Abstract
OBJECTIVES:
Linaclotide and plecanatide are guanylate cyclase-C (GCC) agonists for the treatment of chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). Our objective is to evaluate the efficacy and tolerability of GCC agonists based on data from multiple randomized controlled trials (RCTs).
METHODS:
We searched PubMED, EMBASE, Cochrane databases, clinicaltrials.gov, major conference abstracts, Food and Drug Administration (FDA) websites, and United States Securities and Exchange Commission filings of drug sponsors to identify RCTs of CIC or IBS-C patients. We assessed efficacy based on FDA-approved composite responder endpoints, diarrhea as an adverse event, and study withdrawal owing to diarrhea for each therapy. Trial results were pooled using DerSimonian and Laird random effects model of meta-analysis and exact logistic regression when appropriate with 95% confidence intervals. Meta-regression was performed to compare outcomes between therapies adjusting for placebo event rate.
RESULTS:
Eight linaclotide trials (five CIC; three IBS-C) and seven plecanatide trials (four CIC; three IBS-C) evaluating 10,369 patients met inclusion criteria. FDA publications documented that different definitions for diarrhea were used in linaclotide vs. plecanatide trials. Both drugs were efficacious in treating CIC (linaclotide 72 μg (Odds ratio (OR)=3.11, 95% CI 1.81–5.34); linaclotide 145 μg (OR=3.25, 2.15–4.91); plecanatide 3 mg (OR=1.99, 1.57–2.51)) and IBS-C (linaclotide 290 μg (OR=2.43, 1.48–3.98); plecanatide 3 mg (OR=1.87, 1.47–2.38); plecanatide 6 mg (OR=1.92, 1.48–2.48)). Diarrhea occurred in excess of placebo in treating CIC (linaclotide 72 μg (OR=3.07, 1.97–4.77); linaclotide 145 μg (OR=3.70, 2.69–5.10); plecanatide 3 mg (OR=3.86, 1.83–8.12)) and IBS-C (linaclotide 290 μg (OR=8.02, 5.20–12.37); plecanatide 3 mg (OR=5.55, 1.62–19.00); plecanatide 6 mg (OR=4.13, 1.57–10.83)). Based on meta-regression, there were no statistically significant differences between therapies in odds ratios for efficacy, diarrhea, or diarrhea-related study withdrawals.
CONCLUSIONS:
Both linaclotide and plecanatide demonstrate similar efficacy and tolerability in treating IBS-C and CIC. No differences in odds of diarrhea were seen between linaclotide and plecanatide.
INTRODUCTION
Irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC) are common conditions associated with a significant detriment to quality of life (1–4). Two therapies, linaclotide and plecanatide, target guanylate cyclase-C (GCC) receptors on the lumen of the intestinal epithelium and have been investigated for the treatment of IBS-C and CIC. Linaclotide was approved as the first GCC agonist for the treatment of IBS-C (290 μg daily) and CIC (145 μg daily) by the Food and Drug Administration (FDA) in 2012. In 2017, the FDA-approved a 72 μcg dose of linaclotide for treatment of CIC. Also, in 2017, plecanatide was the second GCC agonist approved for the treatment of CIC (3 mg daily) and the supplemental new drug application was recently accepted for IBS-C (3 mg or 6 mg daily).
Activation of GCC receptors leads to an increase in cyclic guanosine-3’, 5’-monophosphate in both the intracellular and extracellular space. The increase in intracellular cyclic guanosine-3’, 5’-monophosphate activates the cystic fibrosis transmembrane conductance regulator, enhancing the intestinal secretion of chloride and bicarbonate and leading to increased gastrointestinal transit rates (5,6). Animal data indicate that increases in extracellular cyclic guanosine-3’, 5’-monophosphate modulate abdominal pain (7,8). Per prescribing information, “in an animal model of visceral pain, linaclotide reduced abdominal muscle contraction and decreased the activity of pain-sensing nerves by increasing extracellular cyclic guanosine-3’, 5’-monophosphate” (9). Also, per prescribing information, plecanatide “reduced abdominal muscle contractions” in an animal model of visceral pain (10).
Linaclotide binds to GCC receptors in a pH-independent manner in the acidic as well as neutral or basic portions of the small intestine and colon (5), and ~3–5% of active peptide is recovered in the stool. These data suggest that linaclotide may be active throughout the small intestine and colon. Binding of plecanatide to GCC receptors is pH-dependent with increased activity in the acidic portion of the proximal small intestine. It is unclear if any intact plecanatide could be recovered from stool as no excretion studies have been published. It is unclear if these differences may impact the efficacy or tolerability of these agents.
Diarrhea is a common side-effect of this drug class (11) and is attributed to excessive intestinal secretion from activation of GCC receptors. It has been hypothesized that the pH-sensitive binding of plecanatide to GCC receptors in the proximal small intestine may reduce diarrhea (12–14) and numeric rates of plecanatide-associated diarrhea are lower than linaclotide in randomized controlled trials (RCTs). Although limited clinical trial data about plecanatide have been published, recent FDA publications document that Phase III RCTs of plecanatide for CIC used a more stringent definition for diarrhea compared with similar linaclotide trials (15,16). This could account for the numeric differences in diarrhea rates. No prior meta-analysis has collected RCT data for the GCC agonist class of drugs nor used odds ratios to assess efficacy and tolerability compared with controls. Therefore, the aim of this meta-analysis is to evaluate efficacy, frequency of diarrhea as an adverse event, and frequency of study withdrawal owing to diarrhea for linaclotide and plecanatide.
METHODS
Literature search and trial eligibility
This systematic review was conducted and reported in accordance with the PRISMA statement. We searched the literature using PubMED, EMBASE, Cochrane Central Register of Controlled Trials, and clinicaltrials.gov databases (December 28, 2016), as well as abstract books from DDW and ACG meetings (2005–2016). Study inclusion criteria were: (a) double-blind RCTs; (b) study patients defined as having IBS-C or CIC based on modified ROME II or ROME III criteria; (c) ≥2-week duration; (d) for efficacy analysis, comparison of linaclotide or plecanatide vs. placebo using the FDA-approved composite responder endpoint; (e) report frequency of diarrhea as an adverse event and frequency of withdrawal from study due to diarrhea; and (f) English language.
