Abstract
The signaling cascade induced by the interaction of erythropoietin (EPO) with its receptor (EPO-R) is a key event of erythropoiesis. We present here data indicating that Fyn, a Src-family-kinase, participates in the EPO signaling-pathway, since Fyn−/− mice exhibit reduced Tyr-phosphorylation of EPO-R and decreased STAT5-activity. The importance of Fyn in erythropoiesis is also supported by the blunted responsiveness of Fyn−/− mice to stress erythropoiesis. Fyn−/− mouse erythroblasts adapt to reactive oxygen species (ROS) by activating the redoxrelated-transcription-factor Nrf2. However, since Fyn is a physiologic repressor of Nrf2, absence of Fyn resulted in persistent-activation of Nrf2 and accumulation of nonfunctional proteins. ROS-induced over-activation of Jak2-Akt-mTOR-pathway and repression of autophagy with perturbation of lysosomal-clearance were also noted. Treatment with Rapamycin, a mTOR-inhibitor and autophagy activator, ameliorates Fyn−/− mouse baseline erythropoiesis and erythropoietic response to oxidative-stress. These findings identify a novel multimodal action of Fyn in the regulation of normal and stress erythropoiesis.
1 |. INTRODUCTION
Erythropoiesis is a complex multistep process during which committed erythroid progenitors undergo terminal differentiation to produce circulating mature red cells. Erythroid differentiation is characterized by the production of reactive oxygen species (ROS) in response to erythropoietin (EPO) and by the large amount of iron imported into the cells for heme biosynthesis.1 During erythropoiesis, ROS could function as second messenger by modulating intracellular signaling pathways. EPO activates a signaling cascade, involving Jak2, as the primary kinase, and Lyn, a Tyr-kinase of the Src family (SFK), as secondary kinase.2–4 These two kinases target STAT5 transcription factor, one of the key master transcription regulators involved in erythroid maturation events.2–5
Previous studies have shown that the mice genetically lacking Lyn (Lyn−/−) display reduced STAT5 activation and defective response to phenylhydrazine- (PHZ) induced stress erythropoiesis.2–4 Fyn, is another member of the SFKs that is also expressed in hematopoietic cells.6–10 Fyn has been invoked as an additional regulatory kinase for the canonical thrombopoietin/Jak2 pathway in megakaryopoiesis.11 In addition, Fyn has been shown to target STAT5 and to participate to STAT5 activation in mast-cells in response to FCRI engagement.8 Furthermore, Fyn intersects different intracellular signaling pathways such as Toll like receptor in macrophages or in T cells12,13 and participates to the regulation of the redox sensitive transcriptional factor Nrf2.14–16 Following acute phase response, Fyn switches-off active Nrf2, triggering its exit from the nucleus and degradation.14–17 In erythroid maturation events, the activation of Nrf2 is crucial to support stress erythropoiesis induced by the oxidant, PHZ, and in modulating ineffective erythropoiesis in β-thalassemic mice.18,19 In other cellular models, it has been shown that impairment of Nrf2 post-induction regulation results in perturbation of cell homeostasis and in accumula-stresses of poly-ubiquitylated protein aggregates due to deregulated autophagy.16 Autophagy is activated in response to different cellular stresses to ensure cell survival and ensure the clearance of the damaged proteins.18,19 We recently showed that in chorea-acanthocytosis the impairment of autophagy promotes accumulation of proteins, resulting in engulfment of the cells and in perturbation of erythropoiesis combined with increased oxidative stress.20
In present study, we explored the role of Fyn in regulating normal and stress erythropoiesis. We show that in addition to Jak2 and Lyn, Fyn is an additional kinase involved in EPO signaling cascade by targeting STAT5 activation. The absence of Fyn reduces the efficiency of the EPO signal and promotes the generation of ROS and the over-activation of Jak2-Akt-mTOR pathway, with repression of autophagy. The absence of Fyn also results in persistent activation of Nrf2 and accumulation of damaged proteins. This is further amplified by the blockage of autophagy mediated by mTOR activation, which markedly perturbs the response to stress erythropoiesis induced by either phenylhydrazine (PHZ) or Doxorubicin. In Fyn−/− mice, the rescue experiments with Rapamycin, an mTOR inhibitor and autophagy activator, co-administrated to PHZ further validated the importance of autophagy as adaptive mechanism to stress erythropoiesis in presence of perturbation of EPO cascade.
2 |. METHODS
2.1 |. Mouse strains and design of the study
The Institutional Animal Experimental Committee of University of Verona (CIRSAL) and the Italian Ministry of Health approved the experimental protocols. Two-month old female wild-type (WT) and Fyn−/− mice were studied. Where indicated, WT and Fyn−/− mice were treated with EPO (10 U/mouse/day for 5 days by intraperitoneal injection),3 or Phenylhydrazine (PHZ: 40 mg/kg on day 0 by intraperitoneal injection)19 or Doxorubicin (DOXO: 0.25 mg/kg on day 0 by intraperitoneal injection)21 to study stress erythropoiesis. Rapamycin was administrated at the dosage of 10 mg/kg/d by intraperitoneal injection for 1 week, then mice were analyzed. In experiments with PHZ co-administration, Rapamycin was given at the dosage of 10 mg/kg/d by intraperitoneal injection 1 day before PHZ administration (40 mg/kg body; single dose at day 0) and then Rapamycin was maintained for additional 14 days. N-Acetylcysteine (NAC, 100 mg/kg body; intraperitoneally injected) was administrated for 3 weeks as antioxidant treatment.18,19 In mouse strains, hematological parameters, red cell indices and reticulocyte count were evaluated at baseline and at different time points (6, 8, and 11 days after EPO injection; at 2, 4, 8, and 14 days after PHZ injection; at 3, 6, and 9 days after DOXO injection; at 2, 4, 8, 14 days after Rapamycin plus PHZ injection) as previously reported.22,23 Blood was collected with retro-orbital venipuncture in anesthetized mice using heparinized microcapillary tubes. Hematological parameters were evaluated on a Siemens Hematology Analyzer (ADVIA 2120). Hematocrit and hemoglobin were manually determined.24,25
2.2 |. Flow cytometric analysis of mouse erythroid precursors and molecular analysis of sorted erythroid cells
Flow cytometric analysis of erythroid precursors from bone marrow and spleen from WT and Fyn−/− was carried out as previously described using the CD44-Ter119 or CD71-Ter119 strategies.18,26,27 Analysis of apoptotic basophilic, polychromatic and orthochromatic erythroblasts was carried out on the CD44-Ter119 gated cells using the Annexin-V PE Apoptosis detection kit (eBioscience, San Diego, CA) following the manufacturer’s instructions. Erythroblasts ROS levels were measured as previously reported by Matte et al.18 Sorted cells were used for (i) morphological analysis of erythroid precursors on cytospin preparations stained with May Grunwald-Giemsa; (ii) immuno-blot analysis with specific antibodies against anti-PSer473-Akt, anti-Akt, anti-P-Ser2448-mTOR, anti-mTOR, anti-Jak2 (Cell Signaling, Massachusetts); anti-P-Ser40-Nrf2, anti-Nrf2, antip62, anti-Rab5 (Abcam, Cambridge, UK); anti-Keap1 (Proteintech, Manchester, UK); anti-EPO-R (Sigma-Aldrich, Missouri); anti-STAT5, anti-Lyn (Santa Cruz Biotechnology, Texas); anti-GAPDH (Santa Cruz Biotechnology, Texas) and anti-catalase (Abcam, Cambridge, UK) were used as loading control; (iii) immunoprecipitation assay; and (iv) RTPCR analysis. Details of immunoprecipitation, RT-PCR and immunoblot protocols used for the analysis of sorted erythroblasts are described in Supplementary materials and methods.
