Abstract
Alopecia areata (AA) is a highly prevalent autoimmune disease that attacks the hair follicle and leads to hair loss that can range from small patches to complete loss of scalp and body hair. Our previous linkage and genome-wide association studies (GWAS) generated strong evidence for etiological contributions from inherited genetic variants at different population frequencies, including both rare mutations and common polymorphisms. Additionally, we conducted gene expression (GE) studies on scalp sections of 96 patients to establish signatures of active disease. In this study, we performed an integrative analysis on these two datasets, to test the hypothesis that rare CNVs in AA patients could be leveraged to identify drivers of disease in our AA GE signatures. We analyzed copy number variants (CNVs) in a case-control cohort of 16,984 AA patients and controls independent of the case-control cohort of 96 research participants used in our GE study. Using an integrative computational analysis, we identified 14 genes whose expression levels were altered by CNVs in a consistent direction of effect, corresponding to gene expression changes in lesional skin of patients. Four of these genes were affected by CNVs in three or more unrelated AA patients, including ATG4B and SMARCA2, which are involved in autophagy and chromatin remodeling, respectively. Our findings identified new classes of genes with potential contributions to AA pathogenesis.
Keywords: Integrative genomics, alopecia areata, copy number variants, autophagy
Introduction
AA is a common autoimmune disorder with a prevalence of 2.1%.1 In AA, an aberrant interaction between the immune system and the hair follicle leads to non-scarring hair loss that generally begins in patches on the scalp but may progress to affect the entire scalp (alopecia totalis; AT) and body (alopecia universalis; AU). A genetic basis for the disease was first suggested by studies in families and twin pairs that demonstrated an increased risk of disease among family members.2 Our earlier genetic linkage studies in AA families provided definitive evidence for etiological contributions from rare variants, with the identification of several genomic regions harboring strong statistical evidence for co-segregation.3 However, these regions identified by linkage were too large to implicate specific genes in AA disease pathogenesis, and candidate genes were not forthcoming from these studies.
More recently, we conducted a genome-wide association study (GWAS) and a GWAS meta analysis, which identified common variants (i.e. single nucleotide polymorphisms; SNPs) that are associated with AA across 14 genomic regions, which much smaller than linkage intervals (on the order of kilobases rather than megabases). Many of the GWAS regions implicated individual genes or small clusters of functionally related genes, thus providing new and clinically relevant insight into the disease.4–6 For example, immune susceptibility loci included genes that are involved in regulatory T cell activation and proliferation (Cytotoxic T-lymphocyte-associated protein 4; CTLA4, Interleukin 21; IL21, Interleukin-2; IL2, Interleukin-2 receptor alpha chain; IL2RA, Suppressor of Cytokine Signaling 1; SOCS1), IFNγ-producing NKG2D-mediated cytotoxic T cell activity (UL16-Binding Protein 3/6; ULBP3/6, Interleukin 2; IL2), in addition to the human leukocyte antigen (HLA). Furthermore, we identified several genes that were expressed in the hair follicle, including genes involved in autophagy and pigmentation (Syntaxin17; STX17), oxidative stress (Peroxiredoxin 5; PRDX5), and apoptosis (Bcl-2-like protein 11; BCL2L11, also known as BIM). These findings prompted us to initiate functional immunological and pharmacological studies that demonstrated IFNγ-producing CD8+ NKG2D cytotoxic T cells are both necessary and sufficient to induce AA in a mouse model of the disease. Finally, we showed that targeting these cells with topical or systemic JAK inhibitors induces hair regrowth in patients with longstanding moderate-to-severe AA.7–11 Our GWAS were highly fruitful and represented an unusual example of GWAS candidate genes and functional studies that led to new targeted treatment approaches.12
Despite these successes, compelled by findings in other chronic diseases that have found phenotypic contributions from genetic variants across the entire spectrum of allele frequencies, here, we sought to return to the investigation of rare variants suggested by our linkage studies. Importantly, genes that have been implicated in chronic diseases by the presence of rare causal mutations have proven to be relevant to patients without such mutations. For example, the PCSK9 gene was first identified by linkage studies of familial hypercholestermia that identified rare causal mutations.13 While very few patients with elevated LDL have monogenic mutations in PCSK9, broader population relevance was established when GWAS identified common variants in and around PCSK9 that increase risk of elevated LDL, myocardial infarction and coronary artery disease.14 Importantly, clinical relevance was established when it was shown that targeting PCSK9 with a monoclonal antibody could lower blood LDL levels, offering a new treatment strategy for the 10–20% of patients who are statin-intolerant.15
Thus, we revisited our earlier strategy focused on rare variants, with an approach integrating gene expression studies to streamline the identification of causal genes. In parallel with our genetic studies, we previously performed extensive gene expression (GE) studies OF AA in an independent cohort of 96 unrelated individuals.16 This work identified a signature composed of 3445 genes differentially expressed between AA patients and healthy controls, and underscored contributions from the pathways identified in our genetic studies.6,16 While GE studies are the standard experimental approach for detecting effects of changes in transcriptional levels on disease, these studies tend to identify hundreds or thousands of genes that are differentially expressed between diseased and healthy tissue without providing information about causal order. In GE analysis, genes that are a primary cause of disease are not readily distinguishable from genes whose transcript levels are altered secondarily, as a consequence of disease. This distinction becomes critical when developing strategies for therapeutic targeting, which ideally should be directed towards genes that are causal rather than consequential.