Our PubMED search string was as follows: (“linaclotide” OR “MD-1100” OR “MD 1100” OR “MD1100” OR “plecanatide” OR “SP-304” OR “SP 304” OR “SP304” OR “guanylate cyclase”) AND (“Constipation”[Mesh] OR “Irritable Bowel Syndrome”[Mesh] OR “irritable colon” OR “IBS”). Our EMBASE search string was: “linaclotide”/exp OR “linaclotide” OR “md 1100”/exp OR “md 1100” OR “md1100”/exp OR “md1100” OR “plecanatide”/exp OR “plecanatide” OR “sp 304”/exp OR “sp 304” OR “sp304”/exp OR “sp304” OR “guanylate cyclase”/exp OR “guanylate cyclase” AND (“constipation”/exp OR “constipation” OR “irritable bowel syndrome”/exp OR “irritable bowel syndrome” OR “irritable colon”/exp OR “irritable colon”). We searched clinicaltrials.gov using search terms “linaclotide” and “plecanatide”.
When we identified clinical trials not yet published in full manuscript form in peer-reviewed literature, we utilized data published in abstract form and searched relevant drug sponsor websites and EDGAR (published by the United States Securities and Exchange Commission) for 8-K reports from the relevant drug sponsor to assess trial eligibility and to extract relevant data. Data derived from EDGAR were extrapolated from reported percentages of relevant outcomes when raw event numbers were not available. All data were derived from public sources. Study sponsors (Ironwood Pharmaceuticals, Inc., and Synergy Pharmaceuticals, Inc.) reviewed raw event numbers, provided corrections as needed and verified the remaining data. The literature search, application of study eligibility criteria, and data extraction were performed independently by authors ES and PS. Discrepancies in trial eligibility or data extraction were resolved by consensus among authors.
Data extraction
A spreadsheet was created (Microsoft Excel, Microsoft, Redmond, WA, USA) regarding clinical trial phase, country of primary study site, corresponding level of care (primary, secondary, or tertiary), number of study sites, definition of IBS-C or CIC, inclusion and exclusion criteria, baseline patient characteristics (mean age, sex, abdominal pain/discomfort, and frequency of bowel movements), and dosing protocol (dose, frequency, route of administration, duration).
The Cochrane risk of bias tool was used to evaluate the quality of each study. Each eligible trial was evaluated for randomization protocol, methods of blinding and concealment, attrition from study and dataset completeness, and presence of selective reporting (17).
Study endpoints for meta-analysis
Our co-primary endpoints were therapeutic response based on FDA-approved composite responder endpoints and frequency of diarrhea as an adverse event. For IBS-C trials, the FDA-approved composite responder endpoint is decrease in weekly average of worst abdominal pain in past 24 h of ≥30% with concurrent increase in CSBM (complete spontaneous bowel movements) ≥1 per week for at least 50% of the weeks in a 12-week trial of therapy. (Note: For linaclotide IBS-C trials, the FDA-approved composite responder endpoint was not the original primary endpoint because the FDA had not approved a composite responder endpoint at onset of trial. Data for this endpoint were derived from post hoc analysis). The FDA-approved composite responder endpoint for CIC is defined as improvement in complete spontaneous bowel movements (CSBM) ≥1 per week with ≥3 CSBM per week for at least 75% of weeks in a 12-week trial of therapy. (Note: for plecanatide CIC trials, the new FDA-approved composite responder endpoint or sustained responder endpoint was used. This also requires positive response in three of the last 4 weeks of trial).
We also extracted from each study, by study arm, the number of participants with diarrhea. The definition of diarrhea as an adverse event was more stringent in trials assessing plecanatide vs. trials assessing linaclotide. For Phase III plecanatide trials in CIC, “since an increase in the number of BMs from baseline was an expected pharmacodynamic effect of plecanatide and would be coded as diarrhea, sites were instructed to only record an AE of diarrhea if the patient reports that it was bothersome [e.g., watery/mushy stool (BSFS score of 6 or 7) with a sense of urgency, etc.] or if the event required treatment or hospitalization” (15). For Phase III linaclotide trials in CIC and IBS-C, “per protocol, patients were given the opportunity to report adverse events spontaneously. In addition, at each visit following the first visit, patients were questioned (in a non-leading manner) to volunteer information regarding any AEs that had occurred since the previous visit. Examples of questions included, “Have you had any unusual signs or symptoms since your last visit?” All verbatim terms were collected on patient’s eCRF” (16).
As a secondary endpoint, we assessed study withdrawal owing to diarrhea, which may be a better reflection of clinically important diarrhea compared with prevalence of diarrhea at any time during a research trial. Data were extracted on an intention-to-treat basis. Exploratory continuous outcomes regarding abdominal and bowel symptoms were extracted when reported.
Statistical analysis
Data were pooled with respect to treatment indication, therapy, dosing schedule, and clinical trial endpoint for linaclotide as well as plecanatide. Descriptive statistics are reported using percentages and counts for binary data and mean for continuous data. Studies were pooled using a DerSimonian and Laird random-effects method with Forest plots constructed to display odds ratios (ORs) of the primary and secondary endpoints comparing active therapy to placebo. We then used meta-regression to compare the endpoints of linaclotide vs. plecanatide controlling for placebo arm prevalence. For diarrhea and withdrawal data where there were zero events, cells corresponding to zero events were replaced with a 0.5 value in the respective 2×2 table.(18) For events with zero counts, we also used an exact logistic regression model with fixed effects after expanding to patient-level data to confirm findings from random effects meta-analysis. The potential for publication bias was assessed using Harbord’s test where appropriate. An I2 statistic was calculated to assess between-study heterogeneity, and I2≥50% was defined as substantial heterogeneity. Statistical analysis was performed using STATA 14.2 (StataCorp; College Station, TX, USA).
