Abstract
Immunotherapies are rapidly entering the clinic as approved treatments for diverse cancer pathologies. Radiation therapy is an integral partner in cancer therapy, commonly as part of complicated multimodality approaches that optimize patient outcomes. Preclinical studies have demonstrated that the success of radiation therapy in tumor control is due in part to immune mechanisms, and that outcomes following radiation therapy can be improved through combination with a range of immunotherapies. However, preclinical models of cancer are very different from patient tumors, and the way these preclinical tumors are treated is often very different from standard of care treatment of patients. This review examines the preclinical and clinical data for the role of the immune system in radiation therapy outcomes, and how to integrate preclinical findings into clinical trials, using ongoing studies as examples.
Evidence of immune contribution to radiation treatment outcome
The use of radiation therapy (RT) has evolved over the years, integrating developments in technology permitting improved tumor targeting as well as iterative studies examining the integration of radiation treatment in various doses, fractionations, and scheduling with other cancer therapies to improve response rates in patients. Most of the early concepts were related to radiation dose and fractionation to achieve a differential effect that allows normal tissues to survive within acceptable parameters while cancer cells are selectively eliminated. During this earlier era, RT was generally regarded as immunosuppressive mostly due to its cytotoxic effect on leukocytes, and treatment was shown to lead to lymphopenia and impaired leukocyte function1,2. Nevertheless, more recent studies demonstrate a synergy between RT and the immune system, and this has generated significant excitement in the field 3. In this review we will focus on how the preclinical data influences the combination of RT with immunotherapy and how this impacts RT clinical trial design, and use examples to show how treatment realities require different approaches from those used in preclinical models.
Preclinical evidence that the immune status of tumors influences outcome following radiation therapy
Animal models provide the advantage of allowing investigators to rapidly test drug doses and combinations and perform critical mechanistic investigations to understand treatment responses and develop new approaches. However, all preclinical models are a compromise of the central goal of developing therapies for cancer patients. The dominant approach to model immunotherapy of cancer is syngeneic murine cancer cell lines implanted in immune competent mice. These tumors were once maintained by serial passage through mice, but almost all current models are maintained as cell lines grown in vitro and implanted into mice to form tumors. Injection of a bolus of cancer cells into immune competent mice requires rapid recruitment of host cells to establish critical structural features of the tumor including neovasculature. However, not all injected cells survive, and this results in initial immunity to the implanted tumor (Figure 1).
Figure 1.
In preclinical models of cancer, an injected bolus of cancer cells rapidly outstrips the local vascular supply and results in death of a proportion of the cancer cells. Phagocytes including tissue macrophages respond to hypoxia and cell death by driving neovasculature formation thus allowing tumors to continue their growth. At the same time, innate sensing mechanisms can trigger inflammation following exposure to dying cell material, and dendritic cells can mature and travel to the draining lymph node to cross-present tumor associated antigen and establish initial implantation-related anti-tumor immunity. Tumor-specific T cells are amongst those recruited to the establishing tumor via local inflammation, and either cause spontaneous rejection of the tumor or fail to control the tumor and undergo exhaustion and tolerance.
It has long been recognized that cell lines vary in their ‘tumorigenicity’ and ‘immunogenicity’, which can greatly influence results. As a measure of tumorigenicity, the B16 cell line is capable of reliably forming tumors with as few as 500 cells implanted intracranially 4, though since low implantation doses can result in a more variable growth pattern, higher initial doses of 10,000–100,000 cells are often preferred for subcutaneous injection. Engineering this cell line to express a strong model antigen, such as SIY can result in a requirement for 2,000,000 B16-SIY cells to be injected to reliably form a tumor 5. Similarly, the Panc02 pancreatic adenocarcinoma reliably forms tumors with a dose of 200,000 cells injected subcutaneously 6,7, but Panc02-SIY tumors require 1,000,000–5,000,000 cells to form tumors at a similar rate 8,9. However, Panc02-SIY reliably forms tumors with only 200,000 cells in immunodeficient mice, or mice depleted of CD8 T cells (unpublished data). Thus, highly immunogenic tumors can exhibit decreased tumorigenicity due to endogenous immune control.
When immunogenic tumors are rejected by immunocompetent mice, this spontaneous rejection is dependent on classical activation of adaptive immune responses. Putting tumors that are ordinarily rejected into BATF3−/− mice that lack cross-presenting DC can allow tumors to grow at an identical rate to that seen in immunodeficient mice 10. Similarly, loss of innate immune sensors such as STING, which forms part of an endogenous DNA sensing mechanism, also permits immunogenic tumors to grow in otherwise immune competent animals 11. Each of these molecules feed into mechanisms that result in CD8 T cell mediated rejection of immunogenic tumors. Consistent with this, the precursor frequency of tumor antigen-specific CD8 T cells has been shown to directly influence the ability of tumors to grow following implantation 12. However, even when tumors are not rejected and successfully grow in immune competent mice, CD8 T cells are still generated following tumor implantation – they are just unable to prevent progressive tumor growth. This is the underlying principle behind concomitant tumor immunity, where subsequent challenge of a second tumor of the same kind can be rejected while the primary tumor continues to grow 13. Concomitant tumor immunity occurs via the development of a CD8-mediated T cell response following tumor implantation, which is later tempered by a Treg-mediated suppression of anti-tumor immunity 14–18. Thus, in our standard preclinical models of implantation of cancer cell lines into immune competent animals, tumor implantation generates a T cell response to the cancer cells.
This T cell response to tumor implantation has significant impact on subsequent therapy. Immediately following tumor implantation T cell responses are rapidly expanding and are highly responsive to immune therapies. Thus, many models use immunotherapy with agents such as anti-CTLA4, anti-OX40, anti-41BB, and anti-PD1/PD-L1 administered in the first few days following tumor implantation, and in this timeframe they are all effective at tumor control 9,19–22. However, once the initial priming phase is complete and the tumor has established a mature environment, these therapies fail as single agents 9,21,23–25. This is highly impactful to clinical translation. There is likely no equivalent to the “implantation vaccination” event in patients, and all patients present in the established tumor phase. Consistent with preclinical data, agents such as anti-OX40 and anti-41BB are showing appropriate biological responses in patients, but not showing single agent efficacy in clinical studies 26,27. In fact, it is impressive that both anti-CTLA4 and anti-PD1 are showing clinical efficacy as single agents, since in almost all models they are each unable to treat an established murine tumor, and require combination therapies.
Radiation therapy is one such combination, and in mice the addition of radiation to OX40, 4–1BB, CTLA4, and PD1/PDL1 immunotherapy has been shown to re-invigorate responses in established tumors and can result in CD8-mediated tumor cures 28–32. Radiation therapy has widely been proposed as an endogenous cancer vaccine due to dying cancer cells being a source of both endogenous adjuvants and tumor-associated antigens to prime anti-tumor immunity. However, as discussed above, in preclinical model of tumor cell lines implanted into immunocompetent mice, the mice already have antitumor immunity established following tumor implantation. Using approaches to block the immune response to tumor implantation, we identified that the immune-mediated tumor control by RT combined with anti-CTLA4 or anti-PD1 is entirely lost 9. In these models, RT was unable to generate new immune responses capable of controlling residual cancer cells in the absence of implantation-initiated pre-existing antitumor immunity. Tumor cure by radiation and immunotherapy was dependent on the establishment of a T resident memory population of CD103+CD39+ T cells in the tumor9.
