Abstract
Objectives
Using a prospective research design, we evaluated the association between acquisition of diarrhoeagenic Escherichia coli (DEC) and development of reactive arthritis (ReA) and other reactive musculoskeletal (MSK) symptoms among international travellers.
Methods
A total of 526 study participants were asked to provide pretravel and post-travel stool samples and fill in questionnaires (pretravel, post-travel and 3-week follow-up). A multiplex quantitative PCR assay was deployed to detect five DEC comprising enteroaggregative E. coli, enteropathogenic E. coli, enterotoxigenic E. coli, enterohaemorrhagic E. coli and enteroinvasive E. coli and Salmonella, Shigella, Campylobacter, Yersinia, and Vibrio cholerae. Multivariate analysis was employed to identify factors predisposing to MSK symptoms. New post-travel MSK symptoms reported by participants with DEC were assessed by phone interviews and, if needed, clinically confirmed.
Results
From among the total of 224 volunteers who returned all questionnaires and stool specimens, 38 (17.0%) reported MSK symptoms. Multivariate analysis revealed that acquisition of DEC was associated with MSK symptoms (OR 3.9; 95% CI 1.2 to 13.3). Of the 151 with only-DEC, four (2.6%) had ReA, two (1.3%) reactive tendinitis and three (2.0%) reactive arthralgia. ReA was mostly mild, and all patients with ReA were negative for human leucocyte antigen B27. Antibiotic treatment of travellers’ diarrhoea did not prevent development of MSK symptoms.
Conclusion
A total of 17% of volunteers reported post-travel MSK symptoms. DEC acquisition was associated with an increased risk of developing them, yet the ReA incidence remained low and the clinical picture mild. Antibiotic treatment did not protect against development of MSK symptoms.
Keywords: reactive arthritis, infections, arthritis, spondyloarthritis
Key messages.
What is already known about this subject?
Enteropathogenic bacteria triggering reactive arthritis (ReA) are often contracted while visiting (sub)tropical countries in particular. Although diarrhoeagenic Escherichia coli (DEC) are the most common pathogens causing travellers’ diarrhoea (TD), studies are lacking of whether DEC acquisition is associated with musculoskeletal (MSK) symptoms.
Data are needed on how antibiotic treatment of TD influences development of MSK symptoms.
What does this study add?
This is the first prospective study employing multivariate analysis to: (1) investigate whether reactive MSK symptoms are associated with acquisition of DEC and (2) determine incidence of MSK symptoms and ReA among travellers with DEC.
Acquisition of DEC predisposes to development of MSK symptoms.
Development of MSK symptoms does not depend on the severity of TD.
Antibiotic use does not prevent development of MSK symptoms.
How might this impact on clinical practice or future developments?
Acquisition of DEC predisposes to development of MSK symptoms. Antibiotic use does not prevent them. Therefore, concern about potential MSK symptoms does not justify antibiotic treatment for TD.
As no vaccines are available, it appears that, today, the only reasonable approaches to prevent MSK symptoms are those decreasing the rate of exposure to intestinal pathogens.
Introduction
Reactive arthritis (ReA) is a sterile arthritis typically appearing subsequent to a gastrointestinal or genitourinary infection. Salmonella, Yersinia, Shigella and Chlamydia trachomatis are considered the classical causative agents, but also Campylobacter and Escherichia coli can trigger ReA.1–6 These bacteria are usually contracted while visiting (sub)tropical countries. Although diarrhoeagenic E. coli (DEC) are the most common pathogens causing travellers’ diarrhoea (TD),7–11 association between DEC acquisition and musculoskeletal (MSK) symptoms has not been explored to date in prospective studies among travellers recruited before departure.
Up to 78% of travellers with TD contract DEC, but asymptomatic carriage is not rare either.8–11 There are five pathotypes of DEC: enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC) and enterohaemorrhagic E. coli (EHEC).12 EIEC is closely related to Shigella, indistinguishable from it by quantitative PCR (qPCR). While it is well known that Shigella triggers ReA,13 the various DEC require more research.
In ankylosing spondylitis (AS), both microscopic intestinal inflammation14 and increased IgA levels against E. coli 15 16 have been described. AS and ReA both belong to spondylarthritides and may have similar aetiological and pathogenetic mechanisms.17 In animal experiments, immunisation with E. coli O:14 and its lipopolysaccharide (LPS) has induced chronic arthritis, with LPS and interleukin 1 detected in synovial and pannus cells in arthritic joints.18 Furthermore, a higher frequency of E. coli-reactive Th1 cells have been found in synovial fluid among patients with AS than those with rheumatoid arthritis, suggesting involvement of mucosal antigens in the pathogenesis of AS.19
Routine diagnostics of TD previously only covered Salmonella, Shigella, Campylobacter and Yersinia; EHEC 0157 and Clostridium difficile were analysed on request. Consequently, DEC, the most common TD pathogens, often remained unrecognised.20 Recently, however, multiplex qPCR methods covering a large variety of TD pathogens have revolutionised the diagnostics.9 11 20 21 EPEC, EAEC and ETEC are the three most common TD pathogens worldwide, while Campylobacter ranks as major pathogen only among travellers to Southeast Asia; Salmonella and Shigella are less frequent.7 11 21 22
Some studies have described ReA triggered by E. coli following urinary tract3 23 24 and gastrointestinal infections.4–6 25 However, the studies are few, their participants were not recruited prospectively before travel, controls with no pathogen findings were not included and the various DEC (particularly EAEC) were mostly not covered.
