Abstract
Myocardial interstitial fibrosis (MIF) is a histological hallmark of several cardiac diseases that alter myocardial architecture and function and are associated with progression to heart failure. MIF is a diffuse and patchy process, appearing as a combination of interstitial microscars, perivascular collagen fiber deposition, and increased thickness of mysial collagen strands. Although MIF arises mainly because of alterations in fibrillar collagen turnover leading to collagen fiber accumulation, there are also alterations in other nonfibrillar extracellular matrix components, such as fibronectin and matricellular proteins. Furthermore, in addition to an excess of collagen, qualitative changes in collagen fibers also contribute to the detrimental impact of MIF. In this part 3 of a 4-part review series, we review the evidence on the complex mechanisms leading to MIF, as well as its contribution to systolic and diastolic cardiac dysfunction and impaired clinical outcomes in patients with nonischemic heart disease.
Keywords: biomarker, collagen, fibroblast, heart failure, myocardial interstitial fibrosis
As we have described previously in this JACC review series, the myocardial interstitium is the cardiac tissue compartment that contains stromal cells and is supported by a complex extracellular matrix (ECM) composed of a wide array of molecules that include structural proteins (e.g., collagens and elastins) and nonstructural protein-sugar composites (glycoproteins and proteoglycans) and glycosaminoglycans (e.g., hyaluronan), as well as a large reservoir of bioactive signaling molecules (1,2). The ECM is not a passive entity but, rather, a complex and dynamic microenvironment that functions in a highly orchestrated way, providing structural integrity, assisting in force transmission throughout the cardiac cycle, acting as a signaling medium for communication between cells, and executing the repair response after cardiac injury (1–3). However, biomechanical stress imposed on the heart by ischemic and nonischemic injuries can lead to alterations of the cardiac ECM, which, in conjunction with other changes, lead to remodeling of the myocardial structure and subsequent alterations in cardiac function that are important in the progression to heart failure (HF) (4).
From a general point of view, the major alterations in the ECM seen in HF are due to disturbances in collagen turnover (i.e., the balance between the generation and deposition of collagen type I and III fibers and the degradation and removal of these fibers) that, in turn, result in changes in the quantity, quality, and organization of the collagen network (4,5). For instance, when the generation and deposition of collagen fibers predominate over their degradation and removal, the result is excessive collagen fiber deposition leading to either focal (e.g., post-infarct scar) or diffuse (e.g., interstitial) myocardial fibrosis. On the contrary, when collagen fiber degradation and removal predominate over generation and deposition, the disruption and loss of the physiologic collagen scaffold of the perimysium and the endomysium ensue. It is likely that alterations of the collagen network in the failing human heart are dynamic and, thus, may coexist in a single cardiac disease (4–6) (Table 1). This review article will focus on nonischemic cardiac diseases, particularly hypertensive heart disease, aortic stenosis, diabetic cardiomyopathy, and hypertrophic cardiomyopathy, in which myocardial interstitial fibrosis (MIF) arises as the major alteration in the ventricular ECM. The role of fibrosis and the ECM in ischemic heart disease is reviewed in detail in the fourth article in this JACC review series.
TABLE 1.
Histological Characteristics of Myocardial Fibrosis in Distinct Human Cardiac Diseases, and Associated Disturbances in the Turnover of Fibrillary Collagen
| Collagen Synthesis and Deposition > Degradation and Removal |
Collagen Synthesis and Deposition < Degradation and Removal | ||||
|---|---|---|---|---|---|
| Focal Macroscopic Scar | Diffuse Microscars | Perivascular Fibrosis | Perimysial and Endomysial Fibrosis | ||
| Hypertensive heart disease | − | + + + | + + + | + + + | + |
| Aortic stenosis | − | + + + | + + + | + + + | ? |
| Diabetic cardiomyopathy | − | + + + | + + + | + + + | ? |
| Hypertrophic cardiomyopathy | − | + +/+ + + | + +/+ + + | + +/+ + + | ? |
| Nonischemic dilated cardiomyopathy | − | + | + | + | + + + |
| Ischemic heart disease | + + + | + | + | + | + + + |
HISTOLOGICAL BASIS OF MIF
MIF can be defined as excessive collagen deposition that distorts the myocardial interstitial architecture and is quantitatively characterized by an increase of the percentage of total myocardial tissue occupied by collagen fibers (or collagen volume fraction), as determined in myocardial samples with collagen-specific staining (7). Therefore, MIF is a common finding in patients with HF with nonischemic cardiac diseases such as hypertensive heart disease or diabetic cardiomyopathy (8), aortic stenosis (9), hypertrophic cardiomyopathy (10), and nonischemic dilated cardiomyopathy (11). Of note, diabetes is associated with increased MIF in patients with other cardiac conditions such as hypertensive heart disease (8) and aortic stenosis (12).
HISTOLOGICAL TYPES OF MIF
An excess of collagen deposition may appear as microscars that are typical of reparative MIF. Alternatively, MIF may appear as thick fibrotic sheaths located in the perivascular space around intramural coronary arteries and arterioles or as thick bands surrounding the cardiac muscle bundles (i.e., perimysium) and individual cardiomyocytes (i.e., endomysium) that are typical of reactive MIF (13) (Figure 1). It is currently unclear if these 2 histological types of MIF represent different entities (because they coexist in most patients) or if they represent different evolutionary stages of the disease. For instance, recent data obtained in patients with hypertrophic cardiomyopathy suggest that in the initial stages, there is a predominance of perivascular, perimysial, and endomysial deposits and that, with disease progression, fibrosis appears predominantly as replacement scars (14). Although MIF is patchy, the extent of fibrous deposits increases from the outer to the inner third of the ventricular free wall in patients with hypertensive heart disease, aortic stenosis, and hypertrophic cardiomyopathy (15,16). This is probably related to the transmural pressure gradient, wall stress, and coronary micro-circulation alterations causing relative endocardial ischemia.
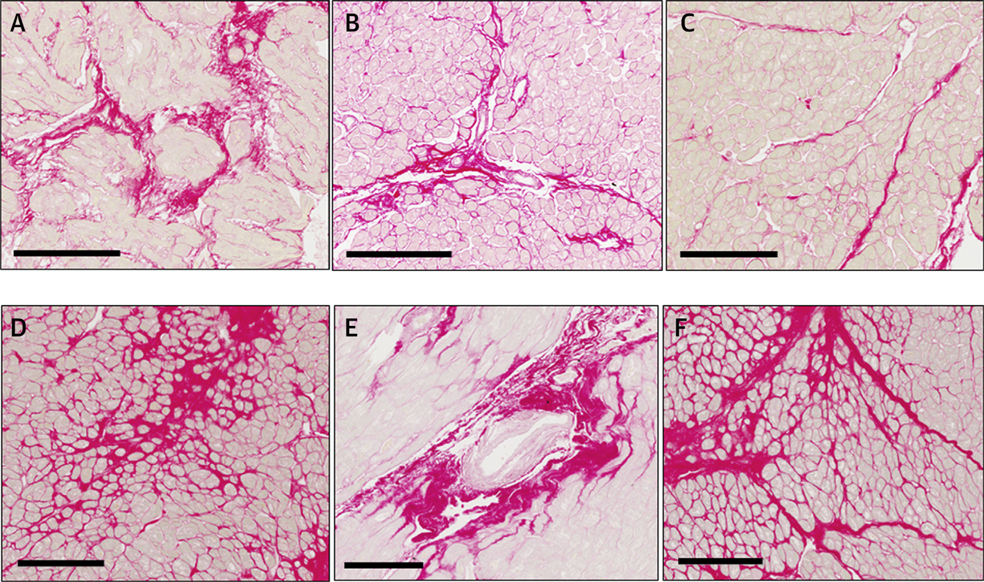
FIGURE 1. Types of Fibrous Deposits in Myocardial Interstitial Fibrosis.
