Abstract
During terrestrial differentiation, the relatively small amount of phosphorus that migrated to the lithosphere was incorporated into igneous rock, predominantly in the form of basic calcium orthophosphate (Ca10(PO4)6(OH,F,Cl)2, apatite). Yet, the highly insoluble nature of calcium apatite presents a significant problem to those contemplating the origin of life given the foundational role of phosphate (PO43-) in extant biology and the apparent requirement for PO43- as a catalyst, buffer and reagent in prebiotic chemistry. Reduced meteorites such as enstatite chondrites are highly enriched in phosphide minerals, and upon reaction with water these minerals can release phosphorus species of various oxidation states. Here, we demonstrate how reduced phosphorus species can be fully oxidized to PO43- simply by the action of ultraviolet light on H2S/HS-. We used low pressure Hg lamps to simulate UV output from the young Sun and 31P NMR spectroscopy to monitor the progress of reactions. Our experimental findings provide a cosmochemically and geochemically plausible means for supply of PO43- that was widely available to prebiotic chemistry and nascent life on early Earth, and potentially on other planets.
Over the past few years, reports have described advances in prebiotic chemistry regarding the terrestrial synthesis of sugars, amino acids, components of RNA and vesicles, the bulk of the constituents of the citric acid cycle and potential means to activate nucleotides.1,2,3,4 Furthermore, the chemistry described in these reactions adheres to a geochemical scenario that has developed in tandem with the chemical discoveries and runs in a progressive, logical sequence of events.2,3,5 This scheme has been outlined several times before and will not be detailed again here,2,3,5 but the key requirements are: cyanide, sulfurous reductants and reagents and ferrous iron at a sunlit location on an emergent landmass which previously experienced meteor impact(s) and was then subjected to fluvial processes. Drainage rivulets are thought to be essential as they allow divergence and confluence of reaction streams, thus enabling separation of subtly different chemistries and remixing of the various products,2,3,5 an idea which has recently garnered support.6,7 Prebiotic sources for all starting materials, reagents and catalysts have been accounted for within the geochemical scheme, with one exception – phosphate. One source of globally significant amounts of phosphorus would have been meteoritic delivery of the mineral schreibersite ((Fe,Ni)3P). When it was demonstrated that PO43- was one of the corrosion products of schreibersite, a plausible source of prebiotic PO43- appeared to have been established.8,9 Although encouraging, the mixture of products obtained from aqueous alteration of (Fe,Ni)3P is highly variable depending upon the conditions employed, and may result in hypophosphite (H2PO2-), phosphite (HPO32-) or PO43- being the major product, although PO43- is never formed as the sole product.8,9,10,11,12 This means that any incorporation of phosphorus into prebiotic molecules would likely be indiscriminate and result in a heterogeneous mixture which would seem to frustrate prebiotic chemistry and its transition to biology.
Ultraviolet light is essential for the prebiotic chemistry that we have described, consequently we wondered if photochemical oxidation of H2PO2- and HPO32- could provide PO43-. We were initially intrigued by a report which identified PO43- as a by-product of a reaction involving HPO32- and formaldehyde (CH2O) under UV irradiation,13 although this appeared incongruous with the results from another study involving irradiations of HPO32- alone in H2O where P2O64- was identified as the major product.14 After re-investigation, we conclude there may have been a misassignment and that PO43- not P2O64- appears to be the product