2.3 |. CFU-E, BFU-E assay
CFU-E and BFU-E assay was carried out using MethoCult as previously reported.28 Details are present in Supplementary Methods.
2.4 |. Immunofluorescence assay for p62 and FOXO3 in sorted erythroblasts
Immunofluorescence assay for p62 and FOXO3 in sorted erythroblasts was carried out as previously described.20,25,29 Details are reported in Supplementary materials and methods.
2.5 |. LysoTracker and MitoTracker analysis in maturating reticulocytes
To obtain reticulocyte enriched RBC fraction, WT and Fyn−/− mice were intraperitoneally injected with PHZ (40 mg/kg) at day 0, 1, 3 to induce reticulocytosis, and blood was collected in heparinized tubes at day 7, as previously described.30 RBCs were washed three times with the maturation medium (60% IMDM, 2 mM L-glutamine, 100 U Penicillin-Streptomicin, 30% FBS, 1% BSA and 0.5 μg/mL Amphotericin), diluted 1/500 in maturation medium and cultured in a cell culture incubator at 37°C, 5% of CO2 for 3 days. Clearance of Lysosome and Mitochondria, on the CD71/Ter119 gated RBC populations, were analyzed at day 0 and 3 of culture using the Lysotracker Green DND26 (ThermoFisher Scientific) and the MitoTracker Deep Red (ThermoFisher Scientific) probes, respectively, following the manufacturer’s instructions. Samples were acquired using the FACSCantoII flow cytometer (Becton Dickinson, San Jose, CA) and data were processed with the FlowJo software (Tree Star, Ashland, OR) as previously described.18,25
2.6 |. Pearl’s analysis of liver and spleen
Immediately following dissection, spleen and liver were formalin-fixed and paraffin-embedded for Pearl’s staining and blinded analyzed.
2.7 |. Molecular analysis of liver
Protocols used for RNA isolation, cDNA preparation and quantitative qRT-PCR have been previously described.31 Detailed primer sequences are available on request and shown in Supporting Information Table 1S. Liver immuno-blot analysis was performed as previously described.18,32
2.8 |. Measurement of heme and heme-oxygenase-1 activity
Liver heme content was measured using a fluorescence assay, as previously reported.33 Details are reported in Supporting Information. HO-1 activity was evaluated in tissue microsomal fractions by spectrophotometric determination of bilirubin produced from hemin added as the substrate, as previously reported.34
2.9 |. Statistical analysis
Data were analyzed using either t-test or the 2-way analysis of variance (ANOVA) for repeated measures between the mice of various genotypes. A difference with a P < .05 was considered significant.
3 |. RESULTS
3.1 |. The absence of Fyn results in decreased efficiency of EPO-signaling pathway
Fyn−/− mice displayed a slight microcytic anemia characterized by a small but significant reduction in hemoglobin, microcytosis and increased reticulocyte counts compared to WT animals (Table 1). To understand whether iron deficiency might account for the observed microcytosis, we evaluated iron accumulation in the liver and spleen. No differences in Pearl’s staining for iron in either liver or spleen of Fyn−/− compared to wild type mice was observed (Supporting Information Figure 1Sa). In agreement, expression levels of H-Ferritin in liver were similar in both mouse strains, whereas expression of L-Ferritin was slightly, but significantly lower in Fyn−/− mice compared to WT mice (Supporting Information Figure 1Sb). Haptoglobin levels were measured to determine the possible contribution of hemolysis to microcytic anemia in Fyn−/− mouse. Up-regulation of haptoglobin mRNA levels was noted in liver from Fyn−/− mice, while plasma haptoglobin levels were similar in both mouse strains (Supporting Information Figure 1Sc, d). These findings suggest that in mice genetically lacking Fyn, the noted mildly compensated anemia is not related to either iron deficiency or chronic hemolysis.
TABLE 1.
Hematological parameters and red cell indices in wild-type and Fyn−/− mice
| Wildtype mice (n = 15) | Fyn−/− mice (n = 15) | |
|---|---|---|
| Hct (%) | 48.2 ± 1.3 | 46.1 ± 0.8* |
| Hb (g/dL) | 15.9 ± 0.6 | 14.3 ± 0.5* |
| MCV (fL) | 50.3 ± 0.4 | 46.5 ± 1.3* |
| MCH (g/dL) | 15.3 ± 0.3 | 14.8 ± 1.1 |
| RDW (%) | 11.6 ± 0.3 | 13.2 ± 0.4* |
| Retics (103 cells/μL) | 451 ± 40.7 | 559 ± 45* |
Abbreviation: Hb, hemoglobin; Hct, hematocrit; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; RDW, red cell distribution width; Retics, reticulocytes.
P<0.05 compared to wild-type mice.
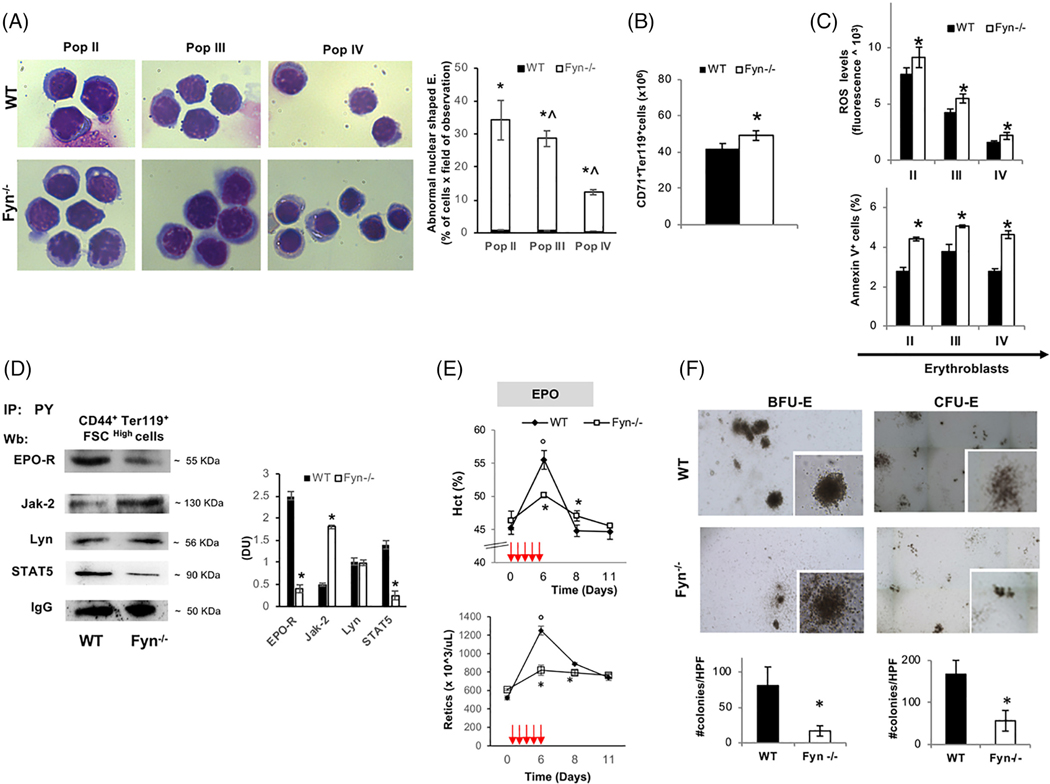