Endomyocardial biopsy of (A-C) a patient with hypertensive heart disease and heart failure and (D-F) a patient with aortic valve stenosis showing myocardial interstitial fibrosis. Sections were stained with picrosirius red, and collagen deposits were identified in red as (A,D) microscars, (B,E) perivascular thick sheaths, and (C,F) thick bands deposited in the perimysium and the endomysium. Scale bars correspond to 200 μm.
COMPOSITION AND ORGANIZATION OF MIF
The available evidence suggests that beyond the extent of fibrous deposits, the collagen composition of the fibers and their physicochemical properties are also relevant in MIF. For instance, in HF due to hypertensive heart disease (17) or aortic stenosis (18), the ratio of collagen type I to type III is abnormally increased due to an excess of collagen type I fibers, whereas in diabetic cardiomyopathy, there is an excess of collagen type III over type I (9), with no differences between the 2 types of collagen in hypertrophic cardiomyopathy (19).
The insolubility, resistance to degradation, and stiffness of collagen fibers depend on the degree of intermolecular covalent linkage (i.e., cross-linking) among their constitutive fibrils (20). The oxidation of specific collagen lysines by enzymes of the lysyl oxidase (LOX) family, acting in concert with members of the lysyl hydroxylase and transglutaminase families, is a major mechanism of collagen cross-linking (21). A second type of collagen cross-linking implies glycation of lysine residues by advanced glycation end products (AGEs) (21). Whereas increased myocardial expression of LOX has been reported in patients with HF with hypertensive heart disease (22) and aortic stenosis (23), accumulation of AGEs has been found in the myocardium of patients with HF with diabetic cardiomyopathy (24). Of note, increased collagen cross-linking (assessed as an increased ratio of insoluble to soluble collagen), in association with increased LOX, has been reported in the myocardium of patients with HF due to hypertensive heart disease (25), namely, in those with HF with preserved ejection fraction (HFpEF) (22,26) and aortic stenosis (23).
HISTOMOLECULAR HETEROGENEITY OF MIF
Our appreciation of the histomolecular heterogeneity of MIF has been advanced by findings from a recent study (27) showing that both the extent of collagen deposition and the degree of collagen cross-linking of deposited collagen allow the identification of diverse MIF phenotypes in patients with HF attributable to hypertensive heart disease. Using this approach, 4 subgroups of patients were identified: those with moderate deposition and normal cross-linking, severe deposition and normal cross-linking, moderate deposition and increased cross-linking, and severe deposition and increased cross-linking. Of clinical relevance, the pulmonary capillary wedge pressure, levels of the amino-terminal pro-peptide of brain natriuretic peptide, and risk of first hospitalization for HF after enrollment or cardiovascular death all showed a progressive and statistically significant increase (i.e., worsening) from the first to the last subgroup, with the greatest risk seen in patients with severe deposition of highly cross-linked fibers (27). Further studies are required to ascertain whether this heterogeneity is also present in patients with other nonischemic cardiac conditions where MIF can arise.
CELLULAR AND MOLECULAR MECHANISMS OF MYOCARDIAL INTERSTITIAL FIBROSIS
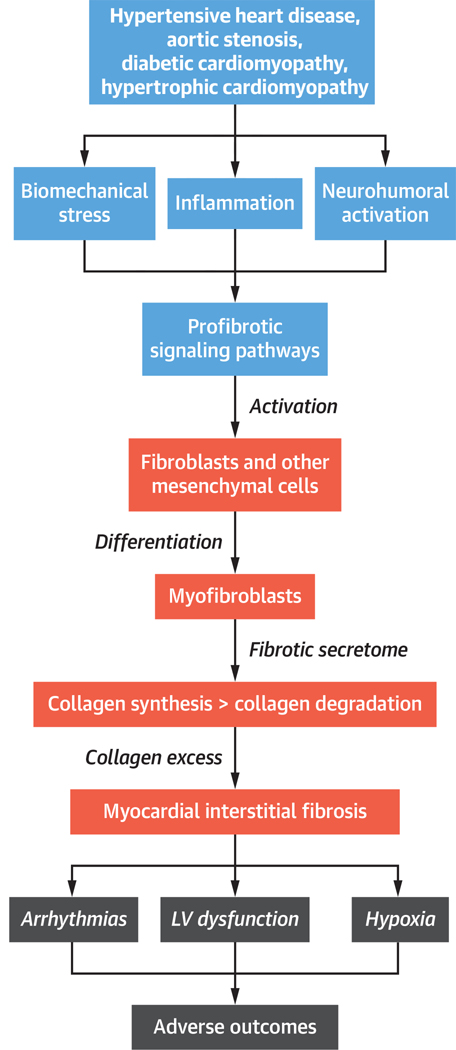
The mechanisms and pathways underlying the disturbances in turnover of cardiac fibrillar collagen leading to organ and tissue fibrosis in general (28) and MIF in particular (29,30) were recently reviewed in detail; therefore, a brief overview of the key steps of the fibrotic process is provided here (Central Illustration).
CENTRAL ILLUSTRATION. The Process of Myocardial Interstitial Fibrosis in Nonischemic Heart Disease.
Steps in the process of myocardial interstitial fibrosis, including associated clinical conditions, major mechanisms, and consequences. LV = left ventricular.
INITIATION OF THE FIBROTIC PROCESS
Cardiomyocyte death is often the triggering event responsible for the initiation of reparative MIF (31). The resulting tissue damage is associated with an inflammatory response, in which local immune cells (mainly macrophages) become activated, and diverse sets of blood cells enter the affected sites of injury. The local and invading immune cells produce a large variety of biologically active soluble mediators (i.e., cytokines and chemokines) that lead to a local activation of mesenchymal cells (namely, fibroblasts), which have the capacity to produce fibrillar collagen and other ECM molecules and to further increase the production of proinflammatory cytokines, chemokines, and growth factors. In reactive MIF, varied stimuli may trigger fibrosis in the absence of cell death through the activation of a diversity of fibrotic signaling pathways in mesenchymal cells, including mechanical stress associated with pressure overload in hypertensive heart disease and aortic stenosis (32,33), defects imparted by the various causal mutations on sarcomere structure and functions in hypertrophic cardiomyopathy (34), metabolic injury associated with hyperglycemia in diabetic cardiomyopathy (35), or coronary microvascular endothelial inflammation in HFpEF (36).
Cardiac fibroblasts express a range of innate immunity pattern-recognition receptors that are stimulated by a host of different damage-associated molecular patterns (DAMPs) that are up-regulated with injury (37). Damage-associated molecular patterns include intracellular molecules released by dying cells (i.e., heat shock proteins), prionflammatory cytokines (i.e., interleukin 1α), ECM molecules up-regulated in response to injury (i.e., fibronectin), or molecules modified by a pathological environment (i.e., AGEs). When activated, fibroblasts proliferate and differentiate into a secretory phenotype, the myofibroblast, with combined ultrastructural and phenotypic characteristics of smooth muscle cells acquired through formation of contractile stress fibers and the de novo expression of α-smooth muscle actin, as well as with an extensive endoplasmic reticulum, a feature of synthetically active fibroblasts (38). In addition, cardiac fibroblasts present membrane receptors for a number of neurohumoral factors, cytokines, and growth factors that regulate signaling pathways. They also express a wide range of ion channels that govern differing ion transport pathways and whose function may be modified by pro-fibrotic factors; for example, angiotensin II-mediated calcium entry facilitates fibroblast extracellular signal-related kinase (ERK) phosphorylation and activation, which enhances fibroblast proliferation (39). Moreover, cardiac fibroblasts have 2 classes of cell surface receptors— integrins and discoidin domain receptors—which mediate mechanosensing and fibroblast interactions with the ECM, resulting in stimulation of multiple cellular responses, including differentiation into myofibroblasts, migration, and proliferation (40).