of photolysis of HPO32- in H2O (Supplementary Discussion 1, Supplementary Figs. 1, 3 and 4). However, in these reports it was assumed that a UV component at 185 nm was responsible for the observed chemistry through photolysis of H2O, although the direct photolysis of HPO32- at 185 nm cannot be ruled out (Supplementary Discussion 1, Supplementary Figs. 5-7).13,14 This mechanism of oxidation of HPO32- to PO43- cannot be considered plausible due to the fact that an early Earth atmosphere containing H2O and CO2 would not be expected to permit light < 200 nm to reach the planet’s surface due to absorption and scattering,15 and even light < 220 nm may have been limited.16 Although an alternate mechanism for the photochemical production of hydroxyl radicals, potentially capable of oxidising H2PO2- and HPO32- to PO43-, may have been possible using light with wavelength > 200 nm, we instead pursued a solution which would be consistent with our prebiotic scheme and operated at a wavelength fully compatible with models of Earth’s early atmosphere.15,16
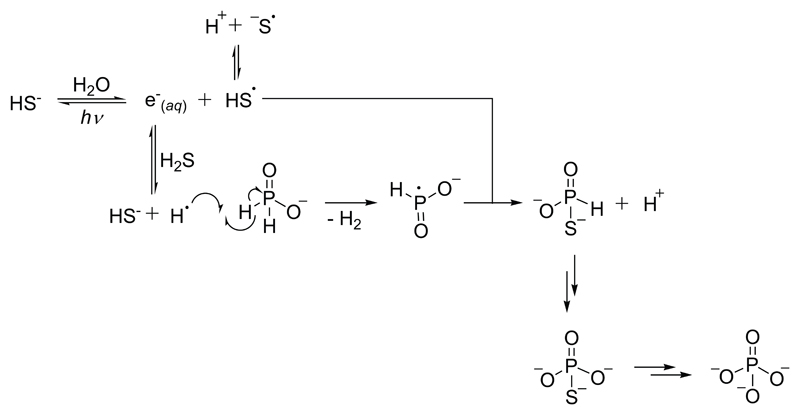
As H2S/HS- had been implicated in our previous work, often under photochemical conditions,1,2 we contemplated chemistry which may result from photolysis of H2S/HS- (a process which has been studied for decades and is known to occur under irradiation at 254 nm17,18). When HS- is irradiated, an electron is detached from the anion, or excited state of the anion, which is then solvated by H2O, resulting in a hydrated electron (e-(aq)) and a hydrosulfidic radical (HS•, Fig. 1).19 Hydrated electrons possess a pKb of ~ 9.6,20 and a general acid (GA-H) can protonate e-(aq) to form a hydrogen atom, H•.21 If this hydrogen atom then abstracted a second such atom from HPO32-, radical recombination of the resulting phosphite radical with the remaining HS• would afford thiophosphate (PSO33-) and thence PO43- (Fig. 1). This pathway was especially attractive as we have found PSO33- to be an extremely versatile prebiotic reagent, although a plausible prebiotic source had remained elusive.22
Fig. 1. Proposed photochemical synthesis of orthophosphate (PO43-) proceeding by way of thiophosphate (PSO33-).
Experimental oxidation of reduced phosphorus species
To investigate this proposal, a solution of NaSH (50 mM) and HPO32- (30 mM) in 10% D2O (pH 6.5) was irradiated under our standard conditions and the sample was inspected using 31P NMR spectroscopy. Gratifyingly, clean formation of PO43- resulted and a significant rate enhancement was observed when compared to the reaction without HS- (Fig. 2, Supplementary Fig. 8). After 8 h irradiation in the presence of HS-, the reaction was near completion, with ~ 94% PO43- present (Fig. 2). Furthermore, PSO33- – as revealed by acidifying the sample before spectroscopy – was also present in the reactions (Supplementary Figs. 9 and 10). Interestingly, there was a lag phase of ca. 1 h when little PO43- was produced. Production of H2 was evident as gas evolution could be seen from the sample, which implied the concurrent formation of polysulfides (HS(S)nS-) and we therefore reconsidered the reaction mechanism (Supplementary Discussion 2, Supplementary Fig. 11). Hence, the oxidation of HPO32- to PO43- appears to be prebiotically plausible and to rely mainly or solely on polysulfide species, which can be produced in situ by the action of UV light on a solution containing HS-.
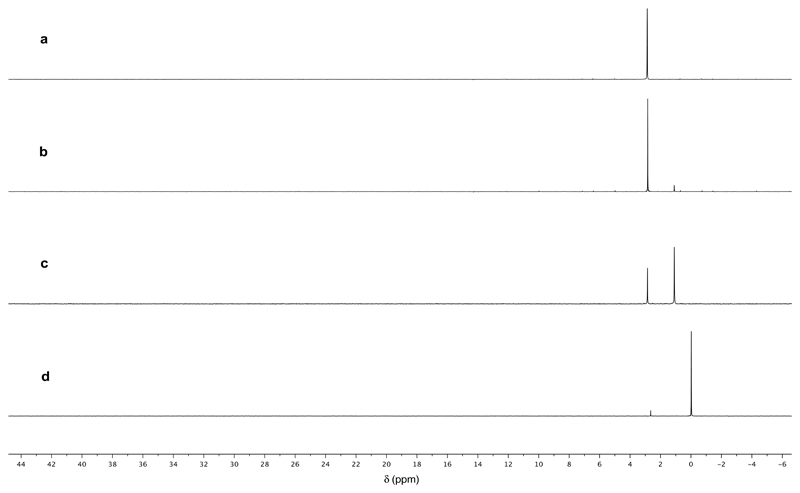
Fig. 2. Photochemical oxidation of phosphite.
a – 31P NMR Spectrum of a commercial sample of phosphite at pH 6.5; b – 31P NMR Spectrum of the irradiation of phosphite (30 mM) in 10% D2O after 4 h; c – 31P NMR Spectrum of the irradiation of phosphite (30 mM) in the presence of NaSH (50 mM) after 4 h according to Procedure 1; d – As spectrum C after 8 h irradiation. Differences in chemical shift are due to slight variations in pH.
