Abstract
Radiation therapy benefits the majority of patients across the spectrum of cancer types. However, both local and distant tumor recurrences limit its clinical success. While departing from the established tenet of fractionation in clinical radiotherapy, ablative-intensity hypo-fractionated radiotherapy, especially stereotactic radiosurgery (SRS) and stereotactic ablative radiotherapy (SABR), has emerged as an alternative paradigm achieving unprecedented rates of local tumor control. Direct tumor cell killing has been assumed to be the primary therapeutic mode of action of such ablative radiation. But with increasing recognition that tumor responses also depend on the immunostimulatory or immunosuppressive status of the tumor microenvironment, the immunologic effect of ablative radiotherapy is emerging as a key contributor to anti-tumor response. More recently, novel radiation modalities, such as spatially fractionated radiotherapy (SFRT) and ultra-high dose rate FLASH irradiation, that venture even further from conventional paradigms have shown promise of increasing the therapeutic index of radiation therapy with the potential of immunomodulation. Here, we review the immunomodulatory impact of novel radiation therapy paradigms, heretofore considered radiobiological heresies, a deeper understanding of which is imperative to realizing fully their potential for more curative cancer therapy.
Introduction
Radiation therapy (RT) has long been a pillar of curative cancer therapy. Over a century of clinical experience has established that fractionation of RT in daily doses protracted over weeks is a primary approach to achieving therapeutic index. For most solid tumors, to reach potentially curative doses while avoiding intolerable collateral damage to normal tissues, typical conventionally fractionated radiation therapy (CFRT) regimens involve small doses of 1.8–2 Gy fractions per day, 5 days per week, over 6–8 weeks with total doses of 60–80 Gy. The emergence of stereotactic radiosurgery (SRS) for intracranial tumors1 and later stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiation therapy (SBRT), for other body sites2,3 defied the conventional wisdom of the time with the use of extremely hypo-fractionated regimens, such as large single-fraction treatments of up to 25–34 Gy or up to 60 Gy in 3–8 fractions over 1–2 weeks. This departure from established fractionation dogma is possible only in the setting of limited volume targets and highly conformal dose delivery, producing therapeutic index through physical separation of ablative doses from normal tissues and achieving unprecedented local tumor control outcomes in this setting 4. Severe or fatal toxicity when excessively intensive SABR dosing is applied too close to critical normal structures or to too large a volume5 highlights the continued need for fundamentally new strategies to improve the therapeutic index.
More recently, and in a much more nascent state of development, novel radiation therapy approaches that challenge tenets of classical radiobiology even further include ultra-rapid FLASH radiation therapy and spatially fractionated radiation therapy (SFRT). That increased therapeutic index can be achieved by the same dose of radiation given in a fraction of a second rather than the conventional several minutes, or by dose distributions in which substantial portions of a tumor are not directly targeted, seemingly defies explanation by conventional models.
Pre-clinically FLASH has been shown to achieve substantial normal organ sparing and equal or improved tumor killing in vivo, when compared to conventional dose rate irradiation, with evidence of changes in the immunologic microenvironment in both tumors and normal tissues 6 FLASH delivers sub-second doses at rates of >40 Gy/second compared to conventional dose rates of 0.01–0.2 Gy/second. This ultra-rapid dose has been achieved using dedicated experimental electron linear accelerators as well as specially configured clinical linear accelerators that generate 4.5–20 MeV electrons suitable for preclinical mouse and in vitro experiments, at a high beam current producing average dose rates as high as >200 Gy/s 7–10 Additional beams adapted for preclinical experiments include synchrotron x-rays11 and protons12,13.