To better define the Fyn−/− mouse hematologic phenotype, we carried out the morphologic analysis of erythroblasts at distinct stages of terminal erythroid differentiation. As shown in Figure 1A, decreased chromatin condensation and larger cellular size was a characteristic feature of different populations of sorted Fyn−/− mouse erythroblasts (pop II: basophilic erythroblasts; pop III: polychromatic erythroblasts and pop IV: orthochromatic erythroblasts; Figure 1A). Furthermore, an increase in number of total erythroblasts in bone marrow was noted (Figure 1B), without evidence of extramedullary erythropoiesis (data not shown). The maturation profile of erythroblasts revealed an accumulation of orthochromatic erythroblasts (Supporting Information Figure 2Sa). When CD44/Ter119 approach was used to characterize erythropoiesis, no major differences in either total erythroblasts or in erythroblasts subpopulations between WT and Fyn−/− mice were observed (Supporting Information Figure 2Sb, c). Up-regulation of EPO gene expression in kidney was found in Fyn−/− mice compared to that of WT animals (Supporting Information Figure 2Sd). In addition, we found increased ROS levels throughout Fyn−/− erythroid maturation from basophilic erythroblasts (pop II) to polychromatic (pop III) and orthochromatic erythroblasts (pop IV) compared to WT cells (Figure 1C, upper panel). This was associated with higher amounts of Annexin V+ cells in the different subpopulation of erythroblasts compared to WT cells (Figure 1C, lower panel). Collectively, these findings indicate a decreased efficiency of EPO signaling pathway in the absence of Fyn. To understand the impact of Fyn on EPO cascade, we evaluated the EPO-Jak2-STAT5 signaling pathway in sorted Fyn−/− erythroblasts. As shown in Figure 1D, reduced activation of EPO-receptor (EPO-R) as reflected by decreased EPO-R Tyr-phosphorylation, was noted in erythroblasts genetically lacking Fyn (Figure 1D). This was associated with increased activation of Jak2 kinase without any change in Lyn activity compared to WT cells (Figure 1D). Total expression of EPO-R was similar in sorted erythroblasts from both mouse strains; whereas Jak2 expression was higher in Fyn−/− erythroblasts compared to healthy cells (Supporting Information Figure 2Se). In agreement with the reduction in EPO-R activation, we observed a significant decrease in STAT5 activity with concomitant down-regulation of Cish expression, a well-documented gene target of STAT5 in sorted Fyn−/− erythroblasts (Figure 1D; Supporting Information Figure 2Sf ). Following treatment with recombinant EPO (10 U/day for 5 days), Fyn−/− mice showed blunted increases in Hct and reticulocyte counts compared to WT animals (Figure 1E).
FIGURE 1.
The absence of Fyn results in perturbation of EPO signaling cascade. A, Left panel. Morphology of sorted erythroid precursors: population II (pop II), corresponding to basophilic erythroblasts; population III (pop III), corresponding to polychromatic erythroblasts and population IV (pop IV), corresponding to orthochromatic erythroblasts, from bone marrow of wild-type (WT) and Fyn−/− mice. Cytospins were stained with may-Grunwald-Giemsa. One representative image from a total of 10 for each mouse strains. Right panel. Abnormal nuclear shaped erythroblasts and binuclear erythroblasts from WT and Fyn−/− mice evaluated on cytospin stained with may-Grunwald-Giemsa. Data are presented as means ±SD (n = 8 from each strain); *P < .05 compared to WT; ^P < .05 compared to pop II−. B, Cyto-fluorimetric analysis of total erythroid precursors from the bone marrow of WT and Fyn−/− mice using the following surface markers: CD71 and Ter119 (see also the Supporting Information Materials and Methods and Figure 2Sa for maturation profile). Data are presented as means ±SD (n = 8); * P < .05 compared to WT. C, Upper panel: ROS levels in erythroid precursors from bone marrow of wild-type (WT) and Fyn−/− mice. Data are presented as means ±SD (n = 10 from each strain); * P < .05 compared to WT. Lower panel: Amount of Annexin V+ cells in pop II, III, and IV from bone marrow of WT and Fyn−/− mice. Data are presented as means ±SD (n = 8 from each strain); * P < .05 compared to WT. D, Total Tyrosin-(Tyr) phosphorylated proteins were immunoprecipitated from 2.5 ^106 bone marrow sorted erythroblasts of WT and Fyn−/− mice and detected with antibody to EPO- receptor (EPO-R), Janus kinase-2 (Jak-2), Lyn kinase (Lyn), signal transducer and activator of transcription 5 (STAT5). The experiment shown is representative of six such experiments. IgG was used as loading control. Right panel: Densitometric analyses of the immunoblot bands similar to those shown are presented at right (DU: Densitometric unit). Data are shown as means ±SD (n = 6; *P < .01 compared to WT). E, Hematocrit (%) and reticulocyte count in (n = 6) and Fyn−/− (n = 6) mice exposed to recombinant erythropoietin injection (EPO 50 U/kg/die, red arrows). Data are presented as means ±SD; *P < .05 compared to WT mice; °P < .05 compared to baseline values. F, The CFU-E and BFU-E from WT and Fyn−/− mice were quantified (#CFU-E or BFU-E/dish; lower panel); data are shown as means ±SD (n = 6; P < .05 compared to WT)
To explore whether the reduced efficiency of EPO cascade might also involve erythroid progenitors, we carried out the in vitro erythroid cell colony forming assay. A lower number of CFU-E and BFUE colony forming cells were found in Fyn−/− bone marrow (Figure 1F). This was associated again with lower activation of EPO-R mediated signaling cascade with a reduced activation of STAT5 but hyper-activation of Jak2 in Fyn−/− CFU-E (Supporting Information Figure 2Sg).
