Abstract
Background
There are limited data on the natural history of antenatal Zika virus (ZIKV) exposure in twin pregnancies, especially regarding intertwin concordance of prenatal, placental, and infant outcomes.
Methods
This prospective cohort study included twin pregnancies referred to a single institution from September 2015 to June 2016 with maternal ZIKV. Polymerase chain reaction (PCR) testing of maternal, placental, and neonatal samples was performed. Prenatal ultrasounds were completed for each twin, and histomorphologic analysis was performed for each placenta. Abnormal neonatal outcome was defined as abnormal exam and/or abnormal imaging. Two- to three-year follow-up of infants included physical exams, neuroimaging, and Bayley-III developmental assessment.
Results
Among 244 pregnancies, 4 twin gestations without coinfection were identified. Zika virus infection occurred at 16–33 weeks gestation. Zika virus PCR testing revealed discordance between dichorionic twins, between placentas in a dichorionic pair, between portions of a monochorionic placenta, and between a neonate and its associated placenta. Of the 8 infants, 3 (38%) had an abnormal neonatal outcome. Of 6 infants with long-term follow-up, 3 (50%) have demonstrated ZIKV-related abnormalities.
Conclusions
Neonatal PCR testing, placental findings, and infant outcomes can be discordant between co-twins with antenatal ZIKV exposure. These findings demonstrate that each twin should be evaluated independently for vertical transmission.
Keywords: congenital Zika syndrome, perinatal infection, TORCH infection, twin, vertical transmission
This prospective cohort study of twins with antenatal Zika virus exposure demonstrated that neonatal PCR testing, placental findings, neonatal outcomes, and long-term outcomes can be discordant. Future studies should evaluate each twin and its associated placenta independently for vertical transmission.
Zika virus (ZIKV) infection in pregnancy places the fetus at risk of developing significant central nervous system (CNS) abnormalities, including microcephaly, seizure activity, hypertonia, visual and auditory deficits, and other neurodevelopmental changes [1–3]. During primary maternal infection, this single-stranded flavivirus passes from the maternal blood stream, across the placenta, and into the fetal circulation, gaining access to the fetal CNS where the virus cause destruction and malformation [4].
Because the vast majority of ZIKV infections occur in singleton pregnancies, little is known about the natural history of ZIKV infection in twin pregnancies. We identified 3 published studies addressing this special circumstance. One report described a single case of maternal ZIKV infection in the first trimester of a dizygotic twin pregnancy, with subsequent delivery of 1 healthy neonate and 1 microcephalic neonate, with no long-term infant follow-up [5]. A case series described 2 dizygotic twin pairs with intertwin ZIKV discordance based on prenatal and postnatal neuroimaging after maternal ZIKV infection in pregnancy, with infant follow-up for 7–12 months; notably, these twin pairs were identified as having had antenatal ZIKV exposure based on positive immunoglobulin M testing on neonatal cerebrospinal fluid (CSF), rather than positive maternal serum testing before delivery [6]. Another case series described 6 of 7 dizygotic pairs with ZIKV discordance based on neuroimaging or serology, without long-term infant follow-up [7]. These previously published reports are limited by their retrospective natures, which may bias towards abnormal outcomes, and by their short-term infant follow-up, because subtle outcomes related to antenatal ZIKV exposure, particularly those related to neurodevelopmental outcomes, may not manifest until months to years after birth [8, 9].
We present a prospective cohort of twin pregnancies complicated by confirmed antenatal ZIKV infection, with a focus on prenatal evaluation, molecular confirmation of vertical transmission, placental pathology, and infant follow-up of at least 25 months. We present evidence of intertwin discordance in these findings.
METHODS
Human Subjects and Study Design
This prospective cohort included all twin pregnancies that were referred to the Instituto Fernandes Figueira (IFF) for suspected maternal ZIKV infection from September 2015 to June 2016, based on clinical symptoms of an acute febrile illness and a rash. As the Ministry of Health referral center for Rio de Janeiro, Brazil, the IFF provides comprehensive prenatal, obstetric, and postnatal care for pregnancies complicated by complex fetal diagnoses, including possible congenital ZIKV infection. This study was approved by the institutional human research ethics committee at IFF (CAAE 52675616.0.0000.5269) and was considered exempt at the University of California, San Francisco.