The photochemical reaction of H2PO2- with HS- was markedly more rapid than the corresponding reaction of HPO32-, and after only 1 h, a signal corresponding to PSO33- was visible which integrated for ~ 56% of total phosphorus containing species (Supplementary Figs. 12 and 13). Oxidation of all species to phosphate was essentially complete in 5.5 h (96% yield, Supplementary Figs. 12 and 13). Clearly, the mechanism of oxidation of H2PO2- is different to that of HPO32- (Supplementary Discussion 2), and considering the immediate reaction of H2PO2- under the conditions, it appears likely that H• is able to abstract a hydrogen atom from H2PO2- (Fig. 3). Radical recombination of HṖO2- with HS• or -S• leads to HPSO22-. The subsequent, rapid formation and accumulation of PSO33- indicates HPSO22- is converted to PSO33- and does not proceed via HPO32- (vide supra and Supplementary Discussion 2). How HPSO22- is converted to PSO33- is unclear, but we note that another intermediate could not be observed by 31P NMR spectroscopy.
Fig. 3. Proposed mechanism for the formation of PSO33- and thence PO43- from the irradiation of H2PO2- and HS-.
Global abundances of HS-/H2S on early Earth are thought to have been low, thus, we repeated the reaction using 400 μM H2PO2- and 800 μM HS-/H2S and found all H2PO2- was consumed in 10 min producing PSO33- in ~ 33% yield, and after 4 h PO43- was the only phosphorus containing species present (Supplementary Fig. 14). Even on present day oxic Earth, low millimolar concentrations of H2S/HS- (< 4.1 mM) have been found in the waters emanating from geothermal fields.23 As noted previously, radical reactions involving e-(aq) and/or H• seem to be little perturbed by dilution in water, making them excellent candidates to perform reactions at geologically relevant concentrations.5 Alternatively, H2S could have accumulated as HS-/S2- in small bodies of alkaline water. Re-running the experiment at pH 9.0 (Supplementary Fig. 15) or pH 10.3 (Supplementary Fig. 16) had little effect on the reaction. As high pH did not perturb the reaction, we wondered if diamidophosphate (DAP ((H2N)2PO2-), a suggested reagent for prebiotic phosphorylations24) could be formed if the reaction was run in the presence of ammonia. After several experiments we were unable to find a prebiotically plausible synthesis of DAP using our oxidative conditions (Supplementary Figs. 17 and 18). However, a recent report has described the synthesis of DAP by reacting the schreibersite surrogate Fe3P with concentrated (> 1 M) ammonia solutions.25 The same study also showed that UV irradiation of HPO32- in ammonia solution provides access to amidophosphate (H2N)PO32-).25
Correlation of experimental results with early Earth environments
It is worth noting that the low pressure mercury lamps used during our experiments emit the majority of their output over a narrow range of light centred at 254 nm (~ 252 – 256 nm), which has been considered a suitable proxy for the emission of light from the young Sun at these wavelengths, but does not represent its expected broadband emission.15 A comparison of the UV flux from the equipment used in our experiments with that from the young Sun at Earth’s surface has been made before and was estimated as being roughly 5 orders of magnitude more powerful.16 However, the photochemical oxidation of HS- (λmax = 230 nm26) presumably takes place over a much broader range of wavelengths than the output of the Hg lamps used in these experiments and we thus consider a 100,000-fold reduction in rate of photochemical oxidation of HS- on Hadean Earth, under analogous conditions to those used in the present manuscript, to be a lower limit (this equates to the reaction taking place on the scale of tens of years rather than hours). Whilst the direct photolysis of HO- may have produced HO•, this would presumably require light < 220 nm (and optimally < 200 nm, λmax ~ 195 nm26). Although the young Sun’s UV output would have provided light of these wavelengths, the viability of such a process would have been heavily dependent upon the shielding properties of Earth’s atmosphere to shorter wavelength light.15,16 It is also possible that Fenton chemistry (Fe2+/H2O2) oxidised reduced phosphorus species to phosphate, a process that may have been operational in the mid-Archaean but was unlikely in the Hadean, particularly so in a highly reduced environment.27