The first part of our bipartite traveller study examined the rate of MSK symptoms, seeking to identify travel-related factors predisposing to/preventing them in a prospective study design encompassing all the various DEC. The second part investigated the incidence and clinical picture of ReA.
Methods
Study design and patient involvement
This research was conducted parallel to a larger prospective investigation of the aetiology and clinical features of TD.11 20 21 The current study is the first to present data on its rheumatological findings. The subjects were recruited at their pretravel visits to the Travel Clinic of Aava Medical Centre, Helsinki, between March 2009 and February 2010. The volunteers were asked to provide pretravel and post-travel stool samples and to complete three questionnaires (pretravel, post-travel and 3-week follow-up). The questionnaires collected information on travel characteristics, symptoms (eg, TD and MSK) and medications (antibiotics, antidiarrhoeals and so on). We have published a detailed description of the questionnaires and data on behaviour and symptoms.26
Stool samples were analysed with a qPCR for nine bacterial pathogens: Salmonella, Yersinia, Campylobacter, Vibrio cholerae, Shigella/EIEC, EHEC, ETEC, EAEC and EPEC.20 The findings were categorised into four groups: (1) no pathogens, (2) only-DEC (no other pathogens but EPEC, EAEC, ETEC or EHEC), (3) only non-DEC (no DEC but Shigella/EIEC, Salmonella, Campylobacter, Yersinia or V. cholerae), and (4) DEC+non-DEC. To ensure that the only-DEC group would include no other pathogens, we categorised Shigella/EIEC (not distinguishable by qPCR) as non-DEC, Shigella a known risk factor of ReA.13 We report separately on the MSK symptoms of those with Shigella/EIEC.
The inclusion criteria comprised providing all three questionnaires and both pretravel and post-travel stool samples. For the first part, we included all such participants, and for the second part, those with only DEC in post-travel specimen and MSK symptoms in 3-week follow-up with questionnaires (figure 1). If no other explanation for MSK symptoms was evident (eg, osteoarthritis and trauma), we contacted the volunteers by telephone for a comprehensive interview and, if needed, invited them to visit a rheumatologist for clinical assessment. The phone interview covered their history of MSK diseases and a detailed account of MSK symptoms. Those requested for an appointment were subjected to clinical examination and analyses of human leucocyte antigen B27 (HLA-B27), rheumatoid factor (RF) and C reactive protein (CRP).
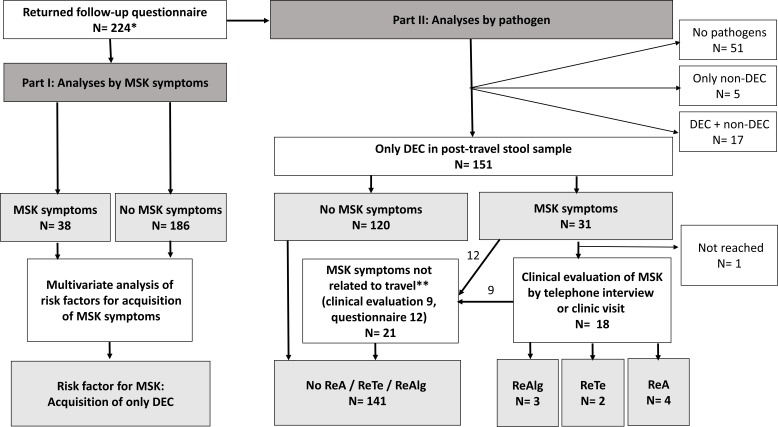
Figure 1.
Study flow chart. *Subjects who returned questionnaire and stool sample before and after journey plus follow-up questionnaire. **Osteoarthritis, similar symptoms before journey. DEC, diarrhoeagenic Escherichia coli; MSK, musculoskeletal; ReA, reactive arthritis; ReTe, reactive enthesitis and tendinitis; ReAlg, reactive arthralgia
Written informed consent was obtained from all participants. The participants were not asked to assess the burden of the intervention and time required to participate in the research.
Diagnostic criteria
ReA was defined as the development of synovitis (swelling accompanied by pain and/or painful movement) in a previously asymptomatic joint within 2 months after gastrointestinal symptoms. Inflammatory back pain (low back pain worsening at night) was also regarded as ReA.27 Reactive tendinitis and enthesopathy (ReTe) were defined as tendinitis, heel or elbow pain, and limitation of joint movement without joint swelling, and reactive arthralgia (ReAlg) as pain in a previously asymptomatic joint not swollen and with a normal movement range. MSK symptoms among participants with previous rheumatological diagnosis were not defined as travel acquired.