EXECUTION OF THE FIBROTIC PROCESS
The myofibroblast’s profibrotic secretome consists of molecules required to alter extracellular fibrillar collagen turnover and facilitate MIF (41), as well as autocrine and paracrine factors that further simulate their proliferation and metabolic activity, perpetuating fibro- genesis in the injured myocardium (42). Among the best-known proteins secreted by the myofibroblast are procollagen types I and III precursors and enzymes that directly intervene in the extracellular synthesis, deposition, and degradation of collagen type I and III fibers (43). For instance, the enzymes procollagen type I amino-terminal proteinase (also known as A disintegrin and metalloproteinase with thrombospondin motifs 2 [ADAMTS2]) and procollagen type I carboxy-terminal proteinase, or PCP (also termed bone morphogenetic protein-1) cleave the terminal pro-peptides of the procollagen precursor secreted by the myofibroblast, converting it into a mature fibril-forming collagen molecule. Subsequently, the enzymes of the LOX family catalyze the formation of covalent bonds between polypeptide chains of adjacent fibrils (i.e., cross-links), forming the final collagen-type fiber, which is deposited in the myocardium. On the other hand, the enzyme matrix metalloproteinase (MMP) 1 initiates the degradation of collagen molecules within fibers, which results in 2 peptides: a small carboxy-terminal telopeptide that is released into the blood stream and a large telopeptide that is further degraded by MMP-2 and MMP-9 to final fragmented peptides termed matrikines. In conditions of MIF, the fibrogenic PCP/LOX axis predominates over the fibrolytic MMP-mediated axis.
Myofibroblasts also contribute to the pro-fibrotic effects of the local angiotensin system. In particular, the fibrogenic axis, comprising the angiotensin-converting enzyme (ACE)/angiotensin II/angiotensin type 1 receptor, represents the most upstream signal that stimulates procollagen type I and III synthesis and secretion by myofibroblasts (42). This axis is mediated through the downstream transforming growth factor (TGF)-β/Smad pathways, and in conditions of MIF, it predominates over a counter-regulatory, fibrolytic ACE2/angiotensin-(1–7)/Mas receptor axis, where ACE2-based hydrolysis of angiotensin II leads to Ang-(1–7) formation. Ang-(1–7)/Mas receptor signaling induces myofibroblast apoptosis through inhibition of antiapoptotic proteins (42). Finally, the myofibroblast also secretes other ECM macromolecules, such as the nonstructural matricellular protein osteopontin and the structural glycoprotein fibronectin, which play an important role in the regulation of MIF (44). For instance, it has been reported that an excess of myocardial osteopontin is associated with increased LOX, insoluble collagen, collagen type I deposition, and left ventricular (LV) stiffness and filling pressures in patients with HF attributable to hypertensive heart disease (45). Because osteopontin up-regulates LOX expression and activity in human cardiac fibroblasts (45), the possibility emerges that this matricellular protein is involved in MIF through the control of LOX-mediated cross-linking of collagen type I, thus leading to degradation-resistant collagen and LV stiffening. On the other hand, it has been shown that the inhibition of fibronectin polymerization or fibronectin gene expression attenuates pathological properties of myofibroblasts in vitro, as well as fibrillar collagen gene expression and collagen fiber deposition in in vivo models of pressure overload (46).
MODULATION OF THE FIBROTIC PROCESS
Although there is experimental evidence supporting myofibroblasts as the major effectors of detrimental MIF in pressure overload-induced fibrosis (47), recent experimental data suggest that they may also play a protective role by suppressing cardiomyocyte injury and macrophage-driven inflammation in the pressure-overloaded heart through Smad3-mediated pathways (48). This functional diversity of myofibroblasts may reflect the phenotypic heterogeneity of cardiac interstitial fibroblasts, which is relevant in ever-changing microenvironments, such as those leading to MIF (2). On the other hand, other cell types beyond fibroblasts and myofibroblasts are also implicated in MIF (e.g., M2 macrophages, mast cells, lymphocytes, cardiomyocytes, and vascular cells). For example, it has been shown that monocyte-derived C-C chemokine receptor 2 (CCR2+) macrophages infiltrate the heart early during pressure overload in mice and that blocking this response, either pharmacologically or with antibody-mediated CCR2+ monocyte depletion, attenuates MIF and LV remodeling and dysfunction (49).
As demonstrated in animal models and human biopsy studies, with aging, not only does the production of fibrillar collagen increase, but collagen degradation also becomes less effective (50). Fibrillar collagen processing and maturation are also altered, and cross-linking appears to increase (50). The triggers for fibrosis in the aging heart are manifold (51,52). In response to cardiomyocyte injury and cell loss, replacement fibrosis may be seen. At the same time, with ongoing inflammation and age-dependent increases in oxidative stress, reactive fibrosis may occur. Therefore, age-dependent MIF will usually develop alongside MIF in response to cardiac injury, which complicates the understanding of what causes and then perpetuates sustained fibrotic processes.
Finally, sex-dependent differences have been found in MIF under conditions of pressure overload due to aortic stenosis (53,54), with male patients having higher expression of collagen types I and III than female patients (54). In this context, recent experimental studies show that the mechanism underlying the sex-specific regulation of collagen I and III in the heart appears to involve 17β-estradiol- mediated differential estrogen receptor (ERa and ERb) signaling in cardiac fibroblasts (55). On the other hand, androgen deficiency can also contribute to excessive MIF after cardiac injury (56).
CLINICAL CONSEQUENCES OF MIF
MIF makes an important contribution to LV dysfunction and a number of other cardiac complications in patients with HF, thus contributing to poor outcomes as reviewed in the following sections (57) (Central Illustration).
LV DYSFUNCTION AND HF
MIF, assessed either histologically or by cardiac magnetic resonance (CMR) imaging, is associated with LV stiffness and diastolic dysfunction in patients with hypertensive heart disease (58), aortic stenosis (18), hypertrophic cardiomyopathy (59), and HFpEF (22,60).
Interestingly, the effect of excess collagen on LV function is modulated by changes in collagen quality. For instance, it has been shown that increased collagen cross-linking results in stiffer fibrous tissue (61) and is associated with LV stiffness and diastolic dysfunction in patients with HF with hypertensive heart disease (22,25,26,45) or aortic stenosis (23). In addition, collagen type I fibers exhibit greater stiffness than type III fibers (62), and an association exists between a predominance of collagen type I over type III fibers and increased LV stiffness with diastolic dysfunction in patients with aortic stenosis and HF (18). Similarly, an increase in the collagen type I:III ratio was found in patients with idiopathic dilated cardiomyopathy (63). However, in dilated cardiomyopathy, newly formed collagen is deficient in forming stable cross-links, which may contribute to ventricular dilatation (64).
On the other hand, collagen reorganization (alignment of fibers relative to cardiomyocytes that occurs in conditions of excess collagen) impairs the transmission of force generated by cardiomyocytes to the ventricular chamber, to the detriment of contractility (4). Of note, an association between the realignment of collagen and muscle fibers with LV systolic dysfunction has been described in patients with aortic stenosis and HF (65). Finally, although advanced loss of the perimysial and endomysial collagen scaffold resulting in slippage of cardiomyocytes is characteristic of nonischemic dilated cardiomyopathies and ischemic heart disease (Table 1), more modest disruptions in the normal collagen fibrillary network can accompany MIF in other cardiomyopathies, contributing to decreased systolic performance (66). For instance, decreased perimysial and endomysial collagen deposition has been described in patients with HF attributable to hypertensive heart disease exhibiting impaired LV systolic function, but not in patients with normal LV systolic function (67).