While many observed transcriptional changes in tissues result from cellular responses to microenvironmental cues (e.g. developmental, homeostatic or pathogenic cellular signaling), GE changes can also result from underlying structural alterations in the genome that affect gene dosage. Copy number variants (CNVs) are genetic variants that involve the duplication or deletion of segments of the genome greater than one kilobase (kb) and represent an important source of variation in the human genome.17,18 CNVs, similar to single nucleotide variants, span the full range of allele frequencies, including de novo, rare, or common (f>1%).
Previous work has established that both rare and common CNVs can influence the development of disease. Rare or de novo CNVs have been studied within the context of rare congenital diseases and syndromes. The rare CNVs identified in these studies tend to be large (usually greater than 100,000 base pairs), and often disrupt multiple protein coding genes and regulatory elements. While CNVs are present in every human genome, burden analyses in cohorts of congenital disorders have shown that affected patients are more likely to harbor large gene- disrupting (genic) CNVs than unaffected individuals.19–22 Likewise, common CNVs have been identified in association studies of autoimmune diseases such as Crohn’s disease,23–25 systemic lupus erythematosus (SLE),26 psoriasis,27 rheumatoid arthritis (RA), type 1 diabetes,25,28 as well as AA.29
CNVs are likely to influence disease through transcriptional dosage effects. Gene transcript levels may be reduced or absent when a gene lies within a deletion CNV, or amplified within a duplication CNV.30–35 Thus, we postulate that a gene whose altered gene expression levels contribute to AA has the potential to be identified in both GE data (comparing diseased tissue to healthy tissue), as well as CNV analysis (comparing a cohort of unrelated patients and healthy controls). Furthermore, whereas GE studies are cross-sectional in nature, identifying transcriptional changes that occur both before and after disease onset, CNV studies, in contrast, identify genetic variants that are present before disease, and thus tend to be causal rather than consequential. Finally, we postulate that large CNVs exerting strong effects on gene expression levels will have a negative impact on health, and are therefore likely to be under purifying selection, remaining at low frequencies in the population.
The goal of this study was to identify large, rare CNVs that are present in the genomes of AA patients and absent from unaffected controls, and then integrate the CNV and GE data to identify genes that are potential drivers of AA pathogenesis. We identified large, rare gene-disrupting CNVs in a cohort of 758 AA cases that were not included in our GE studies, and compared these to CNVs found in 17,769 publicly available unrelated controls.36,37 We first used this data to evaluate the burden of CNVs between cases and controls and found that AA patients harbor more of these CNVs than healthy controls (p=0.002). We next integrated our CNV results with AA GE data and identified 14 genes impacted by large, rare CNVs in AA patients and whose transcript levels are altered in AA skin in a consistent direction (gain or loss of copy number and up- or down-regulated expression levels respectively). Four of the 14 genes were impacted by CNVs in 3 or more patients, including genes that have previously been implicated in hair follicle biology.
Materials and Methods
Subjects
AA patients were ascertained through the National Alopecia Areata Registry with approval from institutional review boards of the contributing sites and genotyped on the Illumina HumanHap 610-Quadv1 or HumanHap 550 chip for our previous GWAS.4 Controls consisted of individuals that were genotyped on HumanHap550, Illumina HumanHap 610-Quadv1, Illumina HumanHap 1M, Illumina HumanHap iM duo chips for 11 genome-wide association case-control or longitudinal studies unrelated to alopecia areata, as described previously.21,22,38,39 Ancestry-specific SNP genotypes called from the arrays were used in principal component analysis to determine genetic ancestry of each case and control. Only genetically European samples were used in our CNV analysis. Relatedness was also assessed with SNP genotypes to insure that cases and controls were unrelated.
CNV calling and quality control assessment
The same analytic pipeline was used for quality control and CNV calling in cases and controls. The CNV calls were generated by PennCNV (version 2011 June).40 To prepare the input files for PennCNV, the raw intensity data for the genotypes of AA patients were first normalized using Illumina’s Genome Studio software. To generate the raw CNV calls, we used the log R ratio (LRR), B allele frequency (BAF) automatically computed from the signal intensity files, and the standard hg18 “all” PennCNV hidden Markov model (hmm) and population frequency of B allele (pfb) files. High quality CNVs (confidence score ≥30; logR ratio SD ≤0.35) were called in 758 cases and 17,769 controls. We limited our analysis to CNVs on autosomes that a) fell within or spanned one or more genes; and b) were within a size range of 100,000 base pairs (bp) and 10Mb.41
CNV validation with quantitative-PCR
We performed quantitative-PCR (q-PCR) to validate the selected CNVs. Primers were designed to target the specific genes contained within the CNVs and q-PCR was performed using Power SYBR® Green Master Mix on ABI PRISM 7300 Real-Time PCR System (Applied Biosystems). All samples were run in triplicate, and the data was established using the relative standard curve method. The data was normalized against the reference gene, GAPDH, and the copy number was determined empirically by calculating the expression fold change relative to the normal control reference DNA, which does not carry the specific CNV. The relative copy number values ≥1.19 and ≤ 0.81 were representative of duplications and deletions, respectively.
CNV Integration with GE Data
AA gene expression signatures were previously identified by comparison of whole scalp skin tissue samples between patients and unrelated unaffected controls. Specifically, three different comparisons generated three signatures and included a comparison of controls to (1) all AA patients, (2) patients with mild disease (transient or patchy AA), or (3) patients with severe disease (universalis or totalis). For our analysis, we combined the three signatures to identify a set of 3658 probes differentially expressed in at least one comparison, representing 3445 genes.