TD was defined according to WHO criteria for diarrhoea.11 28 Mild TD was defined as 1–2, moderate TD as 3–5 and severe TD as 6 or more loose or liquid stools or diarrhoea accompanied by fever and/or bloody stools or requiring hospitalisation.11
Statistical analysis
Data were analysed with SPSS software, V.22.0. Categorical variables were analysed by Pearson’s χ² test, Fisher’s exact test or binary logistic regression analysis when applicable. The following variables were subjected to multivariate analysis: gender, age as categorical variable, pathogen finding in post-travel stool sample (only-DEC, only non-DEC, DEC and non-DEC and no pathogens); severity of TD; and antibiotic use. Statistical significance was defined as p<0.05 or 95% CIs ranging only either above or below 1.
Results
The first part of this bipartite study concerns risk factors of post-travel MSK symptoms, while the second describes the nature and incidence of clinical findings among those with DEC as the only travel-acquired pathogens. Our travellers visited sub-Saharan Africa (48.2%); Southeast Asia (21.4%); South Asia (10.7%); South and Central America and the Caribbean (10.7%); Europe, North America or Australia (5.8%); North Africa and Middle East (2.7%); and East Asia (0.4%).
Pathogen findings among all 224 travellers
Of the 526 subjects initially recruited,21 224 returned all three questionnaires and provided both stool samples. In pretravel specimens, the volunteers had few positive findings: six had (2.7%) EPEC, four (1.8%) EAEC and one (0.5%) Campylobacter. Only the participant with Campylobacter developed MSK symptoms. As for post-travel samples, 173 (77.2%) participants had at least one pathogen and 86 (38.5%) multiple types of pathogens: DEC was detected for 168 (75.0%); Shigella/EIEC for 3 (1.3%); Salmonella for 6 (2.7%); and Campylobacter for 13 (5.8%) (table 1). DEC were the only pathogens identified for 151 (67.4%), only non-DEC were detected for 5 (2.2%), DEC and non-DEC for 17 (7.6%) and no pathogens for 51 (22.8%). Among all of those with MSK and a DEC finding in post-travel stools, the DEC were travel acquired (no DEC in pretravel stools).
Table 1.
Pathogen findings in all 224 post-travel stool samples* and for 38 participants reporting MSK symptoms in the follow-up questionnaire
| Participants with post-travel stool findings N | Participants reporting post-travel MSK symptoms among participants with stool findings indicated, N (%) | |
| Total | 224 | 38 (17.0) |
| No pathogens | 51 | 4 (7.8) |
| Any pathogen | 173 | 34 (19.7) |
| Multiple types of pathogens | 86 | 18 (20.9) |
| EPEC | 99 | 20 (20.2) |
| ETEC | 45 | 8 (17.8) |
| EAEC | 104 | 21 (20.2) |
| EHEC | 20 | 5 (25.0) |
| Shigella/EIEC | 3 | 1 (33.3) |
| Salmonella | 6 | 1 (16.7) |
| Campylobacter | 13 | 1 (7.7) |
*Six (2.7%) pretravel stool samples were positive for EPEC, four (1.8%) for EAEC and one (0.5%) for Campylobacter; of these, solely a participant with Campylobacter in pretravel stools developed MSK. EAEC was found as the sole pathogen in the same individual’s post-travel stools.
EPEC enteropathogenic Escherichia coli; EAEC, enteroaggregative Escherichia coli; EHEC, enterohaemorrhagic Escherichia coli; EIEC, enteroinvasive Escherichia coli; ETEC, enterotoxigenic Escherichia coli; MSK, musculoskeletal.
Part one
Risk factors of post-travel MSK symptoms
Of all 224 participants, 38 (17.0%) reported MSK symptoms in the follow-up questionnaire (tables 1 and 2). In total, 155 (69.2%) contracted TD during the journey and 24 (15.5%) treated it with antibiotics. Scrutinising the relation between MSK symptoms and TD, we found that 18.7% (29/155) of those with and 13.0% (9/69) of those without TD reported MSK symptoms (OR 1.5, 95% CI 0.7 to 3.4; p=0.297). A closer look at antibiotic use among those 109 with TD during travel and only DEC in stools showed MSK symptoms for 27.3% (3/11) of those taking antibiotics and 19.4% (19/98) of those not taking (OR 1.6; 95% CI 0.4 to 6.4, p=0.691).
Table 2.