RESULTS
The flowchart of literature search and study selection is reported in Figure 1. We identified eight trials of linaclotide (evaluating 2,824 patients on active therapy and 1,951 on placebo) (19–25) and seven trials of plecanatide (evaluating 3,617 patients on active therapy and 1,977 on placebo) (14,26–31). Lembo et al. (20) reported pooled results of two phase III linaclotide trials for CIC. The study characteristics of each trial, including registered phase of study, inclusion criteria, dosing protocol, and follow-up length are summarized in Supplementary Table 1 online. Two phase 2a trials (one of linaclotide and one of plecanatide) to treat CIC were not eligible for the efficacy analysis because either the trial length was too short (32) or the outcome was not assessed (26). The evaluated doses for linaclotide were 72 and 145 μg (linaclotide amount; equivalent to 75 or 150 μg peptide amount) per day for chronic constipation and 290 μg (linaclotide amount; equivalent to 300 μg peptide amount) per day for IBS-C. These differences in dosing were owing to bioequivalent reformulation between phase II and phase III studies, thus similar doses were pooled in this analysis. Plecanatide was dosed at 3 or 6 mg per day in CIC and IBS-C trials. Supplementary Table 2 summarizes the risk of bias across studies utilizing the Cochrane Collaboration tool. Low risk of bias was seen in all trials. Figures 2–4 summarize the efficacy and diarrhea-related outcomes for each eligible trial stratified by active vs. placebo arm of study, and additional outcomes are reported in the Supplement.
Figure 1.
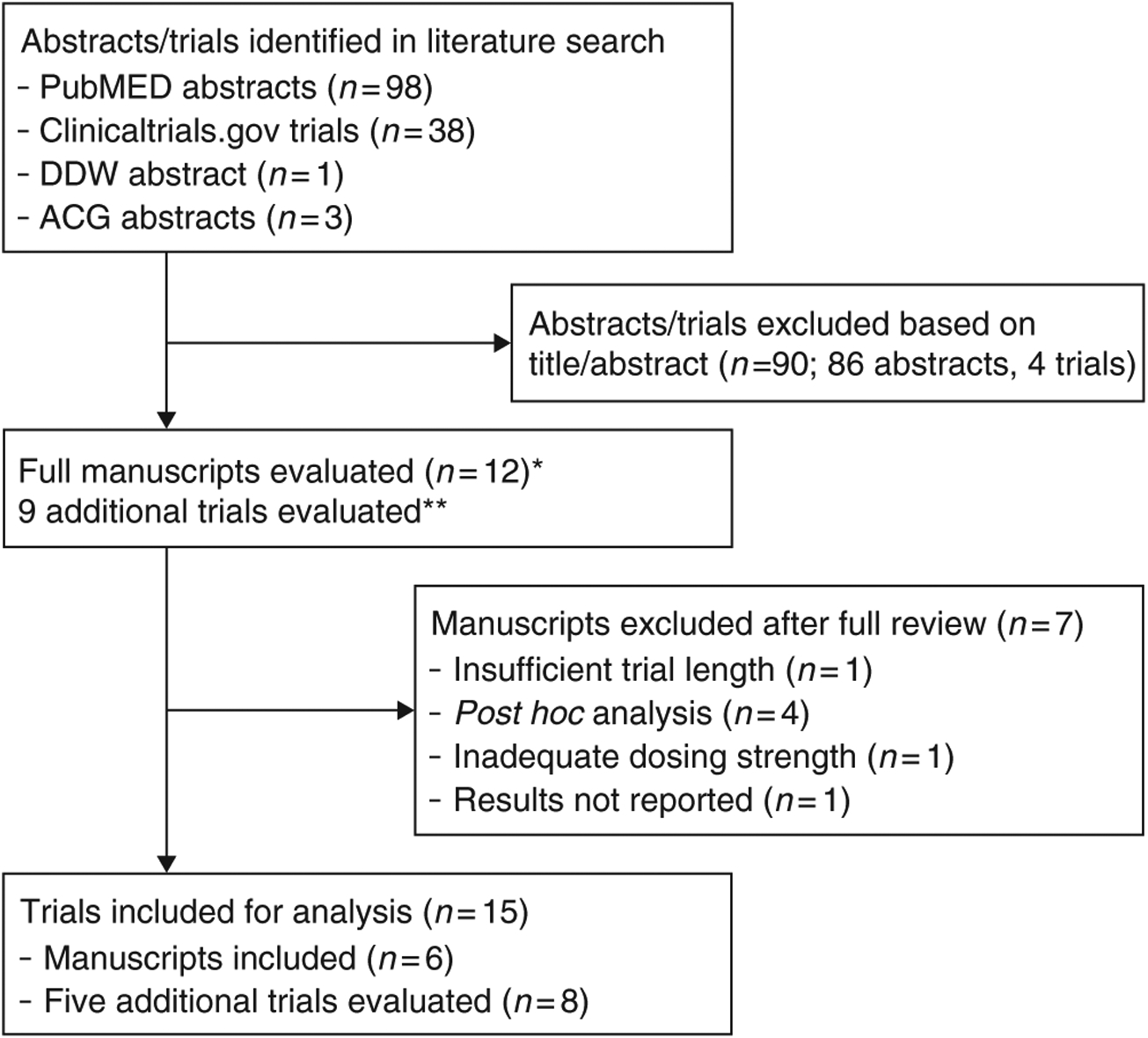
Flowchart of study inclusion. *Seven trials of linaclotide were identified using clinicaltrials.gov; these trials were cross-referenced to PubMED abstracts identified in the literature search. **Four of these trials were identified in DDW and ACG proceedings. Three trials were identified in the clinicaltrials.gov search. None were published in peer-reviewed literature. Data from the abstracts were supplemented by information on clinicaltrials.gov and SEC filings to evaluate trial eligibility for this study and for data extraction.
Figure 2.
