Abstract
PURPOSE
Single-agent purine analog, usually cladribine, has been the standard first-line therapy of hairy cell leukemia (HCL) for 30 years. High complete remission (CR) rates often include minimal residual disease (MRD), leading to relapse and repeated treatments. Rituximab can clear MRD, but long-term results are unknown and optimal timing of rituximab undefined.
PATIENTS AND METHODS
Patients were randomly assigned to first-line cladribine 0.15 mg/kg intravenously days 1-5 with 8 weekly doses of rituximab 375 mg/m2 begun either day 1 (concurrent, CDAR) or ≥ 6 months later (delayed) after detection of MRD in blood. MRD tests included blood and bone marrow (BM) flow cytometry, and BM immunohistochemistry.
RESULTS
Sixty-eight patients with purine analog-naïve classic HCL were randomly assigned 1:1 to concurrent versus delayed arms. At 6 months after CDAR versus cladribine monotherapy, CR rates were 100% versus 88% (P = .11), MRD-free CR rates 97% versus 24% (P < .0001, primary end point), and blood MRD-free rates 100% versus 50% (P < .0001), respectively. At 96 months median follow-up, 94% versus 12% remained MRD free. Compared with CDAR, delayed rituximab after cladribine achieved lower rate (67% of 21 evaluable patients; P = .0034) and durability (P = .0081, hazard radio favoring CDAR, 0.094) of MRD-free CR. Nevertheless, 12 patients in the delayed arm remained MRD free when restaged 6-104 (median, 78) months after last delayed rituximab treatment. Compared with cladribine monotherapy, CDAR led to brief grade 3/4 thrombocytopenia (59% v 9%; P < .0001) and platelet transfusions without bleeding (35% v 0%; P = .0002), but higher neutrophil (P = .017) and platelet (P = .0015) counts at 4 weeks.
CONCLUSION
Achieving MRD-free CR of HCL after first-line cladribine is greatly enhanced by concurrent rituximab and less so by delayed rituximab. Longer follow-up will determine if MRD-free survival leads to less need for additional therapy or cure of HCL.
INTRODUCTION
Hairy cell leukemia (HCL) is an indolent B-cell neoplasm comprising 2% of leukemias, estimated at 1,100-1,240 new cases per year in the United States.1,2 Standard first-line purine analogs cladribine or pentostatin achieve 75%-90% complete remission (CR) rates.3-5 Relapse occurs at a median 4.5-16 years, depending on the population and whether follow-up includes bone marrow (BM) tests6 or just blood counts.3 Repeated courses of purine analogs achieve high but usually declining rates and durations of response, toxicity accumulates with retreatment, and evidence of cure is lacking.3,4,7-9 Because median age at diagnosis is approximately 58 years,10 most can expect to relapse, in many cases repeatedly, after first-line therapy, although follow-up is often too short to document this.
Rituximab has modest single-agent activity, with 13% CRs in relapsed HCL with cytopenias requiring treatment,11 but when combined with purine analog it can increase CR rate and eliminate minimal residual disease (MRD).12 Retrospective data showed second- to seventh-line purine analog-rituximab either concurrently in 20 or sequentially in 6 patients achieved 88% CR.13 In a phase II study from MD Anderson Cancer Center, 8 weekly doses of rituximab 1 month after cladribine in 59 untreated patients achieved 100% CR and 95% 5-year failure-free survival.14,15 MRD, assessed by BM aspirate (BMA) flow cytometry until 3 months after cladribine, just after completion of rituximab, was negative in 22 (76%) of 28 evaluable patients.15
In vitro, cladribine-rituximab synergy involved rituximab increasing the sensitivity of lymphoma/leukemia cells to cladribine.16 However, cladribine is rapidly excreted,17 the active intracellular 2-chlorodeoxyadenosine triphopsphate (CdATP) undetectable 48 hours after the last dose, and thus synergy cannot occur with rituximab begun more than several days after cladribine. We therefore tested concurrent cladribine-rituximab and compared with cladribine followed at least 6 months later by delayed rituximab if/when MRD was detected in blood. To determine whether eradication of MRD might require > 8 weekly doses of rituximab, both concurrent and delayed arms could receive a second rituximab course if MRD was detected in blood at least 6 months after the first.