Specimen Collection and Analysis
At the time of suspected infection, maternal serum and urine specimens were collected and tested with reverse-transcriptase polymerase chain reaction (PCR) assays to confirm ZIKV infection, as previously described [1]. Maternal samples were also collected to rule out other infections, including toxoplasmosis, syphilis, rubella, cytomegalovirus, and herpes simplex virus. Any cases with coinfection were excluded. At birth, placenta, cord blood, neonatal serum, neonatal urine, and/or neonatal CSF specimens were collected as available for ZIKV PCR to confirm cases of vertical transmission.
Placental Specimen Collection and Histopathologic Analysis
Placentas were collected and processed as soon as possible after delivery, within a maximum of 48 hours. Macroscopic evaluation of fresh, unfixed placental specimens was performed according to previously published guidelines [10]. Three placental pieces from the umbilical cord insertion site, measuring 0.5 cm each, were collected for PCR analysis. The placentas were fixed in 10% phosphate-buffered formalin for 24 to 48 hours, and at least 5 samples from the placental disc of each twin, including the umbilical cord insertion site, were collected by cutting a vertical plane through the full thickness of the tissue, making sure to include both the fetal and maternal surfaces. Samples from the umbilical cord and membranes were also taken. After dehydration in alcohol and diaphanization in xylene, placental fragments were embedded in paraffin. Histologic sections (4 μm) were stained with hematoxylin and eosin and analyzed by light microscopy (Olympus, Tokyo, Japan) per standard procedure. For each of the 8 twins, histopathologic analysis was performed on at least 5 samples from the placental disc, 4 samples from the umbilical cord, 2 samples from the smooth chorion, and 1 from the intertwin membrane.
Maternal and Neonatal Variables
Data collected for each case included maternal symptoms, obstetric history, delivery outcomes, ultrasound findings, and other prenatal testing results. All ultrasounds were comprehensive and performed by specialists certified by the Brazilian College of Radiology and the Brazilian Federation of Societies of Gynecology and Obstetrics. Neonatal outcomes included birth measurements such as head circumference (HC), weight, and length. At the time of birth, clinical exams were documented by the IFF pediatrics team, which included specialists in neonatology, infectious diseases, neurology, pediatric ophthalmology, and genetics. Neuroimaging was performed on infants after birth, via transfontanellar ultrasound (TFUS). Those with abnormalities on TFUS or on physical exam underwent a subsequent computerized tomography (CT) of the head. Preterm birth was defined as delivery before 37 weeks of gestation. Microcephaly was defined as an HC more than 2 standard deviations (SD) below expected for gestational age and sex by INTERGROWTH curves, and severe microcephaly was defined as HC more than 3 SD below expected for gestational age and sex. Birth weights were compared with INTERGROWTH birth weight standards by sex and considered small for gestational age if the birth weight Z-score was more than 1.28 SD below expected for gestational age and sex [11]. Abnormal neonatal outcome was defined if any of the following were present: abnormal newborn examination, abnormal imaging studies, and/or perinatal death (defined as intrauterine fetal demise or neonatal death within 30 days of birth).
Long-Term Follow-up
Infants were followed for at least 25 months to screen for developmental, neurologic, orthopedic, auditory, and/or ocular disorders consistent with antenatally acquired ZIKV. These evaluations included routine physical examination, fundoscopic examination, and brainstem evoked response audiometry (BERA) test. Neuroimaging with head CT was performed only if clinically indicated based on presence of seizures, significant developmental delay, and/or signs of autism spectrum disorder. Starting at 6 months of age, infants were evaluated on cognitive, language, and motor development using the Bayley-III Scale of Infant Development, a tool that has previously been validated for cross-cultural use in Brazil [12]. A score between 1 and 2 SD below average (70 to 85) was considered below average and is used as a marker for risk of developmental delay [13]; a score more than 2 SD below average (less than 70) was considered very below average and is generally used as a marker of severe development delay [8, 9, 14].