In mice, substantially decreased normal organ injury has been observed with FLASH compared to the same doses of conventional dose rate irradiation in lung (inflammation and fibrosis) 6, brain (cognition and neuroinflammation) 11,14,15,16, skin (necrosis) 17 and GI tract (intestinal crypt ablation and GI syndrome) 18 These studies collectively suggest that FLASH may provide an effective additional strategy to escalate radiation doses to optimize antitumor control while reducing the complications of RT. Next generation technologies are now under development to deliver ultra-rapid and highly conformal RT to clinically relevant targets, typically large volume and deepseated, simultaneously overcoming the detrimental impact of physiologic motion on RT precision and leveraging the potential biological advantage of FLASH and giving it translational relevance 19
Contrary to the assumption that tumor sterilization requires comprehensive irradiation of the entire tumor to high doses, spatially fractionated radiation therapy (SFRT) is the use of intentionally heterogenous dose delivery comprising high dose peaks, typically much higher doses per fraction than CFRT, separated by low dose valleys within the same tumor target volume20,21. It was originally developed in the early 1900’s as a skin sparing approach, but more recently has demonstrated promising results for achieving tumor responses in bulky tumors too large to treat safely with CFRT, either alone or in combination with lower dose CFRT. SFRT has been delivered as 2-D arrays of pencil beams (GRID) or as 3-D lattices of high-dose vertices (lattice radiation therapy, LRT) using either photons or protons. GRID therapy is the most commonly used SFRT in which blocks or multi-leaf collimators (MLCs) are used to deliver non-uniform radiation to the target volume. GRIDs that have hybrid collimation (one block and one MLC) are faster than MLC based GRIDs 20 Some patients treated with GRID therapy showed induction of TNFα that strongly correlated with complete clinical response 22, An improvement of GRID therapy uses helical tomotherapy (TOMOGRID) and has been shown to achieve superior sparing of normal tissues than commercially available GRID blocks23.
The immunologic effects of radiation therapy are increasingly recognized as critical to its success or failure and may in fact underly the promise of novel radiation therapy paradigms including SABR, FLASH, and SFRT. Immunologic studies in preclinical models indicate that high dose hypo-fractionated RT can be far more immunogenic and efficacious than conventionally fractionated RT 24,25
The Radiation Research branch of the National Cancer Institute organized a workshop in 2018 to encourage approaches that integrate new radiation technologies and biology into therapeutic strategies26.The workshop highlighted the role of immune responses in enhancing the therapeutic response of RT. There is a large body of preclinical data27–29 and promising results from clinical trials that suggest synergy between ablative radiation therapy and immunotherapy 30,31 Here, we discuss the immune-stimulatory effects of ablative radiation that depend on doses and fractionation that seem contradictory to the classical dogma of conventional fractionation. We are optimistic that designing new treatment regimens that integrate technological advances of delivery of ablative RT and immuno-biology of radiation will significantly improve clinical outcomes.
Radiation induced cell death - immunogenic or immunosuppressive
Local tumor control by high dose radiation therapy has classically been considered to be mediated through direct cytotoxic effects on the cancer cells. Radiation induces double strand DNA breaks32, single strand DNA breaks33 and chromosomal aberrations34. These effects lead to cell death through inhibition of mitotic cell cycle or apoptosis, necrosis, autophagy or senescence35. The role of immunologic effects has been appreciated more recently. Pre-clinical studies show that double stranded DNA damage activates innate immune signaling pathways. The activation of cytoplasmic double stranded DNA sensor AIM2 (Absent in Melanoma 2) results in activation of caspase 1 that leads to release of proinflammatory cytokines and pyroptotic cell death36. Radiation produces cytosolic double strand DNA that is sensed by cGAS (cyclic GMP-AMP synthase) and activates STING (stimulator of interferon genes). STING recruits TBK1 (tank-binding kinase 1) to promote transcription of type IFN-I genes. Pre-clinical in vivo studies show that type-I IFN signaling in dendritic cells promotes cross priming of tumor associated antigens to CD8+ T cells which mediate anti-tumor response37,38 In contrast, activation of STING pathway in myeloid derived suppressor cells (MDSCs) that are recruited to tumors through CCR2 chemokine following local ablative radiation (20Gy), are detrimental to anti-tumor responses.39. However, CD8+ T cells can reduce the infiltration of MDSCs into tumors25. Another preclinical study showed radiation dose greater than 24 Gy in 3 fractions (8 Gy/fraction) led to accumulation of cytosolic Three-Prime Repair exonuclease 1 (TREX1) that degrades cytoplasmic double stranded DNA abrogating the immunostimulatory effect of STING40 in tumors cells. Further studies are needed to determine the contribution of STING and TREX1 in optimizing anti-tumor response by different hypo-fractionated SABR radiation regimens.