CNV Burden Analysis
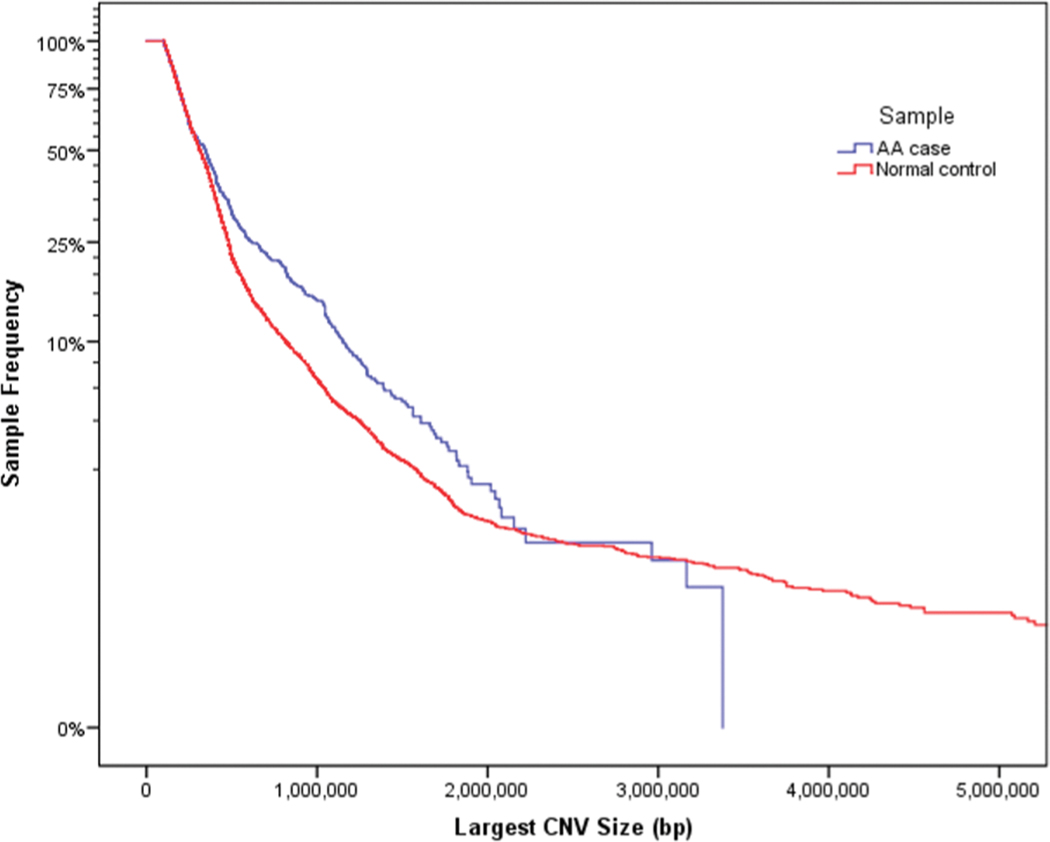
CNV burden analysis was performed by counting large (size greater than or equal to 100 kb), rare (frequency less than 0.1% in the control data set), autosomal CNVs intersecting or containing at least 1 exonic sequence. CNVs larger than 10 Mb were excluded from the burden analysis due to their relative infrequency and the limited sample size for cases. To examine the global CNV burden, the population frequency of the largest CNV per genome was compared in AA cases and normal controls (Fig 1). The population frequencies of the largest CNVs per genome were analyzed using a log-rank test, Wilcoxon test, and Tarone-Ware test (SPSS IBM v.24). P values of less than 0.005 were considered significant for the above analyses.
Fig 1. AA cases have an increased burden of CNVs.
Kaplan-Meier curve showing autosomal CNVs >100Kb and <10Mb (N=2,410) for 673 cases vs. 16,311 controls (p=0.03). Our data suggests that there may be a slight enrichment of CNVs greater than 500Kb in size among AA patients relative to controls. Estimates for CNVs greater than 1.5 Mb in cases may be imprecise due to the small number of observations in our sample.
Immunofluorescence Analysis
Snap frozen human hair follicle sections were post-fixed using 4% (wt/vol) paraformaldehyde fixation for 10 min at room temperature. Samples were washed twice in PBS for 5 minutes and permeabilized for 10 min using PBS with 0.2% Triton X-100. Samples were then washed again in PBS for 5 minutes. Blocking was performed for 20 minutes using 5% (wt/vol) Goat Serum and PBS with 0.1% Triton X-100. The following primary antibodies were used: rabbit anti-ATG4B (1:500; Invitrogen) and rabbit anti-SMARCA2 (1:100, Abcam). Primary antibodies were incubated in blocking solution at RT for 1 hour. Primary antibodies were washed off using two 15 minutes washes in PBS with 0.1% Triton X-100 and then three consecutive 5-minute PBS washes. Secondary antibody (Alexafluor 488 goat anti-rabbit 1:1000; Invitrogen) was applied for 30 minutes at room temperature. Samples were washed using two 15 minutes washes in PBS with 0.1% Triton X-100 and then one more PBS wash. Samples were then mounted using Fluoroshield with DAPI (Sigma) and slides were visualized on a Zeiss Exciter confocal microscope.
This work is approved by Columbia University IRBs AAAA8075 and AAAI0706.
Results
CNV Characterization
Of the 758 cases and 17,769 controls for which CNVs were called, we identified 673 cases and 16,311 controls that contained at least one CNV meeting our inclusion criteria, which included a low frequency in controls (f<0.1%) and a size range of 100kb–10Mb. Among the 673 AA cases, we identified 2888 genes affected by CNVs, 2506 of which were also affected by a CNV in at least one control. However, when we removed CNVs that were present in both cases and controls to identify CNVs only ever observed among our cases, we identified 382 genes that were uniquely impacted by CNVs in AA cases.