Our data indicate that Fyn is involved in EPO signaling cascade and that absence of Fyn lead to increased ROS generation, which may contribute to the hyper-activation of Jak2 in presence of reduced efficiency of EPO signaling pathway.35 Thus, the very mild microcytic anemia phenotype of Fyn−/− mice is likely to be related more to reduced STAT5 activation, as observed in mice genetically lacking STAT5 than to perturbation of iron metabolism.36
3.2 |. Fyn−/− mice display increased sensitivity to PHZ or doxorubicin induced stress erythropoiesis
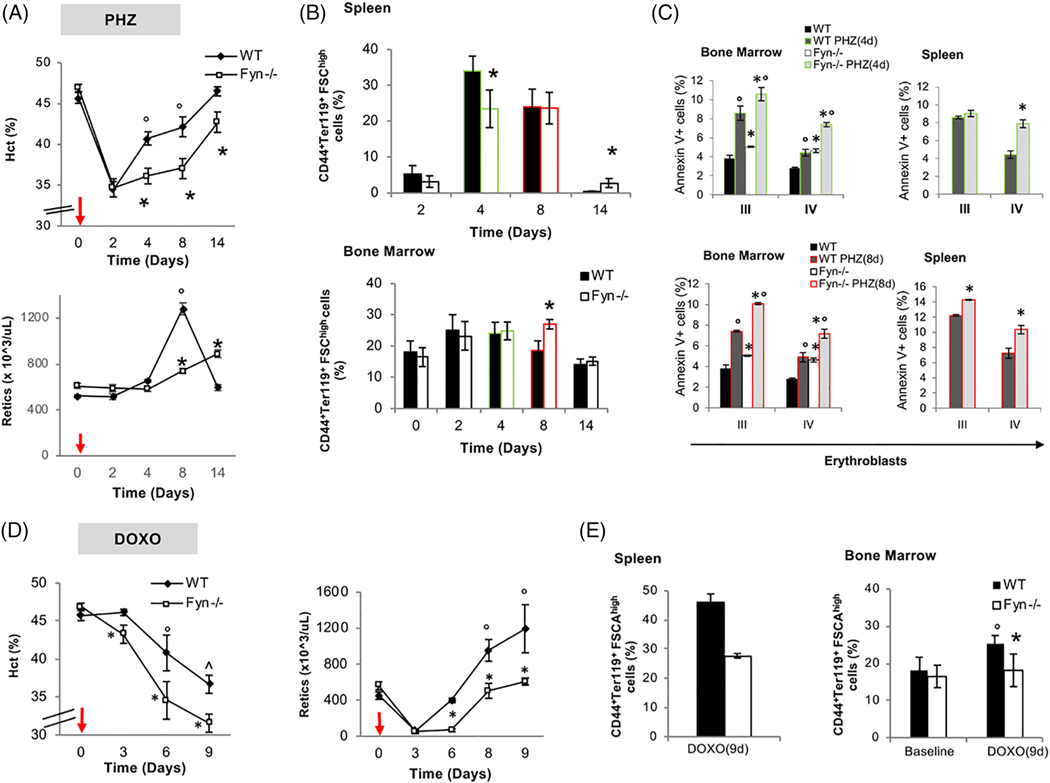
Since EPO is the primary signal in stress erythropoiesis, we treated Fyn−/− mice with either PHZ to induce acute hemolytic anemia due to severe oxidative stress or Doxorubicin that temporary represses erythropoiesis with generation of ROS.19,21 PHZ treatment induced a similar drop in Hct levels in both mouse strains at day 2 following PHZ administration (Figure 2A, upper panel). However, the Hct and reticulocyte recovery were blunted in Fyn−/− mice compared to control animals (Figure 2A, upper and lower panel). Extramedullary erythropoiesis as assessed by increased splenic erythropoiesis showed a blunted response in Fyn−/− mice at day 4 following PHZ treatment with a compensatory increase by day 14 (Figure 2B, upper panel, see also Supporting Information Figure 3Sa for absolute values of number of erythroblasts at day 4 after PHZ). In bone marrow, we observed a mild increase in total erythroblasts in both mouse strains at day 2 and 4 after PHZ injection (Figure 2B, lower panel). It is of interest to note that in Fyn−/− mice at day 8 following PHZ treatment, we observed a significant increase in the total number of bone marrow erythroblasts as possible compensatory mechanism due to the failure in efficient activation of splenic extramedullary erythropoiesis (Figure 2B, lower panel). The amount of Annexin V+ cells following PHZ treatment was higher in Fyn−/− polychromatic and orthochromatic erythroblasts compared to WT cells (Figure 2C).
FIGURE 2.
A blunted response to stress erythropoiesis characterizes Fyn−/− mice. A, Hematocrit (%) and reticulocyte count in WT (n = 6) and Fyn−/− (n = 6) mice exposed to phenylhydrazine injection (PHZ/kg/die, red arrows). Data are presented as means ±SD; *P < .05 compared to WT mice; °P < .05 compared to baseline values. B, Cyto-fluorimetric analysis of total erythroid precursors from the bone marrow and the spleen of WT and Fyn−/− mice using the following surface markers: CD44 and Ter119 (see also the Supporting Information and Methods and Figure 3Sa for absolute values). Data are presented as means ±SD (n = 6); * P < .05 compared to WT. since we focus on day 4 and day 8 after PHZ administration, we highlighted them respectively in green and red. This color code is used also in C. C, Amount of Annexin-V+ cells in population III (pop III), corresponding to polychromatic erythroblasts and population IV (pop IV), corresponding to orthochromatic erythroblasts from either spleen or bone marrow of WT and Fyn−/− mice respectively at 4 (green) and 8 (red) days after PHZ administration. Data are presented as means ±SD (n = 6 from each strain); *P < .05 compared to WT. D, Hematocrit (%) and reticulocyte count in WT (n = 6) and Fyn−/− (n = 6) mice exposed to doxorubicin injection (DOXO 0,25 mg/kg/die, red arrows). Data are presented as means ±SD; *P < .05 compared to WT mice; P < .05 compared to baseline values. E, Cyto-fluorimetric analysis of total erythroid precursors from the bone marrow and the spleen of WT and Fyn−/− mice using the following surface markers: CD44 and Ter119 (see also the Supporting Information and Methods and Figure 3Sb for absolute values) 9 days after doxorubicin injection. Data are presented as means ± SD (n = 6); * P < .05 compared to WT; P < .05 compared to baseline values
Doxorubicin induced a more severe and prolonged anemia in Fyn−/− mice than in WT animals (Figure 2D, left panel). At day 3 and 6 following Doxorubicin treatment, we noted a plateau in reticulocyte count in Fyn−/− mice (Figure 2D), suggesting a substantial impairment in the reticulocyte response compared to Doxorubicin treated WT animals. Enumeration of total number of erythroblasts in spleen and bone marrow at day 9 after Doxorubicin administration, showed a substantial reduction in both bone marrow and splenic erythropoiesis in Fyn−/− mice compared to WT animals (Figure 2E; see also Supporting Information Figure 3Sb for absolute values). Increases in the numbers of Annexin V+ polychromatic and orthochromatic erythroblasts were noted in Fyn−/− mice compared to WT animals at 9 days after Doxorubicin administration (Supporting Information Figure 3Sc). The findings of diminished responsiveness of Fyn−/− mice to stress erythropoiesis induced by PHZ or Doxorubicin, further validate the importance of Fyn in EPO signaling cascade.
3.3 |. Increased activation of Akt in Fyn−/− mice contributes to the modulation of redox cellular response during erythropoiesis
In normal and disordered erythropoiesis, previous studies have shown that Jak2 and oxidation can activate Akt, which affects multiple targets during erythropoiesis (Supporting Information Figure 4Sa).4 Notably, Akt is also important in mediating cellular response to oxidation by the activation of two redox sensitive transcriptional factors, Forkhead box-O3 (FOXO3) and Nrf2, as well as of mTOR, the gatekeeper of autophagy activation.37–39 Fyn−/− mouse erythroblasts displayed higher levels of active Akt (Ser 473) compared to WT cells (Supporting Information Figure 4Sb). The activation of FOXO3 was evaluated by both immunomicroscopy and immunoblot analysis, this latter using a specific antibody against inactive phospho-FOXO3. In Fyn−/− mouse erythroblasts, we found a slight but not significant increase in activation of FOXO3 compared to WT erythroblasts (Supporting Information Figure 4Sc).