Radiation induced tumor cell necrosis releases adenosine triphosphate (ATP) as well as HMGB1 (high mobility group B1) protein, both of which have been shown to activate dendritic cells in tumors and enhance antigen presentation to T cells34. Expression of TLR4 by dendritic cells (DCs) and HMGB1 are necessary for activation of DCs and presentation of tumor antigens released by dying tumor cells after RT 41. Upregulation of DAMPs (damage associated molecular patterns) in dying tumor cells such as calreticulin (CRT) and heat shock proteins (HSP) stimulates phagocytosis in tumor associated dendritic cells and macrophages42,43. In addition, NK cells can also be activated by DAMPs44. The cell death pathways can vary with the dose, fractionation, tumor type and tumor stage. High radiation dose promotes tumor cell necrosis and favors immunogenic cell death through expression of DAMPs while low dose radiation of less than 5 Gy promote non-immunogenic apoptotic cell death 45,46 Uptake of apoptotic bodies by DCs has been shown to prevent DC maturation and induce tolerance47–49. In addition, tumor radiation induced apoptosis has been shown to activate Caspase-3 which facilitates re-population of tumor cells through prostaglandin E2 production50. We have recently shown that 30 Gy in 10 fractions (3 Gy/fraction) radiation of B cell lymphoma caused significantly enhanced tumor cell necrosis and expression of CRT and HSP70 and HSP90 and better tumor control when delivered over 4 days (accelerated schedule) compared with over 10 days (conventional schedule) 51. The inability of the tumor cells to repair endoplasmic reticulum stress within the shorter duration (4 hours) between radiation doses compared with conventional duration (24 hours) may account for higher expression of DAMPs in tumors treated with the accelerated schedule. Thus, ablative radiotherapy regimens that activate immunogenic cell death pathways and promote antigen presentation to CD8+ T cells are likely to induce durable complete remissions.
Immunogenic or immunosuppressive tumor microenvironment following ablative radiation therapy
Cancer cells survive the host immune system attack by creating immuno-suppressive tumor microenvironments including immune cells such as regulatory T cells, myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs) that impair infiltration of CD8+T cells that mediate anti-tumor responses. These immune cells have varying degrees of radiation sensitivity52–54. Monocytes, neutrophils, granulocytes T cells and B cells are radiation sensitive while regulatory T cells, macrophages, dendritic cells and NK cells are radiation resistant.
Studies show that ablative or hypo-fractionated regimens induce superior CD8+ T cell anti-tumor responses compared to conventional fractionation regimens24,25,55. A single high radiation dose of 20Gy upregulates Fas expression on MC38 colon cancer tumors expressing carcinoembryonic antigen. This makes the tumor cells sensitive to killing by CD8+ T cells through a Fas and Fas-ligand mediated mechanism56. Compared to fractionated radiotherapy (15 Gy in 3 fractions, 5 Gy/fraction), a single dose of 15 Gy radiation enhanced infiltration of antigen presenting cells and CD8+ T cells into tumors, and showed superior CD8+ T cell anti-tumor cytolytic activity 57 Addition of fractionated radiation dose (10X3Gy(30 Gy in 10 fractions, 3 Gy/fraction) after a single ablative dose of 30 Gy abrogated the robust anti-tumor immunity observed with a single 30 Gy alone in a CT26 colon cancer tumor model25. This observation suggests that addition of fractionated radiation may result in death of infiltrating T cells that kill tumor cells.