CNV validation with quantitative-PCR
We performed genomic qPCR to experimentally validate a sample of CNVs harbored by AA patients (S1 Table). We validated 47 (92.2%) of 51 CNVs subject to q-PCR. Two of the four CNVs that failed validation were less than 18,000 bp, and the relative lower confidence associated with calling such small CNVs likely generated the unreliable CNV calls.
CNV Burden Analysis
Previous studies of rare, large CNVs found that they tend to contribute to disease burden22. To evaluate if AA patients carry a greater number of CNVs than controls, we conducted a burden analysis by selecting the largest CNV in each individual and examining the distribution of CNVs in AA cases and controls. We observed an excess burden of rare, large, gene-disrupting CNVs in AA cases compared with controls (log-rank test, P = 7.8 × 10–7; Wilcoxon, P = 3.6 × 10–3; Tarone-Ware, P = 4.4 × 10–5). Our data suggests that AA patients tend to carry more CNVs within a discrete size range, specifically between 500Kb and 1.5Mb in size (P= 4.4 × 10–5, Tarone-Ware test) (Fig 1). We did not observe enrichment in AA patients for CNVs greater than 1.5 Mb
CNV Integration with Gene Expression Data
We identified 35 genes (out of 382 genes affected by CNVs in AA cases and never in controls) that were present in at least one of the previously published AA GE signatures (all AA, severe disease, mild disease). Of these 35 genes, only 14 had a consistent direction of effect within the CNV across all experiments (Table 1). Specifically, seven genes were down-regulated in the AA GE signature and were impacted by a CNV deletion (copy number state=1); and seven genes were up-regulated in the AA GE signature and were impacted by a CNV duplication (copy number state=3) (Table 1). The majority of these genes fell within a CNV observed only once in our sample of AA cases. Four of these genes are affected by CNVs in three or more AA patients. The cohort of AA patients used for CNV analysis is independent of the cohort of AA patients used for GE analysis.
Table 1.
Genes identified by CNV analysis as potential contributors to alopecia areata pathogenesis.
| CNV Patient Count | Gene Expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| region | gene | chr | Hg38.Start | Hg38.Stop | CNV deletion | CNV duplication | fold change | Signature | |
| decreased expression | 2q37.3 | BOK | 2 | 241,558,721 | 241,574,138 | 4 | −1.58 | AU | |
| ATG4B | 2 | 241,637,213 | 241,673,857 | 6 | −1.59 | AAP,AA,AU | |||
| 3q25.1 | TM4SF1 | 3 | 149,369,022 | 149,377,865 | 1 | −3.19 | AAP,AA,AU | ||
| 3q29 | NCBP2 | 3 | 196,935,402 | 196,942,597 | 1 | −1.65 | AU | ||
| 9p24.3 | SMARCA2 | 9 | 1,980,290 | 2,193,624 | 3 | −1.56 | AA,AU | ||
| 19q13.43 | ZNF814 | 19 | 57,848,731 | 57,889,074 | 4 | −1.86 | AA,AU | ||
| ZNF274 | 19 | 58,183,029 | 58,213,562 | 1 | −1.59 | AU | |||
| increased expression | 9q33.3 | RALGPS1 | 9 | 126,914,774 | 127,223,166 | 1 | 1.8 | AU | |
| 10p12.1 | YME1L1 | 10 | 27,110,112 | 27,155,266 | 1 | 2.34 | AA,AU | ||
| 19p13.3 | ZNF57 | 19 | 2,900,898 | 2,918,476 | 1 | 1.55 | AU | ||
| 19p13.3 | CELF5 | 19 | 3,224,703 | 3,297,076 | 1 | 1.69 | AU | ||
| 1p36.22 | CTNNBIP1 | 1 | 9,848,276 | 9,910,336 | 1 | 1.77 | AU | ||
| 2q11.1 | FAHD2A | 2 | 95,402,721 | 95,416,616 | 1 | 2.06 | AA,AU | ||
| 2q37.1 | SP140 | 2 | 230,203,110 | 230,313,215 | 1 | 1.96 | AA,AU | ||
Notably, deletions in three genomic regions (affecting a total of four genes) were identified in multiple AA patients, including ATG4B/BOK (chromosome 2q37.3), SMARCA2 (chromosome 9p24.3), and ZNF814 (chromosome 19q13.43). ATG4B and BOK are located in the same genomic region on chromosome 2 (Fig 2). One CNV spans both genes in four patients. Two additional patients have a CNV that spans ATG4B but does not affect BOK. On chromosome 9, SMARCA2 is the only gene affected by CNVs in that region of the genome. On chromosome 19, one patient carries a deletion affecting both ZNF274 and ZNF814, and three patients have a CNV spanning ZNF814, but not ZNF274.
Fig 2. Genes impacted by CNVs in multiple AA patients.

(A) Genomic display of CNV deletions observed in AA patients are shown in red across the ATG4B genomic region. While all of the CNVs span five genes, only ATG4B is differentially expressed in AA lesional skin.16 (B) Genomic display of CNV deletions observed in AA patients are shown as red bars across the SMARCA2 genomic region. (C) ATG4B immunofluorescence staining of a healthy human anagen hair follicle reveals peri-nuclear expression in hair follicle keratinocytes. The dermal papilla shows positive yet diffuse ATG4B staining, with stronger staining in the hair matrix that sporadically tapers off in the hair shaft cortex (HSCx). (D) SMARCA2 staining reveals nuclear protein localization in the human hair follicle. Expression appears throughout the hair follicle in the outer root sheath (ORS), matrix, and dermal papilla with weaker expression in the HSCx and dermal sheath.