We then focused on Nrf2 since Fyn is important in post-induction regulation of Nrf2.16 We found increased activation of Nrf2, as indicated by higher phospho-Nrf2 form in Fyn−/− mouse erythroblasts compared to WT cells (Supporting Information Figure 5Sa). The up-regulation of ARE-genes for anti-oxidant systems such as catalase, Gpx1, HO1, and Prx2 confirmed the increased Nrf2 function in Fyn−/− mouse erythroblasts (Supporting Information Figure 5Sb). Immunoblot analysis with specific antibodies for the corresponding proteins further validated the activation of Nrf2 pathway (Supporting Information Figure 5Sc).
However, the increased expression of anti-oxidant and cytoprotective system related to Nrf2 function does not completely counteract the induced oxidative damage of Fyn−/− mouse erythroblasts. Overall these effects are similar to those observed in both cell and animal models characterized by prolonged Nrf2 activation, which is associated with severe and even lethal phenotype,15,40 mainly related to the accumulation of damaged proteins and the perturbation of autophagy.
Among the many Nrf2 related genes up-regulated in Fyn−/− mouse erythroblasts, we focused on HO-1, the main hemecatabolizing enzyme under stress conditions and a major player in the maintenance of cell homeostasis.41
3.4 |. Heme-oxygenase activity and heme levels are similar in Fyn−/− and WT mice
Since systemic heme homeostasis is orchestrated by the liver, we evaluated the impact of Fyn deficiency on hepatic heme catabolism. First, we confirmed the increased activation of Nrf2 in Fyn−/− liver compared to WT counterpart (Supporting Information Figure 6Sa). When we analyzed HO-1 expression and HO-1 activity in this organ, despite similar levels of HO-1 mRNA in the livers of WT and Fyn−/− mice, HO-1 protein level was higher in the Fyn−/− mice. Similar findings were also noted in erythroid cells (Supporting Information Figure 6Sb). Interestingly, in spite of increased protein levels, hepatic HO activity was unchanged in Fyn−/− animals (Supporting Information Figure 6Sc) suggesting no alteration in heme catabolism. This conclusion was supported by the finding that the heme content in the liver twas similar in WT and Fyn−/− mice (Supporting Information Figure 6Sd). Thus, in the absence of Fyn, HO-1 protein is increased but its activity is unchanged in Fyn−/− mice, suggesting the accumulation of functionally inactive HO-1.16 Autophagy is the master control system regulating protein quality and clearance of damaged proteins.37,39,42 The lysosomal related cargo p62 protein can be used as indirect marker of autophagy and its accumulation correlates with impairment of autophagy.43 In liver from Fyn−/− mice, we found an accumulation of p62, suggesting a blockage of autophagy in the absence of Fyn (Supporting Information Figure 6Se, f ). In agreement, mTOR was more active in liver from Fyn−/− mice compared to WT animals (Supporting Information Figure 6Sg).37,44,45 These data imply impaired autophagy in liver from mice genetically lacking Fyn.
3.5 |. Impaired autophagy related to mTOR activation characterizes Fyn−/− mouse erythropoiesis
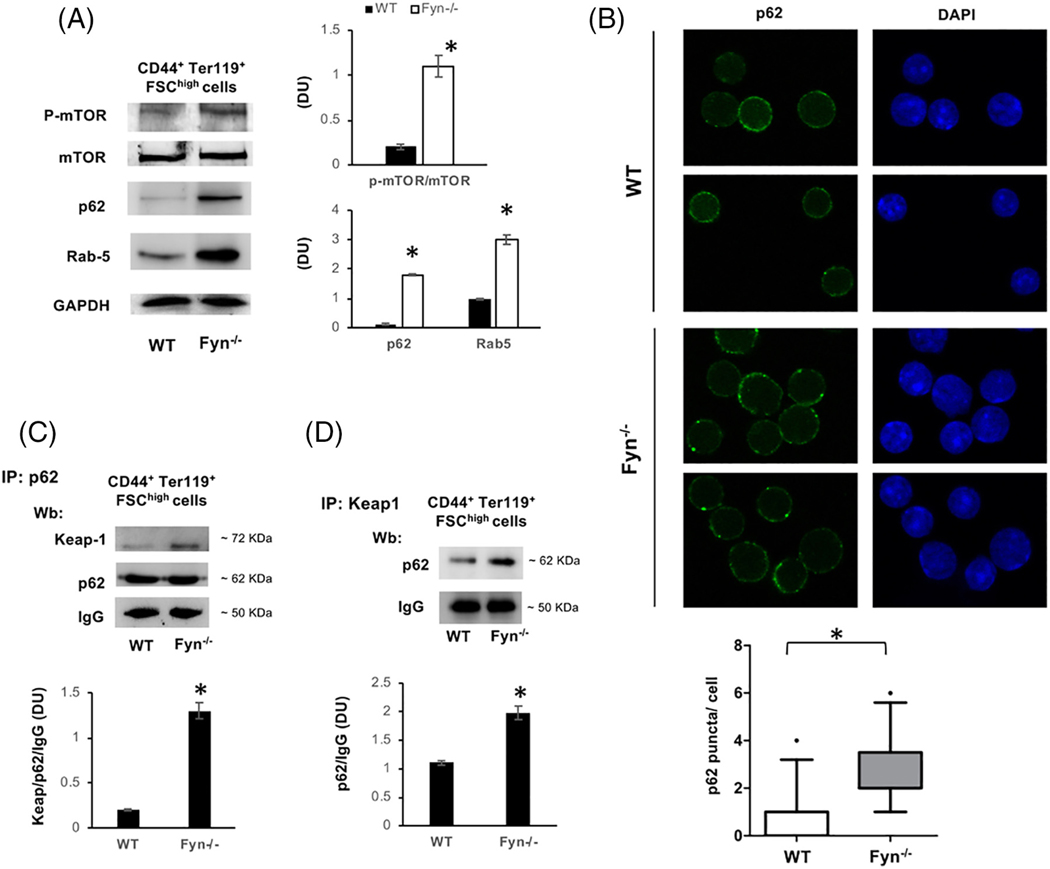
Since autophagy is also important during erythropoiesis,42 we explored mTOR signaling during erythroid maturation in Fyn−/− mouse. As shown in Figure 4A, Fyn−/− mouse erythroblasts displayed increased activation of phosho-mTOR compared to WT cells in association with accumulation of p62, similarly to that noted in Fyn−/− mouse liver (Figure 3A) as well as of Rab5, a small GTP protein involved in the late phases of autophagy (Figure 3A).39 Consistent with impaired autophagy during erythropoiesis in Fyn−/− mouse, we noted accumulation of p62 in large clusters in Fyn−/− erythroblasts compared to WT cells (Figure 3B, Supporting Information Figure 7Sa). Since p62 acts as autophagy adaptor, controlling proteins turnover,15,16 we evaluated Keap1, a known substrate of p62.16,43 In Fyn−/− erythroblasts, we found increased accumulation of Keap1 compared to WT cells (Supporting Information Figure 7Sb). Co-immunoprecipitation with either antibodies to p62 (Figure 3C) or Keap1 (Figure 3D) showed accumulation of p62-Keap1 complex in Fyn−/− mouse erythroblasts. If the perturbation of autophagy in Fyn−/− mice is physiologically relevant to erythroid maturation, it is also likely to affect reticulocyte maturation.30,42,46 To test this possibility, we evaluated the in vitro maturation of reticulocytes from PHZ treated mice.30 Decreased maturation of Fyn−/− mouse reticulocyte was detected by decreased lysosomal clearance with LysoTracker analysis (Supporting Information Figure 7Sc, d). Interesting, no difference in mitochondrial clearance using MitoTracker analysis was noted (Supporting Information Figure 7Sd, lower panel).