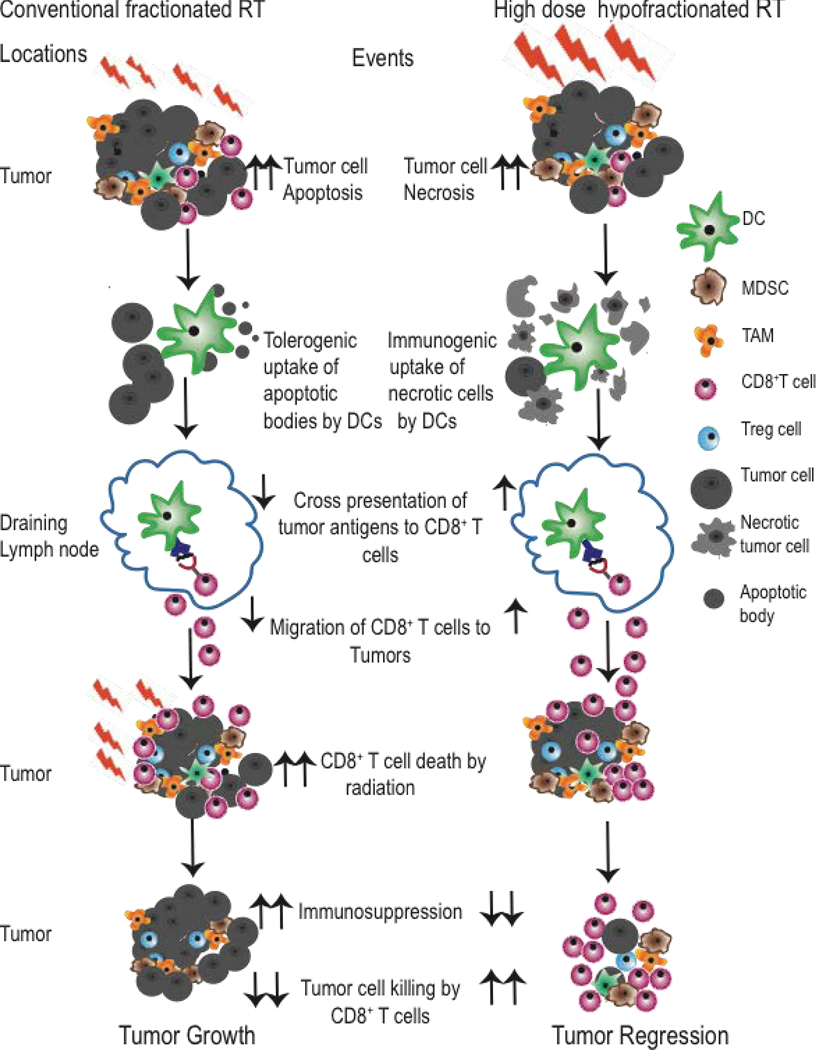
We have recently shown that fractionated radiation (10×3Gy) of A20 lymphoma tumors over 10 days results in reduced CD8+ T cell infiltration as compared to (10×3Gy) over 4 days. The prolonged daily irradiation over 10 days depleted T cells that infiltrated the tumor a few days after the start of local tumor irradiation51. In a pre-clinical model of TSA breast cancer, anti-CTLA4 antibody therapy along with fractionated radiation of 24 Gy in 3 fractions (8 Gy/fraction) was more effective in eliciting an abscopal effect on secondary tumors than either a single dose of 20 Gy or 30 Gy in 5 fractions (6 Gy/fraction)55. This suggests that high dose hypo-fractionated radiation therapy regimens may be more immunogenic than a single dose per fraction. In Figure 1, we summarize the effect of prolonged CFRT and hypo-fractionated accelerated high dose regimens on anti-tumor response.
Figure 1. Schematic diagram of immunomodulatory effects of conventional fractionated radiation and high dose hypo-fractionated radiation therapy on anti-tumor response.
In conventional fractionated RT, small daily radiation doses cause tumor cells to either die by apoptosis or repair of radiation induced DNA damage and recover. High dose hypo-fractionated RT cause cancer cell death by necrosis. Uptake of apoptotic bodies by dendritic cells (DCs) can be tolerogenic while uptake of necrotic cells by dendritic cells is immunogenic. These DCs migrate to the draining lymph node and present tumor antigens to CD8+ T cells. These cross-primed CD8+ T cells migrate to the tumor and kill cancer cells that lead to tumor regression. As conventional RT is administered over prolonged period, radiation kills tumor infiltrating CD8+ T cells while sparing immunosuppressive cells such as MDSCs, Treg cells and TAMS. In contrast, in hypo-fractionated RT the radiation schedule is completed before CD8+ T cell infiltrate the tumor.
Using several tumor models Dovedi et al 58 reported that CFRT induced upregulation of PD-L1 on cancer cells and MDSCs by increasing IFNγ produced by CD8+ T cells. This led to attenuation of anti-tumor responses. Tumor irradiation can induce the chemokine CCL2 that can attract monocytes to the tumor microenvironment and promote their differentiation into tumor associated macrophages. Radiation increases the expression of HIF-1α which promotes the transcription of genes such as monocyte colony stimulating factor 1 (MCSF-1), vascular endothelial growth factor alpha (VEGF-α) and CXCL12. MCSF-1 polarizes tumor associated macrophages to a more immunosuppressive M2 type that secrete transforming growth factor β (TGFβ) that converts CD4+ T cells to Treg cells59. Additionally, VEGF-α facilitates proliferation for Treg cells. A single radiation dose of 15 Gy to glioblastoma tumors induces CXCL12 which promotes influx of bone marrow derived CXCR4+CD11b+ monocytes to tumors60. These myeloid cells promote formation of new blood vessels and tumor recurrences. There was a 10-fold increase in CD11b+ cells in glioblastoma tumor biopsies from patients after radiation therapy60. Thus, radiation may have both pro-inflammatory or anti-inflammatory effects on the tumor microenvironment.