PATIENTS AND METHODS
Eligibility Criteria
The study was registered (ClinicalTrials.gov identifier: NCT00923013) and performed in accordance with the declaration of Helsinki. The National Cancer Institute Institutional Review Board approved the informed consent. Patients required a diagnosis of classic HCL, purine analog and rituximab naïve, and needed treatment due to cytopenias (neutrophils < 1 × 109/L, hemoglobin < 10 g/dL, or platelet < 100 × 106/L), lymphocytosis > 5 × 109/L, symptomatic splenomegaly, or recurrent infections. Patients were not excluded for severe cytopenias, and those with infections under treatment were eligible. The clinical protocol includes other cohorts, including for patients with once-relapsed classic HCL that are still being accrued, and for HCL variant, which was previously reported.18
Study Design and Objectives
Patients were randomly assigned to cladribine 0.15 mg/kg by 2 hour intravenous infusion days 1-5 either concurrently with rituximab 375 mg/m2/wk × 8 (CDAR) or with rituximab delayed ≥ 6 months after cladribine, when blood MRD was detected. Delayed rituximab was also allowed for HCL-related failure of cytopenias to resolve to neutrophils 1.5 × 109/L, hemoglobin 11 g/dL, and platelets 100 × 109/L, as required for CR. Patients in either arm could receive a second course of rituximab ≥ 6 months after the first for the same blood-MRD criteria. MRD tests included BM immunohistochemistry12 and blood and BMA flow cytometry.19 MRD by immunohistochemistry required B cells greater than or equal to T cells and most of the B cells consistent with HCL.12 Consensus polymerase chain reaction (PCR) was also performed prospectively but was less sensitive and specific than flow cytometry, as previously reported.15 We did not find cases where PCR was more sensitive than flow cytometry. The detection limit of flow cytometry was 0.002% (Data Supplement). The primary objective was to determine if concurrent rituximab would significantly lower MRD 6 months after cladribine, before delayed rituximab could be given. Originally, the primary end point included PCR but not BMA studies, because it was anticipated that BMA would be often nonevaluable. However, because 6-month BMA was evaluable in all patients, the amended primary end point included flow cytometry of the blood and BMA and immunohistochemistry of the BM biopsy. BM studies were performed at 4 weeks, 6 months, and yearly for 2.5 years after cladribine and biannually thereafter. Blood flow cytometry was performed 4 weeks after cladribine, quarterly for 1 year, semiannually for 2.5 years, and annually thereafter. Blood counts were performed every 3 months for 2.5 years and semiannually thereafter. Patients are being followed indefinitely on this schedule. Response was assessed as described.4,20 The hemoglobin needed for CR was 11 g/dL, and patients were considered in CR regardless of cytopenias if negative for MRD by all tests.
Statistical Analysis
Fisher’s exact test was used to determine the difference between arms for dichotomized variables. Wilcoxon rank-sum test compared continuous variables. MRD-free duration was defined from the date when all criteria of MRD negativity were fulfilled, with an event noted at first assessment of relapsed MRD and censoring at last follow-up if not. Log-rank compared Kaplan-Meier survival curves. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with 2-sided significance level 5%.
RESULTS
Patient Characteristics
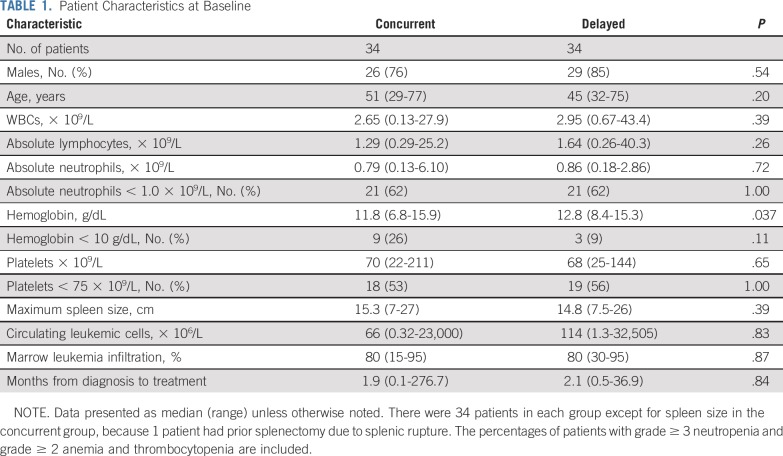
Sixty-eight patients were enrolled in the study from April 2009 to May 2014 and randomly assigned 1:1 to concurrent or delayed rituximab (Fig 1; Data Supplement). Age at enrollment was 29-77 (median, 48) years (Table 1). Most had baseline neutropenia (median, 0.81 × 109/L) and thrombocytopenia (median, 69 × 109/L), without significant differences between arms (Table 1). Baseline hemoglobin was lower in the concurrent arm (P = .037), possibly related to slightly more females in that group, who overall had slightly lower baseline hemoglobin than males (medians, 11.1 v 12.7 g/dL; P = .08). One patient had prior splenectomy after splenic rupture, leaving 67 patients evaluable for baseline spleen diameter, measured by computed tomography. Two patients in the concurrent CDAR arm had active infection (pneumonia and intra-abdominal) at the time of initial treatment (Data Supplement), controlled by antibiotics in one and diagnosed and treated after enrollment in the other.
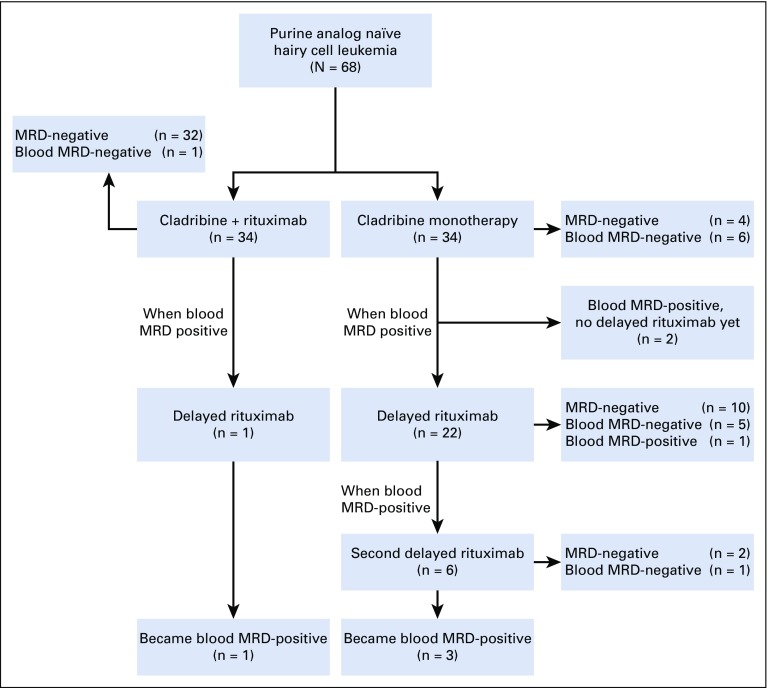
FIG 1.