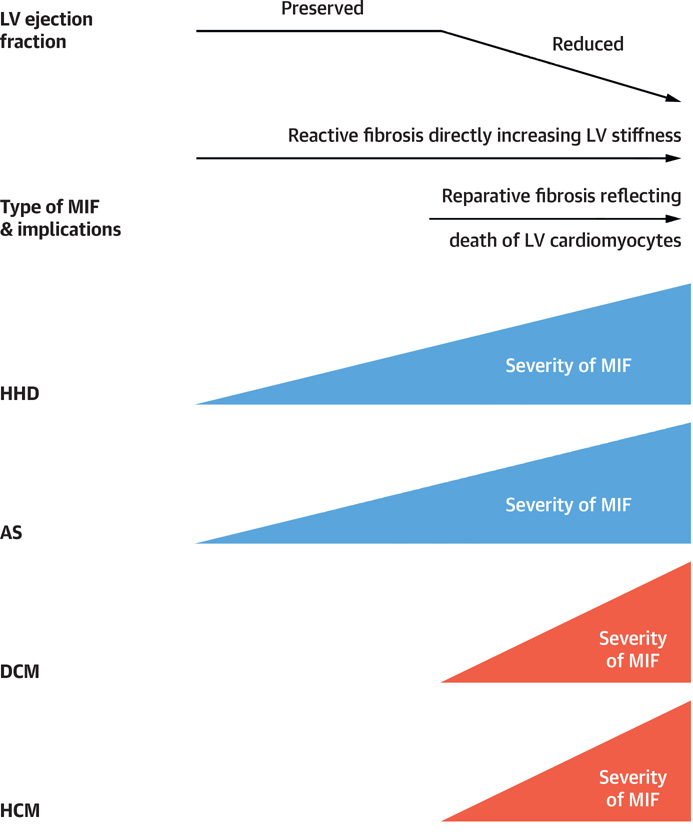
The relations between MIF and the type of HF (with either HFpEF or reduced ejection fraction [HFrEF]) have not been systematically investigated. In 2 studies performed in patients with hypertensive heart disease (67) or aortic stenosis (68) and absence of coronary disease, it was reported that MIF was quantitatively more severe in patients with HFrEF than in patients with HFpEF. In 1 study examining biopsy specimens from patients with HF with diabetic cardiomyopathy and absence of coronary disease, the excess of fibrous tissue was associated with HFrEF but not with HFpEF (24). A similar result was reported in another study examining autopsies of patients with hypertrophic cardiomyopathy and HF (69). Collectively, these findings suggest that variable structure-function relationships may exist between MIF and LV function in patients with HF depending on the etiology and nature of the cardiac disease process (Figure 2). Furthermore, whereas MIF may contribute to LV diastolic dysfunction in HFpEF by directly increasing LV stiffness, in HFrEF the loss of cardiomyocytes and contractile mass may trigger MIF as a form of reparative response (Figure 2).
FIGURE 2. Time Course of Changes in Severity of Myocardial Interstitial Fibrosis.
Functional and clinical impact of myocardial interstitial fibrosis according to its histological type and clinical scenario. AS = aortic stenosis; DCM = diabetic cardiomyopathy; MIF = myocardial interstitial fibrosis; HCM = hypertrophic cardiomyopathy; HHD = hypertensive heart disease; LV = left ventricular.
VENTRICULAR AND ATRIAL ARRHYTHMIAS
There is abundant evidence that MIF impairs myocardial electrophysiology by slowing action potential propagation, initiating re-entry, promoting after-depolarizations, and increasing ectopic automaticity, collectively resulting in increased risk of ventricular arrhythmias (70). For example, the association of MIF with electrophysiological changes mediating ventricular arrhythmias has been described in patients with hypertrophic cardiomyopathy (71). Furthermore, an association of MIF with ventricular arrhythmias independent of LV function has been reported in patients with hypertensive heart disease (72). Indeed, MIF has been proposed as a risk factor for sudden arrhythmic death in patients with hypertrophic cardiomyopathy (73), hypertensive heart disease (74), and nonischemic dilated cardiomyopathy (75).
Although an association between atrial fibrillation and ventricular MIF is less established than for atrial MIF, it is unlikely that the profibrotic cardiac microenvironment (such as in the examples reviewed here) is limited to the ventricles. Rather, in most of the disease states we discuss, the atrial myocardium is also likely to be affected (76). In addition, it is known that in conditions of severe chronic pressure overload, advanced diabetes, or chronic HF, the deposition of atrial collagen may occur, resulting in EHRAS (for EHRA/HRS/APHRS/SOLAECE) class II or III atrial cardiomyopathy with increased risk of atrial fibrillation (77). In this regard, in patients with severe aortic stenosis, a positive graded association of the prevalence of atrial fibrillation with the quantitative severity of LV MIF was reported (78,79).
MYOCARDIAL HYPOXIA
MIF is associated with significant impairment of oxygen delivery to cardiomyocytes. Perivascular fibrosis impairs and is inversely correlated with coronary flow reserve in patients with HF due to hypertensive heart disease or hypertrophic cardiomyopathy (80). Deposition of fibrotic tissue increases oxygen diffusion distance, leading to hypoxia (81). Also, the severity of MIF is associated with the severity of coronary microvascular disease—that is, anatomic abnormalities of the vascular wall and capillary rarefaction—in patients with HF due to hypertrophic cardiomyopathy (14), hypertensive heart disease, or diabetic cardiomyopathy (82). Thus, MIF facilitates ischemia/hypoxia and leads to compromise of the coronary microcirculation, which, in turn, can further aggravate MIF.
MIF IS ASSOCIATED WITH A WORSENED CLINICAL COURSE
The available clinical evidence suggests that the quantity and quality of MIF may influence both the prognosis and response to treatment in patients with HF. The extent of fibrosis (assessed either histologically or by CMR) is associated with all-cause death and adverse cardiovascular events in patients with HF attributable to cardiomyopathies such as hypertensive heart disease, diabetic cardiomyopathy, or hypertrophic cardiomyopathy (83–85) and also with HF hospitalization or mortality in patients with HFpEF (86,87). The chemical nature of deposited fibers also influences outcome. In patients with HF due to hypertensive heart disease, increased collagen type I cross-linking is associated with risk of hospitalization for HF (88). In addition, the combination of increased collagen deposition and increased collagen cross-linking is associated with both hospitalization for HF and cardiovascular mortality in patients with HF with hypertensive heart disease (27). Similarly, the previous extent of fibrotic deposition is associated with mortality, and inversely associated with the degree of LV functional improvement, in patients with severe aortic stenosis who undergo aortic valve replacement (79,89).
DIAGNOSIS OF MIF
Because MIF is fundamental in the development and progression of HF and is related to clinical outcomes, the integration of its assessment into the clinical treatment of these patients may be warranted (90). Although endomyocardial biopsy is relatively safe (91) and MIF on biopsy correlates with MIF in the whole heart (67,92), alternate noninvasive imaging and/or biochemical methods are desirable for routine practice.
TISSUE-IMAGING BIOMARKERS
CMR imaging-derived parameters, particularly late-gadolinium enhancement (LGE) and T1 mapping (including native T1, post-contrast T1, and extracellular volume fraction [ECV]), are currently used as biomarkers for the assessment of fibrotic myocardium. Whereas LGE can be used for identification of focal collagen deposition as seen in large focal post-infarct scars, T1 mapping is useful for identifying diffuse collagen deposition as seen in MIF. Several small clinical studies have been performed, validating ECV against the extent of diffuse collagen deposition (assessed by the collagen volume fraction) (93,94) and demonstrating superiority over LGE (94). However, in a recent large study performed on intraoperative endomyocardial LV biopsy specimens from 133 patients with aortic stenosis, no association was found between ECV and histologically assessed MIF (16). In addition, neither LGE nor ECV can identify qualitative aspects related to the composition and molecular organization of collagen fibers in MIF (95). Therefore, due to these limitations of CMR-derived biomarkers, another biomarker-based approach may be required to capture not only the quantitative but also the qualitative aspects of MIF and, thus, define its histomolecular and phenotypic heterogeneity in patients with HF.