High ablative doses of radiation therapy can reduce the intra-tumoral levels of myeloid derived suppressor cells (MDSCs) while CFRT failed to induce this effect25. Patients with cervical cancer receiving CFRT showed increases in MDSCs in their peripheral blood61. In contrast, hypo-fractionated radiotherapy can decrease hypoxia and VEGF in tumors, and reduce PDL-1 expression and VEGF receptors on MDSCs62. In addition, high dose ablative radiation therapy has been shown to inhibit VEGF/VEGF receptor signaling which is essential for trafficking of MDSCs to tumors63. An important goal of clinical ablative therapy is to optimize radiation doses and fractionation strategies that achieve elimination of immunosuppressive cells such as M2 phenotype tumor associated macrophages and CD11b+ monocytes and MDSCs from tumors and circulation to achieve durable anti-tumor responses while stimulating the infiltration of CD8+ T cells.
Ultra-high dose rate FLASH radiotherapy (≥40 Gy/s) as a single fraction 15 Gy was compared with conventional dose rate (≤0.03 Gy/s) in an orthotopic lung cancer model6. The conventional dose rate caused lung fibrosis along with activation of the immunosuppressive cytokine TGF-β. There was no fibrosis observed below 20 Gy of FLASH radiotherapy. Consequently, it was possible to escalate the dose in FLASH sufficiently to achieve 70% survival in mice with TC-1 Luc+ orthotopic lung tumors whereas it was not possible with conventional dose rate. An in vitro study of normal human lung fibroblasts also found reduced expression of TGFβ after FLASH compared to conventional dose rate irradiation with 4.5 MeV protons 13 Recently, a dose escalation clinical trial in cats found that a single dose of 25–41 Gy FLASH RT achieved complete remission without dose limiting toxicity in 6 domestic cats treated for spontaneous squamous cell cancer of the nasal planum17. However, the effect of FLASH RT on the immune cells in the tumor microenvironment has not been reported. It is speculated that the reduced exposure time of FLASH may spare more circulating lymphocytes than conventional dose rate RT64.
The immunomodulatory role of high dose lattice radiation therapy was evaluated in a murine cancer model65. A dose of 20 Gy LRT was delivered locally to 20% volume of subcutaneous model of LLC1 lung tumor while the second tumor was not irradiated. This 20% volume irradiation demonstrated reduction in volume in both irradiated and unirradiated tumors. There were increases in CD3+ T cell infiltration in both tumors of LRT group compared with untreated tumor in the open field group. The reduced levels CD3+ T cells in un-irradiated tumors suggest that open field radiation kills these circulating CD3+ T cells before they can infiltrate unirradiated tumors. Significant increases in serum Th1 IFNγ and reduction in Th2 cytokines IL-4 and IL-10 compared with untreated group were observed after 3 days of tumor radiation. Using a 67NR breast cancer model, Markovsky et al 66 observed similar growth delays when 100% or 50% of the tumor volume was irradiated with a single dose of 10Gy or 20Gy using X-RAD 225C with a 2X2 collimator. There were Infiltrations of CD8+T cells in both irradiated and non-irradiated parts of the tumor at 24 hours after 10Gy irradiation. The non-irradiated part of the tumor showed a significant increase in endothelial adhesion molecule ICAM that is critical for T cell filtration. Similar results were observed in LLC1 lung tumors that were irradiated with 15Gy.