Any phosphate that was produced, either directly from the corrosion of schreibersite or via the oxidation chemistry we report herein, would have been susceptible to precipitation by Ca2+ ions at moderately high pH or by ferrous ions. For example, plagioclase alteration (albitization) by hot brine yields effluent fluids enriched in CaCl2 and Ca(OH)2.28 Springs, streams or lakes arising from these fluids may have been devoid of PO43- following precipitation of calcium phosphates, but could have contained high concentrations of H2PO2- and/or HPO32- which are far more soluble in the presence of calcium ions.29 However, as albitization proceeded, the concentration of calcium ions in the exiting fluid waters would drop until eventually all calcium ions had been released or the concentration became negligible – a phenomenon indicated previously by the depletion of calcium in source rocks.30 After this point any oxidation of H2PO2- and HPO32- would provide PO43- in an environment devoid of Ca2+ ions. In the presence of Fe2+ ions, PO43- forms the insoluble complex vivianite (Fe[Fe2(PO4)2]). It was pointed out previously, however, that vivianite can be solubilised by cyanide, generating ferrocyanide (Fe(CN)64-) and PO43- in the process2,3 – an idea recently adopted by others.31 As hydrogen cyanide is generated by a variety of mechanisms during meteoritic impacts32,33,34 this method of ‘rescuing’ PO43- from Fe2+ is consistent with the proposed source of PO43- and our previously proposed geochemical and prebiotic scheme.2,3,5
Meteoritic flux of phosphorus to Earth
There is growing understanding that a ‘late heavy bombardment’ spike in impact flux to Earth ~ 3.9 Ga35 did not occur and instead there was a gradual, monotonic decline of impactor frequency.36,37,38 According to the latest models reconciled with cosmochemical tracers, subsequent to the Moon forming impact there was one other colossal impact with Earth by a differentiated body (dubbed Moneta) which re-equilibrated Earth’s silicate reservoirs for the final time (~ 4.48 Ga) and in turn explains both the overabundance and relative chondritic proportions of highly siderophile elements (HSE) in Earth’s mantle.37,39 Others have argued that a larger number of smaller impactors could account for the discrepancy between observed HSE abundances.40 Assuming enstatite chondrite-type composition (Supplementary Discussion 3), we calculated the quantity of reduced phosphorus contained in Moneta’s core to have been ~ 1.69 × 1020 kg (ca. 6 orders of magnitude higher than the combined phosphorus inventory of present day Earth’s oceans, soils and biomass,41 Supplementary Note 1). When a differentiated body of this size travelling at 16 km/s strikes Earth at 45° (statistically the most likely angle42) the majority of the core experiences shock-induced melting followed by fragmentation. Upon re-accretion this material fragments further into ~ 1 mm-sized metallic hail which rains onto the surface of early Hadean Earth.37 The reaction of (Fe,Ni)3P contained in this molten hail with H2O under intensely reducing conditions at such extremes of temperature should have produced substantial amounts of phosphine (PH3), especially considering the propensity of hypophosphorous and phosphorous acid, and oxy anions thereof, towards disproportionation.43,44 If temperatures were high enough, direct evaporation of P2 from (Fe,Ni)3P would have been possible and under the high pressure of H2 a portion of this would have been converted to PH3. Atmospheric oxidation of PH3 e.g. by hydroxyl radicals, leads to the rapid rainout of much less volatile and more hydrophilic phosphorus oxy acids e.g. hypophosphorous acid. If PH3 was not formed on impact, the overwhelming majority of (Fe,Ni)3P and other metallic materials from Moneta’s core would have been subsumed in the ensuing magma ocean. Delivery of reduced phosphorus would then depend on late accretion of enstatite planetesimals and meteorites.
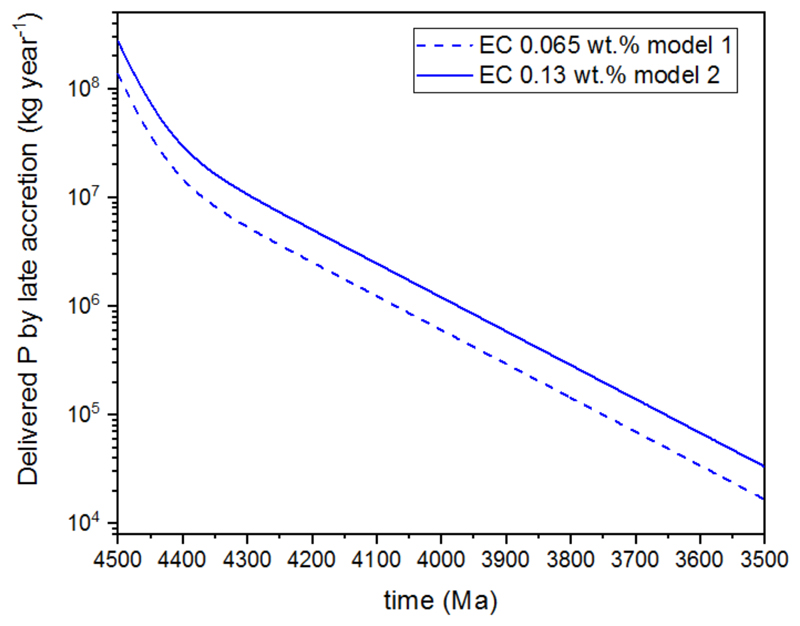
If we assume PH3 production did not occur upon impact or re-accretion of these smaller objects, metallic spherules (and the small amount of fragmented metallic rock around the impact site) would have been subjected to weathering by rainfall and/or groundwater, producing H2PO2- and HPO32- as previously described.8,9,10,11,12 Therefore, based on the late accretion production model developed by Mojzsis et al.,38 we calculated the amount of reduced phosphorus that could have been delivered to Earth during late accretion and subsequent to the Moneta impact i.e. excluding material delivered by Moneta, from 4.50 Ga to 3.50 Ga in million year increments (Fig. 4, Supplementary Discussion 3, Supplementary Table 1). At 4.40 Ga (the point at which Earth is thought to have been habitable38) 2.96 × 107 kg/year of reduced phosphorus could have been expected to be delivered to Earth, by 4.00 Ga this quantity has dropped to 1.20 × 106 kg/year and by 3.50 Ga to 3.30 × 104 kg/year (using 0.13 wt.