RESULTS
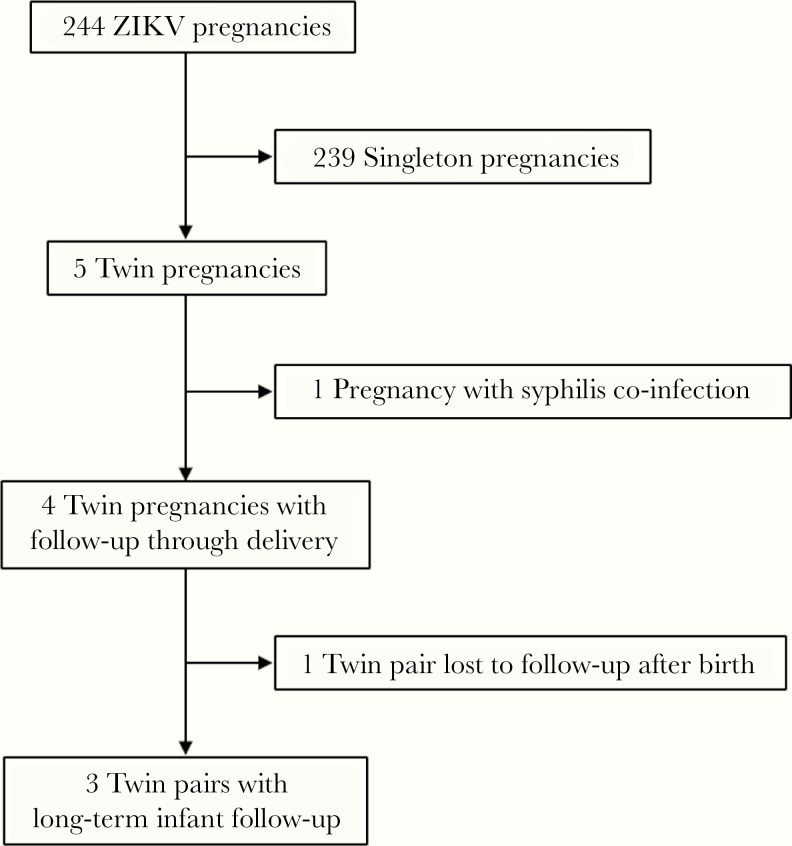
Among 244 cases referred to IFF between September 2015 to June 2016, a total of 5 twin pregnancies were identified (Figure 1). One pregnancy was excluded due to coinfection with syphilis. The remaining 4 twin pregnancies are described in this cohort; 3 were dichorionic, diamniotic twin pregnancies, and 1 was a monochorionic, diamniotic twin pregnancy. Onset of maternal symptoms and subsequent diagnosis ranged from 16 to 33 weeks of gestation. Overall, 3 of the 8 neonates (37.5%) had an abnormal neonatal outcome, with abnormal BERA testing in 2 ZIKV-positive twins in the same pair and abnormal TFUS in 1 ZIKV-positive twin with an unaffected ZIKV-negative co-twin (Table 1).
Figure 1.
Flow diagram of patient inclusion in prospective cohort of twin pregnancies with antenatal Zika virus (ZIKV) exposure.
Table 1.
Details of Maternal Infection, Prenatal Course, Delivery, and Immediate Neonatal Course Among Four Twin Pregnancies Complicated by Antenatal Exposure to ZIKV Infection
| Twin Pair | Maternal Symptoms | WGA at Diagnosis | Maternal ZIKV PCR | WGA at Delivery | Twin | Neonatal ZIKV PCR | Placental ZIKV PCR | BW (Grams) | NICU Admission | Abnormal Neonatal Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Twin pair 1 | Y | 24 | + (serum) | 37 | Twin A | + | + | 2400 | N | N |
| Twin B | + | – | 2590 | N | N | |||||
| Twin pair 2 | Y | 33 | – | 34 | Twin A | + | + | 2190 | N | Y – abnormal BERA at 1 week of life |
| Twin B | + | + | 2240 | N | Y – abnormal BERA at 1 week of life | |||||
| Twin pair 3 | Y | 16 | + (urine) | 35 | Twin A | + | + | 2550 | Y | Y – TFUS with grade 1 IVH |
| Twin B | – | – | 2650 | N | N | |||||
| Twin pair 4 | Y | 21 | + (urine) | 35 | Twin A | N/A | – | 2490 | N | Na |
| Twin B | N/A | – | 2220 | N | Na |
Abbreviations: BERA, brainstem evoked response audiometry; BW, birth weight; IVH, intraventricular hemorrhage; N, no; NICU, neonatal intensive care unit; PCR, polymerase chain reaction; TFUS, transfontanellar ultrasound; WGA, weeks gestational age; Y, yes; ZIKV, Zika virus.
aThese twins did not receive the detailed postnatal examination for suspected antenatal ZIKV exposure, but there were no gross physical or developmental abnormalities noted in the immediate neonatal period.