Consort diagram. Sixty-eight patients were randomly assigned 1:1 to concurrent or delayed rituximab with cladribine. One and 22 patients in concurrent and delayed arms received a first course of delayed rituximab, respectively. Six patients received a second course of delayed rituximab in the delayed arm. Patients labeled as MRD-negative were minimal residual disease (MRD) free by all tests, blood and bone marrow aspirate (BMA) flow cytometry, and bone marrow biopsy immunohistochemistry. Patients labeled as blood MRD-negative were MRD-positive by BMA flow cytometry but negative by blood flow cytometry.
TABLE 1.
Patient Characteristics at Baseline
Status at 4 Weeks and 6 Months
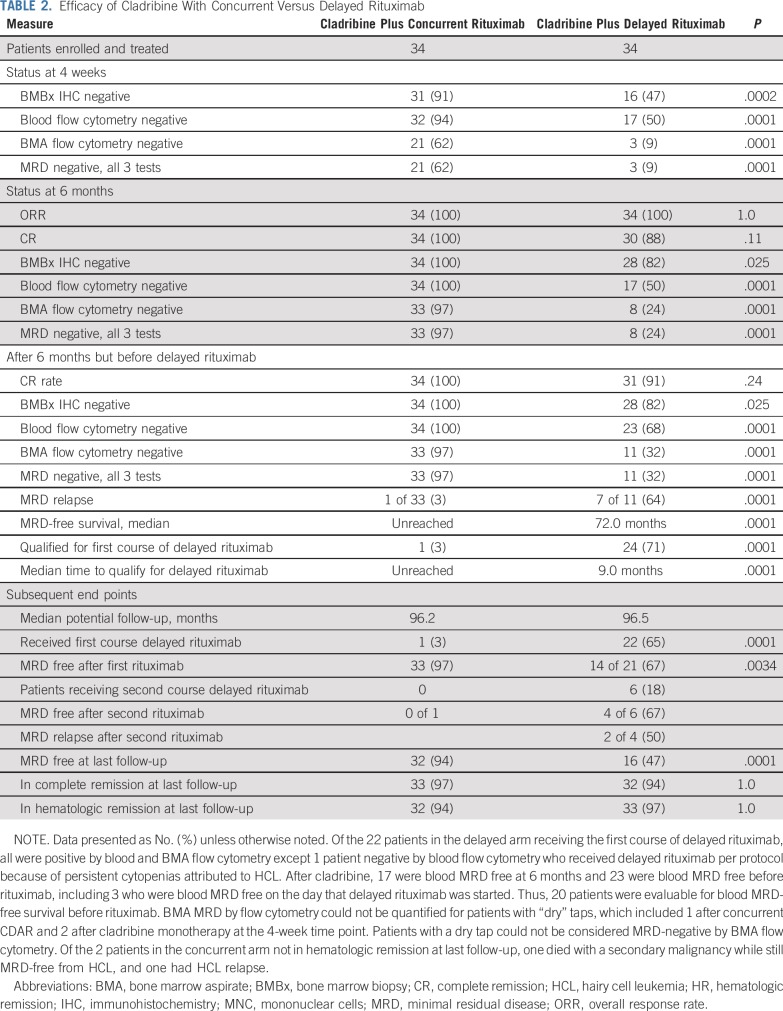
At 4 weeks, 62% and 9% of patients achieved negative MRD after CDAR and cladribine monotherapy, respectively (P < .0001; Table 2). Across the 68 patients in both arms, MRD by BMA flow cytometry was more sensitive than BM immunohistochemistry or blood flow cytometry (P < .0001; Fisher’s exact test), and no patients negative by BMA flow cytometry were positive by these 2 other tests. Thus, MRD-free CR was equal to the rate of patients MRD free by BMA flow cytometry. At 6 months, all but 4 in the delayed arm achieved CR (100% v 88%; P = .11; Table 2). MRD-free CR at 6 months, the primary end point of the study, was achieved by 33 (97%) versus 8 (24%) patients in the concurrent and delayed arms, respectively (P < .0001). The one patient with positive MRD after CDAR was unusual in having both classic (CD25 and CD11c bright positive) and variant (CD25 negative and CD11c-dim) phenotype of HCL cells in the BMA at 1 and 6 months, but only classic HCL at 1.5 years and thereafter.
TABLE 2.