% as baseline model, see Supplementary Discussion 3, Supplementary Table 1). The total mass of reduced phosphorus that late accretion could have delivered to Earth from 4.50 Ga to 3.50 Ga is 1.32 × 1019 kg (Supplementary Note 1, Supplementary Table 1). Previous calculations of the meteoritic delivery of phosphorus to Earth during late accretion gave similar values to those we report here.45,46 Until very recently,38,47 however, there existed no quantitative estimate for late accretion that used cratering data and highly siderophile element abundances combined with dynamics to show enstatite chondrites were a principle source of late accretion to Hadean Earth. Clearly it cannot be expected that the deposition of phosphorus resulting from late accretion occurred evenly over the surface of Earth, hence we provide the flux in kg/year. For the phosphorus content of a meteorite to be useful in the prebiotic chemistry we invoke, its payload must settle on dry land. Although impacts striking the ocean may have been capable of delivering material to dry land (depending on the size, velocity and impact angle of the impactor and depth of the water column), presumably maximal dispersion of a meteorite’s content over a land mass would be by a (near) direct hit. The concentrated deposition of the contents of metal-rich meteorites on dry land has already been described.45 The smallest objects considered in our calculations, and therefore the most abundant and likely to hit a subaerial landmass, were 1 km in diameter and could have individually delivered 3.2 × 109 kg of reduced phosphorus (Supplementary Note 1). Objects smaller than 1 km were not considered in the model as the mass augmentation to Earth from these objects relative to the rest of late accretion is considered negligible. However, this does not discount their potential significance for the origin of life given the inversely proportional relationship of impactor size and their impact frequency.
Fig. 4. Delivery of reduced phosphorus to Earth by late accretion.
Declining flux of reduced phosphorus delivered to Earth by planetesimals (≥1 km in diameter) throughout the Hadean and early Archean and subsequent to the proposed Moneta impact at ca. 4500 Ma (based on the model developed by Mojzsis et al.38).
As reduced phosphorus was delivered throughout the Hadean eon, the weathering of (Fe,Ni)3P and oxidation by UV/HS- could have provided a constant supply of thiophosphate and phosphate for prebiotic chemistry and early life. Furthermore, atmospheric photolysis of H2S resulted in the deposition of polysulfides on Earth’s surface and oceans,48 thus providing the apparent requisite species for phosphite oxidation. In addition, due to the decreased pKa and volatility of polysulfides relative to H2S,49 the accumulation of these species, particularly in oceans, may have been significant i.e. the photochemical oxidation of diagenetically produced phosphite by polysulfides could have provided a persistent low level of oceanic phosphate throughout the mid- to late Hadean and early Archean.50
Methods
General experimental
Reagents and solvents were bought from Sigma-Aldrich, Alfa Aesar and Santa Cruz Biotechnology and were used without further purification. Photochemical reactions were carried out using a Rayonet RPR-200 photochemical reactor chamber, with cooling fans switched on and fitted with low pressure RPR-2537A Hg lamps purchased from Rayonet (principle emission 254 nm). UV Reactions were run in Hellman QS Spectrosil 10.0 mm quartz cuvettes with 4 UV transparent windows. UV-Vis Spectra were acquired using a Varian 6000i UV-Vis-NIR spectrophotometer and Cary winUV software (Agilent Technologies) in Hellman QS Spectrosil quartz cuvettes. A Mettler Toledo SevenMulti pH/mv module fitted with a Thermo Scientific Orion 8103BN pH probe was used to measure pH, and deoxygenation of solvents was achieved by sparging with Ar for 20-30 min before use. 1H And 31P NMR spectra were acquired using a Bruker Ultrashield™ 400 Plus (at 400.1 and 162.0 MHz, respectively) or Bruker Ascend™ 400 (at 400.2 and 162.0 MHz, respectively) using HOD suppression to collect 1H NMR data (reactions were run in 10% D2O-H2O solutions). Yields were calculated by relative integration of NMR signals. Chemical shifts (δ) are given in ppm.