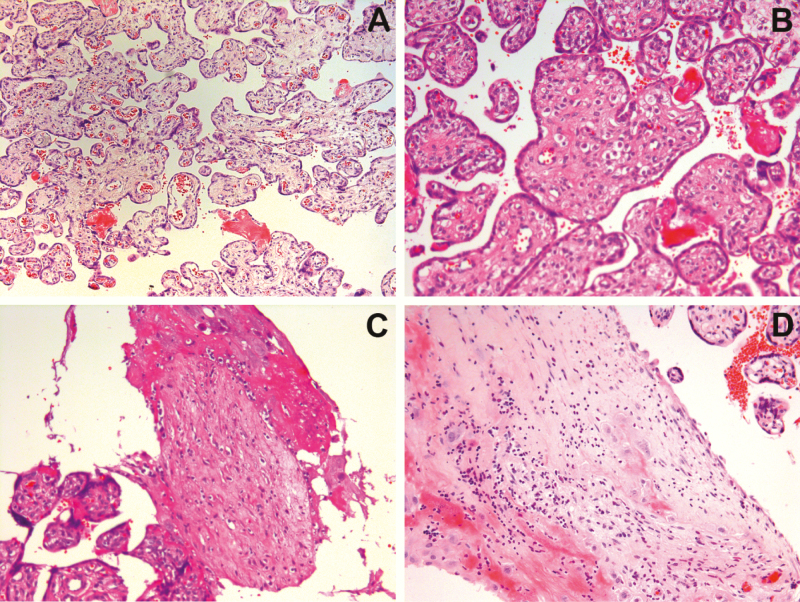
Each placenta underwent detailed histopathologic analysis by a trained pathologist. These findings are summarized in Table 2, and representative findings are shown in Figure 2. Representative images from a normal, noninfected, term placenta are shown in Supplemental Figure 1 for comparison. All placentas demonstrated delayed villous maturation (DVM) (Figure 2A). Other common findings included stromal fibrosis and Hofbauer cell hyperplasia (Figure 2B), basal villitis (Figure 2C), and chronic deciduitis (Figure 2D). Of placental histopathologic findings, basal villitis and lymphocytic deciduitis were commonly discordant between twin pairs. Placental PCR was discordant in 50% of twin pairs, including in one monochorionic placenta.
Table 2.
Details of PCR Testing and Placental Histopathologic Analysis Among Four Twin Pregnancies Complicated by Antenatal Exposure to ZIKV Infection
| Twin Pair | Chorionicity | Twin | ZIKV PCR Neonate | ZIKV PCR Placenta | Specific Features on Placental Histopathology | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV | BVT | CA | Cal | CC | DVM | FCV | HCH | LD | PVF | RVSM | SF | VT | |||||
| Twin pair 1 | Dichorionic | Twin A | + | + | – | + | – | – | – | + | + | + | – | + | – | + | – |
| Twin B | + | – | + | + | – | – | – | + | + | + | + | + | – | + | – | ||
| Twin pair 2 | Dichorionic | Twin A | + | + | – | – | – | – | + | + | + | – | – | + | + | + | – |
| Twin B | + | + | + | – | – | + | + | + | – | – | + | + | + | – | + | ||
| Twin pair 3 | Dichorionic | Twin A | + | + | – | – | – | + | – | + | – | + | – | – | + | + | – |
| Twin B | – | – | – | + | + | + | – | + | – | – | – | – | – | + | – | ||
| Twin pair 4 | Monochorionic | Twin A | – | – | – | – | + | – | + | + | – | – | – | – | – | – | – |
| Twin B | – | – | + | – | + | – | + | + | – | – | – | – | – | – | – |
Abbreviations: BV, basal villitis; BVT, blood vessel thickening; CA, chorioamnionitis; Cal, calcification; CC, circulatory changes; DVM, delayed villous maturation; FCV, focal chronic villitis; HCH, Hofbauer cell hyperplasia; LD, lymphocytic deciduitis; PCR, polymerase chain reaction; PVF, perivascular fibrosis; RVSM, reduction of vasculosyncytial membrane; SF, stromal fibrosis; VT, vascular thrombosis; ZIKV, Zika virus.
Figure 2.
Representative pathological features from Zika virus-infected placentas: (A) delayed villous maturation; (B) stromal fibrosis and Hofbauer cell hyperplasia; (C) basal villitis; and (D) chronic deciduitis.
One twin pair was lost to follow-up after birth, leaving 3 twin pairs available for long-term follow-up of at least 25 months and up to 39 months. Among these 6 infants, 3 (50%) have developed abnormalities on long-term follow-up, with significant strabismus in 1 ZIKV-positive twin, and significant bilateral hearing loss and below average Bayley-III language scores in 2 ZIKV-positive twins from the same pair (Table 3). Further information regarding antepartum course, placental findings, and infant outcomes for each twin pair are detailed in the following paragraphs.
Table 3.