Clinical studies
In early stage non-small cell lung cancer (NSCLC), a study found increases in activated CD4+ and CD8+ T cells with decrease in CD4+ FOXP3+ Treg cells in the circulation of patients who received SABR of 48 Gy in 6–8 fractions (6–8 Gy/fractions) 67 Increases in the number of CD8+ T cells, CD4+ T cells and decreases in CD4+CD25+FOXP3+ Treg were observed after SBRT of the lungs68. A small study investigated radiation induced lymphopenia in patients with stage I - II NSCLC who received small-field hypofractionated stereotactic ablative radiotherapy. The degree of drop in absolute lymphocyte counts after SABR compared with pre-SABR levels were associated with overall survival, disease free survival, distant progression-free survival and local progression free survival 69. Lymphopenia was also observed in NSCLC patients who received SABR 70
The effect of radiation dose to the immune system on local tumor control and overall survival was evaluated in patients stage III NSCLC enrolled in a randomized phase III clinical trial (RTOG0617) with CFRT and cetuximab. Higher radiation dose to the immune system correlated with poorer survival 71. Recently, a secondary dosimetric analyses of patients in the RTOG0617 trial receiving CFRT with chemotherapy for stage III NSCLC showed similar results. Patients who received high estimated doses of radiation to immune cells (EDRIC) had shorter survival than those who received low-estimated doses. This suggests that radiation to circulating immune cells causes lymphopenia, and may be an important predictor of tumor control and survival 72. The clinical outcome of radiation induced lymphopenia has also been reported in breast cancer patients 73
Tumor biopsies from patients with cervical cancer after 10 Gy, 20 Gy and 30 Gy local irradiation showed reduction in CD4+ and CD8+ T cells in tumor tissue as compared to the levels before irradiation. Interestingly, there were no differences in the numbers of FOXP3+Treg cells before and after irradiation, indicating that FOXP3+ Treg cells were more resistant to ionizing radiation than T cells74. This study suggests that radiation induced killing of CD8+ T cells may compromise local-anti-tumor response.
Recently a clinical study evaluated the systemic immune responses following SABR to lung, liver, bone and brain. The study found increases in CD4+ memory T cells in peripheral blood with increased expression of ICOS and CD25 activation markers after SABR to parenchymal sites but not in brain or bone. There were no changes in the memory CD8+ T cell compartment 75 ICOS+CD4+ T cells have been associated with improved clinical outcomes in anti-CTLA4 and anti-OX40 immunotherapies76,77.
The first in human FLASH RT treatment has now been reported, in which patient with multi-resistant CD30+ T-cell cutaneous lymphoma received a single fraction of 15 Gy delivered in 90 ms using 5.6 MeV electrons to a symptomatic forearm tumor. This produced rapid, complete tumor control without clinically significant toxicity at 5 months of follow-up, in contrast to protracted wound healing after prior courses of CFRT 78
A pilot study used GRID therapy as a palliative treatment for sarcomas, recurrent gastrointestinal cancers, liver metastases, melanoma, prostate cancer, renal cell carcinoma and squamous cell carcinoma79. A single radiation dose ranging from 10–15 Gy was given using a grid consisting of 50% open and 50% closed areas. Palliation of symptoms and objective response was observed in 20 out of 22 patients without acute effects. In a follow up study 80, two groups of head and neck cancer patients were treated with a conventional external beam median radiation dose of 70Gy and GRID therapy (15Gy) (Group 1) or 59Gy and GRID therapy (15Gy) to the neck disease followed by planned surgery (Group 2). Local regional control was 93% and disease specific survival was 50% in Group 1 at a median follow up of 10 months, while in Group 2 pathologic complete response and disease specific survival were both 85% and local control was 92% at a median follow up of 38 months. A recent review summarizes clinical trials in SFRT20. In a recent study, 10 patients with voluminous NSCLC were treated with initial LRT fraction of 18 Gy in the vertices and 3 Gy in the periphery followed by conventional radiation of 25 to 29 daily fractions of 1.8 Gy – 2 Gy. There was no LRT related toxicity with 42 % mean decrease in tumor volume81. The immune parameters were not evaluated in this study.
Future perspectives
There is profound clinical interest in optimizing radiation therapy for maximum anti-tumor immune responses. Novel ablative radiation therapy paradigms include stereotactic ablative radiotherapy, FLASH, and spatially fractionated radiation therapy. Perhaps heresies of classical radiobiology, may produce better immunologic synergy than conventional radiation therapy fractionation, dose rate, and targeting. Although preclinical studies have provided insights into the immuno-stimulatory role of ablative radiation therapy, there is a relative lack of clinical studies that correlate radiation fractionation and dose-dependent induction of immune responses with patient outcomes. Hence, there is a compelling need for translational studies to evaluate the anti-tumor responses through immune monitoring of large cohorts of patients treated with SABR, FLASH, and SFRT as they mature or begin to enter clinical use, to determine the immune profile and biomarkers that are associated with long term remissions or disease recurrences. The knowledge obtained from such studies would assist in designing future clinical trials that will ultimately help realize the hope of optimal synergy between radiation therapy and anti-tumor immunity.