CIRCULATING MOLECULAR BIOMARKERS
A number of molecules, detectable in serum or plasma, were recently proposed as biomarkers of MIF (96–98). However, in most cases, demonstration of an association between the biomarker and histologically assessed MIF is lacking or remains inconclusive (Table 2). Among the many proposed circulating molecules, only a limited number have been shown to be associated with histological MIF in humans. The level of carboxy-terminal pro-peptide of procollagen type I (PICP)—formed during the extracellular conversion of procollagen type I into mature fibril-forming collagen type I by the enzyme PCP—was found to be highly correlated with the extent of collagen type I deposition in the myocardium of patients with HF due to hypertensive heart disease (25). The levels of amino-terminal pro-peptide of procollagen type III (PIIINP)—formed during the extracellular conversion of procollagen type III into mature fibril-forming collagen type III by the enzyme procollagen type III amino-terminal proteinase—were also found to be highly correlated with the extent of myocardial collagen type III deposition in patients with HF with ischemic heart disease or idiopathic dilated cardiomyopathy (99). Circulating transcript levels may also hold promise, and an inverse relationship has been shown between levels of circulating microRNA 19b with myocardial collagen cross-linking and LV stiffness in patients with severe aortic stenosis (23).
TABLE 2.
Analysis of the Association of Circulating Biomarkers With Histologic Parameters of Myocardial Interstitial Fibrosis in Clinical Studies
| Biomarkers Related to | Association Confirmed | Association Not Confirmed |
|---|---|---|
| Collagen synthesis | ||
| PICP | With the extent of collagen deposition | |
| PINP | Demonstrated the absence of association | |
| PIIINP | With the extent of collagen deposition | |
| Collagen degradation | ||
| CITP | Inconclusive evidence of association | |
| MMP-1 | Demonstrated the absence of association | |
| MMP-2, -3, -8, -9 | Associations not explored | |
| TIMP-1 | Demonstrated the absence of association | |
| TIMP-4 | Association not explored | |
| Collagen composition/organization | ||
| CITP: MMP-1 ratio | With the degree of collagen cross-linking | |
| microRNA-21 | With the degree of collagen cross-linking | |
| Collagen turnover | With the degree of collagen cross-linking | |
| microRNA-29b, -122, -133a, -499-5p | Associations not explored | |
| Transforming growth factor β | Inconclusive evidence of association | |
| Growth differentiation factor 15 | Inconclusive evidence of association | |
| Fibroblast growth factor 23 | Association not explored | |
| Connective tissue growth factor | Inconclusive evidence of association | |
| Osteopontin | ||
| Osteoglycin | Association not explored | |
| Periostin | Association not explored | |
| Syndecan-1, -4 | Association not explored | |
| Injury that triggers fibrosis | ||
| Galectin-3 | Demonstrated the absence of association | |
| Cardiotrophin-1 | Demonstrated the absence of association | |
| Soluble ST2 | Association not explored | |
| Midregional pro-ANP | Association not explored | |
| Myostatin | Association not explored |
CITP = carboxy-terminal telopeptide of collagen type I; MMP = matrix metalloproteinase; PICP = carboxy-terminal propeptide of procollagen type I; PIIINP = amino-terminal propeptide of procollagen type III; PINP = amino-terminal propeptide of procollagen type I; TIMP = tissue inhibitor of matrix metalloproteinases.
Recently, Ravassa et al. (27) reported that levels of PICP and the ratio of serum carboxy-terminal telopeptide of collagen type I (CITP) to MMP-1, corresponding to severe collagen deposition and increased collagen cross-linking, respectively, allow the stratification of patients with HF attributable to hypertensive heart disease. There were 4 proposed biomarker-based bio-profiles: moderate PICP and normal CITP:MMP-1 ratio, high PICP and normal CITP:MMP-1 ratio, moderate PICP and low CITP:MMP- 1 ratio, and high PCIP and low CITP:MMP-1 ratio. The primary outcome for this study was a composite of first HF hospitalization after enrollment or death from cardiovascular causes. During a median follow-up period of 5.31 years, there was a significant and progressive increase in the incidence of the primary outcome from the first to the last groups. In addition, a secondary composite outcome of first hospitalization for HF or all-cause death also increased significantly from the first to the last groups. Moreover, this classification improved the prognostic performance of important risk factors. Using the same biomarker- based approach, it was recently reported that the fourth bio-profile was associated with higher atrial fibrillation prevalence and incidence in patients with HF attributable to hypertensive heart disease (100), improving the predictive value of relevant atrial fibrillation risk factors. Therefore, the combination of PICP and the CITP:MMP-1 ratio appears to reflect the clinical impact of the histomolecular phenotypes of MIF in patients with HF due to hypertensive heart disease. Furthermore, the bio-profile defined by the coincidence of high serum PICP and low CITP:MMP-1 ratio identifies patients with a high-risk phenotype characterized by increased risk of atrial fibrillation, HF hospitalization, and mortality. Nevertheless, it is important to remark that these biomarkers are not cardiac specific, and changes in their blood levels may represent integrated abnormalities of cardiovascular collagen and/or the influence of comorbidities affecting collagen metabolism in HF. BECAUSE the extent and nature of MIF depend on the etiologic context, their potential clinical value may vary according to the specific underlying cardiac disease process.
TREATMENT OF MYOCARDIAL INTERSTITIAL FIBROSIS
Although the development of fibrosis has long been viewed as a unidirectional process consisting of nonreversible sequelae from chronic injury to fibrosis, and ultimately on to tissue architecture remodeling and destruction, there is evidence that fibrogenesis is likely reversible, even at later stages (101). In this regard, one mechanistic principle of fibrosis regression involves the resolution of chronic tissue injury (102,103). However, not all clinical evidence supports this notion regarding MIF. For instance, the extent of collagen deposition remains unchanged in patients with aortic stenosis after long-term aortic valve replacement and successful avoidance of pressure overload (104), as well as in hypertensive patients with normalization of blood pressure after midterm treatment with the calcium channel blocker amlodipine (105). Thus, the effective treatment of MIF is challenging and requires a broader approach.
USE OF AGENTS WITH PROVEN ANTI-MIF EFFICACY AND SAFETY
The available clinical evidence indicates that MIF is a target of drugs interfering with the renin-angiotensin-aldosterone axis. In fact, treatment of patients with hypertensive heart disease using the angiotensin-converting enzyme inhibitor lisinopril (106) or the angiotensin receptor blocker losartan (58) was associated with reduction in the extent of fibrotic deposits, with corresponding improvement of LV diastolic dysfunction and reduction of LV stiffness, respectively. Similarly, the mineralocorticoid receptor antagonist spironolactone reduced the extent of collagen deposition and LV stiffness and ameliorated diastolic dysfunction in patients with HF (107). Although these drugs directly target MIF and are convenient because of their current widespread clinical use and safety, a residual fibrotic burden remains, thus requiring novel therapeutic approaches for more effective antifibrotic treatment. In this regard, it has been reported that the administration of the loop diuretic torsemide to patients with hypertensive heart disease, in addition to standard HF therapy, is associated with reductions in the extent of collagen deposition, as well as in the degree of cross-linking of the deposited fibers and the expression of LOX in fibrotic areas (108–110). These effects were accompanied by normalization of LV stiffness and improved functional class in most patients. Interestingly, the antifibrotic effect of torsemide was not observed in patients with HF treated with furosemide, suggesting that torsemide may directly target MIF (beyond its renal actions). Although these findings appear promising, they were obtained in studies with a relatively small number of patients who were treated for short periods. Large, long-term clinical trials are required to verify whether torsemide targets MIF in a clinically effective manner.