Protein expression in hair follicle
Immunofluorescence staining of ATG4B and SMARCA2 in healthy human anagen hair follicles revealed distinct expression patterns. ATG4B (Fig 2A) showed peri-nuclear expression, with stronger staining in the hair matrix that tapers off in the hair shaft cortex (HSCx). The dermal papilla showed positive yet diffuse ATG4B staining. SMARCA2 (Fig 2B) showed widespread nuclear protein localization throughout the hair follicle in the outer root sheath (ORS), matrix, and dermal papilla, with an overall weaker expression in the dermal sheath and hair shaft cortex (HSCx).
Discussion
The genotyping data generated for our GWAS and access to a large sample of control genotypes data provided an opportunity to investigate an etiological role for CNVs in AA. Our previous linkage studies in AA families provided evidence that rare genetic variation contributes disease pathogenesis, thus providing a rationale to pursue the investigation of rare CNVs. Furthermore, recent sequencing efforts in common diseases have shown that the genetic architecture of common diseases includes variants across the whole spectrum of population frequencies, including rare variants42–45, and that rare variants can provide clinically relevant mechanistic insight that can be extrapolated to a majority of patients.13,15,46–50
While our cohort size could limit the power to detect rare variants increasing Type I error, our integrative analytic strategy mitigates the chance of false positives. Specifically, we postulated that if a gene influenced AA through altered transcript levels, it would be identified in both CNV data and GE data generated from independent cohorts. We further increased our experimental stringency by restricting our analysis to CNVs observed only in our AA cases, and never in our population controls. This integrative approach enabled us to overcome the main limitation of GE approaches, which are cross-sectional in nature and fail to distinguish causes of disease from consequences.
Our burden analysis indicated that AA patients are more likely to carry large rare CNVs (ranging from 100kb–10Mb) than population controls (Figure 1). However, it remains unknown if or how many AA patients in our cohort have congenital disorders that include AA as a symptom or co-presenting disorder. For example, trisomy 21 is known it increase the risk of AA, and we identified three patients with genetic evidence of trisomy 21.51 Thus it remains unknown if the increased burden that we detected is generalizable to all AA patients. Among AA patients, we did not detect any CNVs greater than 1.5 Mb in size. Larger CNVs tend to disrupt more genes and therefore are more likely to have severe health consequences and remain at low population frequencies. Given the relatively small number of cases included in our study, we may have limited power to detect such rare events of CNVs >1.5 Mb.
While our analysis identified 14 genes residing in 12 genomic regions, only 4 genes were affected by CNVs found in more than 1 patient, thereby increasing our confidence in potentially casual disease effects. While none of these genes have previously been implicated in AA by our GWAS or linkage studies, two of them (ATG4B and SMARCA2) have been previously implicated in hair follicle biology.
ATG4B encodes a cysteine protease and is a core autophagy protein and is located on chromosome 2.52,53 Autophagy is an intracellular mechanism for capturing cytosolic proteins or organelles for transport to the lysosome for degradation or to the plasma membrane for cellular export. ATG4B has been implicated in a wide range of diseases, including cancer, pathogen clearance deficiencies, and other autoimmune diseases.54,55 Atg4b-null mice present with autophagy deficits, differential expression of proinflammatory cytokines implicated in bacterial innate immune responses, and an increased risk for induced colitis.56–58 This mouse model mimics multiple symptoms present in Crohn’s disease and ulcerative colitis, and has been proposed as a novel autophagy-deficient model for testing new therapeutics in IBD and colitis.58 In humans, Crohn’s disease GWAS identified the ATG4B locus as a susceptibility gene (rs35320439, P=9.89×10−10, OR=1.09),59,60 and reduced expression of ATG4B was found in the inflamed colon region of IBD patients.58 ATG4B has also been implicated in prostate cancer by GWAS (rs3771570, P=5 ×10−9, OR=1.12),61 and in chronic myeloid leukemia (CML) where it has been proposed as a potential biomarker for predicting therapeutic response in treatment-naïve CML stem/progenitor cells.62
Several lines of evidence implicate ATG4B in hair follicle biology. In a growth hormone-deficient transgenic rat model for hair follicle regeneration, ATG4B was shown to be markedly upregulated after depilation of telogen-phase hairs.63 Within melanocytes, ATG4B plays a critical role in melanosome trafficking, and earlier studies in primary melanocytes demonstrated that induction of autophagy can increase pigmentation while autophagy inhibition reduces pigmentation64. Recently, ATG4B knockdown was found to disrupt melanosome trafficking within the cell, resulting in the perinuclear accumulation of the melanosomal organelles64. ATG4B knockdown also reduced the co-localization of pre-melanosome protein (PMEL) with LC3 (a core autophagy protein).64 Our staining of human HF with ATG4B revealed peri-nuclear expression with strong staining in the hair matrix.
Taken together, the genetic evidence in other autoimmune diseases, along with functional evidence in hair follicle and melanocyte biology, suggest a role for ATG4B in AA disease pathogenesis. Of note, autophagy has been implicated in AA by our previous GWAS and meta-analysis, specifically by associations with syntaxin17 (STX17) (p=1.09×10−5) and BCL2L11, also known as BIM (p=1.5×−8), both which have known roles in autophagy pathways, as well as PMEL (p= 4.4×−9).4,5,65 The precise mechanisms by which autophagy contributes to AA pathogenesis have yet to be fully elucidated.
In four of the six AA patients with an ATG4B CNV deletion, the CNV also encompasses a neighboring gene, BOK, which is located approximately 100 kb away. Similar to ATG4B, BOK is also downregulated in lesional skin of AA patients. BOK is a pro-apoptotic member of the BCL2 family that also induces autophagy.66,67 Therefore, it is possible that in AA patients the tandem deletion of ATG4B and BOK could result in an additive effect in which multiple autophagy genes are perturbed, causing a more pronounced deficit in the pathway. Future studies are needed to further refine contributions from BOK.