The importance of the tumor-resident tumor antigen-specific T cells agrees with data in models of immunotherapy alone, where tumor cure by combinations of checkpoint inhibitors only needs tumor-resident cells and is eliminated by blocking the immune response on tumor implantation 5,33. Similarly, this agrees with experiments that use surgically implantation of fragments of tumors to avoid the immune response seen with implantation of tumor cell suspensions 34,35. When tumors were implanted as a fragment, the efficacy of RT plus anti-PD1 therapy was lost 8. Mice implanted with tumor fragments required additional antigen-specific vaccination prior to treatment with radiation and immunotherapy for tumor control 8. Again, this agrees with data in poorly immunogenic cancer models with low level pre-existing anti-tumor T cell responses, where tumor-specific vaccination is necessary to generate immunity prior to treatment with immunotherapy combinations 36–38. Together, these data suggest that immunotherapy combinations based around checkpoint inhibitors on existing T cells are reliant on the right mix of pre-existing tumor-specific immune cells present in the tumor, and are not generating sufficient levels of de novo immune responses to cure tumors without these pre-existing immune cells.
It has long been known that the degree of total CD3 or CD8 T cell infiltrate in tumors is highly predictive of patient outcome across an array of malignancies (discussed below); however, many of these cells are likely not specific for tumor antigens. The level of pre-existing tumor-specific immunity in patients has become measurable with the discovery that CD103+CD39+ T resident memory cells are the critical antigen-responsive T cell population in patient tumors 39,40. The number of these tumor-infiltrating cells is highly predictive of patient outcome following conventional cancer therapies 40.
The reason so many non-specific T cells can be found in tumors is because recruitment of T cells through vasculature is not antigen specific. Rather, as in infectious states, T cell recruitment is based on appropriate expression of adhesion molecules by inflamed vasculature, and the pattern of chemokines available to trigger diapedesis (summarized in Figure 2). In close proximity to vasculature there can be a constant flux of T cells entering the tumor, but tumor residency is likely dependent on these T cells meeting their cognate antigen in the tumor (Figure 2). Thus, although an inflamed tumor can have a high T cell infiltrate, it does not necessarily mean that these cells are valuable for tumor control. For example, in the midst of a viral infection to the lungs, T cells are recruited based on their differentiation status, but remain based on their antigen specificity 41. Subsequent differentiation of effector cells into tissue resident memory populations requires the presence of antigen at that tissue site, secondary to recruitment 42. For these reasons, while a more inflamed tumor may have more T cells, it does not necessarily mean that these cells are capable of controlling the tumor, and conversely a highly antigenic tumor is not necessarily inflamed and enriched for T cells 43.
Figure 2.
Recruitment of immune cells to the tumor occurs via vasculature, and the selection of recruited cells is determined by the inflammatory status of the tumor and chemokine secretion pattern, transmitted through effects on tumor vasculature. Initial recruitment of myeloid precursors can result in differentiation into macrophages and DC, according to further triggers in the tumor environment. Activation and retention of recruited T cells is determined by the presence of cognate antigen, and can result in stable differentiation into resident memory populations that co-exist with cognate targets.
Since in preclinical models, tumors with a poor T cell infiltrate, or more importantly a poor infiltrate of CD103+CD39+ T resident memory cells, respond poorly to treatment with RT plus immunotherapy, we may expect similar outcomes in patients. In addition, since any radiation-mediated vaccine effect is insufficient to overcome this limitation in preclinical models 9, we should not expect it to perform better in patients. Therefore, for patients with poor pre-existing anti-tumor immunity novel approaches to generate an anti-tumor immune response may be appropriate to first ensure effective tumor control by radiation and immunotherapy combinations.
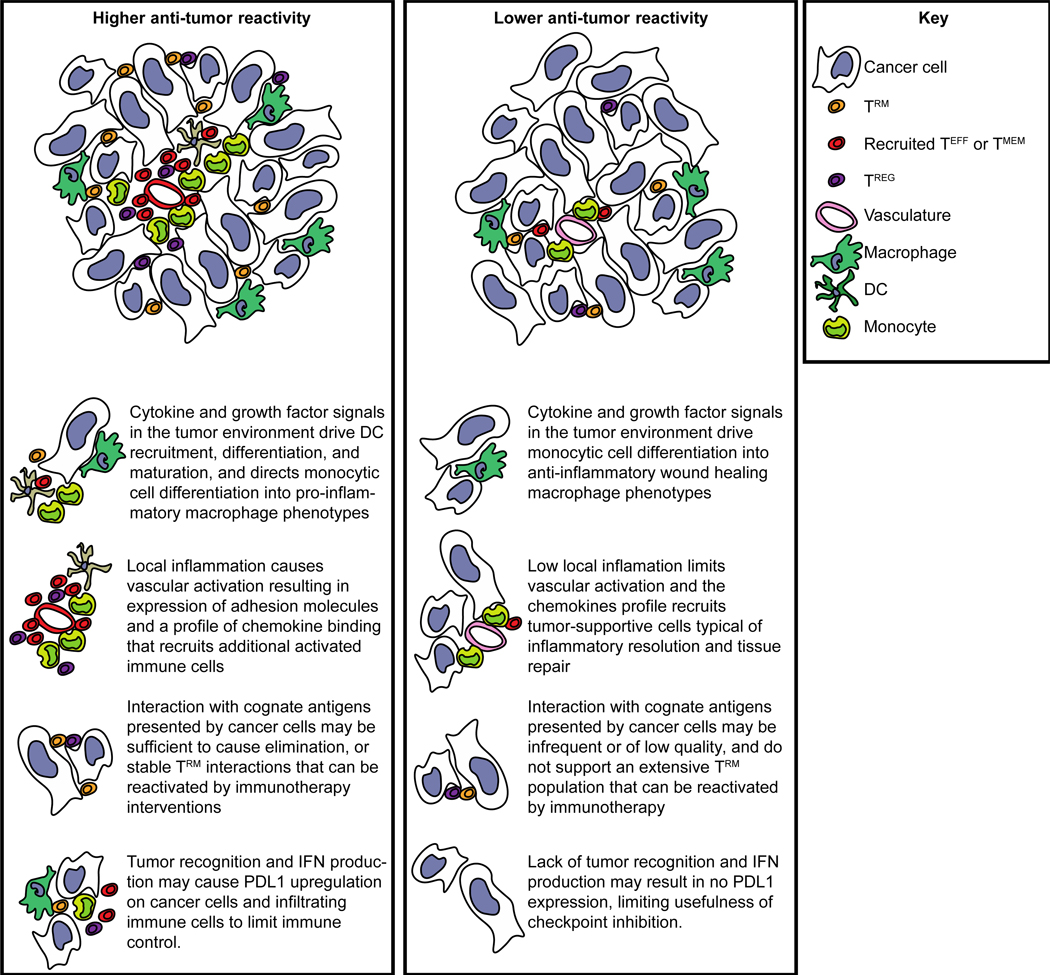
Increasing the degree of tumor recognition by antigen-specific T cells in the tumor environment can have multiple knock-on effects that are associated with good prognosis (Figure 3). T cell recognition of their cognate antigen results in cytokine secretion that can guide differentiation of precursor myeloid cells into pro-inflammatory macrophages and dendritic cells, and the inflammatory activation and chemokine secretion can cause recruitment of additional immune cells to survey the tumor (Figure 3). While T cell activation can generate negative feedback responses including upregulation of PD-L1 in their vicinity, this is generally a good prognostic feature and an opportunity to intervene with blocking antibodies. For those tumors that lack the initial T cell recognition the situation is much worse. Without pro-inflammatory cytokines, myeloid cells differentiate into tumor supportive and T cell suppressive phenotypes, and the quiescent vasculature means that there is little opportunity for potential tumor-reactive T cells to enter and find their target. In this scenario, checkpoint inhibitors may have no role, since they are not the feature that is limiting immune control of the tumor (Figure 3). Patients with this kind of immune environment may need additional immune interventions before the addition of checkpoint immunotherapy to RT could be expected to provide a benefit 8.
Figure 3.
Tumors with increased antitumor reactivity by immune cells can be expected to exhibit a number of features that are prognostic of good outcome, including recruitment and differentiation of dendritic cells, high levels of immune cell recruitment via inflamed vasculature, residency of antigen-specific T cells, and expression of negative feedback molecules such as PDL1 and IDO1.