Risk factors of musculoskeletal (MSK) symptoms
| Total* | MSK +† | MSK –† | Univariate statistics | Multivariable statistics | |||
| P value | OR (95% CI) | P value | aOR (95% CI) | ||||
| n (%) | n (%) | n (%) | |||||
| Total | 224 | 38 (17.0) | 186 (83.0) | ||||
| Gender | |||||||
| Male | 83 (37.1) | 9 (10.8) | 74 (89.2) | reference | 1.0 | reference | 1.0 |
| Female | 141 (62.9) | 29 (20.6) | 112 (79.4) | 0.061 | 2.1 (1.0 to 4.8) | 0.117 | 2.0 (0.8 to 4.9) |
| Age | |||||||
| Mean±SD range | 39.5+/-16.7 (0–72) |
48.1+/-12.7 (21–67) |
37.7+/-16.9 (0–72) |
||||
| Age group (years) | |||||||
| 0–17 | 20 (8.9) | 0 (0) | 20 (100) | n/a | n/a | ||
| 18–30 | 57 (25.4) | 5 (8.8) | 52 (91.2) | 0.656 | 0.7 (0.1 to 3.8) | 0.353 | 0.4 (0.1 to 2.6) |
| 31–50 | 85 (37.9) | 14 (16.5) | 71 (83.5) | 0.691 | 1.4 (0.3 to 6.8) | 0.897 | 1.1 (0.2 to 5.9) |
| 51–64 | 46 (20.5) | 17 (37.0) | 29 (63.0) | 0.083 | 4.1 (0.8 to 20.3) | 0.131 | 3.6 (0.7 to 19.0) |
| Over 65 | 16 (7.1) | 2 (12.5) | 14 (87.5) | reference | 1.0 | reference | 1.0 |
| Pathogen finding | |||||||
| Negative | 51 (22.8) | 4 (7.8) | 47 (92.2) | reference | 1.0 | reference | 1.0 |
| Only-DEC | 151 (67.4) | 31 (20.5) | 120 (79.5) | 0.047 | 3.0 (1.0 to 9.1) | 0.030 | 3.9 (1.1 to 13.2) |
| Only non-DEC | 5 (2.2) | 0 (0) | 5 (100) | n/a | n/a | n/a | n/a |
| DEC+non-DEC | 17 (7.6) | 3 (17.6) | 14 (82.4) | 0.085 | 2.5 (0.5 to 12.6) | 0.111 | 4.5 (0.7 to 27.9) |
| TD during travel | |||||||
| No TD | 69 (30.8) | 9 (13.0) | 60 (87.9) | reference | 1.0 | reference | 1.0 |
| Mild TD | 53 (23.7) | 9 (17.0) | 44 (83.0) | 0.544 | 1.4 (0.5 to 3.7) | 0.571 | 1.4 (0.5 to 4.2) |
| Moderate TD | 62 (27.7) | 14 (22.6) | 48 (77.4) | 0.156 | 1.9 (0.8 to 4.9) | 0.272 | 1.8 (0.6 to 5.1) |
| Severe TD | 40 (17.9) | 6 (15.0) | 34 (85.0) | 0.775 | 1.2 (0.4 to 3.6) | 0.585 | 1.4 (0.4 to 5.3) |
| Antibiotic use during travel‡ | |||||||
| No AB | 195 (87.1) | 32 (16.4) | 163 (87.6) | reference | 1.0 | reference | 1.0 |
| AB for TD | 24 (10.7) | 5 (20.8) | 19 (79.2) | 0.586 | 1.3 (0.5 to 3.9) | 0.311 | 2.0 (0.5 to 7.1) |
| AB other | 5 (2.2) | 1 (20.0) | 4 (80.0) | 0.831 | 1.3 (0.1 to 11.8) | 0.460 | 2.5 (0.2 to 28.4) |
*Percentages refer to proportion of risk factors.
†Percentages refer to proportion of participants with and without MSK symptoms for each risk factor.
‡Antibiotic regimens: 18 fluoroquinolones, 4 macrolides, 2 third-generation cephalosporins, 3 unknown and 5 others.
AB, antibiotic; aOR, adjusted OR; DEC, diarrhoeagenic Escherichia coli; TD, travellers' diarrhoea.
In multivariate analysis, the findings of only-DEC were associated with MSK symptoms (aOR 3.9, 95% CI 1.2 to 13.3). By contrast, no association was observed for TD, severity of TD, antibiotic use, age or gender (table 2).
Part two
MSK symptoms among travellers with only-DEC
Of the 151 participants with only-DEC in post-travel stools, 31 (20.5%) reported MSK symptoms; one was not reached. Perusing the questionnaires, the symptoms of 12 participants were either considered unrelated to travel (eg, similar joint symptoms before journey) or ascribed to something else (eg, trauma and infection). Eighteen were interviewed by phone or invited for a clinic visit. Of these, nine had MSK symptoms related to some other disorder (eg, osteoarthritis). The joint symptoms of nine participants were considered newly acquired.
Three participants with Shigella/EIEC were not included in the only-DEC group, as described above. One reported MSK symptoms and was diagnosed with ReAlg in clinical examination.
Clinical evaluation of nine participants with newly acquired MSK symptoms and DEC as only pathogen
Of the nine participants with reactive MSK symptoms, four met the criteria for ReA, two had ReTe and for the remaining three, ReAlg remained as diagnosis. The symptoms had appeared on average 21.5 (SD 23.5, range 0–56) days after TD. The mean duration of MSK symptoms was 82.8 days (SD 92.9, range 2–300).
The mean age was 47.7 years (SD 7.6, range 30–56). Six (66.7%) were women and two had a history of ReA (one had ReA and the other ReTe in this study). Eight (88.9%) reported TD during travel. None of the nine participants had contacted healthcare due to TD, nor taken antibiotics. One (11.1%) reported urogenital symptoms during travel; none reported extra-articular symptoms like uveitis or erythema nodosum.