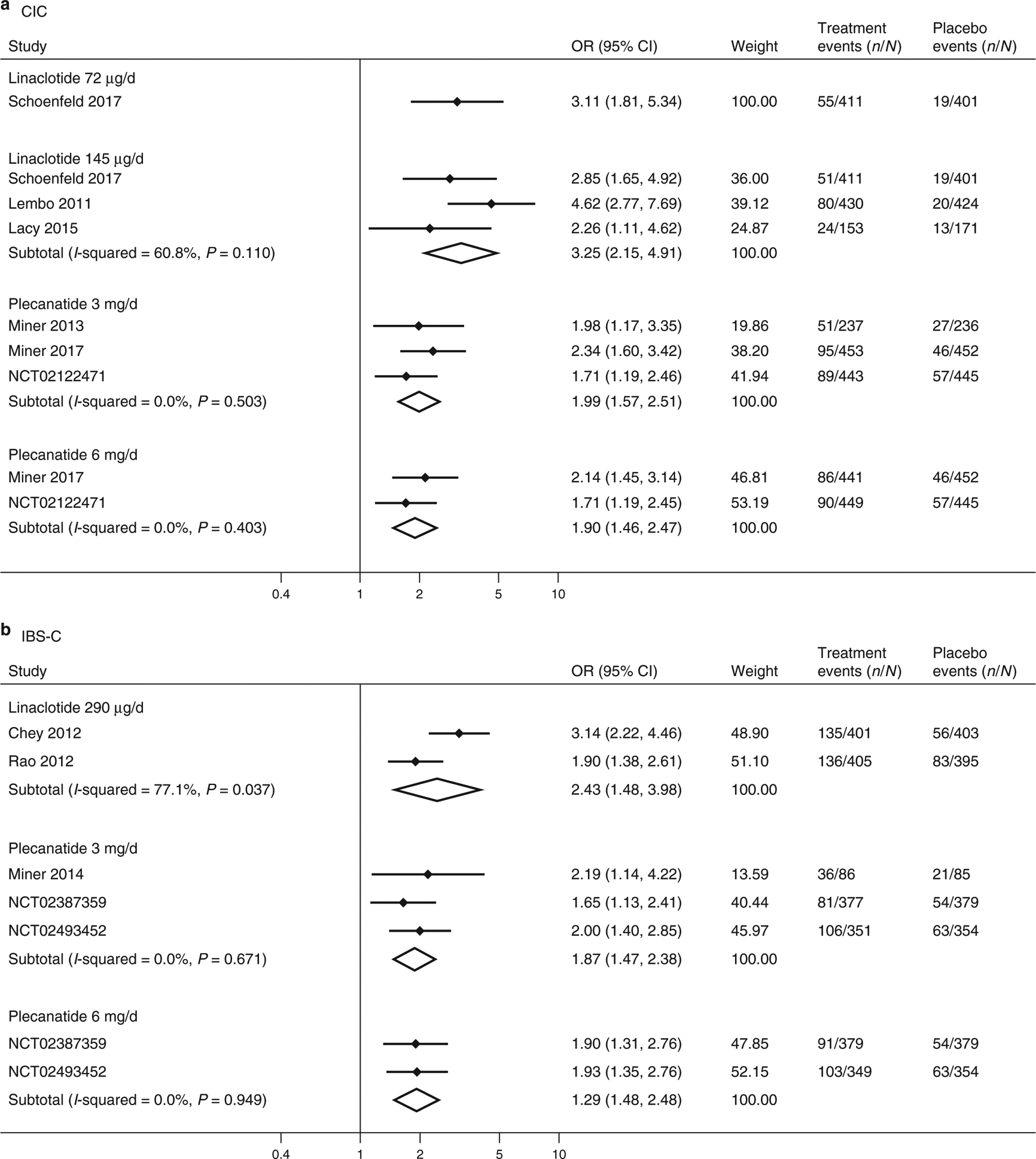
Forest plots for analysis of efficacy based on FDA responder endpoint in treating CIC (a) and IBS-C (b) with linaclotide or plecanatide.
Figure 4.
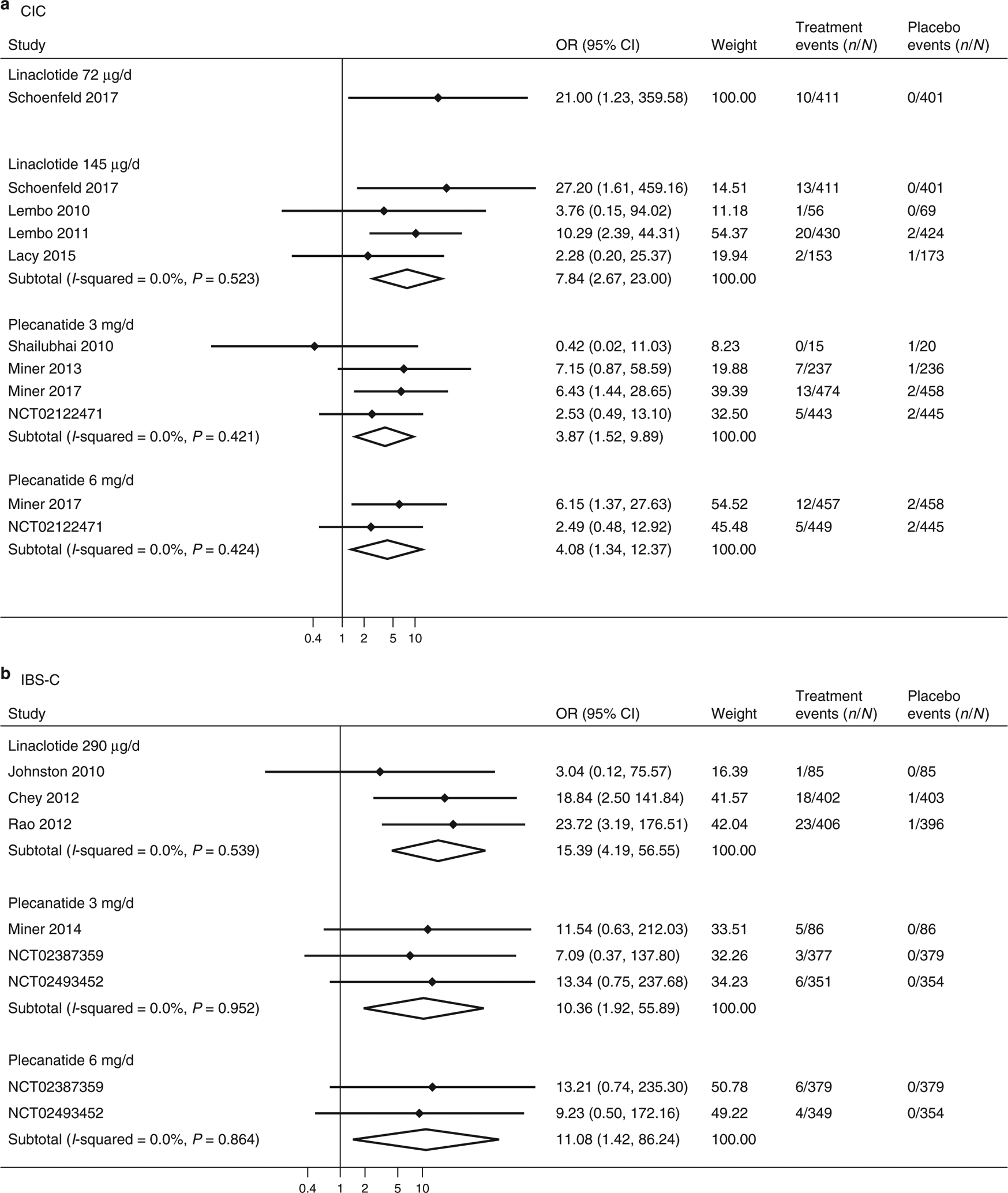
Forest plots for analysis of study withdrawal due to diarrhea in treating CIC (a) and IBS-C (b) with linaclotide or plecanatide.