Translating these findings to patients would require a pre-treatment assessment of the patient’s immune status, and a risk-adapted selection of immune therapies prior to treatment. In a number of clinical studies of radiation and immunotherapy combinations, a range of pre-treatment immune factors were different in patients who went on to respond, as compared to those who did not respond to the treatment 44–46. However, there is little consistency in markers between studies, and at present there is likely insufficient predictive power in these markers to determine that RT and immunotherapy will be successful or futile with any degree of clinical utility. As more data is acquired from the wide range of ongoing studies, we may be able to consider such risk adapted immunotherapy studies, where patients predicted to respond receive the current optimal immunotherapy combination, and the remaining patients are randomized to first receive novel treatments that aim to convert non-responders to responders.
Clinical data demonstrating that the immune status of patients influences outcome following radiation therapy
In contrast to pre-clinical models, addressing the role of the immune response to RT in clinical scenarios is complicated due to significant patient to patient variability and the inability to subject patients to continuous invasive sampling. Contrasting the extensive amount of preclinical evidence indicating an inter-relation between the immune response, RT, and tumor control discussed above, clinical evidence is more limited. If the immune status of the patient and their immune response to RT is critical to outcome, it should be possible to identify key features of responders and of response. While there are limited data, there are some studies that have examined the tumor environment before or after RT. The timing of these samples is likely critical, as more time between treatment and sampling possibly makes it less likely to observe the immune mechanisms of response. In studies of neoaduvant RT, post-treatment samples obtained at resection may be 5–12 weeks following completion of the radiation course. While they may be informative, they may contain little direct information on the effect that radiation had on the tumor immune environment. In studies of adjuvant RT the primary tumor has been resected, and a large portion of outcome assessments is based on growth of distant disease outside the treatment field. Therefore, in conventional treatment settings it is difficult to definitively ascertain that the immune infiltrate in the tumor environment treated with radiation impacted outcome.
In view of the inherent difficulty in acquiring multiple tumor samples from patients over time, multiple investigators have evaluated the peripheral blood and serum for potential immunological biomarkers. Numerous studies and meta-analyses have associated a high neutrophil to lymphocyte ratio (NLR) with poor prognosis in various types of cancer, including breast 47, lung 48, esophageal 49, ovarian 50, cervical 51, and colorectal 52, generally assuming a direct link between circulating and tumor-associated neutrophils. How the NLR affects tumor responses is less clear, but systemic expansions in neutrophils and decreases in T cells are thought to relate to the inflammatory biology of the tumor.
Tumors represent a chronic state of inflammation, during which inflammatory mediators including myeloid growth factors are secreted resulting in altered myelopoeisis. Myeloid cells are frequently expanded in the peripheral blood of cancer patients 53,54 Some mouse models are associated with extreme myeloid expansions detectible in the tumor, spleen and peripheral blood, and these myeloid cells are able to suppress T cell activation in vitro 55–57. Suppressive myeloid cells have been termed myeloid-derived suppressor cells (MDSC) 53. These cells are believed to promote immunosupression at the tumor microenvironment 58, though their definition, function, and relation to circulating neutrophils are still under debate 53,59.
While these data indicate that the tumor impacts the number of circulating neutrophils, since their expansion may be in response to tumor-derived growth factors it also means that circulating myeloid cells can provide insight into the likely inflammatory biology of the tumor. The number of these cells can also be affected by treatment. Following irradiation of murine tumors we have demonstrated a decrease in tumor size, along with a reduction in the number of circulating myeloid cells 60. Similarly, surgical removal of the primary tumor also causes a decrease in circulating myeloid cells 61,62 and gemcitabine and 5-FU chemotherapy have been shown to control the myeloid expansion in the spleens of tumor-bearing mice 62–64 though each of these chemotherapy agents has also been described to have a direct inhibitory effect on myeloid populations in vitro 63,64.
Pre-radiation NLR has been shown to serve as a biomarker of radio-sensitivity in a cohort of rectal cancer patients who underwent pre-operative radiotherapy 65. A high NLR predicted increased local relapse, decreased progression-free survival (DFS), and overall survival (OS), thus potentially identifying responders to RT intensification or neoadjuvant chemotherapy 65. However, expansions of peripheral blood immune cells may not be predictive of their number in the tumor. In a prospective cohort study of resectable pancreatic ductal adenocarcinoma we quantified tumor-infiltrating immune cells (CD3+, CD68+, and CD8+) by immunohistochemistry, and circulating populations (CD3+, CD8+, CD8+/CD25+, CD4+, CD4+/CD25+, monocytes, CD14+ monocytes, and total granulocytes) by flow cytometry immediately prior to surgery and on the first postoperative visit. Though patients exhibited variations in in myeloid populations in the peripheral blood, there was no correlation between the number of a range of circulating immune cells and the number of their corresponding population in the tumor 66. Therefore, in agreement with prior studies, higher levels of tumor-infiltrating CD3+ were associated with an improved prognosis, but the number of these cells in the tumor could not be predicted though peripheral blood analysis 66.
While it is unclear whether circulating cells are predictive of their infiltrate in the tumor, it is possible to measure in the serum other factors generated in the tumor that may be predictive of outcome. In a study of esophageal squamous cell carcinoma, serum and resected tumor samples were analyzed for high-mobility group box 1 protein (HMGB1), an endogenous adjuvant that may play a role in generating T-cell immunity following immunogenic cell death 67. Among the patients who underwent preoperative chemoradiation therapy, higher levels of HMGB1 were found both in the serum and in the tumor microenvironment, three days after completion of chemoradiation therapy and on the day of resection, respectively, as opposed to patients who were treated with surgery alone 68. Higher expression of HMGB1 in the tumor at resection was associated with improved outcome 68. However, it is unclear whether those with elevations in the tumor and those with elevations in the serum are the same patients. In other studies, HMGB1 induction following chemoradiation therapy had no association with tumor control, and no link to T cell infiltration in the tumor 69.
Calreticulin is another endogenous molecule released following cell death, and it has been demonstrated to function as an innate adjuvant 70. This positive role of calreticulin contrasts other data demonstrating that patients with higher levels of calreticulin have no benefit 69, or a worse prognosis than those with lower or absent expression 71, and calreticulin functions to suppress local immunity through interactions with phagocytes 71. Serum calreticulin can be detected in some pathological states, including cancer patients, but correlates with worse prognosis 72. Thus, peripheral immune status including inflammatory biomarkers may provide some information as to the likelihood of response, but there are currently no validated predictive biomarkers of response based on the peripheral immune status of patients.
Since tumor antigen-specific T cells may be a subpopulation of those T cells infiltrating tumors 39,40, there are a number of tools to infer the degree of pre-existing T cell reactivity in the tumor. These include markers of IFN signals in the tumor, along with the negative feedback consequence of IFN signaling such as expression of PD-L1 and IDO1. Under non-cancer scenarios, type I IFN limits the spread of infection through induction of cell-intrinsic antimicrobial and antiviral states and helps generate adaptive immune responses by promoting antigen presentation (reviewed in 73–75). IFN was one of the first immunotherapies, but its potent toxicity on systemic application limits its use in patients. However, greater understanding of the role of an early innate immune response following radiation in the tumor immune environment has led to renewed interest in pathways that activate local interferon responses 76,77.
Radiation-mediated DNA damage to cancer cells can result in micronuclei formation 78, which can permit cGAS recognition of cytoplasmic DNA, endogenous cGAMP synthesis, and STING activation 79,80. When cells are unable to sense DNA damage through the STING pathway, RT and immunotherapy are less effective 78,81,82. Similarly, exogenous administration of STING agonists to the tumor improves immune control of tumors following RT 82,83. These DNA sensing pathways directly activate IFN production in the tumor environment, and are required for optimal immune activation 76,77,84. However, IFN-related signaling pathway components were used to to construct a gene-expression profile that was associated with resistance to RT 85, and this predicts a poor response to chemotherapy and RT in patients with breast cancer 86 and glioblastoma 87. These data do not quite fit with the preclinical data showing that induction of local IFN-pattern responses is positive (reviewed in 76,77), and may instead relate to the rapid negative feedback that follows IFN signaling, including selection for an IFN-resistant state in the cancer cells 88.