Of the nine participants, seven (including all four with ReA) were evaluated at the rheumatology clinic on average 5.9 months (SD 2.0, range 4–8) after the onset of joint symptoms. The clinical picture was mostly mild. The affected joints of the four ReA patients were knees, shoulder, elbow, wrist, fingers and toes (table 3). At the time of clinical examination, only one patient with ReA still had synovitis 8 months after symptom onset and needed an intra-articular corticosteroid injection (left MTP I). Only one patient with ReA reported fever during diarrhoea, and three patients had had mild TD. Only one with ReTe was HLA-B27 positive, while all four with ReA were HLA-B27 negative. The CRP values ranged between 3 mg/L and 6 mg/L. RF was negative for all seven.
Table 3.
Clinical picture of the four patients with reactive arthritis (ReA)
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age, years | 48 | 47 | 50 | 50 |
| Gender (M/F) | M | F | F | F |
| Pathogen | EPEC EAEC |
EPEC | EPEC | EPEC |
| Affected joints | Knees | Toe; finger | Feet; knees; elbows | Shoulder; elbow; wrist |
| Joint swelling | − | + | − | + |
| Arthtralgia | + | + | + | + |
| Low back pain | + | − | + | − |
| HLA-B27 antigen | − | − | − | − |
| Duration of ReA (months) | 2 | 10 | 3 | 1 |
EAEC, enteroaggregative E.coli; EPEC, enteropathogenic Escherichia coli; F, female; M, male; ReA, reactive arthritis.
Seven of the nine (77.8%) participants with ReA/ReTe/ReAlg had EPEC in their post-travel stools (table 4). Two had multiple pathogens, one EPEC and EAEC and the other EPEC and ETEC. All four with ReA had EPEC, one also had EAEC. None had EHEC.
Table 4.
Distribution of bacterial pathogens among patients with reactive musculoskeletal symptoms (n=9)
| ReA n=4* | ReTe n=2 | ReAlg n=3† | |
| EPEC | 4 | 1 | 2 |
| ETEC | 0 | 1 | 1 |
| EAEC | 1 | 0 | 1 |
*One patient with two pathogens (EPEC+EAEC).
†One patient with two pathogens (EPEC+ETEC).
EAEC, enteroaggregative Escherichia coli ; EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli ; ReA, reactive arthritis; ReAlg, reactive arthralgia; ReTe, reactive tendinitis and enthesitis.
Incidence of ReA, ReTe and ReAlg
On the basis of data on the 151 travellers with DEC as the sole pathogen, nine (6.0%) were diagnosed with travel-related ReA, ReTe or ReAlg, yielding for ReA an incidence of 2.6%, for ReTe 1.3% and for ReAIg 2.0%.
Discussion
This is the first prospective study to have employed multivariate analysis in investigating whether reactive MSK symptoms are associated with acquisition of DEC and determining the incidence of enterogenic ReA among travellers with DEC.
Our main finding was that acquiring DEC is, indeed, associated with development of MSK symptoms. This study features three merits not seen in the four largish investigations that have previously examined DEC as a trigger of reactive joint symptoms4–6 29: we collected stool samples prospectively, included control groups, and covered EAEC, one of the most frequently detected pathogens among travellers with TD (table 5). Besides EAEC, we also covered, using the multiplex qPCR assay, EPEC, ETEC, EIEC and EHEC.
Table 5.
Outline of previous studies assessing musculoskeletal symptoms following Escherichia coli infection
| Study (ref) | Number of patients | Type of DEC | MSK symptoms/ReA n (%) |
Clinical picture/duration of symptoms (months) | HLA-B27 status of those with MSK symptoms/ReA |
| Locht and Krogfelt4 | 177 | ETEC | ReA: 10 (5.6) | Not described / 5.5 | Not examined |
| Rees et al 29 | 22 | E. coli O157:H7 | ReA: 1 (4.5)* | Pain in hands and feet | Not examined |
| Schiellerup et al 5 | 290 | All E. coli | 28 (9.7)/ 3 (1.0) | Median VAS joint pain 35.5 mm | 10% HLA-B27 positive |
| EPEC (n=17; 5.8%) | 1 (5.9) | ||||
| ETEC (n=112; 38.6%) | 8 (7.1) | ||||
| A/EEC (n=138; 47.6%) | 16 (11.6) | ||||
| VTEC (n=23; 7.9%) | 3 (13.0) | ||||
| Townes et al 6 | 395 | E. coli O157 | 35 (8.9)/1 (0.25) | Described among other pathogens together | 12% HLA-B27 positive† |
*Infection with Campylobacter and concomitant diagnosis of ulcerative colitis.
†among all participants with ReA tested, comprising findings of Campylobacter, Salmonella, Shigella, E. coli O157 and Yersinia.
A/EEC, attaching and effacing E. coli (considered EPEC), E. coli O157; DEC, diarrhoeagenic E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; HLA-B27, human leucocyte antigen B27; MSK, musculoskeletal; ReA, reactive arthritis; VAS, visual analogue scale 0-100mm; VTEC, verocytotoxin (shigatoxin) producing E. coli.
