), mean (SE) | 32.74 (0.40) | 37.93 (1.06) | 0.000** |
| Physical activity (TMET h/day), mean (SE) | 33.62 (0.20) | 34.44 (0.67) | 0.603 |
| Sex, n (%) | |||
| Male | 702 (22.4) | 123 (3.9) | 0.076 |
| Female | 2026 (64.5) | 289 (9.2) | |
| Obesity, n (%) | |||
| Obese | 704 (23.9) | 138 (4.7) | 0.003† |
| Nonobese | 1841 (62.6) | 258 (8.8) |
*Significant results withP<0.001, **Significant results withP<0.01, †Significant resulted from Chi-square test, P<0.01. Age-adjusted means were calculated using general linear models. Differences in the mean or percentage values of variables between those who had T2DM and those without T2DM. T2DM=Type 2 diabetes mellitus; HDL-C=High-density lipoprotein cholesterol; LDL-C=Low-density lipoprotein cholesterol; BMI=Body mass index; WHR=Waist-to-hip ratio; TG=Triglyceride; Total-C=Total cholesterol; TMET=Total metabolic equivalent; SE=Standard error
The baseline prevalence of high blood pressure, prediabetes, coexisting prehypertension and prediabetes, and coexisting hypertension and prediabetes are shown in Supplementary Table 1. The baseline characteristics of 647 (23.7%) normal participants, 521 (19.1%) patients with only prediabetes, 747 (27.4%) patients with only high blood pressure, 532 (19.5%) patients with prediabetes and prehypertension, and 281 (10.3%) participants with both prediabetes and hypertension are shown in Table 1. All risk factors were raised in normal people, patients with only prediabetes, patients with only prehypertension/hypertension, those with both prediabetes and prehypertension, and participants with both prediabetes and hypertension, consecutively, except physical activity and HDL-C which were declined [Table 1]. Participants with coexisting hypertension and prediabetes were older and had significantly higher age-adjusted mean waist circumference, WHR, BMI, triglyceride, total cholesterol, LDL-C and remnant-cholesterol, and lower age-adjusted mean HDL-C (P < 0.05). Details of post hoc multiple comparisons were presented in Supplementary Table 5.
Table 1.
Baseline characteristics of the participants in the different study groups
| Characteristic | Normal people (n=647) | Patients with only pre-T2DM (n=521) | Patients with only HBP (pre-HTN or HTN) (n=747) | Patients with both pre-T2DM and pre-HTN (n=532) | Patients with both pre-T2DM and HTN (n=281) | P |
|---|---|---|---|---|---|---|
| Age (year), mean (SE) | 41.00 (0.24) | 42.38 (0.29) | 42.91 (0.24) | 44.43 (0.30) | 45.73 (0.42) | <0.001* |
| BMI (kg/m2), mean (SE) | 27.34 (0.17) | 28.53 (0.19) | 29.21 (0.16) | 29.58 (0.19) | 30.70 (0.26) | <0.001* |
| Waist (cm), mean (SE) | 85.03 (0.39) | 87.94 (0.43) | 90.04 (0.36) | 91.05 (0.43) | 93.78 (0.59) | <0.001* |
| WHR, mean (SE) | 0.66 (0.003) | 0.67 (0.004) | 0.69 (0.003) | 0.70 (0.004) | 0.71 (0.005) | <0.001* |
| TG (mg/dL), mean (SE) | 142.18 (4.21) | 155.27 (4.65) | 164.70 (3.85) | 179.39 (4.59) | 203.91 (6.38) | <0.001* |
| Total-C (mg/dL), mean (SE) | 189.58 (1.63) | 195.01 (1.79) | 198.40 (1.48) | 200.94 (1.77) | 207.39 (2.47) | <0.001* |
| HDL-C (mg/dL), mean (SE) | 47.36 (0.50) | 44.86 (0.55) | 45.23 (0.54) | 42.90 (0.76) | 39.80 (1.27) | <0.001* |
| LDL-C (mg/dL), mean (SE) | 113.94 (1.47) | 119.15 (1.62) | 120.34 (1.33) | 119.80 (1.60) | 125.18 (2.25) | <0.001* |
| Remnant cholesterol (mg/dL), mean (SE) | 28.29 (0.83) | 30.97 (0.92) | 32.96 (0.76) | 35.52 (0.91) | 39.80 (1.27) | <0.001* |
| Physical activity (TMET h/day), mean (SE) | 34.09 (0.51) | 33.15 (0.40) | 33.53 (0.44) | 33.76 (0.40) | 33.14 (0.59) | 0.534 |
| Sex, n (%) | ||||||
| Male | 147 (5.4) | 112 (4.1) | 548 (20.1) | 158 (5.8) | 196 (7.2) | 0.006** |
| Female | 500 (18.3) | 409 (15.0) | 199 (7.3) | 374 (13.7) | 85 (3.1) | |
| Obesity, n (%) | ||||||
| Obese | 98 (3.9) | 107 (4.2) | 204 (8.0) | 173 (6.8) | 122 (4.8) | <0.001** |
| Nonobese | 509 (20.0) | 375 (14.7) | 492 (19.3) | 321 (12.6) | 144 (5.7) |
*Significant results from one-way ANOVA for age and ANCOVA for other variables, **Significant results from Chi-square test. BMI: Body mass index, WHR: Waist-to-hip ratio, TMETs: Total metabolic equivalents, Pre-T2DM=Pretype 2 diabetes mellitus; HBP: High blood pressure; HTN=Hypertension; Pre-HTN=Prehypertension; HDL-C=High-density lipoprotein cholesterol; LDL-C=Low-density lipoprotein cholesterol; ANOVA=Analysis of variance; ANCOVA=Analysis of covariance; SE=Standard error; Total-C=Total cholesterol