PD-L1 is a negative regulator of T cell activation by ligating PD-1 on activated T cells 89. While PD-L1 can be expressed at baseline by some cell types, it is highly inducible following exposure to IFN forming part of a negative feedback loop regulating T cell activation 75,90,91. This IFN-inducible expression means that PD-L1 expression in the tumor can be a biomarker of pre-existing immune activation in the tumor environment, and predictive of immunotherapy outcome 90,92.
There are some data suggesting PD-L1 expression is also predictive of RT outcome. Tumor specimens of head and neck squamous cell carcinoma (HNSCC) treated primarily with RT/CRT were stained for p16, c-Met, survivin, PD-1, and PD-L1. Favorable response rates to RT were linked to high p16, and PD-L1 expression levels whereas high survivin and c-Met expression levels indicated unfavorable response rate to RT 93. Higher PD-L1 expression level was associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant RT 94. Similarly, lower PD-L1 expression on immune cells in EBV-positive nasopharyngeal carcinoma was related to an increased chance of local recurrence following RT, and high PD-L1 expression on immune cells or cancer cells was linked to a lower chance of recurrence 95. In 74 locally advanced NSCLC patients receiving concurrent CRT, PD-L1 expression and CD8+ TIL density were evaluated, and the combination of low PD-L1 expression and high CD8+ TIL density was significantly associated with a favorable survival rate 96. In this case the data suggests that more T cells with lower evidence of negative feedback represented a better immune environment. These data suggest that the pre-existing immune status of the tumor is associated with outcome in patients treated with RT, but there is some uncertainty in the optimal immune environment and multiple measures may be necessary.
Indoleamine 2,3 dioxygenase 1 (IDO1) is also induced following exposure to IFN and also acts as a negative regulator of immune responses 97. Pre-treatment anal squamous cell carcinoma specimens of 63 patients undergoing definitive CRT were analyzed for IDO1. Worse OS and high local recurrence rates were associated with >50% of tumor cells expressing IDO198. IDO1 has a range of physiological functions in the tumor, but it is notable that it can directly activate CD4 T regulatory cells (Treg) 99. These cells are defined by expression of the transcription factor FoxP3, have been shown to suppress both conventional CD4 and CD8 T cell activation. Treg are generally enriched in tumors compared to normal tissues and the peripheral circulation. In preclinical settings, depletion of Treg improves outcome in immunogenic tumors 100,101and improves the response to a range of therapies 92,102,103, including radiation 104,105.
In 128 patients with rectal cancer treated with neoadjuvant CRT there was a correlation between a lower density of Tregs in the tumor microenvironment and an improved pathologic response106. In a related study, pretreatment biopsy specimens and post-neoadjuvant CRT resected specimens of 62 rectal cancer patients were analyzed for CD8, CD4, CD56, FOXP3, CD33, CD11b, PD-L1, and CTLA-4. A greater response rate to CRT was associated with a high density of CD8+TILs, CD4+TILs, and low density of MDSC-TILs107. These data are very interesting since as discussed, myeloid populations can be a significant immune suppressive population in tumors and can limit the response to RT in preclinical models 7,108–113. Tumor-associated macrophages were studied in HNSCC patients treated with definitive CRT by analyzing the expression of CD68, CD163, and CD11b in 106 pre-treatment samples. Expression of CD163 proved to be a negative predictor of clinical outcome following definitive CRT, and early local recurrences were correlated to increased infiltration by CD11b+ cells114. Together, these data suggest that many of the same features defined in preclinical models are impacting the response to RT in patients. While there are few examples in patients treated with RT as a single agent due to the limited clinical scenarios where such treatments occur, the preponderance of data supports the scenario where an optimal response to RT is dependent on the immune status of the patient.
Critical issues in translating preclinical studies to patients
Preclinical tumor treatment models are designed to simplify and standardize the treatment scheme so that the effect of additional interventions can be rationally tested. This does not always permit easy translation. Some are more complicated to discuss, such as the fact that much lower energy X-rays are commonly used in preclinical models, and the effect of anatomic location on treatment-related effects. Instead we will discuss a selection of issues that present clear immunological consequences and difficulties in translating preclinical findings to combination clinical trials in patients.
Standard use of hypofractionation in preclinical models
The vast majority of patients treated with RT have some variant of standard fractionation, where daily doses are at or below 2Gy and treatment courses are measured in weeks. For example, standard radiation fractionation for rectal cancer patients incorporates daily dosing of 1.8Gy fractions for 28–30 total fractions. These are delivered Monday to Friday resulting in a roughly 6-week treatment course. However, in preclinical models of cancer, standard fractionation over 6 weeks is rarely tested in combination with immunotherapy. There are some practical considerations, such as the fast growth rate of murine tumors – most experiments are complete within 6 weeks – and the difficulty in scheduling daily treatments with large group sizes over weeks between multiple overlapping experiments. These would not be sufficient justifications alone; however, the murine studies have also been less influenced by historical treatment paradigms and have allowed investigators to evaluate radiation treatments that are optimal for synergy with immunotherapy. Thus, while the current treatments given to patient have been carefully proven to be the most effective over decades of comparative clinical trials, they have not necessarily been optimized with immune responses in mind.
Fractionation makes it possible to achieve a therapeutic dose of radiation to cancer cells while relatively sparing normal tissues from late toxicities. Treatment plans are carefully designed to minimize dose to radiosensitive normal cells outside of the tumor. However, tumor-specific T cells in the tumor are unavoidable, and tumor-draining lymph nodes may be incorporated into the treatment field to target known or potential sites of regional metastases. Tumor antigen specific T cells where present are directly killed by radiation 9, and T cells within tumor-draining lymph nodes play a role in tumor control following RT 115. Since lymphocytes are sensitive to even low doses of RT, repeated local dosing will likely result in repeated killing of critical immune cells. This has been demonstrated in preclinical models. In a therapeutic model where high local doses of radiation (30Gy) resulted in effective local control via CD8 T cells, additional follow-on doses of fractionated radiation (3Gy x10) decreased tumor control 116. These data suggest that extended fractionation should be cautiously considered where immune control of residual cancer cells is the goal.
In a related concern, RT is used as a single agent in very limited set of scenarios. Very commonly, radiation is accompanied by chemotherapy for optimal local and distant control of tumors, but few immunotherapies are tested preclinically with fractionated chemoradiation. One reason is that chemoradiation therapy in patients is known to be lymphotoxic. Fractionated radiation can hinder immune responses 117 in part due to lymphocyte death in the radiation field leading to systemic lymphodepletion 118. Fractionated chemoradiation therapy has been shown to result in lymphocyte loss in patients receiving treatment for a range of cancer types 119–122, and low lymphocyte counts following chemoradiation has been linked to poor treatment outcome 119. In preclinical models, the addition of chemotherapy has been shown to eliminate the immune-mediated benefits of RT 123. Increasingly, hypofractionated regimens of RT are being studied 124,125, and hypofractionated chemoradiation approaches can reduce the lymphodepletion associated with treatment 126, and may be a superior partner for immunotherapy combinations.
The rationale for hypofractionation does not just relate to lymphodepletion. Just as there is a dose threshold for radiation-induced death of cancer cells, radiation-mediated innate immune activation has dose thresholds. Generally, higher radiation doses have been associated with improved outcomes following immune combinations in preclinical models, with a minimum dose in the 5–8Gy per fraction range 123,127,128. However, even low doses of RT can synergize with immunotherapy 129–131, so there is not currently a strict cut-off for immune effects.