Acknowledgments
Financial Support: This work was supported in part by a grant from National Cancer Institute to E Engleman and BW Loo (1R01CA233958-01A1).
Disclosures: BWL receives research funding from Varian Medical Systems, and is a board member of TibaRay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leksell L Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry 46:797–803, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shultz DB, et al. To SABR or not to SABR? Indications and contraindications for stereotactic ablative radiotherapy in the treatment of early-stage, oligometastatic, or oligoprogressive non-small cell lung cancer. Semin Radiat Oncol 25:78–86, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Shah JL, et al. Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. Semin Radiat Oncol 27:218–228, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Lee P, et al. Local Control After Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollom EL, et al. Normal Tissue Constraints for Abdominal and Thoracic Stereotactic Body Radiotherapy. Semin Radiat Oncol 27:197–208, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Favaudon V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 6:245ra293, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Schuler E, et al. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int J Radiat Oncol Biol Phys 97:195–203, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Jaccard M, et al. High dose-per-pulse electron beam dosimetry: Commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys 45:863–874, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Lempart M, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiother Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 10.Lansonneur P, et al. Simulation and experimental validation of a prototype electron beam linear accelerator for preclinical studies. Phys Med 60:50–57, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Montay-Gruel P, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 129:582–588, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Patriarca A, et al. Experimental Set-up for FLASH Proton Irradiation of Small Animals Using a Clinical System. Int J Radiat Oncol Biol Phys 102:619–626, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Buonanno M, et al. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montay-Gruel P, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 124:365–369, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Montay-Gruel P, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A 116:10943–10951, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons DA, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 17.Vozenin MC, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res 25:35–42, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Loo BW, et al. Delivery of Ultra-Rapid Flash Radiation Therapy and Demonstration of Normal Tissue Sparing After Abdominal Irradiation of Mice. International Journal of Radiation Oncology Biology Physics 98:E16–E16, 2017. [Google Scholar]
- 19.Maxim PG, et al. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 20.Billena C, et al. A Current Review of Spatial Fractionation: Back to the Future? Int J Radiat Oncol Biol Phys 104:177–187, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasanna A, et al. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J Thorac Dis 6:287–302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathishkumar S, et al. The impact of TNF-alpha induction on therapeutic efficacy following high dose spatially fractionated (GRID) radiation. Technol Cancer Res Treat 1:141–147, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, et al. Application of Spatially Fractionated Radiation (GRID) to Helical Tomotherapy using a Novel TOMOGRID Template. Technol Cancer Res Treat 15:91–100, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114:589–595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filatenkov A, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 21:3727–3739, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed MM, et al. Workshop Report for Cancer Research: Defining the Shades of Gy: Utilizing the Biological Consequences of Radiotherapy in the Development of New Treatment Approaches-Meeting Viewpoint. Cancer Res 78:2166–2170, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudqvist NP, et al. Radiotherapy and CTLA-4 Blockade Shape the TCR Repertoire of Tumor-Infiltrating T Cells. Cancer Immunol Res 6:139–150, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herter-Sprie GS, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 1:e87415, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliru ML, et al. Radiation therapy and immunotherapy: what is the optimal timing or sequencing? Immunotherapy 10:299–316, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker J, et al. Radiotherapy and Immunotherapy-Shining Further Together. JAMA Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 31.Grassberger C, et al. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 32.Toulany M Targeting DNA Double-Strand Break Repair Pathways to Improve Radiotherapy Response. Genes (Basel) 102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baskar R, et al. Biological response of cancer cells to radiation treatment. Front Mol Biosci 1:24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, et al. The renaissance of anti-neoplastic immunity from tumor cell demise. Immunol Rev 280:194–206, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Lauber K, et al. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol 2:116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu B, et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354:765–768, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng L, et al. From DNA Damage to Nucleic Acid Sensing: A Strategy to Enhance Radiation Therapy. Clin Cancer Res 22:20–25, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 41:843–852, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang H, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun 8:1736, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 8:15618, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Frey B, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem 19:1751–1764, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Deng L, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaipl US, et al. Kill and spread the word: stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy 6:597–610, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Rainaldi G, et al. Induction of apoptosis or necrosis by ionizing radiation is dose-dependent in MG-63 osteosarcoma multicellular spheroids. Anticancer Res 23:2505–2518, 2003. [PubMed] [Google Scholar]