Experimental
Procedure 1: Oxidation of phosphite
Sodium phosphite pentahydrate (0.060 mmol, 13 mg) and NaSH.xH2O (>60%, 0.100 mmol, 9 mg) were dissolved in degassed 10% D2O (1 mL) in an Eppendorf tube and the pH was adjusted to 6.5 with degassed HCl. The volume was made up to 2 mL with degassed 10% D2O then the solution was transferred to a quartz cuvette and irradiated for the desired amount of time, after which the reaction was analysed by 31P NMR spectroscopy.
Procedure 2: Oxidation of hypophosphite
Hypophosphorus acid (50% wt., 0.060 mmol, 6.2 μL) was dissolved in degassed 10% D2O (1 mL) in an Eppendorf tube and the pH was adjusted to near neutrality with degassed NaOH. NaSH.xH2O (>60%, 0.100 mmol, 9 mg) was added and the pH was adjusted to 6.5, then the volume was made up to 2 mL with degassed 10% D2O. The solution was transferred to a quartz cuvette and irradiated for the desired amount of time, after which its contents were analysed by 31P NMR spectroscopy.
Procedure 3: Oxidation of hypophosphite at low concentration
Hypophosphorus acid (50% wt., 1 μL) was dissolved in degassed 10% D2O (1 mL, 9.6 mM solution) and 83 μL of this solution was added to degassed 10% D2O (1 mL) in an Eppendorf tube. The pH was adjusted to near neutrality with degassed NaOH. NaSH.xH2O (>60%, 1.9 mg) was dissolved in degassed 10% D2O (1 mL, 20.3 mM solution) and 80 μL was added to the reaction then the pH was adjusted to 6.5. The volume was made up to 2 mL with degassed 10% D2O and the solution was transferred to a quartz cuvette and irradiated for the desired amount of time, after which the reaction was analysed by 31P NMR spectroscopy.
Procedure 4: Oxidation of hypophosphite at high pH
Hypophosphorus acid (50% wt., 0.060 mmol, 6.2 μL) was dissolved in degassed 10% D2O (1 mL) in an Eppendorf tube and the pH was adjusted to near neutrality with degassed NaOH. NaSH.xH2O (>60%, 0.100 mmol, 9 mg) was added and the pH was adjusted to 9.0, then the volume was made up to 2 mL with degassed 10% D2O. The solution was transferred to a quartz cuvette and irradiated for the desired amount of time, after which its contents were analysed by 31P NMR spectroscopy.
Supplementary Material
Acknowledgements
The authors would like to thank P. B. Rimmer for helpful discussions about atmospheric shielding from UV light and photochemical reactions and R. Brasser and O. Abramov for informative discussions about dynamical models and Earth’s formation. D.J.R Would like to thank T. Rutherford for assistance with NMR spectroscopy. This work was supported by the Medical Research Council (Grant No. MC_UP_A024_1009 to J.D.S), a grant from the Simons Foundation (Grant No. 290362 to J.D.S) and S.J.M thanks the Collaborative for Research in Origins (CRiO) at the University of Colorado, which was supported by The John Templeton Foundation (principal investigator: Steven Benner/FfAME): the opinions expressed in this publication are those of the authors, and do not necessarily reflect the views of the John Templeton Foundation. S.J.M also acknowledges the NASA Solar System Workings Program, grant 80NSSC17K0732 (principle investigator: Oleg Abramov/PSI).
Footnotes
Data availability
The authors declare that all data associated and supporting this study are available in the published article and supplementary information. We have chosen not to make the data available in a publicly accessible repository at this time.