Our analysis also identified SMARCA2 (also known as Brahma, BRM)68 located on chromosome 9 as a potential contributor to AA pathogenesis. This gene encodes a transcriptional activator protein that functions as a subunit of the Switch/Sucrose non-fermentable (SWI/SNF) complex.69,70 The SWI/SNF complex is crucial in regulating cellular growth, differentiation and division through chromatin remodeling.71 SMARCA2 and other SWI/SNF subunits have essential roles in tumor suppression and are implicated in many types of cancer when dysregulated.72–74 Furthermore, SMARCA2 was proposed as a potential therapeutic candidate to target specific cancers with Brahma-related gene-1 (BRG1) mutations, such as non-small-cell lung carcinoma, Burkitt’s Lymphoma, and childhood medulloblastoma.74,75
SMARCA2 mutations were previously associated with diseases characterized by hair phenotypes.76–78 For example, SMARCA2 mutations have been identified in Nicolaides-Baraitser Syndrome (NBS), which manifests with hypotrichosis 77,78. Substantial phenotypic overlap exists with NBS and another rare genetic disease, Coffin-Siris syndrome (CSS), a condition that exhibits hypotrichosis, as well as facial hypertrichosis.76,77 SMARCA2 sequencing in 44 NBS patients revealed causative missense mutations in ~80% of patients, and functional analysis suggested a dominant-negative effective of these mutations.78,79 In a CNV analysis, 4 CSS patients were identified to have a chromosome 9p duplication encompassing 43 protein-coding genes, one of which was SMARCA2.76 It was postulated that the increase in SMARCA2 gene dosage was contributing to the CSS phenotype.76 The implication of SMARCA2 in cancer and hair-related conditions provide a rationale for the plausible involvement of SMARCA2 in AA pathogenesis. Our staining showed expression of SMARCA2 throughout the human hair follicle in the dermal papilla, outer root sheath (ORS), and matrix.
Zinc Finger Protein 814 (ZNF814) on chromosome 19 has not previously been studied within the context of skin or hair biology. It has been reported to be differentially expressed in parous vs. nulliparous breast tissue, and may play a role in pancreatic cancer and squamous cell carcinoma.80–85 In the 4 AA patients with a CNV deletion of ZNF814, the neighboring gene, ZNF274, was also deleted in 1 patient. ZNF274 is also a zinc finger protein that acts as a transcriptional factor and complexes with chromatin-remodeler ATRX (Alpha Thalassemia/Mental Retardation Syndrome X-linked), a SWI/SNF-family protein.86 Depletion of ZNF274 results in cell cycle dysfunction, DNA damage, and decreased H3K9 trimethylation.86
Our work demonstrates that rare CNVs can be leveraged to identify and prioritize drivers of disease within GE datasets in patients with AA. These findings provide further evidence for etiological contributions from rare variants in AA in support of our previous linkage analysis. Our integrative analysis of CNV and GE data uncovered evidence that implicated at least two new genes in AA (ATG4B and SMARCA2), suggesting a role for dysregulated autophagy in AA pathogenesis.
Supplementary Material
S1 Table. Report of all CNVs found in AA patients and not in controls.
Acknowledgements
We thank the patients and their family members who participated in this study. We are grateful to David Norris, Maria Hordinsky, Madeleine Duvic, Julian Mackay-Wiggan, and Vera Price. This work was supported in part by NIH/NIAMS P50AR070588 Alopecia Areata Center for Research Translation (AACORT), NIH/NIAMS R01AR065963 Functional Genomics of Alopecia Areata, and NIH/NIAMS U01AR067173 Developing an Alopecia Areata Disease Activity Index (ALADIN), R01AR52579 Genetic Analysis of Alopecia Areata, and R01AR056016 Genome Wide Association Studies in Alopecia Areata. We are grateful for the support of the National Alopecia Areata Foundation for funding initial studies. The patient cohort was collected and maintained by the National Alopecia Areata Registry (N01AR62279).