- 46.Wu Q, et al. Modulating Both Tumor Cell Death and Innate Immunity Is Essential for Improving Radiation Therapy Effectiveness. Front Immunol 8:613, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423–434, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol 9:353–363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman RM, et al. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 191:411–416, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Q, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17:860–866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutt S, et al. Accelerated, but not conventional, radiotherapy of murine B-cell lymphoma induces potent T cell-mediated remissions. Blood Adv 2:2568–2580, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heylmann D, et al. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta 1846:121–129, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Deloch L, et al. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front Oncol 6:141, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manda K, et al. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol 2:102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakraborty M, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 64:4328–4337, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 174:7516–7523, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Dovedi SJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74:5458–5468, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Fujimura T, et al. Tumor-Associated Macrophages: Therapeutic Targets for Skin Cancer. Front Oncol 8:3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Meir H, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 6:e1267095, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostrand-Rosenberg S, et al. Radiotherapy Both Promotes and Inhibits Myeloid-Derived Suppressor Cell Function: Novel Strategies for Preventing the Tumor-Protective Effects of Radiotherapy. Front Oncol 9:215, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan J, et al. Targeting Myeloid-derived Suppressor Cells and Programmed Death Ligand 1 Confers Therapeutic Advantage of Ablative Hypofractionated Radiation Therapy Compared With Conventional Fractionated Radiation Therapy. Int J Radiat Oncol Biol Phys 101:74–87, 2018. [DOI] [PubMed] [Google Scholar]
- 64.Durante M, et al. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol 91:20170628, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanagavelu S, et al. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat Res 182:149–162, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Markovsky E, et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. Int J Radiat Oncol Biol Phys 103:697–708, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Goeje PL, et al. Stereotactic Ablative Radiotherapy Induces Peripheral T-Cell Activation in Patients with Early-Stage Lung Cancer. Am J Respir Crit Care Med 196:1224–1227, 2017. [DOI] [PubMed] [Google Scholar]
- 68.Rutkowski J, et al. Changes in systemic immune response after stereotactic ablative radiotherapy. Preliminary results of a prospective study in patients with early lung cancer. Pol Arch Intern Med 127:245–253, 2017. [DOI] [PubMed] [Google Scholar]
- 69.Kuo P, et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin Cancer Res 20:5558–5569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maehata Y, et al. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int 2013:731346, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin JY, et al. Higher Radiation Dose to Immune System is Correlated With Poorer Survival in Patients With Stage III Non-small Cell Lung Cancer: A Secondary Study of a Phase 3 Cooperative Group Trial (NRG Oncology RTOG 0617). International Journal of Radiation Oncology Biology Physics 99:S151–S152, 2017. [Google Scholar]
- 72.Ladbury CJ, et al. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys 2019. [DOI] [PubMed] [Google Scholar]
- 73.Meyer KK Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg 101:114–121, 1970. [DOI] [PubMed] [Google Scholar]
- 74.Qinfeng S, et al. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res 179:584–589, 2013. [DOI] [PubMed] [Google Scholar]
- 75.McGee HM, et al. Stereotactic Ablative Radiation Therapy Induces Systemic Differences in Peripheral Blood Immunophenotype Dependent on Irradiated Site. Int J Radiat Oncol Biol Phys 101:1259–1270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng Tang D, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 1:229–234, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Metzger TC, et al. ICOS Promotes the Function of CD4+ Effector T Cells during Anti-OX40-Mediated Tumor Rejection. Cancer Res 76:3684–3689, 2016. [DOI] [PubMed] [Google Scholar]
- 78.Bourhis J, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 79.Mohiuddin M, et al. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation. A pilot study. Cancer 66:114–118, 1990. [DOI] [PubMed] [Google Scholar]
- 80.Huhn JL, et al. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat 5:607–612, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Amendola BE, et al. Safety and Efficacy of Lattice Radiotherapy in Voluminous Non-small Cell Lung Cancer. Cureus 11:e4263, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]