Author contributions
D.J.R And J.D.S designed the chemical experiments, D.J.R carried out the chemical experiments and D.J.R and J.D.S analysed the data. S.J.M Calculated the quantities of reduced phosphorus delivered to Earth from dynamical models. D.J.R Wrote the manuscript with input from J.D.S and S.J.M, all authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
References and Notes
- 1.Ritson DJ, Sutherland JD. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew Chem Int Edw. 2013;52:5845–5847. doi: 10.1002/anie.201300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat Chem. 2015;7:301–307. doi: 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland JD. The origin of life – out of the blue. Angew Chem Int Ed. 2016;55:104–121. doi: 10.1002/anie.201506585. [DOI] [PubMed] [Google Scholar]
- 4.Mariani A, Russell DA, Javelle T, Sutherland JD. A light-releasable potentially prebiotic nucleotide activating agent. J Am Chem Soc. 2018;140:8657–8661. doi: 10.1021/jacs.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritson DJ, Battilocchio C, Ley SV, Sutherland JD. Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04147-2. Article number: 1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker S, et al. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat Commun. 2018;9 doi: 10.1038/s41467-017-02639-1. Article number: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker S, et al. Unified prebiotically plausible synthesis of pyrimidine and purine RNA ribonucleotides. Science. 2019;366:76–82. doi: 10.1126/science.aax2747. [DOI] [PubMed] [Google Scholar]
- 8.Pasek MA, Lauretta DS. Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiol. 2005;5:515–535. doi: 10.1089/ast.2005.5.515. [DOI] [PubMed] [Google Scholar]
- 9.Bryant DE, Kee TP. Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem Commun. 2006:2344–2346. doi: 10.1039/b602651f. [DOI] [PubMed] [Google Scholar]
- 10.Pasek MA, Dworkin JP, Lauretta DS. A Radical pathway for organic phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim Cosmochim Acta. 2007;71:1721–1736. [Google Scholar]
- 11.Bryant DE, et al. Electrochemical studies of iron meteorites: phosphorus redox chemistry on the early Earth. Int J Astrobiol. 2009;8:27–36. [Google Scholar]
- 12.Bryant DE, et al. Hydrothermal modification of the Sikhote-Alin iron meteorite under low pH geothermal environments. A plausibly prebiotic route to activated phosphorus on the early Earth. Geochim Cosmochim Acta. 2013;109:90–112. [Google Scholar]
- 13.de Graaf RM, Visscher J, Schwartz AW. A plausibly prebiotic synthesis of phosphonic acids. Nature. 1995;378:474–477. doi: 10.1038/378474a0. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AW, van der Veen M. Synthesis of hypophosphate by ultraviolet irradiation of phosphite solutions. Inorg Nucl Chem Lett. 1973;9:39–41. [Google Scholar]
- 15.Ranjan S, Sasselov DD. Influence of the UV environment on the synthesis of prebiotic molecules. Astrobiol. 2016;16:68–88. doi: 10.1089/ast.2015.1359. [DOI] [PubMed] [Google Scholar]
- 16.Rimmer PB, et al. The origin of RNA precursors on exoplanets. Sci Adv. 2018;4 doi: 10.1126/sciadv.aar3302. eaar3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volman DH, Wolstenholme J, Hadley SG. Photochemical formation of free radicals from hydrogen sulfide, mercaptans, and cysteine. J Phys Chem. 1967;71:1798–1803. doi: 10.1021/j100865a037. [DOI] [PubMed] [Google Scholar]
- 18.Das TN, Huie RE, Neta P, Padmaja S. Reduction potential of the sulfhydryl radical: pulse radiolysis and laser flash photolysis studies of the formation and reactions of •SH and HSSH•- in aqueous solutions. J Phys Chem A. 1999;103:5221–5226. [Google Scholar]
- 19.Sauer MC, Crowell RA, Shkrob IA. Electron photodetachment from aqueous anions. 1. Quantum yields for generation of hydrated electron by 193 and 248 nm laser photoexcitation of miscellaneous inorganic anions. J Phys Chem A. 2004;108:5490–5502. [Google Scholar]
- 20.Hart EJ, Gordon S, Fielden EM. Reaction of the hydrated electron with water. J Phys Chem. 1966;70:150–156. [Google Scholar]
- 21.Karmann W, Meissner G, Henglein A. Pulsradiolyse des schwefelwasserstoffs in wäßriger lösung. Z Naturforsch B. 1967;22:273–282. [Google Scholar]
- 22.Ritson DJ, Xu J, Sutherland JD. Thiophosphate – a versatile prebiotic reagent? Synlett. 2017;28:64–67. [Google Scholar]
- 23.Kaasalainen H, Stefánsson A. Sulfur speciation in natural hydrothermal waters, Iceland. Geochim Cosmochim Acta. 2011;75:2777–2791. [Google Scholar]
- 24.Gibard C, Bhowmik S, Karki M, Kim E-K, Krishnamurthy R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat Chem. 2018;10:212–217. doi: 10.1038/nchem.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibard C, et al. Geochemical sources and availability of amidophosphates on the early Earth. Angew Chem Int Ed. 2019;58:8151–8155. doi: 10.1002/anie.201903808. [DOI] [PubMed] [Google Scholar]
- 26.Zuman P, Szafranski W. Ultraviolet spectra of hydroxide, alkoxide, and hydrogen sulfide anions. Anal Chem. 1976;48:2162–2163. [Google Scholar]
- 27.Pasek MA, Kee TP, Bryant DE, Pavlov AA, Lunine JI. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew Chem Int Ed. 2008;47:7918–7920. doi: 10.1002/anie.200802145. [DOI] [PubMed] [Google Scholar]
- 28.For example, see: Harigane Y, Michibayashi K, Ohara Y. Deformation and hydrothermal metamorphism of gabbroic rocks within the Godzilla Megamullion, Parece Vela Basin, Philippine Sea. Lithos. 2011;124:185–199.