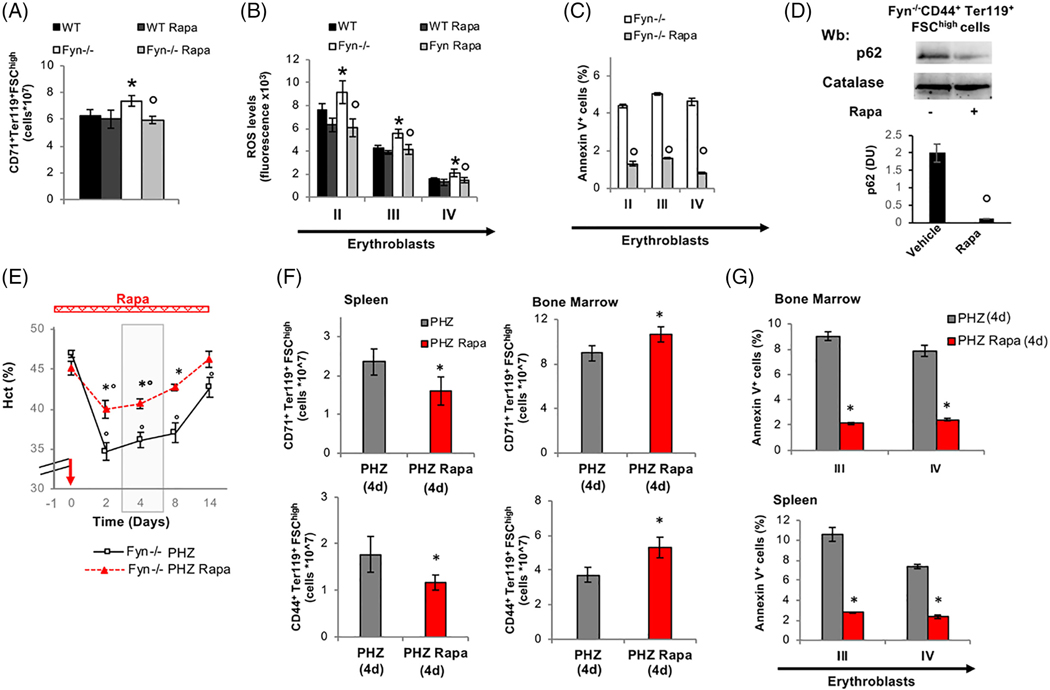
FIGURE 4.
The mTOR inhibitor, Rapamycin rescues the abnormal response of Fyn−/− erythroblasts to stress erythropoiesis. A, Effect of treatment with Rapamycin (Rapa) on total erythroid precursors (CD71-Ter119) from the bone marrow of WT and Fyn−/− mice. Data are presented as means ±SD (n = 6); *P < .05 compared to WT; °P < .05 compared to vehicle treated animals. B and C, Effect of Rapamycin treatment (Rapa) on ROS levels and Annexin V+ cells in erythroid precursors: Population II (pop II), corresponding to basophilic erythroblasts; population III (pop III), corresponding to polychromatic erythroblasts and population IV (pop IV), corresponding to orthochromatic erythroblasts from bone marrow of WT and Fyn−/− mice. Data are presented as means ±SD (n = 6 from each strain); * P < .05 compared to WT; P < .05 compared to vehicle treated animals. D, Western-blot (Wb) analysis of p62 in 1.5 ^106 sorted CD44+Ter119+FSChigh bone marrow cells with and without Rapamycin from Fyn−/− mice. Catalase was used as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right (DU: Densitometric unit). Data are shown as means ±SD (n = 4; *P < .01 compared to WT). E, Hematocrit (%) in Fyn−/− (n = 6) mice treated with either phenylhydrazine alone (PHZ) or combined with Rapamycin (Rapa- red bar is the time of treatment with it). The red arrow indicates the injection of PHZ. Data are presented as means ±SD; *P < .05 compared to PHZ treated animals; °P < .05 compared to baseline values. The gray area identifies the window time for characterization of the stress erythropoiesis in Fyn−/− mice. F, Cyto-fluorimetric analysis of total erythroid precursors from either bone marrow or spleen of Fyn−/− mice treated with either PHZ alone or Rapamycin (Rapa) plus PHZ (4 days after injection). Results from CD44-Ter119 (lower panel) or CD71Ter119 (upper panel) strategies are shown. Data are presented as means ±SD (n = 4); *P < .05 compared to PHZ treated animals; °P < .05 compared to baseline values. (G) Amount of Annexin V+ cells in population III (pop III), corresponding to polychromatic erythroblasts and population IV (pop IV), corresponding to orthochromatic erythroblasts from either spleen or bone marrow of Fyn−/− mice. Data are presented as means ±SD (n = 4 from each strain); *P < .05 compared to WT)
FIGURE 3.
Activation of mTOR and impaired autophagy characterize Fyn−/− mouse erythroblasts. A, Western-blot (Wb) analysis of phospho-mTOR (p-mTOR), m-TOR, p62 and Rab 5 in sorted 1.5 ^106 CD44+Ter119+FSCHigh bone marrow cells from WT (n = 4) and Fyn−/− mice. GAPDH was used as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right (DU: Densitometric unit). Data are shown as means ±SD (n = 4; *P < .01 compared to WT). B, p62 immunostained cytospin preparations of sorted CD44+Ter119+FSCHigh bone marrow cells from WT and Fyn−/− mice counterstained with DAPI. Lower panel: The puncta mean fluorescence was measured using image J software. Data are presented as means ±SD (n = 3); *P < .05 compared to WT. C, Immunoprecipitates (IP) containing equal amounts of p62 were obtained from 2.5 ^106 sorted CD44+Ter119+FSCHigh bone marrow cells from WT and Fyn−/− mice, then subjected to immunoblot with anti-Keap1 or p62 antibody (Wb: Western-blot). The experimental results shown are representative of four similar separate experiments. IgG was used as loading control.Densitometric analyses of the immunoblot bands similar to those shown are presented at lower panel (DU). Data are shown as means ±SD (n = 4; *P < .01 compared to WT). D, IP of Keap 1 were obtained from sorted 2.5 ^106 CD44+Ter119+FSChigh bone marrow cells from WT and Fyn−/− mice, then subjected to immunoblot with anti- p62 antibody (Wb: Western-blot). The experimental results shown are representative of 4 similar separate experiments. IgG was used as loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at lower panel (DU). Data are shown as means ±SD (n = 4; *P < .01 compared to WT)
Documentation of increased accumulation of p62 in Fyn−/− reticulocyte further supports the impairment of autophagy during erythroid maturation in Fyn−/− mice (Supporting Information Figure 7Se).