In addition to this broad pathogen coverage, our prospective study design with pathogen-positive and pathogen-negative travellers allowed multivariate analysis of predisposing factors. Contrary to previous studies, we found no association between MSK symptoms and age, female sex or severity of TD, all of which have previously been associated with the development of MSK symptoms.2 5 30 31 We also looked at antibiotic treatment of TD, as some travel medicine researchers have speculated that it could prevent postinfectious sequelae such as ReA or irritable bowel syndrome.30 31 We found no association between development of MSK symptoms and antibiotic treatment of TD. This accords with research showing that antibiotic treatment of infections caused by Campylobacter, ETEC or non-typhoidal Salmonellae does not prevent postinfectious joint symptoms.32–35 To our knowledge, the present investigation is the first to scrutinise the relation between antibiotic treatment of TD and MSK symptoms among travellers.
The prospective study design allowed the very first evaluation of the incidence of various MSK symptoms among individuals with travel-acquired DEC. MSK symptoms were recorded for a considerable proportion (20.5%) of those with DEC as the sole post-travel pathogen; the incidence of ReA was 2.6%, ReTe 1.3% and ReAlg 2.0%. These figures resemble those reported for volunteers only recruited after acquisition of some pathogen. For postinfectious joint symptoms, Schiellerup et al 5 report an incidence of 9.7% among patients with DEC—none had definite and three (1.0%) had probable ReA. Townes et al 6 describe ReA for 0.25%, and Rees et al 29 report prolonged joint symptoms for 4.5% of participants with E. coli O157. Locht and Krogfelt4 show joint symptoms among 16% of patients with Campylobacter (n=173) and 6% with ETEC (n=177) over a 6-month follow-up. As for incidence of MSK symptoms among patients with other pathogens, in their systematic review, Ajene et al 36 found ReA in the samples of 0.9%–1.2% of subjects with Salmonella, Shigella or Campylobacter,36 a rate according with that among our participants who had DEC. Finnish studies exploring ReA following infection with Salmonella, Shigella and Campylobacter have reported incidences of 4.5%, 7% and 7%, respectively.2 13 37 In our investigation, the symptoms of ReA mostly remained mild, although one patient had arthritis persisting over 8 months. All our patients with ReA were negative for HLA-B27, a finding according with a previous study, which found no relation between HLA-B27 and MSK symptoms following an infection with E. coli.5 Low prevalence of HLA-B27 and mild clinical course have also been reported among other pathogens triggering ReA in population-based studies.2 6 13 37
In previous studies, the microbe triggering ReA has often remained unidentified. Fendler et al 38 report a causative pathogen in 56% of ReA cases and, on the other hand, among 47% of patients with undifferentiated oligoarthritis without preceding symptoms of infection. Our data show a bacterial pathogen for 87.9% of travellers with MSK symptoms and all of those with ReA. Interestingly, the development of MSK symptoms was not related to the severity of TD but, by contrast, those with MSK symptoms or ReA reported mostly mild or moderate TD. The pathogens were identified only because of participation in this study; travellers mostly do not seek medical care for mild TD symptoms.26 Furthermore, with the exception of EHEC, traditional stool diagnostics fails to detect species of DEC, and therefore, the pathogens would have remained unidentified in laboratories not using qPCR assays with a broad pathogen coverage.9 20 25
In our data, EPEC was the pathogen most frequently detected among those with new MSK symptoms (77.8%) and ReA (100%). Its role in adult TD has remained disputable,9 11 21 yet for this study population, we have previously reported EPEC more frequently among those with than those without TD.11 Despite the considerable prevalence of EAEC among travellers with TD, it has not been covered in the previous studies reporting association between various DEC and ReA or other postinfectious joint symptoms (table 5). We show EAEC for 55.3% (21/38) of the participants with MSK symptoms versus 44.6% (83/186) without them (table 1), and for 25.0% (1/4) with ReA versus 20.0% (1/5) with ReTe and ReAlg (table 4). ETEC deserves special attention, since it is often considered to cause more severe TD than the other DEC.7 It was detected in the specimens of 21.1% of the participants with MSK symptoms and none of those with ReA.
Limitations and strengths
First, as we could not collect stool samples while abroad, the primary TD pathogen may in some cases have already disappeared before sampling. Second, our data may slightly overestimate the incidence of MSK symptoms, since those with symptoms after travel may have been more eager to return the follow-up questionnaire. Third, the time period between primary pathogen acquisition and clinical examination was rather long. Naturally, our lengthy follow-up also enabled us to evaluate the patients’ recovery. However, we may have missed some ReA cases where the joint symptoms appeared later than 3 weeks after travel.
The main merits of our investigation are its prospective design and multiplex qPCR method with a wide coverage of pathogens. They allowed us to compare symptomatic versus asymptomatic participants with and without DEC, to look for an association between MSK symptoms and travel-acquired DEC and estimate the incidence of MSK symptoms. Furthermore, data on antibiotic use afforded a rare opportunity to estimate whether antibiotic treatment of TD could prevent MSK symptoms.