DEVELOPMENT OF NOVEL ANTI-MIF CLINICAL APPROACHES
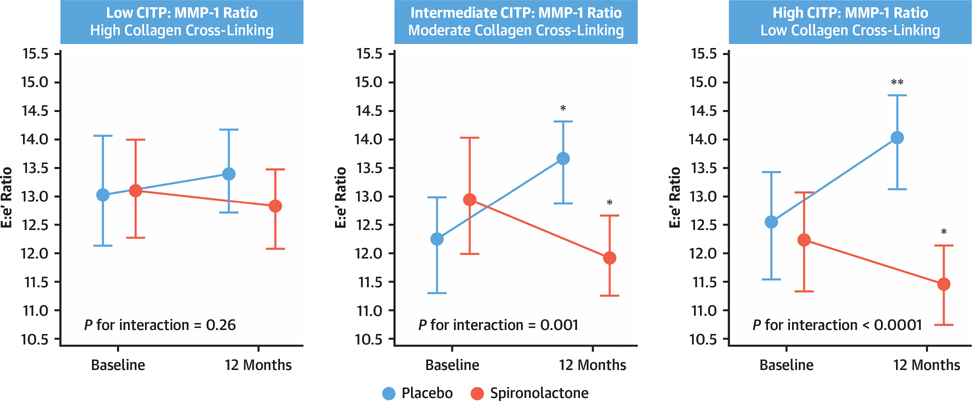
By taking into account the fundamentals of precision medicine, it can be anticipated that by defining MIF at a deeper biological level, patients can be treated based on an understanding of the molecular underpinnings of their presentation, rather than grouping them into a single broad category with one-size-fits-all treatment (111). This is illustrated by the recent observation that biomarker-based phenotyping of the histomolecular characteristics of MIF (e.g., serum CITP:MMP-1 ratio-based phenotyping of the degree of collagen cross-linking) identifies a subgroup of patients with HFpEF with high collagen type I cross-linking in whom spironolactone fails to improve LV function (112) (Figure 3). These findings support the notion that a precise biomarker-based phenotyping of MIF will be critical to advance the field of HFpEF therapy.
FIGURE 3. Effect of Spironolactone on Patients With Heart Failure With Preserved Ejection Fraction.
Peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity ratio (E:e′) according to study treatment in tertiles of patients with low, intermediate, and high serum carboxy-terminal telopeptide of collagen type I to matrix metalloproteinase-1 ratio (CITP:MMP-1), which are equivalent to high, moderate, and low myocardial collagen cross-linking, respectively. Data are expressed as mean values and 95% confidence intervals at baseline and at 12 months in patients with heart failure with preserved ejection fraction treated with placebo or spironolactone. *p < 0.05 versus baseline. **p < 0.01 versus baseline. Reprinted with permission from Ravassa et al. (112).
Data from experimental studies suggest that pharmacological agents already used in clinical practice with proven safety may be of interest to treat MIF through novel mechanisms (111). For instance, the neprilysin inhibitor and angiotensin receptor blocker combination of sacubitril/valsartan reduced MIF with improvement of LV function in HF mice with diabetes (113) and cardiac pressure overload (114). Additional data suggest that the anti- fibrotic effect of sacubitril/valsartan may be due to the specific inhibition of neprilysin, beyond the angiotensin receptor blocker effect. However, in a recent phase 3 clinical study of sacubitril/valsartan in 4,822 patients with HF with relatively preserved LV function (LV ejection fraction: >45%), as compared with valsartan monotherapy, there was only a borderline beneficial effect seen in terms of a reduction of the primary composite endpoint of total hospitalizations for HF and death from cardiovascular causes (p = 0.06) (115).
On the other hand, it has been shown that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin reduces MIF and is linked to improved diastolic dysfunction in diabetic mice (116). Because the antifibrotic effect was not linked to metabolic and/or hemodynamic changes and SGLT2 is not expressed in the heart, it has been suggested that it likely reflects direct pleiotropic effects of the drug on the myocardium (117). At the present time, it remains to be tested whether SGLT2 inhibitors are also effective in reducing MIF in humans and if this effect is merely related to their proven safety and effectiveness to prevent and ameliorate HF. Nevertheless, in a recent large phase 3 clinical study among patients with HF and reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received the SGLT2 inhibitor dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes (118). Although it must be noted that the majority of these participants (approximately 55%) had ischemic heart disease and only approximately 35% had a nonischemic etiology for their HF, subgroup analyses appear to indicate that the primary composite endpoint remained significantly different between dapagliflozin and placebo treatment when the analysis was restricted to either the ischemic or the nonischemic subgroups (118).
Finally, several experimental studies have documented the cardiac antifibrotic effects of pirfenidone and tranilast, both clinically approved drugs that inhibit TGF-β signaling, in several models of MIF (119). Because prolonged dosages of either of these drugs can have hepatic toxicity and may lead to liver failure (120), more research is warranted to explore alternative methods that can safely, but efficaciously, target TGF-p signaling for reduction of MIF.
SEARCH FOR NEW ANTI-MIF STRATEGIES
Ongoing preclinical research for reversing nonischemic MIF is based on the prevention of excessive fibrous tissue deposition. As specific examples, this may be via the deactivation of inflammatory pathways and establishment of an anti-inflammatory microenvironment, and/or the deactivation and elimination of myofibroblasts, and/or fibrolysis of excess collagen. In this conceptual framework, novel druggable molecular targets (e.g., noncoding RNAs and epigenetic modifiers) are potential new anti-MIF strategies (121). However, although many new therapies targeting MIF appear to be promising in the pre-clinical setting, the translation to the clinical arena remains challenging for several reasons. First, the injured heart is a volatile microenvironment with cardiomyocyte death, infiltration of immune and inflammatory cells, and activation of mesenchymal cells that may hinder the efficacy of delivering antifibrosis therapies (29,30). Second, it is important to rationalize drug discovery by using a meaningful step-by-step process to efficiently discard irrelevant and inefficacious molecules or those with unfavorable pharmacokinetic and toxicological profiles (122). Finally, it is important to rationalize anti-MIF strategy testing in clinical studies by pursuing those therapies with a high degree of correspondence between the properties of the new therapy under investigation and the specific patient population and their cardiac disease process (120).
SUMMARY AND CONCLUSIONS
MIF is a critical component of myocardial remodeling in patients with HF that occurs secondary to a number of nonischemic cardiac diseases. The cell biology underlying MIF is complex, and significant difficulty has arisen in translating results from animal studies to humans. Furthermore, the triggers, dynamics, and characteristics of the fibrotic process vary among the differing etiologies of MIF and depend also on the contribution of aging. Correspondingly, at the present time, the diagnosis and treatment of MIF suffer from a lack of precision, and strategies that allow for the differentiation of MIF subtypes in a disease-specific way are needed, ideally combining noninvasive imaging and molecular biomarkers. These biomarkers must be useful not only for establishing and phenotyping MIF, but also for the predicting and monitoring its response to intervention. Finally, it is likely that a combination of various therapies will be necessary to address the complexity of MIF. Despite these challenges, the rapid progress in our understanding of MIF and the concurrent heightened clinical awareness of the importance of HFpEF serve as a solid platform for ongoing and future research that tackles these issues and that drives toward novel clinical biomarkers and therapies to treat MIF.
HIGHLIGHTS.
Myocardial interstitial fibrosis (MIF) is a histological hallmark of several cardiac diseases that alter myocardial architecture and function.
MIF is a diffuse and patchy process, appearing as a combination of interstitial microscars, perivascular collagen fiber deposition, and increased thickness of mysial collagen strands.
MIF plays an important role in systolic and diastolic cardiac dysfunction, as well as impaired clinical outcomes in patients with nonischemic heart disease.
Ongoing pre-clinical research for reversing nonischemic MIF is aiming to prevent excessive fibrous tissue deposition; however, although many new therapies targeting MIF appear promising in the pre-clinical setting, their translation to the clinical arena remains challenging.
Acknowledgments
This project was funded by the Spanish Ministry of Science, Innovation, and Universities (Instituto de Salud Carlos III: CIBERCV CB16/11/00483and PI18/01469 cofinanced by FEDER funds) and the ERA-CVD Joint Transnational Call 2016 LYMIT-DIS (AC16/00020), and LIPCAR-HF (AC16/00016). Jason C. Kovacic acknowledges research support from the National Institutes of Health (R01HL130423, R01HL135093).