Efficacy of Cladribine With Concurrent Versus Delayed Rituximab
Status After 6 Months but Before Delayed Rituximab
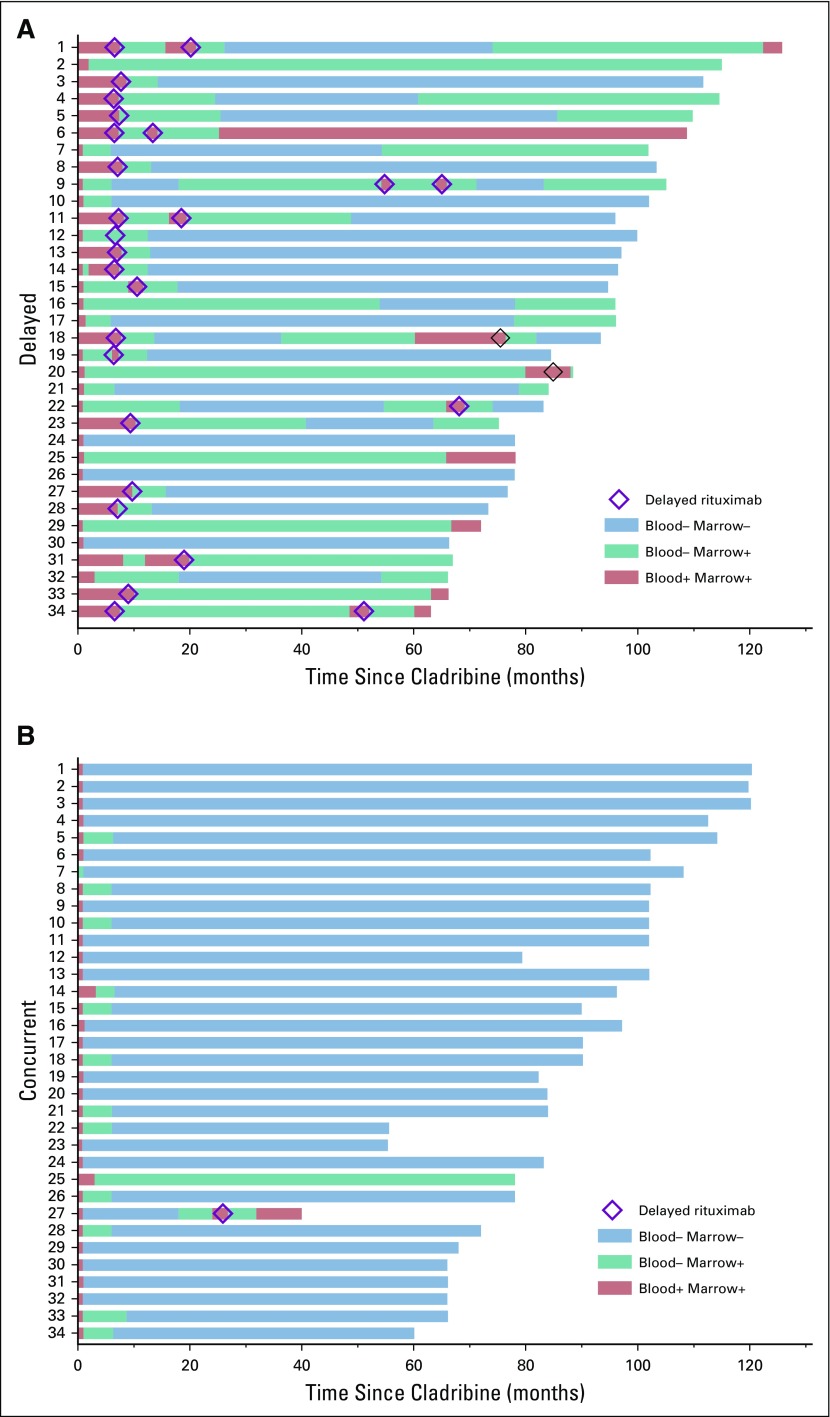
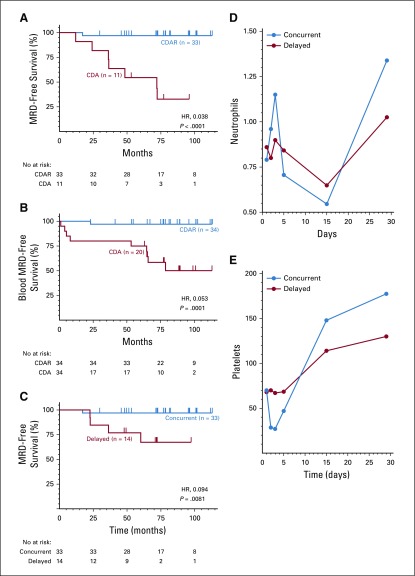
In the delayed arm, after the 6-month restaging time point but before delayed rituximab, 1 patient first achieved CR at 1.5 years with persistently positive BM immunohistochemistry; 4 additional patients became negative by blood flow cytometry at 7, 9, 7 and 10 months; and 3 additional patients achieved MRD-free CR at 54, 18, and 18 months (Fig 2A; patients 31, 5, 27, 28, 31, 16, 22, and 32, respectively). Consistent with Figure 2A, patients 5, 27, and 28 became blood MRD-free on the day that delayed rituximab was begun. These 3 patients were blood MRD positive at only 0.781, 0.241, and 0.118 cells/mm3 1-2 months before delayed rituximab. Overall, 33 (97%) of 34 after CDAR versus 11 (32%) of 34 after cladribine monotherapy achieved MRD-free CR (Table 2; P < .0001). As shown in Figure 3A, without delayed rituximab, 1 (3%) of 33 patients in the concurrent arm versus 7 (64%) of 11 patients in the delayed arm (P < .0001) became MRD positive by BMA flow cytometry. Median MRD-free survival was 72.0 months after cladribine monotherapy and not reached after CDAR (hazard ratio [HR], 0.038; 95% CI, 0.005 to 0.31; P < .0001). The one relapse after CDAR was the patient diagnosed 23 years before with prior interferon treatment and higher baseline disease burden than others; he was MRD free at 1 and 6 months but not at 18 months. Of 34 patients after CDAR and 20 after cladribine monotherapy who became blood MRD free and were followed without delayed rituximab, the median duration of blood MRD free status was longer after CDAR compared with cladribine monotherapy (unreached v 78.7 months; HR, 0.053; 95% CI, 0.007 to 0.42; P = .0001; Fig 3B). Thus, both rate and durability of MRD-free remissions were superior after CDAR versus cladribine monotherapy, whether patients are followed by blood and BM or peripheral blood alone.