Conclusion
This was the first prospective study to explore whether travel-acquired DEC are associated with reactive MSK symptoms and to provide incidences for such development. Multivariate analysis showed acquisition of DEC to be connected with risk of developing MSK symptoms yet the risk did not depend on severity of TD symptoms. The incidence of ReA proved fairly low and the course mild; no association was seen with HLA-B27. Further investigations are needed to confirm whether ReA triggered by DEC remains milder than that caused by the conventionally known bacteria. Antibiotic treatment of TD did not prevent post-travel MSK symptoms.
Acknowledgments
We wish to express our debt of gratitude to the late Dr Jukka Riutta for recruiting the volunteers. We would also like to thank the nurses at the Travel Clinic of Aava Medical Centre for help, and the personnel of Helsinki University Hospital Laboratory (HUSLAB) for assistance with the stool specimens.
Footnotes
Handling editor: Josef S Smolen
Contributors: Study concept and design: AK and JK; acquisition of data: RT, SHP, JK and AK; analysis and interpretation of results: RT, TL and AK; drafting of manuscript: RT, TL and AK; statistical analysis: TL; critical comments: TH, SHP and ML-R; final approval of version published: all authors.
Funding: The work was supported by the Finnish Governmental Subsidy for Health Science Research, the SSAC Foundation, the Paulo Foundation, the Sigrid Jusélius Foundation, the Finnish Cultural Foundation and the Finnish Society for Rheumatology.
Disclaimer: The funding sources had no involvement in study design, data collection, analysis, interpretation of data, writing of report, and decision to submit the article for publication.
Competing interests: AK has received honorary for lectures (Valneva and Immuron) and investigator-initiated grants (Pfizer and Valneva), none of these relevant to the current manuscript. JK is an employee of Mobidiag developing diagnostics test for infectious diseases. No commercial tests, however, are used in the study.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the Ethics Committee of Helsinki University Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. Any other data are available from the corresponding author.
References
- 1. Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol 2011;25:347–57. 10.1016/j.berh.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 2. Hannu T, Mattila L, Rautelin H, et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology 2002;41:312–8. 10.1093/rheumatology/41.3.312 [DOI] [PubMed] [Google Scholar]
- 3. Laasila K, Leirisalo-Repo M. Recurrent reactive arthritis associated with urinary tract infection by Escherichia coli. J Rheumatol 1999;26:2277–9. [PubMed] [Google Scholar]
- 4. Locht H, Krogfelt KA. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann Rheum Dis 2002;61:448–52. 10.1136/ard.61.5.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiellerup P, Krogfelt KA, Locht H. A comparison of self-reported joint symptoms following infection with different enteric pathogens: effect of HLA-B27. J Rheumatol 2008;35:480–7. [PubMed] [Google Scholar]
- 6. Townes JM, Deodhar AA, Laine ES, et al. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann Rheum Dis 2008;67:1689–96. 10.1136/ard.2007.083451 [DOI] [PubMed] [Google Scholar]
- 7. Steffen R, Hill DR, DuPont HL. Traveler's diarrhea: a clinical review. JAMA 2015;313:71–80. 10.1001/jama.2014.17006 [DOI] [PubMed] [Google Scholar]
- 8. Jiang Z-D, Dupont HL, Brown EL, et al. Microbial etiology of travelers' diarrhea in Mexico, Guatemala, and India: importance of enterotoxigenic Bacteroides fragilis and Arcobacter species. J Clin Microbiol 2010;48:1417–9. 10.1128/JCM.01709-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lertsethtakarn P, Silapong S, Sakpaisal P, et al. Travelers' diarrhea in Thailand: a quantitative analysis using TaqMan® array card. Clin Infect Dis 2018;67:120–7. 10.1093/cid/ciy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paschke C, Apelt N, Fleischmann E, et al. Controlled study on enteropathogens in travellers returning from the tropics with and without diarrhoea. Clin Microbiol Infect 2011;17:1194–200. 10.1111/j.1469-0691.2010.03414.x [DOI] [PubMed] [Google Scholar]
- 11. Lääveri T, Antikainen J, Pakkanen SH, et al. Prospective study of pathogens in asymptomatic travellers and those with diarrhoea: aetiological agents revisited. Clin Microbiol Infect 2016;22:535–41. 10.1016/j.cmi.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 12. Croxen MA, Law RJ, Scholz R, et al. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 2013;26:822–80. 10.1128/CMR.00022-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hannu T, Mattila L, Siitonen A, et al. Reactive arthritis attributable to Shigella infection: a clinical and epidemiological nationwide study. Ann Rheum Dis 2005;64:594–8. 10.1136/ard.2004.027524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mielants H, Veys EM, Cuvelier C, et al. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol 1988;27 Suppl 2:95–105. 10.1093/rheumatology/XXVII.suppl_2.95 [DOI] [PubMed] [Google Scholar]