3.6 |. The mTOR inhibitor Rapamycin unblocks autophagy defect and ameliorates erythropoiesis in Fyn−/− mice
We next tested whether Rapamycin, a known mTOR inhibitor, may modulate Fyn−/− mouse erythropoiesis as reported for other models of pathologic erythropoiesis.37,38,42,47 In Fyn−/− mice, Rapamycin administration reduced total erythroblasts, while no significant effects were observed in control animals as previously reported37,38,42,47 (Figure 4A). In Fyn−/− mice treated with Rapamycin, this was associated with amelioration of the terminal phase of erythropoiesis (Supporting Information Figure 8Sa). Rapamycin significantly reduced the generation of ROS and the amount of Annexin- V+ positive cells only in erythroid precursors from Fyn−/− mice (Figure 4B, C). In agreement with Rapamycin induced activation of autophagy, we found a significant reduction in levels of p62 in erythroblasts from Rapamycin treated Fyn−/− mice compared to vehicle treated animals (Figure 4D).
We also evaluated the impact of anti-oxidant treatment with Nacetylcysteine (NAC), which has been shown to indirectly modulate autophagy by reducing intracellular oxidation.48,49 In Fyn−/− mice, NAC reduced total number of erythroblasts, increased orthochromatic erythroblasts and reduced the amount of Annexin V+ cells, indicating an amelioration of erythropoiesis in Fyn−/− mice (Supporting Information Figure 8Sb, c, d). The findings indicate that Rapamycin unblocks autophagy, allowing the degradation of accumulated proteins and ameliorating erythropoiesis in Fyn−/− mice.
3.7 |. Rapamycin rescues the abnormal response of Fyn−/− erythroblasts to stress erythropoiesis
Next, we investigated whether Rapamycin may alleviate the PHZ-induced stress erythropoiesis in FYN−/− mice. As shown in Figure 4F, the administration of Rapamycin in PHZ treated Fyn−/− mice resulted in milder anemia and faster recovery compared to PHZ treated Fyn−/− mice. At day 4 following PHZ administration, a plateau in Hct value is evident in both Fyn−/− mouse groups; we focused therefore on this time point to carry out a detailed analysis of. As shown in Figure 4G, we found decreased extramedullary splenic erythropoiesis and increased bone marrow erythropoiesis in PHZ-Rapamycin treated Fyn−/− mice compared to PHZ treated animals.
Annexin-V+ cells were also markedly reduced by the co-administration of Rapamycin and PHZ in Fyn−/− mouse erythroblasts from both bone marrow and spleen compared to PHZ alone (Figure 4H). It is interesting to note that, in WT animals the co-administration of Rapamycin worsened the PHZ induced stress erythropoiesis as previously reported38 (Supporting Information Figure 9Sa-c). Collectively, these data support the idea that by activating autophagy it is possible to rescue the altered response of Fyn−/− mice to stress erythropoiesis.
4 |. DISCUSSION
We have identified Fyn as a new kinase involved in EPO signaling cascade during normal and stress erythropoiesis. Previous studies have documented a similar role for Jak2 and Lyn kinases in erythropoiesis.2–4,50,51 The reduction in EPO induced phosphorylation of STAT5 noted in Fyn−/− mouse erythroid cells implies a role for Fyn downstream of STAT5 and suggests that Fyn is implicated in EPO signaling pathway by modulating the activation of EPO-R through STAT5. Similar findings have been reported for mice genetically lacking Lyn,50 supporting the important and nonredundant role of Fyn in the EPO-mediated signaling cascade. Our findings also suggest that multiple kinases may be important in coordinating stress erythropoiesis in health and disease.
In Fyn−/− mice, the reduced effectiveness of EPO signaling is associated with increase ROS generation and cell apoptosis. The attempt of Fyn−/− mouse erythroblasts to adapt to oxidative stress is indicated by the activation of the redox related transcription factor Nrf2.35,37 However, the persistent activation of Nrf2 due to the absence of its physiologic repressor Fyn, resulted in accumulation of damaged/nonfunctional proteins that further amplified the intracellular oxidative stress.15,16 Indeed, Fyn−/− mice display impaired functioning of several cytoprotective systems, such as HO-1 indicating an impairment of the protein quality control process mediated by autophagy.
Previous studies have shown that perturbation of autophagy is detrimental for erythroid maturation and has been documented in condition with disordered erythropoiesis such as β-thalassemic syndromes or chorea-acanthocytosis or during iron deficiency.20,37,46,52,53 In Fyn−/− mice, the ROS mediated activation of Jak2-Akt–mTOR pathway represses autophagy and thereby contributes to the ineffective erythropoiesis of Fyn−/− mice.4,39 Consistent with this hypothesis, in Fyn−/− mouse erythroblasts we found accumulation of the autophagy cargo protein p62, a marker of autophagy inhibition. In addition, p62 was also complexed with Keap1, the Nrf2 shuttle protein,16 further supporting the dysregulation of Nrf2 and the blockage of autophagy during Fyn−/− erythropoiesis. The accumulation of Rab5, a small GTPase involved in endocytic vesicular transport,54 suggested a possible perturbation of the autophagy lysosomal system in Fyn−/− mice. Indeed, we found a reduction in lysosome clearance during Fyn−/− mouse reticulocyte maturation in presence of preserved clearance of mitochondria compartment.
The ability of Rapamycin, a known mTOR inhibitor and autophagy activator, to ameliorate Fyn−/− mouse baseline erythropoiesis and to prevent accumulation of p62 support the notion of impaired autophagy in Fyn−/− mice. This is further corroborated by the observation that Rapamycin co-administrated with PHZ restored the erythropoietic response in Fyn−/− mice. These finding shed a new light on the link between dysregulation of Nrf2 and impairment of autophagy in stress erythropoiesis, demonstrating the multimodal action of Fyn in establishing the developmental program of erythropoiesis.