FIG 2.
Swimmer plot. Times after enrollment are shown when minimal residual disease (MRD) by flow cytometry was positive in both marrow and blood (red), positive in marrow but negative in blood (green), and negative in both (blue). Delayed rituximab courses are shown by diamonds. (A) Patients 24, 26, and 30 were MRD negative before the 6-month time point, and patients 7, 9, 10, 17, and 21 became MRD negative at the 6-month time point, making 8 patients in the delayed arm MRD negative by 6 months, as indicated in Table 2. (B) Patient 33 at 6 months was bone marrow biopsy negative, but bone marrow aspirate flow was unevaluable; the repeat at 8.7 months was negative, so it was included in the 6-month results.
FIG 3.
Kaplan-Meier curves showing minimal residual disease (MRD)-free survival. (A) After concurrent cladribine-rituximab (CDAR, blue) versus cladribine (CDA, red) monotherapy. (B) Thirty-four patients receiving concurrent CDAR (blue) versus 20 receiving CDA (red) becoming MRD free in blood by 6 months are compared for blood MRD-free survival. (C) MRD-free survival is shown for the 14 patients in the delayed arm achieving MRD-free complete remission after the first course of delayed rituximab (red), compared with the same group as in A receiving concurrent rituximab (blue). Two patients in the delayed arm became positive at similar times, 22.6 and 22.8 months after delayed rituximab. (D and E) Median neutrophil and platelet counts are shown for concurrent CDAR (blue) and delayed CDA (red) groups, with the first day of cladribine given on day 1. At each time point for D and E, 68 patients were evaluable, except 1 in the concurrent arm and 2 in the delayed arm on day 2, and 1 in the concurrent arm and 1 in the delayed arm each on days 3 and 15. Day 15 was ± 4 days. Median neutrophil and platelet counts for CDAR versus CDA arms were 0.96 versus 0.8 (P = .60) and 29 versus 70 (P < .0001) for day 2, 0.705 versus 0.842 (P = .67) and 47 versus 69 (P = .0028) for day 5, 0.634 versus 0.616 (P = .98) and 143 versus 113 (P = .062) for 2 weeks, and 1.339 versus 1.025 (P = .017) and 178 versus 130 (P = .0015) for 4 weeks.
Effect of Delayed Rituximab
In addition to 1 patient in the concurrent arm, 24 patients in the delayed arm qualified for delayed rituximab, in one case because of persistent thrombocytopenia attributed to HCL, and in the other 23 because of positive blood flow cytometry. Median time to qualify for first delayed rituximab was 9.0 months in the delayed arm. Two patients are in the process of receiving first delayed rituximab, which was so far received by 1 and 22 patients in concurrent and delayed arms, respectively. Twenty-one patients in the delayed arm are so far evaluable for MRD after delayed rituximab. A total of 35 and 28 courses of rituximab have been administered to patients in the concurrent and delayed arms, respectively. The one patient in the concurrent arm remained MRD positive after delayed rituximab and had hematologic relapse 1 year later, requiring next treatment.
Of 21 evaluable patients in the delayed rituximab arm, 14 (67%) became MRD-free, significantly less than the 33 of 34 who became MRD free after concurrent rituximab (P = .0034; Table 2). Four (29%) of 14 delayed MRD-free patients had MRD relapse with median follow-up of 65.5 months after achieving MRD-free CR, resulting in shorter durability of MRD free after delayed than after concurrent rituximab (HR, 0.094; 95% CI, 0.010 to 0.84; P = .0081; Fig 3C). So far, of 6 patients in the delayed arm who received a second course of delayed rituximab, 4 became and 2 remain MRD free, and 3 became blood MRD positive without yet requiring treatment (Table 2; Fig 1). Figure 2 shows the timing of delayed rituximab and MRD status of patients showing MRD-free CR by CDAR more frequent and durable, summarized in Table 2 and quantified in the Data Supplement. With a median follow-up of 96 months, 32 (94%) patients in the concurrent arm versus 16 (47%) patients in the delayed arm were MRD-free (P < .0001; Table 2). Only 4 patients (12%) remain MRD free after cladribine monotherapy. Of the 18 patients in the delayed arm with MRD, 9 have not yet had delayed rituximab because of negative blood flow cytometry. Not including these 9 patients, a still-higher percentage of patients in the concurrent arm (94% of 34) versus the delayed arm (64% of 25) were MRD free at last follow-up (P = .0055). These results will change once these patients become eligible for and receive delayed rituximab.