Details of Long-Term Infant Follow-up Among Three Twin Pregnancies Complicated by Antenatal Exposure to ZIKV
| Twin Pair | Twin | Eye Exam | BERA | Most Recent Bayley-III Scale of Infant Development Scores | |||
|---|---|---|---|---|---|---|---|
| Timing | Cognitive | Language | Motor | ||||
| Twin pair 1 | Twin A | Normal | Normal | 25 months | 90 | 89 | 91 |
| Twin B | Normal | Normal | 25 months | 90 | 91 | 91 | |
| Twin pair 2 | Twin A | Normal | Bilateral hearing loss at 28 months | 37 months | 95 | 83a | 91 |
| Twin B | Normal | Bilateral hearing loss at 28 months | 37 months | 90 | 77a | 91 | |
| Twin pair 3 | Twin A | Significant strabismus | Normal | 39 months | 100 | 94 | 85 |
| Twin B | Normal | Normal | 39 months | 100 | 94 | 88 |
Abbreviations: BERA, brainstem evoked response audiometry; ZIKV, Zika virus.
aBelow average score, which indicates a value between 1 and 2 standard deviations below the norm.
Twin Pair 1
A 40-year-old woman was diagnosed with maternal ZIKV infection by PCR from maternal serum at 24 weeks after presenting with symptoms of acute ZIKV infection (Table 1). Prenatal ultrasounds were unremarkable. She delivered liveborn dichorionic twins at 37 weeks in the setting of preeclampsia. Neonatal birth weights were appropriate for gestational age (2400 grams and 2590 grams). Zika virus was identified from urine and CSF samples of both neonates. Both twins had normal neonatal findings. Placentas were discordant for ZIKV PCR testing (ie, one was positive, while the other was negative). On histopathology, both placentas had evidence of DVM, Hofbauer cell hyperplasia, focal chronic villitis, stromal fibrosis, and perivascular fibrosis (Table 2). The placentas were discordant for other histopathological features, including basal villitis and deciduitis. On long-term follow-up, both ZIKV-positive twins from this pair have had normal vision, hearing, and Bayley-III evaluations at 25 months of follow-up.
Twin Pair 2
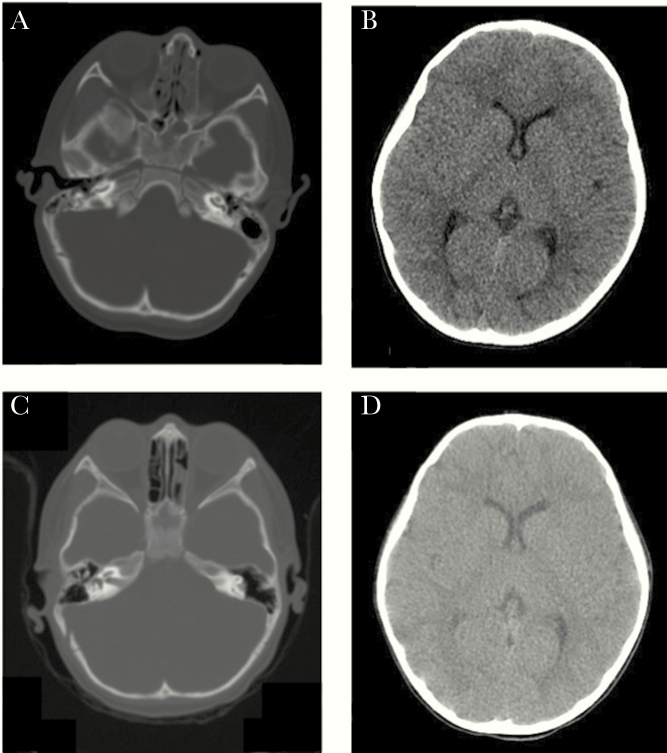
A 24-year-old woman presented with symptoms of acute ZIKV infection at 33 weeks, but her urine and serum samples tested negative for ZIKV by PCR (Table 1). Prenatal ultrasounds were notable for transient abnormalities on middle cerebral artery (MCA) Doppler interrogation of both twins. She delivered liveborn dichorionic twins at 34 weeks in the setting of oligohydramnios. Both twins had birth weights that were appropriate for gestational age (2190 grams and 2240 grams). Zika virus was identified in the cord blood and in the placenta of each twin. Both twins had abnormal BERA tests at 1 week of life. On histopathology, both placentas had evidence of DVM, perivascular fibrosis, and a reduction of the vasculosyncytial membrane (Table 2). The placentas were discordant for basal villitis, focal chronic villitis, stromal fibrosis, deciduitis, vascular thrombosis, and calcifications. On long-term follow up, both ZIKV-positive twins from this pair have developed bilateral hearing loss and demonstrated below average Bayley-III language scores at 37 months. On cerebral, mastoid, and temporal CT imaging of both twins at 37 months, there were no anatomical abnormalities (Figure 3), suggesting a functional sensorineural etiology of the severe hearing loss.