Supplementary Table 5.
Results of multiple pairwise comparisons between the groups at baseline
| Variable | Group (A) | Group (B) | P* |
|---|---|---|---|
| BMI (kg/m2) | Normal | Only pre-T2DM | <0.001 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.07 | |
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.99 | |
| With both pre-T2DM and HTN | <0.001 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.005 | |
| Waist (cm) | Normal | Only pre-T2DM | <0.001 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.002 | |
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.70 | |
| With both pre-T2DM and HTN | <0.001 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.007 | |
| WHR | Normal | Only pre-T2DM | 0.07 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | <0.001 | |
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.99 | |
| With both pre-T2DM and HTN | 0.004 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.07 | |
| TG (mg/dl) | Normal | Only pre-T2DM | 0.37 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.99 | |
| With both pre-T2DM and pre-HTN | 0.002 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.14 | |
| With both pre-T2DM and HTN | <0.001 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.02 | |
| Total-C (mg/dl) | Normal | Only pre-T2DM | 0.25 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.99 | |
| With both pre-T2DM and pre-HTN | 0.19 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.99 | |
| With both pre-T2DM and HTN | 0.02 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.33 | |
| HDL-C (mg/dl) | Normal | Only pre-T2DM | 0.007 |
| Only HBP | 0.01 | ||
| With both pre-T2DM and pre-HTN | 0.04 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.99 | |
| With both pre-T2DM and pre-HTN | 0.99 | ||
| With both pre-T2DM and HTN | 0.37 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.71 | |
| With both pre-T2DM and HTN | 0.88 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.93 | |
| LDL-C (mg/dl) | Normal | Only pre-T2DM | 0.17 |
| Only HBP | 0.01 | ||
| With both pre-T2DM and pre-HTN | 0.08 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.99 | |
| With both pre-T2DM and pre-HTN | 0.99 | ||
| With both pre-T2DM and HTN | 0.30 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.99 | |
| With both pre-T2DM and HTN | 0.99 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.50 | |
| Remnant cholesterol (mg/dl) | Normal | Only pre-T2DM | 0.30 |
| Only HBP | <0.001 | ||
| With both pre-T2DM and pre-HTN | <0.001 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only pre-T2DM | Only HBP | 0.95 | |
| With both pre-T2DM and pre-HTN | 0.005 | ||
| With both pre-T2DM and HTN | <0.001 | ||
| Only HBP | With both pre-T2DM and pre-HTN | 0.30 | |
| With both pre-T2DM and HTN | <0.001 | ||
| With both pre-T2DM and pre-HTN | With both pre-T2DM and HTN | 0.08 |
*Adjustment for age, multiple comparisons based on Bonferroni correction. Pre-T2DM=Pretype 2 diabetes mellitus; HBP: High blood pressure; HTN=Hypertension; Pre-HTN=Prehypertension; HDL-C=High-density lipoprotein cholesterol; LDL-C=Low-density lipoprotein cholesterol; BMI=Body mass index; WHR=Waist-to-hip ratio; TG=Triglyceride; Total-C=Total cholesterol; TMET=Total metabolic equivalent
Resultsss of the longitudinal assessment
The results of univariate and bivariate models that were used to measure the causal-effect of potential risk factors on prediabetes and high blood pressure [Table 2] indicated that age, obesity index and remnant-cholesterol were associated significantly with prediabetes and its coexistence with high blood pressure during follow-ups. These figures were also associated significantly with high blood pressure and its coexistence with prediabetes. In the bivariate model, a joint significant positive association between prediabetes and high blood pressure was estimated to be 0.26 (95% CI: 0.16–0.35) during the follow-up period.