Efficacy endpoints and patient-reported outcomes in CIC trials
Linaclotide at 72 μg (OR=3.11, 95% confidence interval (CI) 1.81–5.34; number needed to treat (NNT) =12, 95% CI 6–29) and 145 μg (OR=3.25, 95% CI 2.15–4.91; NNT=10, 95% CI 6–19) doses, as well as plecanatide at 3 mg (OR=1.99, 95% CI 1.57–2.51; NNT=11, 95% CI 8–19) and 6 mg (OR=1.90, 95% CI 1.46–2.47; NNT=12, 95% CI 8–23) doses, were more likely than placebo to meet the FDA responder endpoint for CIC (Figure 2). Heterogeneity in ORs in linaclotide 145 μg trials were moderate (I2 =33.9%), and no study heterogeneity was identified in plecanatide trials at either dose (I2 =0.0%).
Diarrhea-related outcomes in CIC trials
Linaclotide at 72 μg (OR=3.07, 95% CI 1.97–4.77; number needed to harm (NNH) =9, 95% CI 6–18) and 145 μg (OR=3.70, 95% CI 2.69–5.10; NNH=9, 95% CI 6–13) doses, and plecanatide at 3 mg (OR=3.86, 95% CI 1.83–8.12; NNH=27, 95% CI 11–89) and 6 mg (OR=3.96, 95% CI 2.08–7.52; NNH=27, 95% CI 13–72) doses, were more likely than placebo to be associated with diarrhea as an adverse event in CIC trials (Figure 3). There was no heterogeneity in ORs in linaclotide 145 μg trials or in plecanatide 6 mg trials (I2 =0.0%); however, there was moderate heterogeneity in plecanatide 3 mg trials (I2 =33.2%). There was no evidence of publication bias in linaclotide 145 μg trials (P =0.20). No assessment of publication bias can be made with the plecanatide trials, because there are not adequately available fully published manuscripts.
Figure 3.
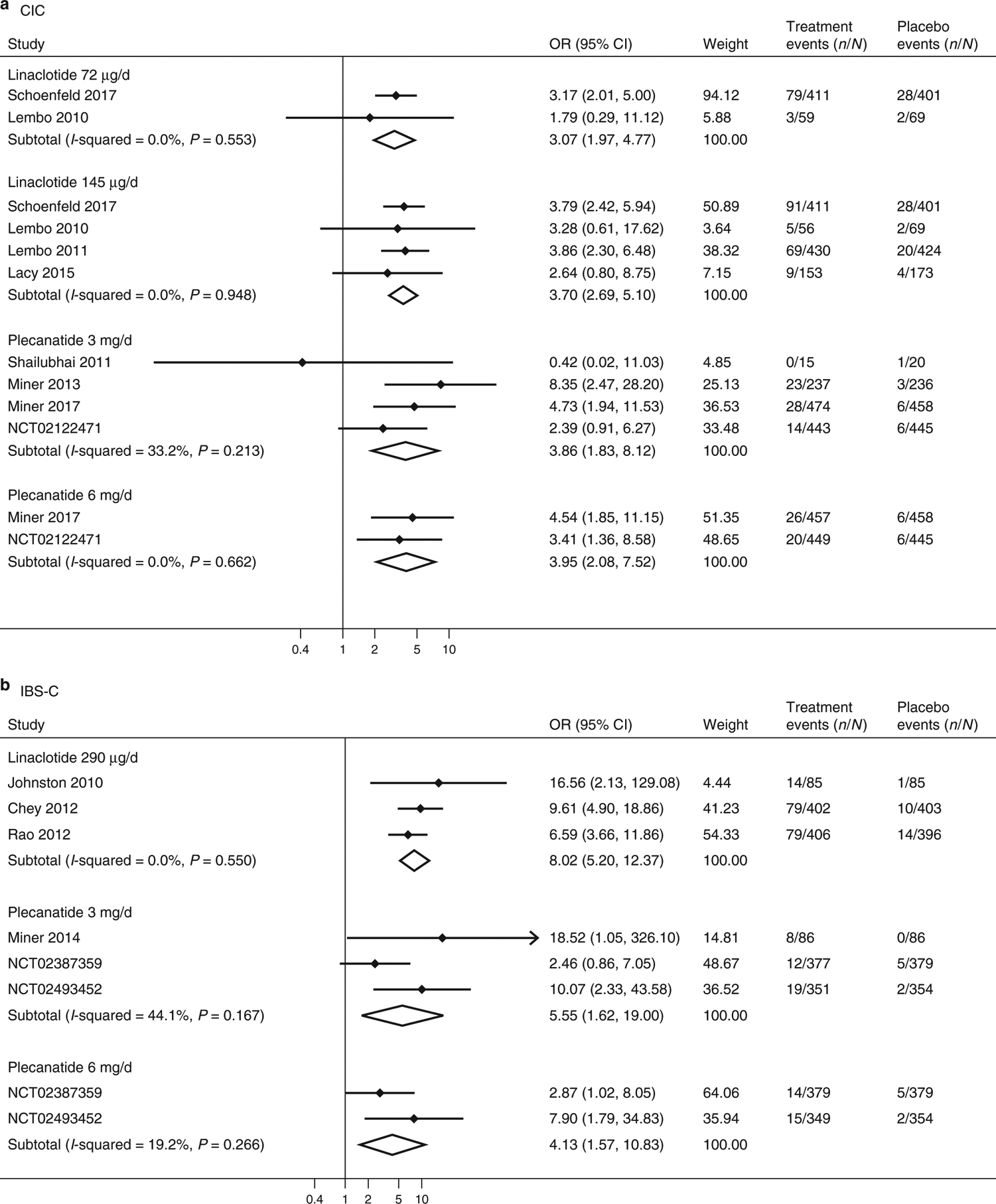
Forest plots for analysis of diarrhea as an adverse event in treating CIC (a) and IBS-C (b) with linaclotide or plecanatide.