Figure 3.
Cerebral, temporal, and mastoid computerized tomography imaging of twin pair 2: (A) and (B) normal imaging of twin A with severe hearing loss; (C) and (D) normal imaging of twin B with severe hearing loss.
Twin Pair 3
A 28-year-old woman was diagnosed with maternal ZIKV infection by PCR from maternal urine at 16 weeks after presenting with symptoms of acute ZIKV infection (Table 1). Prenatal ultrasounds were notable for transient abnormalities on MCA Doppler interrogation of only 1 fetus, with normal MCA Doppler interrogation in the other. She delivered liveborn dichorionic twins at 35 weeks in the setting of preterm premature rupture of membranes. Both twins had birth weights that were appropriate for gestational age (2550 grams and 2650 grams). Zika virus was detected in only 1 twin on neonatal urine specimen. The placentas were discordant for ZIKV PCR testing, with the positive placenta corresponding to the positive twin, and the negative placenta corresponding to the negative twin. The ZIKV-positive twin was admitted to the neonatal intensive care unit (NICU), where postnatal TFUS demonstrated grade 1 intraventricular hemorrhage; follow-up TFUS 1 month later demonstrated normal findings. The ZIKV-negative twin had no abnormal neonatal findings. On histopathology, both placentas had evidence of DVM, stromal fibrosis, and calcifications. The placentas were discordant for Hofbauer cell hyperplasia, blood vessel thickening, a reduction of the vasculosyncytial membrane, and chorioamnionitis (Table 2). On long-term follow-up, the ZIKV-positive twin from this pair has been noted to have significant strabismus at 39 months; the ZIKV-negative co-twin has had normal findings on all follow-up testing, including ophthalmologic examination.
Twin Pair 4
A 20-year-old woman was diagnosed with maternal ZIKV infection by PCR from maternal urine at 21 weeks after presenting with symptoms of acute ZIKV infection (Table 1). Prenatal ultrasounds were notable only for growth restriction in 1 twin at 32 weeks. She delivered liveborn monochorionic, diamniotic twins at 35 weeks in the setting of preeclampsia. Both twins had birth weights that were appropriate for gestational age (2220 grams and 2490 grams). These twins did not receive the detailed postnatal examination for suspected antenatal ZIKV exposure, but there were no gross physical or developmental abnormalities noted in the immediate neonatal period. Zika virus PCR testing was not performed on neonatal specimens, but both placentas were negative for ZIKV on PCR testing. On histopathology, placental portions from each twin had evidence of DVM, chorioamnionitis, reduction of the vasculosyncytial membrane, and small areas of abruption. Basal villitis was associated with only 1 side of the placenta (Table 2). This twin pair was lost to follow-up after birth.
DISCUSSION
This prospective cohort describes the natural history of antenatal ZIKV exposure in twin pregnancies, with a spectrum of mild to severe ZIKV-related outcomes, even in the absence of microcephaly. These findings demonstrate that transmission of maternal ZIKV infection in twin pregnancies can be discordant at multiple levels—between twins in a dichorionic pair (twin pair 3), between placentas in a dichorionic pair (twin pairs 1 and 3), between placental portions of a monochorionic pair (twin pair 4), and between the neonate and its associated placenta (twin pair 3). These findings add to the limited literature regarding discordant ZIKV infection in twin pregnancies, including 1 case report of 1 dizygotic pair with ZIKV discordance based on postnatal microcephaly, another report of 2 of 2 dizygotic pairs with ZIKV discordance based on prenatal and postnatal neuroimaging, and a third report of 6 of 7 dizygotic pairs with ZIKV discordance based on neuroimaging or serology [5–7].