Table 2.
Result of Bayesian binary/ordinal logistic regression with random subject effect and handling missingness
| Independent variables | |||||
|---|---|---|---|---|---|
| Dependent variables | Sex‡ | Age at baseline (year) | Obesity§ | Remnant cholesterol (mg/dL) | Physical activity (TMET h/day) |
| OR (95% Credible intervals) | |||||
| Univariate model | |||||
| Pre-T2DM | 0.844 (0.630,1.072) | 1.027 (1.010,1.045)* | 1.140 (1.033,1.390)* | 1.009 (1.005,1.015)* | 0.997 (0.972,1.019) |
| HBP (pre-HTN or HTN) | 1.076 (1.056,1.104) | 1.312 (1.062,1.776)* | 2.251 (1.803,2.969)* | 1.0129 (1.010,1.020)* | 0.999 (0.972,1.027) |
| Bivariate model | |||||
| Pre-T2DM | 0.956 (0.743,1.238) | 1.055 (1.040,1.068)* | 1.399 (1.129,1.730)*,† | 1.086 (1.043,1.119)*,† | 0.990 (0.970,1.012) |
| HBP (pre-HTN or HTN) | 1.084 (1.067,1.102) | 1.419 (1.077,1.877)* | 2.443 (1.978,3.031)*,† | 1.093 (1.067,1.121)*,† | 0.999 (0.972,1.027) |
*Significant results based on 95% credible intervals, †Significant difference between univariate and bivariate logistic regressions, ‡Female is the reference group, ¦Nonobese is the reference group. Pre-T2DM=Pretype 2 diabetes mellitus; HBP=High blood pressure; HTN=Hypertension; Pre-HTN=Prehypertension; OR=Odds ratio; TMET=Total metabolic equivalent
In univariate analysis, the odds of prediabetes alone in obese participants was 14% (95% CI: 3.3–39) higher than nonobese ones. In bivariate model, the odds of coexisting prediabetes and high blood pressure were also 39.9% (95% CI: 12.9–73) higher than obese participants [Table 2], with significantly stronger effects than that in the univariate model (P < 0.05). For every one unit raise in remnant-cholesterol, the likelihood of having prediabetes was increased by 0.9% (95% CI: 0.5–1.5) and 8.6% (95% CI: 4.3–11.9) in univariate and bivariate models, respectively [Table 2]. The effect of increasing remnant-cholesterol on the odds of prediabetes and high blood pressure comorbidity was significantly stronger than that of prediabetes alone (P < 0.05).
The results of univariate and bivariate models that were used to measure the causal-effect of potential risk factors on high blood pressure disorders [Table 2] showed that the odds of high blood pressure for obese participants was 125.1% (95% CI: 80.3–196.9) for only high blood pressure and was 144.3% (95% CI: 97.8–203.1) for high blood pressure coexisting with prediabetes much higher than nonobese participants. These risks were significantly higher than that those in univariate model. For one units raise in remnant-cholesterol value, the odds of high blood pressure were increased to 1.29% (95% CI: 1–2) when the high blood pressure was considered alone and reached to 9.3% (95% CI: 6.7–12.1) when the high blood pressure was coexisted with prediabetes [Table 2]. The remnant-cholesterol showed significantly the stronger effects on high blood pressure in bivariate model than in univariate model (P < 0.05).