Linaclotide 72 μg (OR=21.00, 95% CI 1.23 to >100; NNH>100.0) and 145 μg (OR=7.84, 95% CI 2.67–23.00; NNH=53, 95% CI 17–213) doses, as well as plecanatide at 3 mg (OR=3.87, 95% CI 1.52–9.90; NNH=69, 95% CI 23–370) and 6 mg (OR=4.08, 95% CI 1.35–12.37; NNH=75; 95% CI 21–667) doses, were more likely than placebo to be associated with study withdrawal due to diarrhea in CIC trials (Figure 4). The numerically large odds ratio for linaclotide 72 μg is partly explained by zero-events in the placebo arm of the study. There was no heterogeneity identified among trials in these analyses (I2 =0.0%). Th ere was no evidence of publication bias in linaclotide 145 μg trials (P=0.07). Analysis of study withdrawal using exact logistic regression similarly identified excess study withdrawal on linaclotide 72 μg (OR=13.88, 95% CI 2.22 to >100), linaclotide 145 μg (OR=12.36, 95% CI 3.89–62.96), plecanatide 3 mg (OR=4.20, 95% CI 1.68–12.58), and plecanatide 6 mg (OR=4.31, 95% CI 1.40–17.67).
Efficacy endpoints and patient-reported outcomes in IBS-C trials
Linaclotide 290 μg (OR=2.43, 95% CI 1.48–3.98; NNT=6, 95% CI 4–16) and plecanatide 3 mg (OR=1.87, 95% CI 1.47–2.38; NNT=9, 95% CI 6–16) and 6 mg (OR=1.92, 95% CI 1.48–2.48; NNT=9, 95% CI 6–17) were more likely than placebo to meet the FDA responder endpoint for IBS-C. There was substantial study heterogeneity identified in the linaclotide analysis (I2=77.1%), but no study heterogeneity (I2=0.0%) identified in the plecanatide analysis.
Diarrhea-related outcomes in IBS-C trials
Linaclotide 290 μg (OR=8.02, 95% CI 5.20–12.37; NNH=6, 95% CI 4–10) and plecanatide 3 mg (OR=5.55, 95% CI 1.62–19.00; NNH=27, 95% CI 8–192) and 6 mg (OR=4.13, 95% CI 1.57–10.83; NNH=35, 95% CI 12–185) were more likely than placebo to be associated with diarrhea as an adverse event in IBS-C trials (Figure 3). There was no study heterogeneity identified in the linaclotide analysis (I2=0.0%) or 6 mg plecanatide (I2=19.2%), but moderate heterogeneity was identified in analyses of 3 mg plecanatide (I2=44.1%).
Linaclotide 290 μg (OR=15.39, 95% CI 4.19–56.55; NNH=32, 95% CI 9–141) and plecanatide 3 mg (OR=10.36, 95% CI 1.92–55.89; NNH>100) and 6 mg (OR=11.08, 95% CI 1.42–86.24; NNH>100) were more likely than placebo to be associated with study withdrawal due to diarrhea in IBS-C trials (Figure 4). There was no evidence of study heterogeneity in any analysis (I2 =0.0%). One linaclotide trial and three plecanatide trials had zero events in the placebo arm of study. Analysis of study withdrawal using exact logistic regression was similar for linaclotide (OR=21.73, 95% CI 5.62 to >100), plecanatide 3 mg (OR=20.18, 95% CI 3.38 to >100), and plecanatide 6 mg (OR=14.17, 95% CI 2.28 to >100).
Meta-regression of efficacy and diarrhea-related outcomes
We used meta-regression of aggregate data to compare the endpoints of efficacy, diarrhea and study withdrawal owing to diarrhea between the two therapies in treating IBS-C or CIC. This was performed to control for differences in event rates in the placebo arms of trials. There were no statistically significant differences in any analysis (Table 1).
Table 1.
Results of meta-regression as an indirect comparison between linaclotide and plecanatide in treating CIC and IBS-C
| Disease | Chronic idiopathic constipation | Irritable bowel syndrome with constipation | ||
|---|---|---|---|---|
| Dosing | Linaclotide 72 μg/day vs. Plecanatide 3 mg/day | Linaclotide 145 μg/day vs. Plecanatide 3 mg/day | Linaclotide 290 μg/day vs. Plecanatide 3 mg/day | Linaclotide 290 μg/day vs. Plecanatide 6 mg/day |
| Efficacy | OR=0.77 (P=0.77) | OR=0.78 (P=0.66) | OR=1.28 (P=0.45) | OR=1.38 (P=0.34) |
| Diarrhea as an adverse event | OR=0.95 (P=0.97) | OR=0.93 (P=0.90) | OR=5.20 (P=0.13) | OR=4.72 (P=0.19) |
| Study withdrawal owing to diarrhea | OR=3.51 (P=0.51) | OR=1.58 (P=0.57) | OR=0.29 (P=0.55) | OR=0.27 (P=0.57) |
There were no statistically significant differences in outcomes in any analysis.
Additional outcomes
We identified additional exploratory outcomes evaluating objective improvements in bowel symptoms, as well as additional patient-reported outcomes and global improvement measures. Owing to heterogeneity in reporting, measures are reported descriptively only and no statistical analysis was performed (Supplementary Tables 3–7).