Discordance in vertical transmission is not unique to ZIKV, because prior case series have reported intertwin discordance for other antenatally acquired infections, including parvovirus B19 [15], toxoplasmosis [16], and cytomegalovirus [17]. The precise factors that contribute to discordant vertical transmission of congenital infections remain unknown but may include placental alterations that promote versus prevent acquisition of infection. In this cohort, Hofbauer cell hyperplasia was a common placental finding. A normal component of the intervillous stroma, the Hofbauer cells can undergo hyperplasia in the presence of congenital infections and villitis of unknown etiology [18–20]. One case report of a placenta infected with ZIKV at 11 weeks noted marked villous enlargement due to Hofbauer cell hyperplasia [21], and 2 in vitro studies demonstrated Hofbauer cell infection by ZIKV, with intracellular viral replication leading to subsequent activation of these cells [22, 23]. In our series, the placentas from Case 3 were discordant for Hofbauer cell hyperplasia, which was present only in the ZIKV-positive placenta (which corresponded to the ZIKV-positive neonate). However, Hofbauer cell hyperplasia was absent in both placentas of Case 2, in which the 2 neonates and their placentas tested positive for ZIKV, indicating that there must be other mechanisms and/or markers of vertical transmission. Another common placental finding in this cohort was the presence of DVM. Delayed villous maturation is characterized by a homogeneous population of villi that resemble the villi of early pregnancy, with increased stromal tissue, decreased vasculosyncytitial membranes, and centrally located capillaries [24]. Compared with mature, terminal villi with thin vasculosyncytial membranes and peripherally located capillaries, villi with DVM are characterized by an increased distance between the maternal and fetal circulations. This inefficiency in circulatory exchange has been postulated as the mechanism underlying the previously reported associations between DVM and intrauterine fetal demise, neonatal demise, admission to NICUs, and hypoxic-ischemic neonatal encephalopathy [25–27]. Delayed villous maturation has also been implicated in other congenital infections; for example, cytomegalovirus has been shown to infect progenitor cells and inhibit trophoblast differentiation, an important step in normal villous maturation [28, 29]. Our study reports only on the presence or absence of DVM, which limits the interpretation of DVM being present in all placentas in this cohort. A working group of perinatal and placental pathologists recently suggested that all pathologists grade the degree of DVM [24]. With a more nuanced grading of DVM, it may be possible to identify an association between the degree of DVM and the risk of vertical transmission and/or the risk of adverse neonatal outcomes in ZIKV-affected pregnancies, but further research is needed to evaluate this hypothesis.
This study is limited by the sample size, which was made even smaller by the loss of 1 twin pair to long-term follow-up. Another limitation of the study is the exclusive use of head CT for advanced neuroimaging. Magnetic resonance imaging may be more sensitive in characterizing mild structural variations but was unfortunately not routinely available due to the limited resources inherent to an epidemic setting.
Despite these limitations, this study has many strengths, particularly its prospective nature, which yields itself to accurate collection of the outcomes of interest via thorough evaluation and testing of each subject. Extending from at least 25 months to up to 39 months, the length of follow-up in this study is another major strength. Prolonged follow-up is critical in evaluating for more subtle ZIKV-related neurodevelopmental abnormalities that may not be obvious at the time of immediate neonatal assessment. Another strength of this study is the thoroughness of placental histopathological reporting. We hope that this may serve as a framework for future studies that will yield additional information regarding placental lesions associated with vertical transmission of ZIKV.
CONCLUSIONS
Our findings of twin discordance suggest that factors beyond the mother can modulate vertical transmission and infant outcomes in cases of antenatal ZIKV exposure. These factors may be at the level of the placenta and/or the fetus. To better understand these factors, each neonate and its placenta in a ZIKV-affected twin pregnancy should be evaluated separately. We also report on a monochorionic twin pregnancy, for which there was discordance for basal villitis between the 2 portions of the placenta. Future studies examining ZIKV-affected monochorionic twins and placentas to determine the potential contributions of monozygosity (ie, genetic identicalness) and intertwin vascular connections (via placental anastomoses) in mediating the placental response to ZIKV infection and the likelihood of vertical transmission would provide further insights.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded by the following: National Institute of Allergy and Infectious Diseases (NIAID) (K08AI141728) and the Foundation for the Society of Maternal-Fetal Medicine Queenan Fellowship for Global Health (to S. L. G.); generous support from Marc and Lynne Benioff (to S. J. F.); National Institute of Allergy and Infectious Diseases (R21AI28697 and R21AI129534) and National Eye Institute (NEI) (R21EY028318) (to K. N.-S.); the Brazilian Ministry of Health (to P. B. and M. E. M.); Wellcome Trust and the UK’s Department for International Development (205377/Z/16/Z; to M. E. M. and E. B.); the European Union’s Horizon 2020 Research and Innovation Programme under ZikaPLAN grant agreement number 734584 and FAPERJ (to M. E. M.); and the Program for Research Incentives (PIP)/Fernandes Figueira Institute/FIOCRUZ (EAP).
Potential conflicts of interest. S. J. F. is a consultant for Novo Nordisk. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 38th Annual Meeting of the Society of Maternal-Fetal Medicine, February 2018, Dallas, TX.