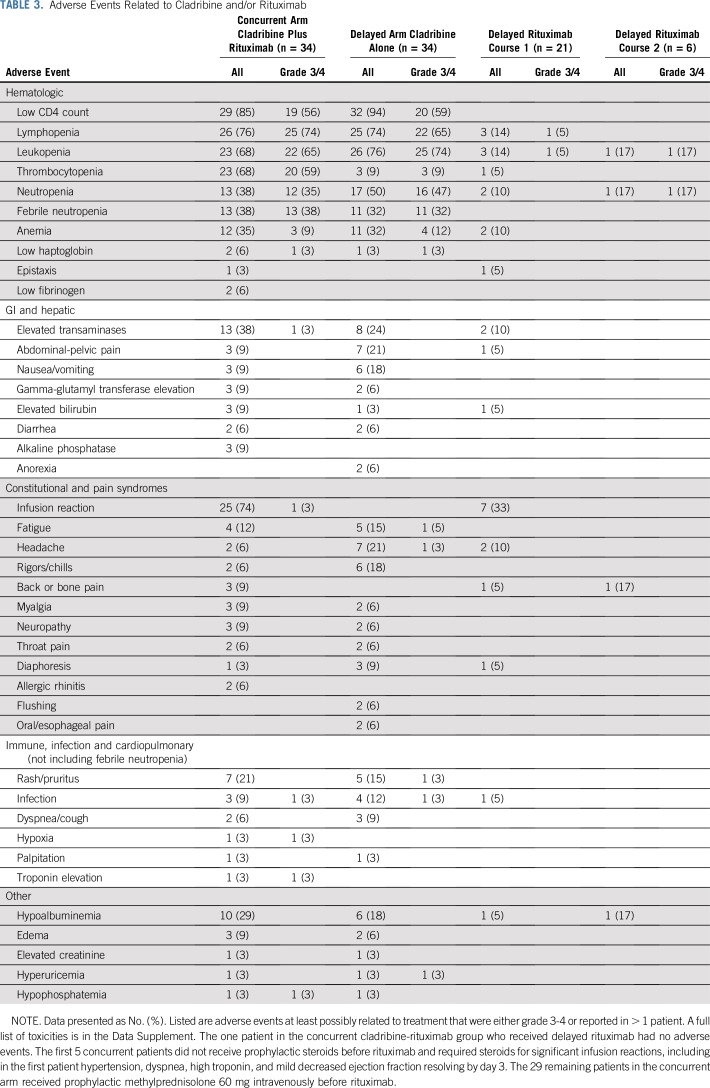
Toxicity
All patients experienced some treatment-related toxicity, but, as shown in Table 3 and the Data Supplement, most nonhematologic toxicity was grade 1-2. Despite the rituximab, there were no significant differences in cytopenias other than thrombocytopenia. As shown in Figure 3D, neutrophils decreased in both arms to median nadirs of 0.546-0.649 × 109/L by 2 weeks (P = .81) but rapidly recovered. To prevent or treat infusion reactions with rituximab, patients in the concurrent arm received methylprednisolone 60 mg intravenously, consistent with increasing neutrophils from days 1-5. Filgrastim was received by 12 patients in the concurrent arm and 14 patients in the delayed arm. Platelets dropped on day 2 to a median of 29 × 109/L in the CDAR arm (Fig 3E), resulting in more frequent grade 3/4 thrombocytopenia versus cladribine monotherapy (59% v 9%; P < .0001). Though rapidly reversible without significant bleeding, platelets were transfused prophylactically only in patients in the concurrent arm, 12 (35%) of 34, for platelet counts below 8-10. By 4 weeks, however, counts were higher after CDAR versus cladribine monotherapy for both neutrophils (P = .017) and platelets (P = .0015). One patient in the concurrent arm was diagnosed with metastatic cutaneous squamous cell carcinoma 4 years after treatment and died at age 82 years of pulmonary fibrosis or metastases but remained HCL MRD-free. The other 67 patients are being followed.
TABLE 3.
Adverse Events Related to Cladribine and/or Rituximab
DISCUSSION
To test the synergy of cladribine and rituximab in HCL, patients receiving first-line cladribine were randomly assigned between concurrent CDAR and delayed rituximab, with the latter group receiving rituximab ≥ 6 months later if/when MRD was evident in blood. Although the potential advantage of CDAR was synergy, the potential advantages of the delayed group included receiving rituximab with minimal HCL burden, with partially recovered immunity including B and T lymphocytes, with less risk of thrombocytopenia and platelet transfusions, with less or no need for steroid prophylaxis of infusion reactions, and in some patients, avoiding rituximab altogether. We found that CDAR was superior to cladribine monotherapy with respect to both rate (97% v 33%) and durability (median, unreached v 72 months) of MRD-free CR. MRD-free CR could usually be achieved by delayed rituximab, although the rates and durability of MRD-free CR were less and shorter than those achievable by CDAR. Toxicity was comparable in both arms, the main difference being rapid-transient thrombocytopenia in the concurrent arm not associated with significant bleeding. By 4 weeks, both neutrophils and platelets were significantly higher in patients receiving concurrent treatment than in those receiving delayed treatment.