Case study examples of preclinical to clinical translation
To illustrate how preclinical studies can be used to design human clinical trials of radiation and immunotherapy combinations, we will present three examples from our institution. We explain how these relate to the wider literature and how current clinical practice influenced trial design and the compromises necessary to fit preclinical observations into best patient care. To provide a diversity of scenarios, the examples focus on 1) local control; 2) integration with fractionated chemoradiation, or 3) treatment of metastatic disease.
Neoadjuvant anti-PD1 and radiation therapy for local control of HNSCC
The roughly 15% overall response rate observed for PD-1 blockade in platinum-refractory recurrent/metastatic HNSCC 132–134 leading to two new FDA approvals in late 2016 (Keytruda, Opdivo), contributed to a surge of interest in the combination of radiation and PD-1 blockade in recurrent/metastatic HNSCC 135–137. The rationale for most studies of RT and PD-1 checkpoint blockade in HNSCC is that addition of radiation will incrementally improve on this 15% systemic response seen with PD-1 blockade. Based on our preclinical data, we designed a study based on the concept that the addition of PD-1 blockade to RT will exert a far greater influence in terms of local response to boost and prolong existing immune responses in the irradiated field and ultimately enhance local control achieved with RT 9.
As discussed above, much of the therapeutic efficacy of checkpoint inhibitors can be achieved with immune cells that already exist in the tumor at the time of treatment 5,33. Similarly, we have demonstrated that these pre-existing immune cells are required for the efficacy of RT and checkpoint inhibitor combinations 9. We saw no evidence of new immune responses generated by RT at any dose or fraction where pre-existing immunity was blocked. This is critical information, since of course CTLA4 and PD-1 are not present on naïve T cells, and their mechanisms of action include restoring the function of existing suppressed cells. The critical tumor-resident cells that are required for the response to RT and PD-1/PD-L1 responses exhibit T resident memory phenotypes 9, and cells with this phenotype represent the antigen-reactive cells in patient tumors, and also are prognostic of outcome in patients 39,40.
Pioneering studies in the field have highlighted a critical role of radiation on the cancer cell phenotype 138–140, which renders the cancer cells more susceptible to immune killing. Importantly, many cancer cell lines exhibit very low MHCI expression, which limits recognition by CD8 T cells. MHCI expression by cancer cells is increased in a dose-dependent manner following RT 138 and radiation treatment permits T cell mediated control of residual cancer cells 138–140. Cancer cells and other cells in the tumor stroma also upregulate PD-L1 following RT, and in settings where neither RT nor anti-PD-1/PD-L1 therapy are curative, the combination can result in local control of tumors in murine models 31,141. We believe these data explain the ability of RT to improve control of tumors in T cell dependent and site-specific manner, as well as the role for pre-existing T cells in tumor control in this setting. In this scenario, resident memory T cells are present in tumors, but antigen presentation by the cancer cells is poor due to low level expression of MHCI. Radiation therapy upregulates MHCI on cancer cells in the treatment field, but also upregulates PD-L1, limiting the function of the resident T cells. The combination of RT and anti-PD-1/PD-L1 permits activation of resident T cells with enhanced local control. This occurs without requiring any endogenous vaccine effect of RT, and new T cells generated following RT are not required for this local response. This mechanism is clearly distinct from the mechanism of distant tumor control following RT, since MHCI and PD-L1 upregulation in the treatment field will not affect distant, untreated tumors. We designed a clinical trial in HNSCC on the basis that immunotherapy will increase the sensitivity of tumors to RT.
Given the significant acute morbidity associated with 6 to 7 weeks of definitive chemoradiation, or 6 weeks of adjuvant radiation plus or minus chemotherapy, and the critical importance of local control in head and neck cancer, the neoadjuvant setting may be ideal for assessing a therapeutically beneficial in field interaction between hypofractionated radiation and PD-1 blockade. As discussed, hypofractionated radiation may have particularly favorable properties over standard fractionation for activating immune mediated clearance of residual cancer cells 142. Notably, conventional linear-quadratic models of cell killing may not accurately predict response at higher dose ranges as they do not account for the tumor microenvironment, consisting of vasculature, immune and stromal cells, which are implicated in radiotherapy failure, but conversely may contribute to improved local control when appropriate immune targeting agents are combined 110,143–145.
Since we are not considering RT as a vaccine, we need not consider our immunotherapy timing to help newly generated T cells following RT. In preclinical studies, we have found that treatment with immunotherapy starting prior to RT is optimal to improve local control 104,141,146. In our study (ClinicalTrials.gov Identifier: NCT03247712), patients with HPV+ HNSCC who require radiation either in the definitive or adjuvant setting based on upfront factors such as tumor size or number of involved nodes received neoadjuvant anti-PD1 (Nivolimumab) followed by 8Gy x5 (5 patients) or 8Gy x3 (5 patients) (Figure 4a). The accessibility of HNSCC presents opportunities to obtain biopsies to monitor the response to therapies, and the neoadjuvant design provides a final surgical specimen. Therefore, this study was designed to incorporate pretreatment biopsies and on treatment biopsies 3d post the last RT dose. The timing of the post-RT biopsy was determined based on interest in evaluating specific immunologic parameters. In addition, all patients proceed to surgical resection which permits a more extensive investigation of the treated tumor. This study has completed accrual with the initial design, and analysis ongoing.
Figure 4.
General schematics of clinical trials discussed in this review
TGFb inhibition (integration with chemotherapy and fractionation)
Loss of epithelial TGFβ signaling components occurs in approximately 50% of colorectal tumors 147, which results in TGFβ overexpression within the tumor microenvironment 148. Resultant immunosuppression from TGFβ renders antitumor immune responses less effective. Furthermore, following radiation, TGFβ is upregulated in response to the radiation “wound”. Based on these data, we used preclinical models to test a small molecule inhibitor of the TGFβ type I receptor (TGFβRI) prior to delivery of a single dose of 20Gy directed to the tumor. The goal was to improve the tumor immune environment at treatment, and improve immune mediated clearance of tumor following radiation. We demonstrated in a murine model of colorectal cancer that TGFβ inhibition prior to radiation improved survival over either modality alone 146. Further, we demonstrated that this effect was not mediated by known TGFβ effects on EMT, angiogenesis, or stromal remodeling, but was dependent on CD8 T cell clearance of residual cancer cells 146. Given the 75% survival observed in our preclinical model, we were motivated to translate our findings to the clinic.
Clinical translation of our data presented logistical barriers. Standard of care treatment of colon cancer utilizes surgical resection and chemotherapy as the backbone modalities; radiation is rarely utilized for colon cancer due to the dose constraints on nearby small bowel. Therefore, we focused on rectal cancer, for which radiation is routinely used in the neoadjuvant setting for locally advanced disease. While in Europe it is common for patients to undergo 5Gy x 5 consecutive daily fractions followed 1 week later by surgical resection, it is not common practice in the United States. Furthermore, integrating into this treatment paradigm would necessitate an endpoint of progression free survival, which is unappealing for early phase trials needing a timely endpoint. At our institute, common practice was for standard fractionated radiation 1.8Gy x 28 fractions over 5.5 weeks to 50.4Gy with concurrent 5-flourouracil based chemotherapy, followed by recovery over 6–10 weeks, then total mesorectal surgical resection.
While we believed higher dose per fraction radiation to be more immunomodulatory, in order to capture patients on study, we designed our clinical trial to integrate into this standard of care schedule (Figure 4b). One benefit to utilizing this schema was that it allowed for our primary endpoint to be pathologic complete response rate, and therefore a timely endpoint. Others have demonstrated that pathologic response to neoadjuvant chemoradiation in rectal cancer, as measured by tumor regression grade, strongly correlates with progression free survival 149. Therefore, we powered our single arm Phase II study to detect a 35% pathologic complete response rate, a >20% improvement from historical control 149,150. This trial is ongoing and recruiting patients (ClinicalTrials.gov Identifier: NCT02688712).