References
- 1. Brasil P, Pereira JP Jr, Moreira ME, et al. . Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 2016; 375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Melo AS, Aguiar RS, Amorim MM, et al. . Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol 2016; 73:1407–16. [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 4. Chimelli L, Melo AS, Avvad-Portari E, et al. . The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol 2017; 133:983–99. [DOI] [PubMed] [Google Scholar]
- 5. Zuanazzi D, Arts EJ, Jorge PK, et al. . Postnatal identification of Zika virus peptides from saliva. J Dent Res 2017; 96:1078–84. [DOI] [PubMed] [Google Scholar]
- 6. Linden VV, Linden HV Junior, Leal MC, et al. . Discordant clinical outcomes of congenital Zika virus infection in twin pregnancies. Arq Neuropsiquiatr 2017; 75:381–6. [DOI] [PubMed] [Google Scholar]
- 7. Caires-Junior LC, Goular E, Melo US, et al. . Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat Commun 2018; 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopes Moreira ME, Nielsen-Saines K, Brasil P, et al. . Neurodevelopment in infants exposed to Zika virus in utero. N Engl J Med 2018; 379:2377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nielsen-Saines K, Brasil P, Kerin T, et al. . Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019; 25:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khong TY, Mooney EE, Ariel I, et al. . Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 2016; 140:698–713. [DOI] [PubMed] [Google Scholar]
- 11. INTERGROWTH 21st Standards/References for Newborn Biometry Available at: http://intergrowth21.ndog.ox.ac.uk/. Accessed 1 July 2018.
- 12. Madaschi V, Mecca TP, Macedo EC, Paula CS. Bayley -III scales of infant and toddler development: transcultural adaptation and psychometric properties. Paiedéia (Ribeirã Preto) 2016; 26:189–97. [Google Scholar]
- 13. Ballot DE, Ramdin T, Rakotsoane D, et al. . Use of the Bayley scales of infant and toddler development, third edition, to assess developmental outcome in infants and young children in an urban setting in South Africa. Int Sch Res Notices 2017; 2017:1631760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayley N. Comparisons of mental and motor test scores for ages 1-15 months by sex, birth order, race, geographical location, and education of parents. Child Dev 1965; 36:379–411. [PubMed] [Google Scholar]
- 15. Dickinson JE, Keil AD, Charles AK. Discordant fetal infection for parvovirus B19 in a dichorionic twin pregnancy. Twin Res Hum Genet 2006; 9:456–9. [DOI] [PubMed] [Google Scholar]
- 16. Thapa R, Banerjee P, Akhtar N, Jain TS. Discordance for congenital toxoplasmosis in twins. Indian J Pediatr 2009; 76:1069–70. [DOI] [PubMed] [Google Scholar]
- 17. Ahlfors K, Ivarsson SA, Nilsson H. On the unpredictable development of congenital cytomegalovirus infection. A study in twins. Early Hum Dev 1988; 18:125–35. [DOI] [PubMed] [Google Scholar]
- 18. Kim JS, Romero R, Kim MR, et al. . Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology 2008; 52:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reyes L, Golos TG. Hofbauer cells: their role in healthy and complicated pregnancy. Front Immunol 2018; 15:9.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol 2004; 35:536–45. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer Cell) proliferation and hyperplasia. Arch Pathol Lab Med 2017; 141:43–8. [DOI] [PubMed] [Google Scholar]
- 22. Quicke KM, Bowen JR, Johnson EL, et al. . Zika virus infects human placental macrophages. Cell Host Microbe 2016; 20:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tabata T, Petitt M, Puerta-Guardo H, et al. . Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016; 20:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khong TY, Mooney EE, Ariel I, et al. . Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016; 140:698–713. [DOI] [PubMed] [Google Scholar]
- 25. Higgins M, McAuliffe FM, Mooney EE. Clinical associations with a placental diagnosis of delayed villous maturation: a retrospective study. Pediatr Dev Pathol 2011; 14:273–9. [DOI] [PubMed] [Google Scholar]
- 26. Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015; 213:S21–8. [DOI] [PubMed] [Google Scholar]
- 27. Harteman JC, Nikkels PG, Benders MJ, Kwee A, Groenendaal F, de Vries LS. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J Pediatr 2013; 163:968–95.e2. [DOI] [PubMed] [Google Scholar]
- 28. Tabata T, Petitt M, Zydek M, et al. . Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J Virol 2015; 89:5134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira L, Tabata T, Petitt M, Fang-Hoover J. Congenital cytomegalovirus infection undermines early development and functions of the human placenta. Placenta 2017; 59Suppl 1:S8–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.