DISCUSSION
The current longitudinal study showed a high prevalence of high blood pressure, prediabetes, and the coexistence of both, as well as their long-term risk assessments in the FDRs of T2DM patients using relevant statistical methods. According to our results, age, remnant-cholesterol, and obesity were significant predictors of prediabetes, high blood pressure and the coexistence of both in the FDRs of diabetic patients during the follow-up period. The effects of the above-mentioned predictors are discussed as follow.
The results of both univariate and bivariate models suggest that increasing age significantly increases the risk (ORs) of high blood pressure and/or prediabetes in the FDRs of T2DM patients [Table 2]. A similar result was obtained for hypertension and prediabetes without considering the coexistence of both conditions in the Iranian FDRs of patients with T2DM.[14,15] Our findings were in agreement with the cross-sectional study from northern and northeastern China, showing the prevalence of prehypertension and prediabetes comorbidity was increased with age among healthy adults.[22]
At baseline, the concentrations of triglyceride, total cholesterol, LDL-C and also remnant-cholesterol as a novel risk factor for high blood pressure and prediabetes were higher for groups with high blood pressure, prediabetes, and combined conditions than the normal population [Table 1]. This was in agreement with parallel results for lipid profile of patients with only hypertension or prediabetes in previous studies.[7,13] However, in those studies, remnant-cholesterol was not considered as a risk factor. Our results showed that the long-term risk of high blood pressure and/or prediabetes in the FDRs of T2DM patients was higher in participants with increasing remnant-cholesterol [Table 2]. Similarly, remnant-cholesterol was previously indicated as a strong causal risk factor for CVD.[23] The effect of remnant-cholesterol on the odds of prediabetes and high blood pressure comorbidity was noticeably stronger than that of high blood pressure or prediabetes separately. However, the relationship between remnant-cholesterol and high blood pressure, prediabetes, and coexistence of both, was not previously investigated.
Our results showed that there were the age-adjusted mean differences of BMI, waist circumference, and WHR between normal participants and patients with high blood pressure and/or prediabetes in the FDRs of T2DM patients at baseline [Table 1]. Although these findings were in agreement with the previous study,[13] the high blood pressure and its coexistence with prediabetes were not previously considered. On the other hand, the cross-sectional study of the Chinese healthy adults showed that the prevalence of coexisting prediabetes and prehypertension was increased with BMI.[22] The waist circumference, WHR, and BMI showed heterogeneous associations with metabolic disorders and thus played different roles as preferable disease predictors.[24,25] Therefore, it was suggested that these three indices should be considered simultaneously as a useful obesity index for predicting different metabolic diseases. According to this recommendation and observed multicollinearity between obesity-related metabolic disorders, waist circumference, WHR and BMI, in our study [Supplementary Table 3], we combined the 85 percentile cutoff points for waist circumference, BMI and WHR variables to generate a new obesity index. This index provided us with a more reliable risk assessment for high blood pressure, prediabetes, and the coexistence of both conditions. Our longitudinal study on a high-risk group indicated the causal role of obesity on these conditions, with stronger effects on coexisting high blood pressure and prediabetes than on each separate condition, specifically based on our new obesity index [Table 1]. This is in consistent with the results of a cross-sectional study on general population which showed a strong association of prediabetes and high blood pressure with obesity.[26] It is worth noting that the obesity was only defined by BMI and waist circumference in previous reports, while we used our newly introduced obesity index.
Strengths of the study
The strengths of the current study included the use of samples, consisting of both men and women, conducting gold standard OGTT to diagnose prediabetes and diabetes, and collecting the information on potential determinants of high blood pressure and prediabetes. All participants were examined in one site, all measurements were performed by trained staff, and the same methods were used to obtain both baseline and follow-up laboratory data. We excluded patients with T2DM who were treated at enrollment. There was no difference between the potential risk factors of high blood pressure and/or prediabetes in participants who were examined once, non-attendees, and those with follow-up. Our database was one of the few that followed the FDRs of patients with T2DM, thus enabling us to simultaneously control the genetic factors that may predict high blood pressure and prediabetes.