To better model the clinical trial, and confirm that these changes would not compromise efficacy, we returned to our preclinical murine model of colorectal carcinoma and evaluated fractionated chemoradiation with the addition of TGFβRI inhibitor. To mimic the clinical schema, we delivered 1 week of TGFβRI prior to three weeks of chemoradiation with 2Gy x 15 fractions with concurrent 5-flourouracil delivered at 25mg/kg i.p. three times per week. TGFβRI was also delivered during the second week of chemoradiation, consistent with the dosing regimen in the clinical trial. We evaluated for changes in the immune microenvironment and response to therapy.
We again observed that addition of TGFβRI improved response to chemoradiation, even with standard fractionation and the addition of chemotherapy, and that this too was dependent on CD8 T cells (manuscript in preparation). These data provide two important considerations: 1) our bias that high-dose per fraction radiation is needed for immunomodulation may limit our clinical translation when standard fractionation may provide another avenue for combinatorial study; and 2) our preclinical modeling utilizes tumor-directed radiation using the Small Animal Radiation Research Platform, while our clinical practice continues to target larger pelvic fields that encompass “at risk lymph nodes” which further compromises our ability to directly extrapolate our findings to the clinical setting. Recent data in preclinical models suggests that routine inclusion of tumor-draining lymph nodes with the tumor results in inferior tumor control 115. It remains difficult to balance the clinical concern for microscopic lymph node metastases with the immunologic concern for critical radiosensitive immune cells where both are within the treatment field. With the goal of patient care foremost, we have not altered the radiation treatment fields in our protocol and lymphatics are included.
A benefit to our rectal cancer study design is that we are able to obtain tissue over the course of treatment. We are evaluating the tumor microenvironment at baseline, day 15, and at surgery using multiplex immunohistology to quantify the immune infiltrate, and evaluate the spatial relationships between cell types. We have also included investigational multiparametric MRI sequences to evaluate non-invasive biomarkers of treatment response. Further, we are obtaining peripheral blood samples for immunologic monitoring every 2 weeks. While there may not be a close correlation with peripheral immune responses and those in the tumor 66, as discussed above, peripheral immune monitoring is particularly relevant in patients treated with chemoradiation therapy. We anticipate that consistent with our data in neoadjuvant chemoradiation therapy of pancreatic adenocarcinoma 126 we will also observe marked peripheral lymphopenia with chemoradiation in rectal cancer patients. It remains to be seen whether peripheral lymphopenia has any reflection on changes in tumor immune infiltrate over the course of treatment.
IL-2 combinations (RT dose and issues with immune monitoring)
High dose Interleukin 2 (IL-2) is a cytokine therapy that activates adaptive immune responses and has been approved for the treatment of metastatic melanoma and renal cell carcinoma. In a 270-patient study of patients with metastatic melanoma, response rates of 16–18% were demonstrated with a median duration of response of 40 months 151. Despite the addition of PD-1 and CTLA-4 blockade as alternative treatments for patients with metastatic melanoma and renal cell carcinoma, IL-2 remains a viable and effective therapy for these patients 152. Our institute maintains an effective biotherapy program with excellent outcomes for patients with high dose IL-2 153; however, while the duration and magnitude of the response seen with high-dose IL-2 is dramatic, the number of responding patients is a limiting factor to this approach.
The initial investigation of radiation combinations leads from the observation that melanoma or RCC patients who had radiation for urgent palliation in the week before IL-2 therapy had surprisingly high systemic response rates. IL-2 is a potent T cell growth factor and can support immune control of systemic disease following vaccination or adoptive transfer in preclinical models and in patients 154,155. If delivered following radiation, IL-2 may support T cell responses generated by any endogenous vaccine effect of RT, to clear additional metastatic disease. In order to study the potential synergy between high-dose focal radiation and immunotherapy we conducted a Phase I study of safety and immunogenicity of SBRT and high-dose IL-2 in patients with metastatic melanoma and renal cell carcinoma. There is a prior published report of RT in combination with IL-2 that described no enhancement of the IL-2 response 156. This study treated 14 melanoma and 12 renal cell cancer patients with 500 cGy BID to a total of 10 Gy- 20 Gy over 1–2 days, 2–24 hours prior to the initiation of IL-2 with no improvement in patient outcomes. There are several possible reasons why this radiation and IL-2 combination was ineffective. One possibility is that the radiation dose was itself suboptimal and did not synergize well with the high dose IL-2. Since as discussed above, larger doses of RT may be a superior partner for immunotherapy combinations, we evaluated the combination of IL-2 with Stereotactic Body Radiotherapy (SBRT).
Focal RT for metastatic disease in the liver and lung has been rapidly evolving over the past 15 years. Stereotactic Body Radiotherapy (SBRT) employs 6–10 times the daily dose of radiation used in standard fractionated radiation resulting in shorter treatment courses of 1–5 fractions 157. Use of these higher ablative doses of radiation has resulted in excellent local control rates in both primary and metastatic disease (70–90%) 158,159.
Therefore, we evaluated whether SBRT could synergize with high-dose IL-2 therapy. Patients were enrolled into three consecutive cohorts of 1, 2, or 3 doses of SBRT (20Gy) to one and up to 3 metastatic lesions in the lung and liver followed two days later by their first cycle of IL-2 (ClinicalTrials.gov Identifier: NCT01416831). IL-2 was administered at 600,000 international units per kg via intravenous bolus infusion every 8 hours for a maximum of 14 doses per cycle. A second cycle of IL-2 was repeated after a 2-week rest period. Four weeks after the second IL-2 cycle restaging studies were repeated and patients with regressing disease received up to 6 cycles of IL-2 therapy. All lesions treated with SBRT responded and regressed without recurrence. For the non-irradiated target lesions, Response Evaluation Criteria in Solid Tumors criteria was utilized. Eight of 12 patients (66.6%) achieved a complete or partial response 45, far in excess of historical data for IL-2 alone. The expected toxicities of high-dose IL-2 were observed, but there was no increase in the severity or frequency of IL-2 toxicity associated with the addition of SBRT 45. A maximum tolerated dose of SBRT was not reached.
Given the promise shown in the phase I study of high-dose IL-2 in combination with SBRT, a follow-up clinical trial was designed where patients were randomized to IL-2 alone or SBRT+IL-2 (Figure 4c). Thus far, there are very few randomized clinical studies of radiation and immunotherapy combinations that can demonstrate a positive role of radiation in treatment efficacy. However, in view of the high response rate it was difficult to exclude patients from a group with a significantly higher potential outcome. For this reason, the trial design allowed patient cross-over. Patients assigned to the IL-2 arm who have disease progression or mixed response after the first two IL-2 cycles have the option to receive SBRT followed by 2 additional cycles of IL-2. SBRT and IL-2 were delivered as in the Phase I study with 2 doses of 20Gy followed by cycles of IL-2 (ClinicalTrials.gov Identifier: NCT02306954). This trial has completed accrual and has ongoing analysis.
Conclusions on study design according to agent and standard of care
While clinical translation of preclinical data is the goal, the feasibility of such translation is often more complicated. Careful consideration of standard of care, practice patterns, and limiting potential harm are primary considerations. In our case studies, careful attention was given to these foundational concepts. By necessity, therefore, more iterative trials may result, and limit the speed of translation.
In addition, it is reasonable that preclinical modeling should adapt to evaluate immunotherapies alongside standard fractionation, or aim to identify whether there are any immune therapies that are particularly suited for combination with fractionated chemoradiation. This can only come from further careful monitoring of the immune consequences of these treatments to identify opportunities for synergy. Similarly, in patients, potential immune consequences should be considered when selecting radiation regimens and chemotherapy partners. It is potentially worthwhile to accept a less effective initial treatment while providing scope for immunotherapies to control residual local and distant disease. It seems likely that our first attempts will not all be successful, and that no single treatment combination will work for all patients. Collaborative scientist-physician scientist research will be key to fairly model actual treatment regimens in preclinical studies, and they will need to be creative to optimally integrate immunotherapies to the clinic while ensuring patients receive the best possible treatment.