ABBREVIATIONS AND ACRONYMS
- ACE
angiotensin-converting enzyme
- AGE
advanced glycation end product
- CCR2
C-C chemokine receptor 2
- CITP
carboxy-terminal telopeptide of collagen type I
- CMR
cardiac magnetic resonance
- ECM
extracellular matrix
- ECV
extracellular volume fraction
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LGE
late-gadolinium enhancement
- LOX
lysyl oxidase
- LV
left ventricular
- MIF
myocardial interstitial fibrosis
- MMP
matrix metalloprotease
- PCP
procollagen type I carboxy-terminal proteinase (also termed bone morphogenetic protein-1)
- PICP
carboxy-terminal pro-peptide of procollagen type I
- SGLT2
sodium-glucose cotransporter 2
- TGF-β
transforming growth factor-β
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
CITP = carboxy-terminal telopeptide ofcollagen type I; MMP = matrix metalloproteinase; PICP = carboxy-terminal propeptideofprocollagen type I; PIIINP = amino-terminal propeptide of procollagen type III; PINP = amino-terminal propeptide of procollagen type I; TIMP = tissue inhibitor of matrix metalloproteinases.
REFERENCES
- 1.Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol 2010;48:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res 2014;114:872–88. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 2017;127:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brower GL, Gardner JD, Forman MF et al. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 2006;30:604–10. [DOI] [PubMed] [Google Scholar]
- 5.Eckhouse SR, Spinale FG. Changes in the myocardial interstitium and contribution to the progression of heart failure. Heart Fail Clin 2012;8:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li AH, Liu PP, Villarreal FJ, Garcia RA. Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res 2014;114:916–27. [DOI] [PubMed] [Google Scholar]
- 7.Hoyt RH, Ericksen E, Collins SM, Skorton DJ. Computer-assisted quantitation of myocardial fibrosis in histologic sections. Arch Pathol Lab Med 1984;108:280–3. [PubMed] [Google Scholar]
- 8.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990;82:848–55. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Umeda K, Sugihara N et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 1993;46:32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000;35:36–44. [DOI] [PubMed] [Google Scholar]
- 11.Brooks A, Schinde V, Bateman AC, Gallagher PJ. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart 2003;89:1255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcao-Pires I, Hamdani N, Borbely A et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 2011;124:1151–9. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol 1979;128:79–85. [DOI] [PubMed] [Google Scholar]
- 14.Galati G, Leone O, Pasquale F et al. Histological and histometric Characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy: a clinical-pathological study of 30 explanted hearts. Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Hamashima Y, Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J 1986;55:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treibel TA, López B, González A et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López B, González A, Querejeta R, Larman M, Rabago G, Díez J. Association of cardiotrophin-1 with myocardial fibrosis in hypertensive patients with heart failure. Hypertension 2014;63:483–9. [DOI] [PubMed] [Google Scholar]
- 18.Echegaray K, Andreu I, Lazkano A et al. Role of myocardial collagen in severe aortic stenosis with preserved ejection fraction and symptoms of heart failure. Rev Esp Cardiol (Engl Ed) 2017;70:832–840. [DOI] [PubMed] [Google Scholar]
- 19.Boerrigter G, Mundhenke M, Stark P, Schulte HD, Strauer BE, Schwartzkopff B. Immunohistochemical video-microdensitometry of myocardial collagen type I and type III. Histochem J 1998;30:783–91. [DOI] [PubMed] [Google Scholar]
- 20.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem 2009;78:929–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J 1992;6:2439–49. [DOI] [PubMed] [Google Scholar]
- 22.Kasner M, Westermann D, López B et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol 2011;57:977–85. [DOI] [PubMed] [Google Scholar]
- 23.Beaumont J, López B, Ravassa S et al. Micro-RNA-19b is a potential biomarker of increased myocardial collagen cross-linking in patients with aortic stenosis and heart failure. Sci Rep 2017;7:40696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51. [DOI] [PubMed] [Google Scholar]
- 25.López B, Querejeta R, González A, Larman M, Díez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension 2012;60:677–83. [DOI] [PubMed] [Google Scholar]
- 26.Zile MR, Baicu CF, Ikonomidis JS et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravassa S, López B, Querejeta R, et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J Hypertens 2017;35:853–861. [DOI] [PubMed] [Google Scholar]
- 28.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol Aspects Med 2019;65:2–15. [DOI] [PubMed] [Google Scholar]
- 29.Cowling RT, Kupsky D, Kahn AM, Daniels LB, Greenberg BH. Mechanisms of cardiac collagen deposition in experimental models and human disease. Transl Res 2019;209:138–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019;65:70–99. [DOI] [PubMed] [Google Scholar]
- 31.Piek A, de Boer RA, Sillje HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev 2016;21:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González A, Ravassa S, López B et al. Myocardial Remodeling in Hypertension. Hypertension 2018;72:549–558. [DOI] [PubMed] [Google Scholar]
- 33.Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG. Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg 2012;143:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 2017;121:749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 2016;90:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Li R, Ross RS, Manso AM. Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol 2016;93:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furtado MB, Costa MW, Rosenthal NA. The cardiac fibroblast: origin, identity and role in homeostasis and disease. Differentiation 2016;92:93–101. [DOI] [PubMed] [Google Scholar]
- 39.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension 2008;51:704–11. [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith EC, Bradshaw AD, Zile MR, Spinale FG. Myocardial fibroblast-matrix interactions and potential therapeutic targets. J Mol Cell Cardiol 2014;70:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013;10:15–26. [DOI] [PubMed] [Google Scholar]
- 42.Bomb R, Heckle MR, Sun Y et al. Myofibroblast secretome and its auto-/paracrine signaling. Expert Rev Cardiovasc Ther 2016;14:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricard-Blum S The collagen family. Cold Spring Harb Perspect Biol 2011;3:a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 2012;92:635–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López B, González A, Lindner D et al. Osteopontin-mediated myocardial fibrosis in heart failure: a role for lysyl oxidase? Cardiovasc Res 2013;99:111–20. [DOI] [PubMed] [Google Scholar]
- 46.Konstandin MH, Volkers M, Collins B et al. Fibronectin contributes to pathological cardiac hypertrophy but not physiological growth. Basic Res Cardiol 2013;108:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil H, Kanisicak O, Prasad V et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017;127:3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russo I, Cavalera M, Huang S et al. Protective effects of activated myofibroblasts in the pressure-overloaded myocardium are mediated through smad-dependent activation of a matrix-preserving program. Circ Res 2019;124:1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel B, Bansal SS, Ismahil MA et al. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. J Am Coll Cardiol Basic Transl Science 2018;3:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horn MA, Trafford AW. Aging and the cardiac collagen matrix: novel mediators of fibrotic remodelling. J Mol Cell Cardiol 2016;93:175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev 2010;15:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 2012;16:1492–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM. Sex-dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J 1995;16:1410–9. [DOI] [PubMed] [Google Scholar]
- 54.Kararigas G, Dworatzek E, Petrov G et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 2014;16:1160–7. [DOI] [PubMed] [Google Scholar]
- 55.Dworatzek E, Mahmoodzadeh S, Schriever C et al. Sex-specific regulation of collagen I and III expression by 17beta-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc Res 2019;115:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung CC, Kao YH, Chen YJ, Chen YJ. Androgen modulates cardiac fibrosis contributing to gender differences on heart failure. Aging Male 2013;16:22–7. [DOI] [PubMed] [Google Scholar]
- 57.González A, Schelbert EB, Díez J, Butler J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J Am Coll Cardiol 2018;71:1696–1706. [DOI] [PubMed] [Google Scholar]