DISCUSSION
This is the first meta-analysis evaluating GCC agonists, linaclotide and plecanatide, for the treatment of CIC and IBS-C. Comprehensive data about the odds of achieving therapeutic response and diarrhea-associated adverse events are provided. Also, detailed data on study design and secondary efficacy endpoints are presented systematically. Based on meta-analysis, both linaclotide and plecanatide RCTs are well-designed and demonstrate efficacy for CIC and IBS-C. Odds ratios for efficacy endpoints overlap in linaclotide and plecanatide RCTs for both CIC and IBS-C. Also, no differences in efficacy were observed between linaclotide and plecanatide in meta-regression. Odds ratios for diarrhea as an adverse event also overlap in linaclotide and plecanatide RCTs for both CIC and IBS-C. We conclude that numeric differences in plecanatide-associated diarrhea and linaclotide-associated diarrhea are probably owing to differences in definitions of diarrhea used in these trials.
Plecanatide is a uroguanylin analog and its binding to GCC receptors is pH-dependent. Therefore, most activity is confined to the acidic portion of the proximal small intestine. Linaclotide binds to GCC receptors in a pH-independent manner (5) and active peptide is recovered in the stool, suggesting that linaclotide could be active throughout the small intestine and colon. It has been hypothesized that these differences in mechanism of action may produce lower rates of diarrhea with plecanatide (12–14).
Rates of diarrhea as an adverse event are numerically low in plecanatide Phase III RCTs, ranging from 3.2%–5.9% (Supplementary Table 8), whereas higher numeric rates of diarrhea, 5.9%–22.1%, are observed Phase III/Phase IIIb RCTs of linaclotide. However, when making these comparisons, it is important to take into account rates of diarrhea in patients treated with placebo. In RCTs of plecanatide, placebo rates were low at 0.6–1.3%, whereas the placebo rates were higher in linaclotide trials at 2.3%–7.0%. In this meta-analysis, our use of ORs can account for numeric differences in placebo rates of diarrhea. As odds ratios overlap for primary efficacy endpoints, diarrhea, and study discontinuation owing to diarrhea, these data suggest no differences between linaclotide and plecanatide in efficacy nor tolerability. Also, meta-regression, which can control for differences in event rates in the placebo arm, show no significant difference between the two medications. Interestingly, we did not identify a dose-dependent effect on incidence of diarrhea or study withdrawal owing to diarrhea for either therapy at evaluated doses. Though there is little evidence to support a data-driven approach at this time, a practical approach toward managing patients experiencing diarrhea on guanylate cyclase-C agonists could include dose reduction.
Based on our review of FDA documents (15,16), the numerically lower rates of diarrhea for plecanatide-treated (and corresponding placebo-treated patients) is most likely due to the definition of diarrhea as an adverse event: “since an increase in the number of BMs from baseline was an expected pharmacodynamics effect of plecanatide and would be coded as diarrhea, sites were instructed to only record an AE of diarrhea if the patient reports that it was bothersome [e.g., watery/mushy stool (BSFS score of 6 or 7) with a sense of urgency, etc.] or if the event required treatment or hospitalization.” Whereas, in Phase III linaclotide trials in CIC and IBS-C, diarrhea was recorded as an adverse event utilizing the system for recording all types of adverse events: “per protocol, patients were given the opportunity to report adverse events spontaneously. In addition, at each visit following the first visit, patients were questioned (in a non-leading manner) to volunteer information regarding any AEs that had occurred since the previous visit. Examples of questions included, “Have you had any unusual signs or symptoms since your last visit?” All verbatim terms were collected.” Also, in plecanatide RCTs, patients had three site visits (weeks 4, 8, and 12), whereas patients in linaclotide RCTs had four site visits (weeks 2, 4, 8, and 12). The additional site visit at week 2 in linaclotide trials provides an additional opportunity to report diarrhea and may contribute to a higher reported rate of diarrhea in linaclotide-treated and placebo-treated patients in these trials.
This study has several important strengths and limitations. First, our efforts to use drug sponsor websites, FDA documents, and publicly available SEC filings to gather data is uncommon. Second, due to differences in events in the placebo-treated population, we used meta-regression to assess for differences in efficacy and tolerability. With respect to study limitations, there was statistically significant heterogeneity in analyses of linaclotide efficacy for both CIC and IBS-C indications. This may be partly explained by differences in eligibility criteria in a Phase IIIb RCT of CIC patients with moderate-severe bloating (21). Finally, the senior author is active as a consultant, advisory board member, and speaker on this topic for the pharmaceutical industry, which is a potential source for bias. This may be partly resolved because the senior author works as an advisory board member/consultant with the manufacturers of both linaclotide and plecanatide. Also, no industry funding was used to perform this study, and established meta-analysis principles were used to ensure an accurate presentation of data.
In conclusion, this study demonstrates efficacy for both GCC agonists in the treatment of CIC and IBS-C. No differences in efficacy or adverse events were identified between linaclotide and plecanatide. The systematic presentation of study design and secondary endpoint results in this review may facilitate future research about the efficacy, tolerability, and safety of this drug class.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Linaclotide (72 μcg and 145 μcg doses) and plecanatide (3 mg) are GCC agonists FDA-approved for CIC. Linaclotide is approved for IBS-C and approval for plecanatide is pending.
Diarrhea rates are lower for both plecanatide and placebo in RCTs compared with linaclotide RCTs. Prior publications opine that plecanatide may produce less diarrhea than linaclotide because of its mechanism of action.
No prior meta-analysis has assessed RCT data for linaclotide and plecanatide.
WHAT IS NEW HERE
In meta-analysis, both agents are efficacious in treating CIC and IBS-C. Odds ratios for efficacy, diarrhea rates, and study withdrawal due to diarrhea overlap, suggesting no significant differences between medications.
In meta-regression, there is no statistically significant difference between medications for these endpoints.
Per FDA reports, a more stringent definition of diarrhea as adverse event was used in RCTs evaluating plecanatide compared with RCTs of linaclotide. This is a likely reason for lower numeric rates of diarrhea associated with plecanatide.