In conclusion, our data indicate that Fyn kinase is a novel and relevant regulator of erythropoiesis, contributing to activation of the EPO signaling cascade, and further increasing the complexity of this pathway. The absence of Fyn and the reduced efficiency of EPO signal generate a “domino effect” affecting several mechanisms associated with response to an increased oxidative stress (Supporting Information Figure 9Sd). The dysregulation of post-induction regulation of Nrf2 due to the absence of Fyn, results in accumulation of aggregated proteins, which further increase cellular oxidative stress. A concomitant activation of Jak2-Akt-mTOR pathway results in repression of autophagy (Supporting Information Figure 9Sd), which can by rescued with Rapamycin, further reinforce the importance of autophagy as adaptive mechanism to stressful conditions associated with perturbations of the EPO signaling pathway. Future studies will be required to fully characterize the role Fyn in cellular signaling in pathologic erythropoiesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by FUR-UNIVR (LDF).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Chiabrando D, Mercurio S, Tolosano E. Heme and erythropoieis: more than a structural role. Haematologica. 2014;99:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108:1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingley E, McCarthy DJ, Pore JR, et al. Lyn deficiency reduces GATA-1, EKLF and STAT5, and induces extramedullary stress erythropoiesis. Oncogene. 2005;24:336–343. [DOI] [PubMed] [Google Scholar]
- 4.Oikonomidou PR, Rivella S. What can we learn from ineffective erythropoiesis in thalassemia? Blood Rev. 2017;32(2):130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harder KW, Quilici C, Naik E, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–3910. [DOI] [PubMed] [Google Scholar]
- 6.He X, Deng Y, Yue W. Investigating critical genes and gene interaction networks that mediate cyclophosphamide sensitivity in chronic myelogenous leukemia. Mol Med Rep. 2017;16:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geahlen RL, Handley MD, Harrison ML. Molecular interdiction of Src-family kinase signaling in hematopoietic cells. Oncogene. 2004;23:8024–8032. [DOI] [PubMed] [Google Scholar]
- 8.Pullen NA, Barnstein BO, Falanga YT, et al. Novel mechanism for fc {epsilon}RI-mediated signal transducer and activator of transcription 5 (STAT5) tyrosine phosphorylation and the selective influence of STAT5B over mast cell cytokine production. J Biol Chem. 2012;287:2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsygankov AY, Spana C, Rowley RB, Penhallow RC, Burkhardt AL, Bolen JB. Activation-dependent tyrosine phosphorylation of Fyn-associated proteins in T lymphocytes. J Biol Chem. 1994;269:7792–7800. [PubMed] [Google Scholar]
- 10.Laurenzana I, Caivano A, Trino S, et al. A Pyrazolo[3,4-d]pyrimidine compound inhibits Fyn phosphorylation and induces apoptosis in natural killer cell leukemia. Oncotarget. 2016;7:65171–65184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lannutti BJ, Shim MH, Blake N, Reems JA, Drachman JG. Identification and activation of Src family kinases in primary megakaryocytes. Exp Hematol. 2003;31:1268–1274. [DOI] [PubMed] [Google Scholar]
- 12.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009; 228:9–22. [DOI] [PubMed] [Google Scholar]
- 13.Tarabra E, An Lee TW, Zammit VA, et al. Differential activation of Fyn kinase distinguishes saturated and unsaturated fats in mouse macrophages. Oncotarget. 2017;8:86634–86645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27:6334–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD . p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JE, Roh E, Lee MH, et al. Fyn is a redox sensor involved in solar ultraviolet light-induced signal transduction in skin carcinogenesis. Oncogene. 2016;35:4091–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matte A, De Falco L, Federti E, et al. Peroxiredoxin-2: a novel regulator of iron homeostasis in ineffective erythropoiesis. Antioxid Redox Signal. 2018;28:1–14. [DOI] [PubMed] [Google Scholar]
- 19.Matte A, De Falco L, Iolascon A, et al. The interplay between Peroxiredoxin-2 and nuclear factor-Erythroid 2 is important in limiting oxidative mediated dysfunction in beta-Thalassemic erythropoiesis. Antioxid Redox Signal. 2015;23:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupo F, Tibaldi E, Matte A, et al. A new molecular link between defective autophagy and erythroid abnormalities in chorea-acanthocytosis. Blood. 2016;128:2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Franceschi L, Turrini F, Honczarenko M, et al. In vivo reduction of erythrocyte oxidant stress in a murine model of beta-thalassemia. Haematologica. 2004;89:1287–1298. [PubMed] [Google Scholar]
- 23.Matte A, Low PS, Turrini F, et al. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med. 2010;49:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalle Carbonare L, Matte A, Valenti MT, et al. Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood. 2015;126:2320–2328. [DOI] [PubMed] [Google Scholar]
- 25.Franco SS, De Falco L, Ghaffari S, et al. Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2014;99:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang J, Ginzburg Y, et al. Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood. 2013;121:e43–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstantinidis DG, Giger KM, Risinger M, et al. Cytokinesis failure in RhoA-deficient mouse erythroblasts involves actomyosin and midbody dysregulation and triggers p53 activation. Blood. 2015;126:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117:3435–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matte A, Pantaleo A, Ferru E, et al. The novel role of peroxiredoxin-2 in red cell membrane protein homeostasis and senescence. Free Radic Biol Med. 2014;76:80–88. [DOI] [PubMed] [Google Scholar]
- 30.Gothwal M, Wehrle J, Aumann K, Zimmermann V, Grunder A, Pahl HL. A novel role for nuclear factor-erythroid 2 in erythroid maturation by modulation of mitochondrial autophagy. Haematologica. 2016;101:1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrillo S, Chiabrando D, Genova T, et al. Heme accumulation in endothelial cells impairs angiogenesis by triggering paraptosis. Cell Death Differ. 2018;25:573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalish BT, Matte A, Andolfo I, et al. Dietary omega-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica. 2015;100:870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorito V, Forni M, Silengo L, Altruda F, Tolosano E. Crucial role of FLVCR1a in the maintenance of intestinal Heme homeostasis. Antioxid Redox Signal. 2015;23:1410–1423. [DOI] [PubMed] [Google Scholar]
- 34.Ingoglia G, Sag CM, Rex N, et al. Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress. Free Radic Biol Med. 2017;108:452–464. [DOI] [PubMed] [Google Scholar]
- 35.Marty C, Lacout C, Droin N, et al. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013;27:2187–2195. [DOI] [PubMed] [Google Scholar]
- 36.Zhu BM, McLaughlin SK, Na R, et al. Hematopoietic-specific Stat5-null mice display microcytic hypochromic anemia associated with reduced transferrin receptor gene expression. Blood. 2008;112:2071–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Camprecios G, Rimmele P, et al. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am J Hematol. 2014;89:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knight ZA, Schmidt SF, Birsoy K, Tan K, Friedman JM. A critical role for mTORC1 in erythropoiesis and anemia. Elife. 2014;3:e01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakabayashi N, Itoh K, Wakabayashi J, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. [DOI] [PubMed] [Google Scholar]
- 41.Chiabrando D, Vinchi F, Fiorito V, Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014;5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang YA, Sanalkumar R, O’Geen H, et al. Autophagy driven by a master regulator of hematopoiesis. Mol Cell Biol. 2012;32:226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. [DOI] [PubMed] [Google Scholar]
- 44.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Zhang Y, Ni M, et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat Cell Biol.2017;19:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Tran J, Wang H, et al. mTOR inhibition improves anaemia and reduces organ damage in a murine model of sickle cell disease. Br J Haematol. 2016;174:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Federti E, Matte A, Ghigo A, et al. Peroxiredoxin-2 plays a pivotal role as multimodal cytoprotector in the early phase of pulmonary hypertension. Free Radic Biol Med. 2017;112:376–386. [DOI] [PubMed] [Google Scholar]
- 49.Filomeni G, Desideri E, Cardaci S, Rotilio G, Ciriolo MR. Under the ROS … thiol network is the principal suspect for autophagy commitment. Autophagy. 2010;6:999–1005. [DOI] [PubMed] [Google Scholar]
- 50.Ingley E Integrating novel signaling pathways involved in erythropoiesis. IUBMB Life. 2012;64:402–410. [DOI] [PubMed] [Google Scholar]
- 51.Tilbrook PA, Palmer GA, Bittorf T, et al. Maturation of erythroid cells and erythroleukemia development are affected by the kinase activity of Lyn. Cancer Res. 2001;61:2453–2458. [PubMed] [Google Scholar]
- 52.Zhang S, Macias-Garcia A, Velazquez J, Paltrinieri E, Kaufman RJ, Chen JJ. HRI coordinates translation by eIF2alphaP and mTORC1 to mitigate ineffective erythropoiesis in mice during iron deficiency. Blood. 2018;131:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lithanatudom P, Wannatung T, Leecharoenkiat A, Svasti S, Fucharoen S, Smith DR. Enhanced activation of autophagy in beta-thalassemia/Hb E erythroblasts during erythropoiesis. Ann Hematol. 2011;90:747–758. [DOI] [PubMed] [Google Scholar]
- 54.Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.