- 58.Díez J, Querejeta R, López B, González A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 2002;105:2512–7. [DOI] [PubMed] [Google Scholar]
- 59.Maragiannis D, Alvarez PA, Ghosn MG et al. Left ventricular function in patients with hypertrophic cardiomyopathy and its relation to myocardial fibrosis and exercise tolerance. Int J Cardiovasc Imaging 2018;34:121–129. [DOI] [PubMed] [Google Scholar]
- 60.Rommel KP, von Roeder M, Latuscynski K et al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2016;67:1815–1825. [DOI] [PubMed] [Google Scholar]
- 61.van der Slot-Verhoeven AJ, van Dura EA, Attema J et al. The type of collagen cross-link determines the reversibility of experimental skin fibrosis. Biochim Biophys Acta 2005;1740:60–7. [DOI] [PubMed] [Google Scholar]
- 62.Collier P, Watson CJ, van Es MH et al. Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J Mol Cell Cardiol 2012;52:148–53. [DOI] [PubMed] [Google Scholar]
- 63.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol 1995;25:1263–72. [DOI] [PubMed] [Google Scholar]
- 64.Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF Jr. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 1996;148:1639–48. [PMC free article] [PubMed] [Google Scholar]
- 65.Villari B, Campbell SE, Hess OM et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol 1993;22:1477–84. [DOI] [PubMed] [Google Scholar]
- 66.Baicu CF, Stroud JD, Livesay VA et al. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol 2003;284:H122–32. [DOI] [PubMed] [Google Scholar]
- 67.López B, González A, Querejeta R, Larman M, Díez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol 2006;48:89–96. [DOI] [PubMed] [Google Scholar]
- 68.Hein S, Arnon E, Kostin S et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003;107:984–91. [DOI] [PubMed] [Google Scholar]
- 69.Melacini P, Basso C, Angelini A et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J 2010;31:2111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac Fibrosis and Arrhythmogenesis. Compr Physiol 2017;7:1009–1049. [DOI] [PubMed] [Google Scholar]
- 71.Kawara T, Derksen R, de Groot JR et al. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 2001;104:3069–75. [DOI] [PubMed] [Google Scholar]
- 72.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 1990;3:735–40. [DOI] [PubMed] [Google Scholar]
- 73.Bockstall KE, Link MS. A primer on arrhythmias in patients with hypertrophic cardiomyopathy. Curr Cardiol Rep 2012;14:552–62. [DOI] [PubMed] [Google Scholar]
- 74.Shenasa M, Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol 2017;237:60–63. [DOI] [PubMed] [Google Scholar]
- 75.Gulati A, Jabbour A, Ismail TF et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 76.Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol 2015;66:943–59. [DOI] [PubMed] [Google Scholar]
- 77.Goette A, Kalman JM, Aguinaga L et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrmann S, Fries B, Salinger T et al. Myocardial fibrosis predicts 10-year survival in patients undergoing aortic valve replacement. Circ Cardiovasc Imaging 2018;11:e007131. [DOI] [PubMed] [Google Scholar]
- 79.Weidemann F, Herrmann S, Stork S et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009;120:577–84. [DOI] [PubMed] [Google Scholar]
- 80.Dai Z, Aoki T, Fukumoto Y, Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol 2012;60:416–21. [DOI] [PubMed] [Google Scholar]
- 81.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 1995;147:29–34. [DOI] [PubMed] [Google Scholar]
- 82.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aoki T, Fukumoto Y, Sugimura K et al. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. Comparison between preserved and reduced ejection fraction heart failure. Circ J 2011;75:2605–13. [DOI] [PubMed] [Google Scholar]
- 84.Wong TC, Piehler KM, Kang IA et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014;35:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015;101:1406–11. [DOI] [PubMed] [Google Scholar]
- 86.Duca F, Kammerlander AA, Zotter-Tufaro C et al. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 2016;9. [DOI] [PubMed] [Google Scholar]
- 87.Schelbert EB, Fridman Y, Wong TC et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.López B, Ravassa S, González A et al. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol 2016;67:251–60. [DOI] [PubMed] [Google Scholar]
- 89.Azevedo CF, Nigri M, Higuchi ML et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010;56:278–87. [DOI] [PubMed] [Google Scholar]
- 90.de Boer RA, De Keulenaer G, Bauersachs J et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail 2019;21:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation 2013;128:1531–41. [DOI] [PubMed] [Google Scholar]
- 92.Ishibashi-Ueda H, Matsuyama TA, Ohta-Ogo K, Ikeda Y. Significance and value of endomyocardial biopsy based on our own experience. Circ J 2017;81:417–426. [DOI] [PubMed] [Google Scholar]
- 93.Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 2016;71:768–78. [DOI] [PubMed] [Google Scholar]
- 94.Diao KY, Yang ZG, Xu HY et al. Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson 2016;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.López B, González A, Ravassa S et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol 2015;65:2449–56. [DOI] [PubMed] [Google Scholar]
- 97.de Jong S, van Veen TA, de Bakker JM, Vos MA, van Rijen HV. Biomarkers of myocardial fibrosis. J Cardiovasc Pharmacol 2011;57:522–35. [DOI] [PubMed] [Google Scholar]
- 98.Passino C, Barison A, Vergaro G et al. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin Chim Acta 2015;443:29–38. [DOI] [PubMed] [Google Scholar]
- 99.Klappacher G, Franzen P, Haab D et al. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol 1995;75:913–8. [DOI] [PubMed] [Google Scholar]
- 100.Ravassa S, Ballesteros G, López B et al. Combination of circulating type I collagen-related biomarkers is associated with atrial fibrillation. J Am Coll Cardiol 2019;73:1398–1410. [DOI] [PubMed] [Google Scholar]
- 101.Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest 2018;128:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tacke F, Trautwein C. Mechanisms of liver fibrosis resolution. J Hepatol 2015;63:1038–9. [DOI] [PubMed] [Google Scholar]
- 103.Moore KJ, Koplev S, Fisher EA et al. Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD Series (Part 2). J Am Coll Cardiol 2018;72:2181–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Villari B, Vassalli G, Monrad ES, Chiariello M, Turina M, Hess OM. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation 1995;91:2353–8. [DOI] [PubMed] [Google Scholar]
- 105.López B, Querejeta R, Varo N et al. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation 2001;104:286–91. [DOI] [PubMed] [Google Scholar]
- 106.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000;102:1388–93. [DOI] [PubMed] [Google Scholar]
- 107.Izawa H, Murohara T, Nagata K et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation 2005;112:2940–5. [DOI] [PubMed] [Google Scholar]
- 108.López B, Querejeta R, González A, Sánchez E, Larman M, Díez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004;43:2028–35. [DOI] [PubMed] [Google Scholar]
- 109.López B, González A, Beaumont J, Querejeta R, Larman M, Díez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol 2007;50:859–67. [DOI] [PubMed] [Google Scholar]
- 110.López B, Querejeta R, González A, Beaumont J, Larman M, Díez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension 2009;53:236–42. [DOI] [PubMed] [Google Scholar]
- 111.Johnson KW, Shameer K, Glicksberg BS et al. Enabling precision cardiology through multiscale biology and systems medicine. JACC Basic Transl Sci 2017;2:311–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ravassa S, Trippel T, Bach D et al. Eur J Heart Fail 2018;20:1290–1299. [DOI] [PubMed] [Google Scholar]
- 113.Suematsu Y, Miura S, Goto M et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail 2016;18:386–93. [DOI] [PubMed] [Google Scholar]
- 114.Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail 2019;12:e005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solomon SD, McMurray JJV, Anand IS et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- 116.Habibi J, Aroor AR, Sowers JR et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol 2017;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2017;2:1025–1029. [DOI] [PubMed] [Google Scholar]
- 118.McMurray JJV, Solomon SD, Inzucchi SE et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–08. [DOI] [PubMed] [Google Scholar]
- 119.Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-beta. Cardiovasc Ther 2012;30:e30–40. [DOI] [PubMed] [Google Scholar]
- 120.Fang L, Murphy AJ, Dart AM. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front Pharmacol 2017;8:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park S, Nguyen NB, Pezhouman A, Ardehali R. Cardiac fibrosis: potential therapeutic targets. Transl Res 2019;209:121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gyongyosi M, Winkler J, Ramos I et al. Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail 2017;19:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]