This study supports the data from Chihara et al14 and Ravandi et al15 that 100% CR rates can be achieved by addition of rituximab 1 month after cladribine, as all patients in both arms of the current study did initially or eventually achieve CR. The 79% of 28 patients MRD free by all tests at 3 months15 was intermediate between the 97% and 24% in the current study assessed at 6 months after cladribine (Table 2). Recently published guidelines recommend purine analog monotherapy as first-line treatment and rituximab combined with purine analog for relapsed patients.20,21 The long-term efficacy and safety data from the current study, combined with data from Chihara et al,14 support the use of combination chemo-immunotherapy with cladribine and rituximab, at least as an option for first-line treatment of HCL. Concurrent cladribine-rituximab is preferred over purine analog monotherapy for HCL variant because of the poor complete and overall response rates to purine analog monotherapy in that disease, versus high CR rates with CDAR.14,15,18
Although CDAR proved superior to cladribine and delayed rituximab in eradicating MRD, it is notable that many patients in the delayed group became MRD free, with 10 (71%) of 14 MRD-free patients remaining MRD free at a median of 65.5 months (Figs 2A and 3C). Together with two patients remaining MRD free after 2 courses of delayed rituximab, 12 patients in the delayed arm remain MRD free at a median of 71.3 months after their last course of delayed rituximab. Thus, if one received cladribine monotherapy and has MRD by blood flow cytometry, delayed rituximab is an option supported by this trial with the goal to become MRD free. One patient was eligible for delayed rituximab only because of thrombocytopenia attributed to residual disease despite negative blood flow cytometry. However, residual post-treatment thrombocytopenia and splenomegaly may occur from fibrosis rather than residual disease,22 so such patients can likely be followed without additional therapy. It is possible that patients with MRD only by BMA flow cytometry could become negative more durably if delayed rituximab were started before positive blood cytometry. Some patients negative by blood flow cytometry also became negative by marrow flow cytometry after the 6-month time point without additional therapy, although they eventually became MRD positive (Table 2; Fig 2A; patients 16, 22, and 32). Thus, although blood MRD as a trigger for delayed rituximab leads to frequent clearing of MRD, and assessment of blood flow cytometry is a convenient method to follow patients, earlier time points for delayed rituximab could be investigated.
Before this trial we could not find prospective data on rates of MRD after cladribine monotherapy, using multicolor flow cytometry in addition to immunohistochemistry of BM. Chihara et al14 reported an MRD-free rate of 19% by flow cytometry 1 month after cladribine and before rituximab, which is close to our rate of 9% (Table 2). Sigal et al23 reported 9 (2.5%) out of 358 patients treated with cladribine monotherapy were MRD free a median of 16 years later, and there were probably additional long-term MRD-free patients from this cohort. We found 4 patients still MRD free 53-96 (median, 65) months from cladribine monotherapy, and MRD relapses as late as 78 months. Our data are consistent with those of Sigal et al23 in that there may be subset of patients with long-term MRD-free CR by cladribine monotherapy, although the percentage appears limited.
A central question is whether MRD affects long-term outcome in HCL like other hematologic malignancies.24-28 We reported that duration of CR to moxetumomab pasudotox was greatly enhanced by eradication of MRD,29 although those patients were multiply relapsed, and first relapse would occur more slowly. Most trials of first-line purine analogs in HCL do not report a median progression-free or relapse-free survival because of limited follow-up. Else et al3 reported median progression-free survivals of 10.5, 9, and 6.5 years, and relapse-free survivals of 16, 11, and 6.5 years after purine analog monotherapy in first, second, and third lines, respectively. Thus, if MRD is not eradicated, HCL is expected to relapse and require additional treatment, in some cases several times, and patients today still die of resistant multiply relapsed HCL. In that study, of 90 CRs with baseline platelets < 100 × 109/L or hemoglobin < 10 g/dL, 28% clinically relapsed by 6.5 and 50% by 10.5 years.3 Because 94% of CRs remain MRD free 2.5-9.5 (median, 6.5) years after CDAR (Fig 3A), and 78% of these 32 patients had baseline platelets < 100 × 109/L, their outcome with respect to clinical relapse may soon appear obviously superior to historical patients treated with cladribine monotherapy. Because of the effectiveness of delayed rituximab, it will take much longer to determine a difference in clinical relapse between randomly assigned groups, and the difference between the delayed group and historical patients receiving cladribine monotherapy may become obvious before that time. If elimination of MRD can prevent or delay eventual relapse and need for additional therapies, future efforts may be directed toward eliminating MRD early using anti-CD20 antibodies combined with a nonchemotherapy agent, such as vemurafenib or moxetumomab pasudotox.30,31
ACKNOWLEDGMENT
We thank Barbara Debrah for analyzing MRD data, research nurses and nurse practitioners involved with the protocol since its inception, nurses and oncology fellows involved in treatment, referring physicians, and the patients and their families. We thank the Hairy Cell Leukemia Foundation for valuable discussions and referrals.