Meanwhile, we used univariate/bivariate mixed-effects (with random subject effects) logistic regression. This statistical method is an appropriate and efficient method for the analysis of natural history longitudinal data with dropout.[27] Bayesian inference was practically feasible for mixed-effect logistic regression, as well as for model-based handling missing covariates at random with higher statistical power than complete case method (eliminated cases with missingness).[28]
Limitations of the study
Our study was limited to a cohort of individuals who were at increased risk of high blood pressure and prediabetes because they were FDRs of patients with T2DM; this selection bias might result in an overestimation of associations. We were also unable to include several possible confounding variables that were known risk factors of high blood pressure and prediabetes including plasma insulin, alcohol and salt consumption (sodium-intake), habitual dietary intakes, sleeping disorder, and socioeconomic status.
CONCLUSION
The prevalence and the long-term risk of high blood pressure, prediabetes, and the coexistence of both were increased significantly by obesity, age, and remnant-cholesterol as a new predictor, among the FDRs of T2DM patients as a high-risk group. Furthermore, obesity index and remnant-cholesterol showed stronger effects on coexisting high blood pressure and prediabetes than on each condition separately. These findings could help healthcare providers to identify individuals at increased risk of high blood pressure and prediabetes and consequently reduce their chance of developing type 2 diabetes and CVD.
Financial support and sponsorship
This research was financially supported by the Student Research Center, School of Health, Isfahan Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran (No. 394909).
Conflicts of interest
There are no conflicts of interest.
Flow diagram of inclusion data in presented longitudinal study
Description of participants' number of visits during the follow-up period in the open cohort study
| Number | Percentage |
|---|---|
| 1340 of FDRs of T2DM patients who were visited once* | 49.12% |
| 709 of FDRs of T2DM patients who were visited twice | 25.99% |
| 373 of FDRs of T2DM patients who were visited three | 13.67% |
| 151 of FDRs of T2DM patients who were visited four | 5.54% |
| 73 of FDRs of T2DM patients who were visited five | 2.66% |
| 50 of FDRs of T2DM patients who were visited six | 1.83% |
| 29 of FDRs of T2DM patients who were visited seven | 1.06% |
| 3 of FDRs of T2DM patients who were visited eight | 0.11% |
| 2728 of FDRs of T2DM who entered into the open cohort study | 100% |
*They did not attend any follow-up examination. FDRs = First-degree relatives; T2DM = Type 2 diabetes mellitus
Acknowledgments
The authors would like to thank all participants. We are also grateful to the staff of Isfahan Endocrine and Metabolism Research Centre for their assistance with collecting clinical data. We would like to thank the Student Research Center, School of Health, Isfahan Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran, for funding support (No. 394909).