Footnotes
Conflicts of Interest
We have no conflicts of interest to declare
References
- 1.Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. The Journal of investigative dermatology. 2014;134(4):1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M. Alopecia areata and cytomegalovirus infection in twins: genes versus environment? Journal of the American Academy of Dermatology. 1998;38(3):418–425. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Mir A, Zlotogorski A, Gordon D, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. American journal of human genetics. 2007;80(2):316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petukhova L, Christiano AM. Functional Interpretation of Genome-Wide Association Study Evidence in Alopecia Areata. The Journal of investigative dermatology. 2016;136(1):314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nature medicine. 2014;20(9):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Z, Xing L, Cerise J, et al. CXCR3 Blockade Inhibits T Cell Migration into the Skin and Prevents Development of Alopecia Areata. J Immunol. 2016;197(4):1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Experimental dermatology. 2016;25(8):642–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins FS. Reengineering translational science: the time is right. Sci Transl Med. 2011;3(90):90cm17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nature genetics. 2003;34(2):154–156. [DOI] [PubMed] [Google Scholar]
- 14.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12(8):581–594. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–2506. [DOI] [PubMed] [Google Scholar]
- 16.Jabbari A, Cerise JE, Chen JC, et al. Molecular signatures define alopecia areata subtypes and transcriptional biomarkers. EBioMedicine. 2016;7:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman JL, Perry GH, Feuk L, et al. Copy number variation: new insights in genome diversity. Genome Res. 2006;16(8):949–961. [DOI] [PubMed] [Google Scholar]
- 18.Khaja R, Zhang J, MacDonald JR, et al. Genome assembly comparison identifies structural variants in the human genome. Nature genetics. 2006;38(12):1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper GM, Coe BP, Girirajan S, et al. A copy number variation morbidity map of developmental delay. Nature genetics. 2011;43(9):838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenway SC, Pereira AC, Lin JC, et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nature genetics. 2009;41(8):931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanna-Cherchi S, Kiryluk K, Burgess KE, et al. Copy-number disorders are a common cause of congenital kidney malformations. American journal of human genetics. 2012;91(6):987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbitsky M, Sanna-Cherchi S, Fasel DA, et al. Genomic imbalances in pediatric patients with chronic kidney disease. The Journal of clinical investigation. 2015;125(5):2171–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fellermann K, Stange DE, Schaeffeler E, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. American journal of human genetics. 2006;79(3):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarroll SA, Huett A, Kuballa P, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nature genetics. 2008;40(9):1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craddock N, Hurles ME, Cardin N, et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464(7289):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Chung EK, Wu YL, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. American journal of human genetics. 2007;80(6):1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollox EJ, Huffmeier U, Zeeuwen PL, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nature genetics. 2008;40(1):23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinney C, Merriman ME, Chapman PT, et al. Evidence for an influence of chemokine ligand 3-like 1 (CCL3L1) gene copy number on susceptibility to rheumatoid arthritis. Annals of the rheumatic diseases. 2008;67(3):409–413. [DOI] [PubMed] [Google Scholar]
- 29.Fischer J, Degenhardt F, Hofmann A, et al. Genomewide analysis of copy number variants in alopecia areata in a Central European cohort reveals association with MCHR2. Exp Dermatol. 2016. [DOI] [PubMed] [Google Scholar]
- 30.Wei R, Zhao M, Zheng CH, Zhao M, Xia J. Concordance between somatic copy number loss and down-regulated expression: A pan-cancer study of cancer predisposition genes. Scientific reports. 2016;6:37358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster-Bockler B, Conrad D, Bateman A. Dosage sensitivity shapes the evolution of copy-number varied regions. PloS one. 2010;5(3):e9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stranger BE, Forrest MS, Dunning M, et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science (New York, NY). 2007;315(5813):848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack JR, Sorlie T, Perou CM, et al. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12963–12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henrichsen CN, Chaignat E, Reymond A. Copy number variants, diseases and gene expression. Human molecular genetics. 2009;18(R1):R1–8. [DOI] [PubMed] [Google Scholar]
- 35.Cahan P, Li Y, Izumi M, Graubert TA. The impact of copy number variation on local gene expression in mouse hematopoietic stem and progenitor cells. Nature genetics. 2009;41(4):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mailman MD, Feolo M, Jin Y, et al. The NCBI dbGaP database of genotypes and phenotypes. Nature genetics. 2007;39(10):1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tryka KA, Hao L, Sturcke A, et al. NCBI’s Database of Genotypes and Phenotypes: dbGaP. Nucleic acids research. 2014;42(Database issue): D975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Rivera E, Liu YP, Verbitsky M, et al. Genetic Drivers of Kidney Defects in the DiGeorge Syndrome. The New England journal of medicine. 2017;376(8):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westland R, Verbitsky M, Vukojevic K, et al. Copy number variation analysis identifies novel CAKUT candidate genes in children with a solitary functioning kidney. Kidney Int. 2015;88(6):1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Li M, Hadley D, et al. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome research. 2007;17(11):1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itsara A, Cooper GM, Baker C, et al. Population analysis of large copy number variants and hotspots of human genetic disease. American journal of human genetics. 2009;84(2):148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Consortium UK, Walter K, Min JL, et al. The UK10K project identifies rare variants in health and disease. Nature. 2015;526(7571):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Kraja AT, Smith JA, et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nature genetics. 2016;48(10):1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature genetics. 2016;48(2):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair DR, Lyttle CS, Mortensen JM, et al. A nondegenerate code of deleterious variants in Mendelian loci contributes to complex disease risk. Cell. 2013;155(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell. 2017;169(7):1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund MK, Burch KS, Shi H, et al. Phenotype-Specific Enrichment of Mendelian Disorder Genes near GWAS Regions across 62 Complex Traits. American journal of human genetics. 2018;103(4):535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupski JR, Belmont JW, Boerwinkle E, Gibbs RA. Clan genomics and the complex architecture of human disease. Cell. 2011;147(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timpson NJ, Greenwood CMT, Soranzo N, Lawson DJ, Richards JB. Genetic architecture: the shape of the genetic contribution to human traits and disease. Nat Rev Genet. 2018;19(2):110–124. [DOI] [PubMed] [Google Scholar]