- 15. Nissilä M, Lahesmaa R, Leirisalo-Repo M, et al. Antibodies to Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in ankylosing spondylitis: effect of sulfasalazine treatment. J Rheumatol 1994;21:2082–7. [PubMed] [Google Scholar]
- 16. Mäki-Ikola O, Nissilä M, Lehtinen K, et al. Antibodies to Klebsiella pneumoniae, Escherichia coli and Proteus mirabilis in the sera of patients with axial and peripheral form of ankylosing spondylitis. Br J Rheumatol 1995;34:413–7. 10.1093/rheumatology/34.5.413 [DOI] [PubMed] [Google Scholar]
- 17. Stolwijk C, Boonen A, van Tubergen A, et al. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am 2012;38:441–76. 10.1016/j.rdc.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noyori K, Okamoto R, Takagi T, et al. Experimental induction of arthritis in rats immunized with Escherichia coli 0:14 lipopolysaccharide. J Rheumatol 1994;21:484–8. [PubMed] [Google Scholar]
- 19. Syrbe U, Scheer R, Wu P, et al. Differential synovial Th1 cell reactivity towards Escherichia coli antigens in patients with ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 2012;71:1573–6. 10.1136/annrheumdis-2012-201404 [DOI] [PubMed] [Google Scholar]
- 20. Antikainen J, Kantele A, Pakkanen SH, et al. A quantitative polymerase chain reaction assay for rapid detection of 9 pathogens directly from stools of travelers with diarrhea. Clin Gastroenterol Hepatol 2013;11:1300–7. 10.1016/j.cgh.2013.03.037 [DOI] [PubMed] [Google Scholar]
- 21. Lääveri T, Vilkman K, Pakkanen SH, et al. A prospective study of travellers' diarrhoea: analysis of pathogen findings by destination in various (sub)tropical regions. Clin Microbiol Infect 2018;24:908.e9–908.e16. 10.1016/j.cmi.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 22. Connor BA, Riddle MS. Post-Infectious sequelae of travelers' diarrhea. J Travel Med 2013;20:303–12. 10.1111/jtm.12049 [DOI] [PubMed] [Google Scholar]
- 23. Thomas DG, Roberton DM. Reiter's syndrome in an adolescent girl. Acta Paediatr 1994;83:339–40. 10.1111/j.1651-2227.1994.tb18110.x [DOI] [PubMed] [Google Scholar]
- 24. Singh Sangha M, Wright ML, Ciurtin C. Strongly positive anti-CCP antibodies in patients with sacroiliitis or reactive arthritis post-E. coli infection: A mini case-series based review. Int J Rheum Dis 2018;21:315–21. 10.1111/1756-185X.13113 [DOI] [PubMed] [Google Scholar]
- 25. Houtman PM, Spoorenberg A, Schuurs TA, et al. Reactive arthritis: does screening stools by polymerase chain reaction for Shiga toxin-producing Escherichia coli (STEC) make sense? Scand J Rheumatol 2012;41:237–9. 10.3109/03009742.2012.656700 [DOI] [PubMed] [Google Scholar]
- 26. Vilkman K, Pakkanen SH, Lääveri T, et al. Travelers' health problems and behavior: prospective study with post-travel follow-up. BMC Infect Dis 2016;16:328 10.1186/s12879-016-1682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev 2014;13:546–9. 10.1016/j.autrev.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization Health topics: diarrhoea. Available: http://www.who.int/topics/diarrhoea/en/
- 29. Rees JR, Pannier MA, McNees A, et al. Persistent diarrhea, arthritis, and other complications of enteric infections: a pilot survey based on California FoodNet surveillance, 1998-1999. Clin Infect Dis 2004;38 Suppl 3:S311–7. 10.1086/381601 [DOI] [PubMed] [Google Scholar]
- 30. Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers' diarrhea: a graded expert panel report. J Travel Med 2017;1:S57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yates JA, Stetz LC. Reiter's syndrome (reactive arthritis) and travelers' diarrhea. J Travel Med 2006;13:54–6. 10.1111/j.1708-8305.2006.00009.x [DOI] [PubMed] [Google Scholar]
- 32. Locht H, Mølbak K, Krogfelt KA. High frequency of reactive joint symptoms after an outbreak of Salmonella enteritidis. J Rheumatol 2002;29:767–71. [PubMed] [Google Scholar]
- 33. Schmitt SK. Reactive arthritis. Infect Dis Clin North Am 2017;31:265–77. 10.1016/j.idc.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 34. Schönberg-Norio D, Mattila L, Lauhio A, et al. Patient-Reported complications associated with Campylobacter jejuni infection. Epidemiol Infect 2010;138:1004–11. 10.1017/S0950268809991099 [DOI] [PubMed] [Google Scholar]
- 35. Esan OB, Pearce M, van Hecke O, et al. Factors associated with sequelae of Campylobacter and Non-typhoidal Salmonella infections: a systematic review. EBioMedicine 2017;15:100–11. 10.1016/j.ebiom.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ajene AN, Fischer Walker CL, Black RE. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella-associated reactive arthritis. J Health Popul Nutr 2013;31:299–307. 10.3329/jhpn.v31i3.16515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tuompo R, Hannu T, Mattila L, et al. Reactive arthritis following Salmonella infection: a population-based study. Scand J Rheumatol 2013;42:196–202. 10.3109/03009742.2012.739201 [DOI] [PubMed] [Google Scholar]
- 38. Fendler C, Laitko S, Sörensen H, et al. Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann Rheum Dis 2001;60:337–43. 10.1136/ard.60.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]