- 29.Gulick A. Phosphorus as a factor in the origin of life. Am Sci. 1955;43:479–489. [Google Scholar]
- 30.Cates NL, Ziegler K, Schmitt AK, Mojzsis SJ. Reduced, reused and recycled: detrital zircons define a maximum age for the Eoarchean (ca. 3750-3780 Ma) Nuvvuagittuq supracrustal belt, Québec (Canada) Earth Planet Sci Lett. 2013;362:283–293. [Google Scholar]
- 31.Burcar B, et al. A stark contrast to modern Earth: phosphate mineral transformation and nucleoside phosphorylation in an iron- and cyanide-rich early Earth scenario. Angew Chem Int Ed. 2019;58:16981–16987. doi: 10.1002/anie.201908272. [DOI] [PubMed] [Google Scholar]
- 32.Kurosawa K, et al. Hydrogen cyanide production due to mid-sized impacts in a redox-neutral N2-rich atmosphere. Orig Life Evol Biosph. 2013;43:221–245. doi: 10.1007/s11084-013-9339-0. [DOI] [PubMed] [Google Scholar]
- 33.Ferus M, et al. High energy radical chemistry formation of HCN-rich atmospheres on early Earth. Sci Rep. 2017;7 doi: 10.1038/s41598-017-06489-1. Article number: 6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkos D, Pikus A, Alexeenko A, Melosh HJ. HCN Production via impact ejecta during reentry during the late heavy bombardment. J Geophys Res Planets. 2018;123:892–909. [Google Scholar]
- 35.Tera F, Papanastassiou DA, Wasserburg GJ. Isotopic evidence for a terminal lunar cataclysm. Earth Planet Sci Lett. 1974;22:1–21. [Google Scholar]
- 36.Brasser R, Mojzsis SJ, Werner SC, Matsumura S, Ida S. Late veneer and late accretion to the terrestrial planets. Earth Planet Sci Lett. 2016;455:85–93. [Google Scholar]
- 37.Genda H, Brasser R, Mojzsis SJ. The terrestrial late veneer from core disruption of a lunar-sized impactor. Earth Planet Sci Lett. 2017;480:25–32. [Google Scholar]
- 38.Mojzsis SJ, Brasser R, Kelly NM, Abramov O, Werner SC. Onset of giant planet migration before 4480 million years ago. Astrophys J. 2019;881 doi: 10.3847/1538-4357/ab2c03. [DOI] [Google Scholar]
- 39.Walker RJ. Highly siderophile elements in the Earth, Moon and Mars: update and implications for planetary accretion and differentiation. Chem Der Erde – Geochemistry. 2009;69:101–125. [Google Scholar]
- 40.Kraus RG, et al. Impact vaporization of planetesimal cores in the late stages of planet formation. Nat Geosci. 2015;8:269–272. [Google Scholar]
- 41.Smil V. Phosphorus in the environment: Natural flows and human interfaces. Annu Rev Energy Environs. 2000;25:53–88. [Google Scholar]
- 42.Shoemaker EM. Interpretation of Lunar Craters. In: Kopal Z, editor. Physics and Astronomy of the Moon. Academic Press; New York: 1962. [Google Scholar]
- 43.Bassett J. Inorganic Chemistry: A Concise Text. Pergamon Press Ltd; Oxford: 1966. pp. 280–281. [Google Scholar]
- 44.Liu D, et al. Disproportionation of hypophosphite and phosphite. Dalton Trans. 2017;46:6366–6378. doi: 10.1039/c7dt00243b. [DOI] [PubMed] [Google Scholar]
- 45.Pasek M, Lauretta D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig Life Evol Biosph. 2008;38:5–21. doi: 10.1007/s11084-007-9110-5. [DOI] [PubMed] [Google Scholar]
- 46.Pasek MA. Schreibersite on the early Earth: scenarios for prebiotic phosphorylation. Geosci Front. 2017;8:329–335. [Google Scholar]
- 47.Brasser R, Werner SC, Mojzsis SJ. Impact bombardment chronology of the terrestrial planets from 4.5 Ga to 3.5 Ga. Icarus. 2020;338 113514. [Google Scholar]
- 48.Mojzsis SJ. Sulphur on the Early Earth. In: van Kranendonk MJ, Smithies RH, Bennett VC, editors. Earth’s Oldest Rocks. Developments in Precambrian Geology. Vol. 15. Elsevier; 2007. pp. 923–970. [Google Scholar]
- 49.Steudel R. Inorganic Polysulfanes H2Sn with n > 1. In: Steudel R, editor. Elemental Sulfur and Sulfur-rich Compounds II. Topics in Current Chemistry. Vol. 231. Springer; Berlin, Heidelberg: 2003. pp. 99–126. [Google Scholar]
- 50.Herschy B, et al. Archean phosphorus liberation induced by iron redox geochemistry. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03835-3. Article number: 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.