- 51.Dominguez-Cruz J, Delgado B. Dermatological Manifestations of Down Syndrome. In: Genetics and Etiology of Down Syndrome. IntechOpen; 2011. [Google Scholar]
- 52.Satoo K, Noda NN, Kumeta H, et al. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28(9):1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z, Wilkie-Grantham RP, Yanagi T, Shu CW, Matsuzawa S, Reed JC. ATG4B (Autophagin-1) phosphorylation modulates autophagy. J Biol Chem. 2015;290(44):26549–26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meijer AJ, Codogno P. Autophagy: regulation and role in disease. Crit Rev Clin Lab Sci. 2009;46(4):210–240. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Goronzy JJ, Weyand CM. Autophagy in autoimmune disease. J Mol Med (Berl). 2015;93(7):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marino G, Fernandez AF, Cabrera S, et al. Autophagy is essential for mouse sense of balance. J Clin Invest. 2010;120(7):2331–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Read R, Savelieva K, Baker K, Hansen G, Vogel P. Histopathological and neurological features of Atg4b knockout mice. Vet Pathol. 2011;48(2):486–494. [DOI] [PubMed] [Google Scholar]
- 58.Cabrera S, Fernandez AF, Marino G, et al. ATG4B/autophagin-1 regulates intestinal homeostasis and protects mice from experimental colitis. Autophagy. 2013;9(8):1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prescott NJ, Lehne B, Stone K, et al. Pooled sequencing of 531 genes in inflammatory bowel disease identifies an associated rare variant in BTNL2 and implicates other immune related genes. PLoS Genet. 2015;11(2):e1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eeles RA, Olama AA, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–391, 391e381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothe K, Lin H, Lin KB, et al. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood. 2014;123(23):3622–3634. [DOI] [PubMed] [Google Scholar]
- 63.Umeda-Ikawa A, Shimokawa I, Doi K. Time-course expression profiles of hair cycle-associated genes in male mini rats after depilation of telogen-phase hairs. Int J Mol Sci. 2009;10(5):1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramkumar A, Murthy D, Raja DA, et al. Classical autophagy proteins LC3B and ATG4B facilitate melanosome movement on cytoskeletal tracks. Autophagy. 2017;13(8):1331–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Einsele-Scholz S, Malmsheimer S, Bertram K, et al. Bok is a genuine multi-BH-domain protein that triggers apoptosis in the absence of Bax and Bak. J Cell Sci. 2016;129(11):2213–2223. [DOI] [PubMed] [Google Scholar]
- 67.Kalkat M, Garcia J, Ebrahimi J, et al. Placental autophagy regulation by the BOK-MCL1 rheostat. Autophagy. 2013;9(12):2140–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, Wang F, Du C, et al. BRM/SMARCA2 promotes the proliferation and chemoresistance of pancreatic cancer cells by targeting JAK2/STAT3 signaling. Cancer Lett. 2017;402:213–224. [DOI] [PubMed] [Google Scholar]
- 69.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993;12(11):4279–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruijtenberg S, van den Heuvel S. G1/S Inhibitors and the SWI/SNF Complex Control Cell-Cycle Exit during Muscle Differentiation. Cell. 2015;162(2):300–313. [DOI] [PubMed] [Google Scholar]
- 71.Roberts CW. The SWI/SNF complex--chromatin and cancer. Nat Rev Cancer. 2004;4(2):133–142. [DOI] [PubMed] [Google Scholar]
- 72.Kadoch C, Hargreaves DC, Hodges C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013;8(1):e55119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson BG, Helming KC, Wang X, et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol Cell Biol. 2014;34(6):1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffman GR, Rahal R, Buxton F, et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc Natl Acad Sci U S A. 2014;111(8):3128–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyake N, Abdel-Salam G, Yamagata T, et al. Clinical features of SMARCA2 duplication overlap with Coffin-Siris syndrome. Am J Med Genet A. 2016;170(10):2662–2670. [DOI] [PubMed] [Google Scholar]
- 77.Bramswig NC, Ludecke HJ, Alanay Y, et al. Exome sequencing unravels unexpected differential diagnoses in individuals with the tentative diagnosis of Coffin-Siris and Nicolaides-Baraitser syndromes. Hum Genet. 2015;134(6):553–568. [DOI] [PubMed] [Google Scholar]
- 78.Van Houdt JK, Nowakowska BA, Sousa SB, et al. Heterozygous missense mutations in SMARCA2 cause Nicolaides-Baraitser syndrome. Nat Genet. 2012;44(4):445–449, S441. [DOI] [PubMed] [Google Scholar]
- 79.Sousa SB. Phenotype and genotype in Nicolaides-Baraitser syndrome. Am J Med Genet C Semin Med Genet. 2014;166C(3):302–314. [DOI] [PubMed] [Google Scholar]
- 80.Karasaki T, Nagayama K, Kawashima M, et al. Identification of Individual Cancer-Specific Somatic Mutations for Neoantigen-Based Immunotherapy of Lung Cancer. J Thorac Oncol. 2016;11(3):324–333. [DOI] [PubMed] [Google Scholar]
- 81.Lemire M, Zaidi SH, Zanke BW, Gallinger S, Hudson TJ, Cleary SP. The effect of 5-fluorouracil/leucovorin chemotherapy on CpG methylation, or the confounding role of leukocyte heterogeneity: An illustration. Genomics. 2015;106(6):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peri S, de Cicco RL, Santucci-Pereira J, et al. Defining the genomic signature of the parous breast. BMC Med Genomics. 2012;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stirzaker C, Song JZ, Ng W, et al. Methyl-CpG-binding protein MBD2 plays a key role in maintenance and spread of DNA methylation at CpG islands and shores in cancer. Oncogene. 2017;36(10):1328–1338. [DOI] [PubMed] [Google Scholar]
- 84.Davidson K, Livingstone S, McArthur K, Dickson L, Gumley A. An integrative complexity analysis of cognitive behaviour therapy sessions for borderline personality disorder. Psychol Psychother. 2007;80(Pt 4):513–523. [DOI] [PubMed] [Google Scholar]
- 85.Meszaros B, Zeke A, Remenyi A, Simon I, Dosztanyi Z. Systematic analysis of somatic mutations driving cancer: uncovering functional protein regions in disease development. Biol Direct. 2016;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valle-Garcia D, Qadeer ZA, McHugh DS, et al. ATRX binds to atypical chromatin domains at the 3’ exons of zinc finger genes to preserve H3K9me3 enrichment. Epigenetics. 2016;11(6):398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Table. Report of all CNVs found in AA patients and not in controls.