ACKNOWLEDGMENTS
This material is the result of work supported in part by resources from the Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr Schoenfeld is supported by K24-DK1K24DK084208.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: Philip Schoenfeld.
Specific author contributions: Eric Shah and Philip Schoenfeld conducted the study and collected and interpreted data. Eric Shah drafted the manuscript and both authors edited the manuscript. Eric Shah and Hyung-Jin Myra Kim conducted statistical analysis. All authors approve the final draft submitted.
Financial support: There was no financial support for this study.
Potential competing interests: Dr Shah and Dr Kim have no conflicts of interest to declare. Dr Schoenfeld has served as a consultant and advisory board member for Ironwood Pharmaceuticals, Allergan Pharmaceuticals, Synergy Pharmaceuticals, and Salix Pharmaceuticals. He has served on the speakers bureau for Ironwood Pharmaceuticals, Allergan Pharmaceuticals, and Salix Pharmaceuticals. He is on the Board of Trustees for the GI Health Foundation. He was formerly a partner in MD-Evidence, LLC, a medical education and consulting firm.
REFERENCES
- 1.El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther 2002; 16: 1171–85. [DOI] [PubMed] [Google Scholar]
- 2.Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol 2000; 95: 67–71. [DOI] [PubMed] [Google Scholar]
- 3.Sun SX, Dibonaventura M, Purayidathil FW et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci 2011; 56: 2688–95. [DOI] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721.e4. [DOI] [PubMed] [Google Scholar]
- 5.Busby RW, Bryant AP, Bartolini WP et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 2010; 649: 328–35. [DOI] [PubMed] [Google Scholar]
- 6.Linaclotide (Linzess) for constipation. Med Lett Drugs Ther 2012; 54: 91–2. [PubMed] [Google Scholar]
- 7.Silos-Santiago I, Hannig G, Eutamene H et al. Gastrointestinal pain: unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 2013; 154: 1820–30. [DOI] [PubMed] [Google Scholar]
- 8.Hannig G, Tchernychev B, Kurtz CB et al. Guanylate cyclase-C/cGMP: an emerging pathway in the regulation of visceral pain. Front Mol Neurosci 2014; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drugs@FDA. Label for New Drug Application 202811. US Food Drug Adm. 11 2017, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202811s013lbl.pdf. [Google Scholar]
- 10.Drugs@FDA. Label for New Drug Application 208745. US Food Drug Adm. 6 2017, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208745lbl.pdf. [Google Scholar]
- 11.Sonu I, Triadafilopoulos G, Gardner JD. Persistent constipation and abdominal adverse events with newer treatments for constipation. BMJ Open Gastroenterol 2016; 3: e000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patwa V, Joshi A, Th adi A et al. 967 plecanatide, like uroguanylin, activates guanylate cyclase-C signaling in a pH-dependent manner in T84 cells, and in murine intestinal epithelial cells and tissues. Gastroenterology 2016; 150: S193–S194. [Google Scholar]
- 13.Brancale A, Shailubhai K, Ferla S et al. Mo1316 structural and dynamic features of plecanatide: insights from molecular dynamics simulations. Gastroenterology 2016; 150: S695. [Google Scholar]
- 14.Miner PB Jr, Koltun WD, Wiener GJ et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017; 112: 613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanes LS. Clinical review (Application Number 208745Orig1s000). US Food Drug Adm 2016; 140: 140. [Google Scholar]
- 16.Wynn EL. Clinical review (Application Number 202811Orig1s000). US Food Drug Adm 2012; 169: 169. [Google Scholar]
- 17.Higgins JPT, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23: 1351–75. [DOI] [PubMed] [Google Scholar]
- 19.Lembo AJ, Kurtz CB, Macdougall JE et al. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology 2010; 138: 886–895.e1. [DOI] [PubMed] [Google Scholar]
- 20.Lembo AJ, Schneier HA, Shiff SJ et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med 2011; 365: 527–36. [DOI] [PubMed] [Google Scholar]
- 21.Lacy BE, Schey R, Shiff SJ et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: a randomized, controlled trial. PloS ONE 2015; 10: e0134349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston JM, Kurtz CB, Macdougall JE et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 2010; 139: 1877–1886.e2. [DOI] [PubMed] [Google Scholar]
- 23.Chey WD, Lembo AJ, Lavins BJ et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107: 1702–12. [DOI] [PubMed] [Google Scholar]
- 24.Rao S, Lembo AJ, Shiff SJ et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–24. quiz p.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenfeld P, Lacy BE, Chey WD et al. Low-dose linaclotide (72 μg) for chronic idiopathic constipation: a 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2018; 113: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shailubhai K et al. plecanatide, a guanylate cyclase C agonist, improves bowel habits and symptoms associated with chronic constipation in a phase IIa clinical study. Am J Gastroenterol 2011; 106: S502. [Google Scholar]
- 27.Miner PB et al. 925g plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12 week, multi-center trial. Gastroenterology 2013; 144: S–163. [Google Scholar]
- 28.Miner P et al. Plecanatide, a novel uroguanylin analog: a 12-week, randomized, double-blind, placebo-controlled, dose-ranging trial to evaluate efficacy and safety in patients with irritable bowel syndrome with constipation (IBS-C). Am J Gastroenterol 2014; 109: S541. [Google Scholar]
- 29.Synergy Pharmaceuticals Announces Positive Results in First Phase 3 Trial of Plecanatide in Patients with Irritable Bowel Syndrome with Constipation (IBS-C). Form 8-K, Exhibit 99.1 Accession No. 0001104659-16-161411 2016.
- 30.Synergy Pharmaceuticals Announces Positive Results in Second Phase 3 Trial of Plecanatide in Patients with Irritable Bowel Syndrome with Constipation (IBS-C). Form 8-K, Exhibit 99.1 Accession No. 0001104659-16-163508 2016.
- 31.Synergy Pharmaceuticals Announces Positive Results in the Second Phase 3 Trial of Plecanatide in Patients with Chronic Idiopathic Constipation (CIC). Form 8-K, Exhibit 99.1 Accession No. 0001104659-15-054706 2015.
- 32.Johnston JM, Kurtz CB, Drossman DA et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol 2009; 104: 125–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