Acknowledgments
Supported in part by: NIH R01CA182311 (MJG), NIH R01CA208644 (MRC), NIH R21AI126151 (MRC).
Conflict of interest: MJG and MRC have research support from Mavupharma, Bristol Myers Squibb, and Jounce that is relevant to the subject of this manuscript. KHY has research support from Eli Lilly that is relevant to the subject of this manuscript. The funders had no part in the subject or content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J. Gough, Earle A. Chiles Research Institute, Providence Cancer Institute, Providence Portland Medical Center, Portland, OR..
Shay Sharon, Department of Oral and Maxillofacial Surgery, Hadassah and Hebrew University Medical Center, Jerusalem, ISRAEL.
Marka R. Crittenden, Earle A. Chiles Research Institute, Providence Cancer Institute, Providence Portland Medical Center, Portland, OR, The Oregon Clinic, Portland, OR.
Kristina H. Young, Earle A. Chiles Research Institute, Providence Cancer Institute, Providence Portland Medical Center, Portland, OR. The Oregon Clinic, Portland, OR.
References
- 1.Wara WM Immunosuppression associated with radiation therapy. International journal of radiation oncology, biology, physics 2, 593–596. [DOI] [PubMed] [Google Scholar]
- 2.Walle T, et al. Radiation effects on antitumor immune responses: current perspectives and challenges. Therapeutic advances in medical oncology 10, 1–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharabi AB, Lim M, DeWeese TL & Drake CG Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The Lancet. Oncology 16, e498–509 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Kong LY, et al. Inhibition of p-STAT3 enhances IFN-alpha efficacy against metastatic melanoma in a murine model. Clinical cancer research : an official journal of the American Association for Cancer Research 16, 2550–2561 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spranger S, et al. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PDL1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priebe TS, Atkinson EN, Pan BF & Nelson JA Intrinsic resistance to anticancer agents in the murine pancreatic adenocarcinoma PANC02. Cancer chemotherapy and pharmacology 29, 485–489 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Crittenden MR, et al. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PloS one 7, e39295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng W, et al. Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget 7, 43039–43051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crittenden MR, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Scientific reports 8, 7012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzuto GA, et al. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. The Journal of experimental medicine 206, 849–866 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon RK, Carter RL & Kondo K. On concomitant immunity in tumour-bearing hamsters. Nature 213, 674–676 (1967). [DOI] [PubMed] [Google Scholar]
- 14.Bursuker I. & North RJ Immunological consequences of tumor excision: from active immunity to immunological memory. International journal of cancer. Journal international du cancer 37, 275–281 (1986). [DOI] [PubMed] [Google Scholar]
- 15.North RJ & Dye ES Ly 1+2- suppressor T cells down-regulate the generation of Ly 1–2+ effector T cells during progressive growth of the P815 mastocytoma. Immunology 54, 47–56 (1985). [PMC free article] [PubMed] [Google Scholar]
- 16.Mills CD & North RJ Ly-1+2- suppressor T cells inhibit the expression of passively transferred antitumor immunity by suppressing the generation of cytolytic T cells. Transplantation 39, 202–208 (1985). [DOI] [PubMed] [Google Scholar]
- 17.Bursuker I & North RJ Suppression of generation of concomitant antitumor immunity by passively transferred suppressor T cells from tumor-bearing donors. Cancer immunology, immunotherapy: CII 19, 215–218 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North RJ & Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2-suppressor T cells down-regulate the generation of Ly-1–2+ effector T cells. The Journal of experimental medicine 159, 1295–1311 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg AD, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. Journal of immunology 164, 2160–2169 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Melero I, et al. Monoclonal antibodies against the 4–1BB T-cell activation molecule eradicate established tumors. Nature medicine 3, 682–685 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Kocak E, et al. Combination therapy with anti-CTL antigen-4 and anti-4–1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res 66, 7276–7284 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America 99, 12293–12297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough MJ, Killeen N & Weinberg AD Targeting macrophages in the tumour environment to enhance the efficacy of alphaOX40 therapy. Immunology 136, 437447 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belcaid Z, et al. Focal radiation therapy combined with 4–1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PloS one 9, e101764 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selby MJ, et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PloS one 11, e0161779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal NH, et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clinical cancer research: an official journal of the American Association for Cancer Research 23, 1929–1936 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Curti BD, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 73, 7189–7198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gough MJ, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother 33, 798–809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi W & Siemann DW Augmented antitumor effects of radiation therapy by 4–1BB antibody (BMS-469492) treatment. Anticancer research 26, 3445–3453 (2006). [PubMed] [Google Scholar]
- 30.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 11, 728–734 (2005). [PubMed] [Google Scholar]
- 31.Deng L, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation 124, 687–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. International journal of radiation oncology, biology, physics 86, 343–349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow MT, et al. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 50, 1498–1512 e1495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochsenbein AF, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 411, 1058–1064 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Ochsenbein AF, et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proceedings of the National Academy of Sciences of the United States of America 96, 2233–2238 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curran MA, Kim M, Montalvo W, Al-Shamkhani A & Allison JP Combination CTLA-4 blockade and 4–1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PloS one 6, e19499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran MA & Allison JP Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res 69, 7747–7755 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quezada SA, Peggs KS, Curran MA & Allison JP CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. The Journal of clinical investigation 116, 1935–1945 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoni Y, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Duhen T, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nature communications 9, 2724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Topham DJ, Castrucci MR, Wingo FS, Belz GT & Doherty PC The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. Journal of immunology 167, 6983–6990 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Khan TN, Mooster JL, Kilgore AM, Osborn JF & Nolz JC Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. The Journal of experimental medicine 213, 951–966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spranger S, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proceedings of the National Academy of Sciences of the United States of America 113, E7759–E7768 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Formenti SC, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nature medicine 24, 1845–1851 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seung SK, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Science translational medicine 4, 137ra174 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Golden EB, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. The Lancet. Oncology 16, 795–803 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Ethier J-L, Desautels D, Templeton A, Shah PS & Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Research 19, 2–2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Y, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics 70, 524–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yodying H, et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Annals of Surgical Oncology 23, 646–654 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Huang Q.-t., et al. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cellular Physiology and Biochemistry 41, 2411–2418 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Chen M, Liang C & Su W. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Oncotarget 8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li M-X, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. International Journal of Cancer 134, 2403–2413 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res 67, 425; author reply 426 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy : CII 58, 49–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111, 4233–4244 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Bayne LJ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell 21, 822–835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabrilovich DI & Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. Immunology 9, 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umansky V, Blattner C, Gebhardt C & Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 4, 36–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaul ME & Fridlender ZG Cancer-related circulating and tumor-associated neutrophils - subtypes, sources and function. The FEBS journal 285, 4316–4342 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Crittenden MR, et al. The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. PloS one 8, e69527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinha P, Clements VK & Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. Journal of immunology 174, 636–645 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Sinha P, Clements VK, Bunt SK, Albelda SM & Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. Journal of immunology 179, 977–983 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Vincent J, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70, 3052–3061 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Suzuki E, Kapoor V, Jassar AS, Kaiser LR & Albelda SM Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical cancer research: an official journal of the American Association for Cancer Research 11, 6713–6721 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Vallard A, et al. Outcomes prediction in pre-operative radiotherapy locally advanced rectal cancer: leucocyte assessment as immune biomarker. Oncotarget 9, 22368–22382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang ES, et al. Association of Immunologic Markers With Survival in Upfront Resectable Pancreatic Cancer. JAMA surgery 153, 1055–1057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine 13, 1050–1059 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Suzuki Y, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 72, 3967–3976 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Aoto K, et al. Immunogenic tumor cell death induced by chemotherapy in patients with breast cancer and esophageal squamous cell carcinoma. Oncol Rep 39, 151–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature medicine 13, 54–61 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Science translational medicine 2, 63ra94 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molica S, et al. Serum levels of soluble calreticulin predict for time to first treatment in early chronic lymphocytic leukaemia. British journal of haematology 175, 983–985 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Schneider WM, Chevillotte MD & Rice CM Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32, 513–545 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theofilopoulos AN, Baccala R, Beutler B & Kono DH Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 23, 307–336 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Ivashkiv LB & Donlin LT Regulation of type I interferon responses. Nature Reviews Immunology 14, 36–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Medler T, et al. Activating the Nucleic Acid-Sensing Machinery for Anticancer Immunity. in International Review of Cell and Molecular Biology (Academic Press, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baird JR, et al. Stimulating Innate Immunity to Enhance Radiation Therapy-Induced Tumor Control. International journal of radiation oncology, biology, physics 99, 362–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harding SM, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mackenzie KJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartsch K, et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Human molecular genetics 26, 3960–3972 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications 8, 15618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 41, 843–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baird JR, et al. Radiotherapy Combined with Novel STING-Targeting Oligonucleotides Results in Regression of Established Tumors. Cancer Res 76, 50–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim JY, Gerber SA, Murphy SP & Lord EM Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer immunology, immunotherapy: CII 63, 259–271 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khodarev NN, et al. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proceedings of the National Academy of Sciences 101, 1714–1719 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proceedings of the National Academy of Sciences of the United States of America 105, 18490–18495 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duarte CW, et al. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PloS one 7, e29653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benci JL, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 167, 1540–1554 e1512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riley JL PD-1 signaling in primary T cells. Immunological reviews 229, 114–125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishimura H & Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 22, 265–268 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Khagi Y, Kurzrock R & Patel SP Next generation predictive biomarkers for immune checkpoint inhibition. Cancer metastasis reviews 36, 179–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fiedler M, et al. Biological predictors of radiosensitivity in head and neck squamous cell carcinoma. Clinical Oral Investigations 22, 189–200 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Jiang C, et al. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncology letters 17, 1626–1634 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y-J, et al. Low PD-L1 Expression Strongly Correlates with Local Recurrence in Epstein-Barr Virus-Positive Nasopharyngeal Carcinoma after Radiation-Based Therapy. Cancers 10, 374–374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tokito T, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. European Journal of Cancer 55, 7–14 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Munn DH & Mellor AL IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol 37, 193–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitra D, et al. High IDO1 Expression Is Associated with Poor Outcome in Patients with Anal Cancer Treated with Definitive Chemoradiotherapy. The oncologist, theoncologist.2018–0794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of clinical investigation 117, 2570–2582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Onizuka S, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59, 3128–3133 (1999). [PubMed] [Google Scholar]
- 101.Yu P, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. The Journal of experimental medicine 201, 779–791 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Son C-H, et al. Combination Effect of Regulatory T-Cell Depletion and Ionizing Radiation in Mouse Models of Lung and Colon Cancer. International Journal of Radiation Oncology*Biology*Physics 92, 390–398 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Keenan BP, et al. A Listeria Vaccine and Depletion of T-Regulatory Cells Activate Immunity Against Early Stage Pancreatic Intraepithelial Neoplasms and Prolong Survival of Mice. Gastroenterology 146, 1784–1794.e1786 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young KH, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PloS one 11, e0157164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kachikwu EL, et al. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics 81, 1128–1135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCoy MJ, et al. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. British journal of cancer 113, 1677–1686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng F, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Translational research : the journal of laboratory and clinical medicine 166, 721–732.e721 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Crittenden MR, et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 7, 78653–78666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crittenden MR, et al. Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiation research 182, 182–190 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Gough MJ, Young K & Crittenden M. The impact of the myeloid response to radiation therapy. Clinical & developmental immunology 2013, 281958 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 73, 2782–2794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shiao SL, et al. TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res 3, 518–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahn GO, et al. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proceedings of the National Academy of Sciences of the United States of America 107, 8363–8368 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balermpas P, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. British journal of cancer 111, 1509–1518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marciscano AE, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 5058–5071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Filatenkov A, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clinical cancer research : an official journal of the American Association for Cancer Research 21, 3727–3739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stewart CC & Perez CA Effect of irradiation on immune responses. Radiology 118, 201–210 (1976). [DOI] [PubMed] [Google Scholar]
- 118.Rosen EM, Fan S, Rockwell S & Goldberg ID The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer investigation 17, 56–72 (1999). [PubMed] [Google Scholar]
- 119.Wild AT, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. American journal of clinical oncology 38, 259–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Savage AM, Pritchard JA, Deeley TJ & Davies BH Immunological state of patients with carcinoma of the bronchus before and after radiotherapy. Thorax 35, 500–505 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schuler PJ, et al. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 19, 6585–6596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ellsworth S, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology 3, e27357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herman JM, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 121, 1128–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chuong MD, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. International journal of radiation oncology, biology, physics 86, 516–522 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Crocenzi T, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer 4, 45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. Journal of immunology 174, 7516–7523 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 5379–5388 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dovedi SJ, et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 5514–5526 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Dovedi SJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74, 5458–5468 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Klug F, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer cell 24, 589–602 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Ferris RL, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine 375, 1856–1867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chow LQM, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34, 3838–3845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seiwert TY, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet. Oncology 17, 956–965 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Karam I, et al. Survey of current practices from the International Stereotactic Body Radiotherapy Consortium (ISBRTC) for head and neck cancers. Future oncology (London, England) 13, 603–613 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Karam I, et al. Stereotactic body radiotherapy for head and neck cancer: an addition to the armamentarium against head and neck cancer. Future oncology (London, England) 11, 2937–2947 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Bernstein MB, Krishnan S, Hodge JW & Chang JY Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 13, 516–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reits EA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine 203, 1259–1271 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garnett CT, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64, 7985–7994 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Chakraborty M, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 64, 4328–4337 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Friedman D, et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology 47, 702–714 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Gough MJ & Crittenden MR Combination approaches to immunotherapy: the radiotherapy example. Immunotherapy 1, 1025–1037 (2009). [DOI] [PubMed] [Google Scholar]
- 143.Tormoen GW, Crittenden MR & Gough MJ Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol 3, 520–526 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gough MJ, Crittenden MR & Young KH Comparing equals when evaluating immunotherapy with different doses and fractions of radiation therapy. Immunotherapy 7, 847–849 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Gough MJ & Crittenden MR Immune system plays an important role in the success and failure of conventional cancer therapy. Immunotherapy 4, 125–128 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Young KH, et al. TGFbeta inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer Immunol Res 2, 1011–1022 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Coates RF, et al. Significance of positive and inhibitory regulators in the TGF-beta signaling pathway in colorectal cancers. Human pathology 66, 34–39 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Lu SL, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev 20, 1331–1342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rodel C, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23, 8688–8696 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30, 1926–1933 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 17, 2105–2116 (1999). [DOI] [PubMed] [Google Scholar]
- 152.Alva A, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer immunology, immunotherapy : CII 65, 1533–1544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Payne R, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer 2, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith FO, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clinical cancer research : an official journal of the American Association for Cancer Research 14, 5610–5618 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother 25, 243–251 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lange JR, et al. A pilot study of the combination of interleukin-2-based immunotherapy and radiation therapy. J Immunother (1991) 12, 265–271 (1992). [DOI] [PubMed] [Google Scholar]
- 157.Chang BK & Timmerman RD Stereotactic body radiation therapy: a comprehensive review. American journal of clinical oncology 30, 637–644 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Schefter TE, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. International journal of radiation oncology, biology, physics 62, 1371–1378 (2005). [DOI] [PubMed] [Google Scholar]
- 159.Herfarth KK, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 19, 164–170 (2001). [DOI] [PubMed] [Google Scholar]