REFERENCES
- 1.Qiu M, Shen W, Song X, Ju L, Tong W, Wang H, et al. Effects of prediabetes mellitus alone or plus hypertension on subsequent occurrence of cardiovascular disease and diabetes mellitus: Longitudinal study. Hypertension. 2015;65:525–30. doi: 10.1161/HYPERTENSIONAHA.114.04632. [DOI] [PubMed] [Google Scholar]
- 2.Khosravi A, Gharipour M, Nezafati P, Khosravi Z, Sadeghi M, Khaledifar A, et al. Pre-hypertension, pre-diabetes or both: Which is best at predicting cardiovascular events in the long term? J Hum Hypertens. 2017;31:382–7. doi: 10.1038/jhh.2016.42. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Brashear MM, Johnson WD. Coexisting prehypertension and prediabetes in healthy adults: A pathway for accelerated cardiovascular events. Hypertens Res. 2011;34:456–61. doi: 10.1038/hr.2010.267. [DOI] [PubMed] [Google Scholar]
- 4.Moradi S, Haji Ghanbari MJ, Ebrahimi H. Comparison of optimal cardiovascular risk factor management in patients with type 2 diabetes who attended urban medical health center with those attended a tertiary care center: Experiences from Tehran, Iran. Int J Prev Med. 2016;7:113. doi: 10.4103/2008-7802.191440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrannini E, Cushman WC. Diabetes and hypertension: The bad companions. Lancet. 2012;380:601–10. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- 6.Farrell C, Moran J. Comparison of comorbidities in patients with pre-diabetes to those with diabetes mellitus type 2. Ir Med J. 2014;107:72–4. [PubMed] [Google Scholar]
- 7.Gholi Z, Heidari-Beni M, Feizi A, Iraj B, Askari G. The characteristics of pre-diabetic patients associated with body composition and cardiovascular disease risk factors in the Iranian population. J Res Med Sci. 2016;21:20. doi: 10.4103/1735-1995.179888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S, et al. Within-visit blood pressure variability is associated with prediabetes and diabetes. Sci Rep. 2015;5:7964. doi: 10.1038/srep07964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National survey of risk factors for non-communicable diseases of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 10.Janghorbani M, Amini M, Gouya MM, Delavari A, Alikhani S, Mahdavi A, et al. Nationwide survey of prevalence and risk factors of prehypertension and hypertension in Iranian adults. J Hypertens. 2008;26:419–26. doi: 10.1097/HJH.0b013e3282f2d34d. [DOI] [PubMed] [Google Scholar]
- 11.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: Implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–91. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 12.Wagner R, Thorand B, Osterhoff MA, Müller G, Böhm A, Meisinger C, et al. Family history of diabetes is associated with higher risk for prediabetes: A multicentre analysis from the German center for diabetes research. Diabetologia. 2013;56:2176–80. doi: 10.1007/s00125-013-3002-1. [DOI] [PubMed] [Google Scholar]
- 13.Janghorbani M, Bonnet F, Amini M. Glucose and the risk of hypertension in first-degree relatives of patients with type 2 diabetes. Hypertens Res. 2015;38:349–54. doi: 10.1038/hr.2015.10. [DOI] [PubMed] [Google Scholar]
- 14.Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first-degree relatives of patients with type 2 diabetes in Isfahan, Iran: Prevalence and risk factors. Rev Diabet Stud. 2007;4:169–76. doi: 10.1900/RDS.2007.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iraj B, Salami R, Feizi A, Amini M. The profile of hypertension and dyslipidemia in prediabetic subjects; results of the Isfahan diabetes prevention program: A large population-based study. Adv Biomed Res. 2015;4:27. doi: 10.4103/2277-9175.150415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. (2) classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalter D, Thomas A, Best N, Lunn D. OpenBUGS User Manual, Version 30 2. Cambridge: MRC Biostatistics Unit; 2007. [Google Scholar]
- 22.Wu J, Yan WH, Qiu L, Chen XQ, Guo XZ, Wu W, et al. High prevalence of coexisting prehypertension and prediabetes among healthy adults in Northern and Northeastern China. BMC Public Health. 2011;11:794. doi: 10.1186/1471-2458-11-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37:1944–58. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC, et al. Body mass index and obesity-related metabolic disorders in Taiwanese and US whites and blacks: Implications for definitions of overweight and obesity for Asians. Am J Clin Nutr. 2004;79:31–9. doi: 10.1093/ajcn/79.1.31. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Wang WS, Chang HY, Liu JS, Chen YJ. Heterogeneity of body mass index, waist circumference, and waist-to-hip ratio in predicting obesity-related metabolic disorders for Taiwanese aged 35-64 y. Clin Nutr. 2009;28:543–8. doi: 10.1016/j.clnu.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, Johnson WD. Prediabetes and prehypertension in disease free obese adults correlate with an exacerbated systemic proinflammatory milieu. J Inflamm (Lond) 2010;7:36. doi: 10.1186/1476-9255-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamey JD, Bekele BN, Powers S. Bayesian modeling of follow-up studies with missing data. Ann Epidemiol. 2009;19:416–22. doi: 10.1016/j.annepidem.2009.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of inclusion data in presented longitudinal study
Description of participants' number of visits during the follow-up period in the open cohort study