PRIOR PRESENTATION
Presented in part at 2019 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported by the intramural program of the National Cancer Institute and by Genetech.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Evgeny Arons, Seth M. Steinberg, Wyndham Wilson, Robert J. Kreitman
Provision of study material or patients: Hong Zhou, Raul C. Braylan, Robert J. Kreitman
Collection and assembly of data: Dai Chihara, Evgeny Arons, Maryalice Stetler-Stevenson, Constance M. Yuan, Hao-Wei Wang, Hong Zhou, Liqiang Xi, Julie Feurtado, Lacey James, Raul C. Braylan, Katherine R. Calvo, Irina Maric, Alina Dulau-Florea, Robert J. Kreitman
Data analysis and interpretation: Dai Chihara, Evgeny Arons, Maryalice Stetler-Stevenson, Constance M. Yuan, Mark Raffeld, Seth M. Steinberg, Raul C. Braylan, Robert J. Kreitman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Study of First-Line Cladribine With Concurrent or Delayed Rituximab in Patients With Hairy Cell Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mark Raffeld
Stock and Other Ownership Interests: GlaxoSmithKline, Pfizer, Bristol-Myers Squibb
Robert J. Kreitman
Employment: Regional Cancer Care Associates
Research Funding: Genentech (Inst), Teva (Inst), Novartis (Inst), AstraZeneca/MedImmune (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 3.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 4.Saven A, Burian C, Koziol JA, et al. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–1926. [PubMed] [Google Scholar]
- 5.Grever M, Kopecky K, Foucar MK, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: An intergroup study. J Clin Oncol. 1995;13:974–982. doi: 10.1200/JCO.1995.13.4.974. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg JD, Burian C, Waalen J, et al. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: A single-institution series. Blood. 2014;123:177–183. doi: 10.1182/blood-2013-06-508754. [DOI] [PubMed] [Google Scholar]
- 7.Tadmor T. Purine analog toxicity in patients with hairy cell leukemia. Leuk Lymphoma. 2011;52(suppl 2):38–42. doi: 10.3109/10428194.2011.565097. [DOI] [PubMed] [Google Scholar]
- 8.Goodman GR, Burian C, Koziol JA, et al. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21:891–896. doi: 10.1200/JCO.2003.05.093. [DOI] [PubMed] [Google Scholar]
- 9.Seymour JF, Kurzrock R, Freireich EJ, et al. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood. 1994;83:2906–2911. [PubMed] [Google Scholar]
- 10.Dores GM, Matsuno RK, Weisenburger DD, et al. Hairy cell leukaemia: A heterogeneous disease? Br J Haematol. 2008;142:45–51. doi: 10.1111/j.1365-2141.2008.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102:810–813. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 12.Tallman MS, Hakimian D, Kopecky KJ, et al. Minimal residual disease in patients with hairy cell leukemia in complete remission treated with 2-chlorodeoxyadenosine or 2-deoxycoformycin and prediction of early relapse. Clin Cancer Res. 1999;5:1665–1670. [PubMed] [Google Scholar]
- 13.Else M, Dearden CE, Catovsky D. Long-term follow-up after purine analogue therapy in hairy cell leukaemia. Best Pract Res Clin Haematol. 2015;28:217–229. doi: 10.1016/j.beha.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chihara D, Kantarjian H, O’Brien S, et al. Long-term durable remission by cladribine followed by rituximab in patients with hairy cell leukaemia: Update of a phase II trial. Br J Haematol. 2016;174:760–766. doi: 10.1111/bjh.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravandi F, O’Brien S, Jorgensen J, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood. 2011;118:3818–3823. doi: 10.1182/blood-2011-04-351502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow KU, Sommerlad WD, Boehrer S, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: Role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 17.Albertioni F, Lindemalm S, Reichelova V, et al. Pharmacokinetics of cladribine in plasma and its 5′-monophosphate and 5′-triphosphate in leukemic cells of patients with chronic lymphocytic leukemia. Clin Cancer Res. 1998;4:653–658. [PubMed] [Google Scholar]
- 18.Kreitman RJ, Wilson W, Calvo KR, et al. Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemia. Clin Cancer Res. 2013;19:6873–6881. doi: 10.1158/1078-0432.CCR-13-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sausville JE, Salloum RG, Sorbara L, et al. Minimal residual disease detection in hairy cell leukemia. Comparison of flow cytometric immunophenotyping with clonal analysis using consensus primer polymerase chain reaction for the heavy chain gene. Am J Clin Pathol. 2003;119:213–217. doi: 10.1309/G629-9513-NGLC-UB1K. [DOI] [PubMed] [Google Scholar]
- 20.Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129:553–560. doi: 10.1182/blood-2016-01-689422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wierda WG, Byrd JC, Abramson JS, et al. Hairy cell leukemia, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1414–1427. doi: 10.6004/jnccn.2017.0165. [DOI] [PubMed] [Google Scholar]
- 22.Sarid N, Ahmad HN, Wotherspoon A, et al. An unusual indication for splenectomy in hairy cell leukaemia: A report of three cases with persistent splenomegaly after chemoimmunotherapy. Br J Haematol. 2015;171:784–787. doi: 10.1111/bjh.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigal DS, Sharpe R, Burian C, et al. Very long-term eradication of minimal residual disease in patients with hairy cell leukemia after a single course of cladribine. Blood. 2010;115:1893–1896. doi: 10.1182/blood-2009-10-251645. [DOI] [PubMed] [Google Scholar]
- 24.Hilal T, Prasad V. Eliminating MRD - FDA approval of blinatumomab for B-ALL in complete remission. Nat Rev Clin Oncol. 2018;15:727–728. doi: 10.1038/s41571-018-0087-y. [DOI] [PubMed] [Google Scholar]
- 25.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378:1189–1199. doi: 10.1056/NEJMoa1716863. [DOI] [PubMed] [Google Scholar]
- 26.Dimier N, Delmar P, Ward C, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood. 2018;131:955–962. doi: 10.1182/blood-2017-06-792333. [DOI] [PubMed] [Google Scholar]
- 27.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood. 2013;122:515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 28.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–2464. doi: 10.1182/blood-2018-06-858613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreitman RJ, Tallman MS, Robak T, et al. Minimal residual hairy cell leukemia eradication with moxetumomab pasudotox: Phase 1 results and long-term follow-up. Blood. 2018;131:2331–2334. doi: 10.1182/blood-2017-09-803072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiacci E, Carolis LD, Simonetti E, et al. The BRAF inhibitor vemurafenib plus rituximab produces a high rate of deep and durable responses in relapsed/refractory hairy cell leukemia: Updated results of a phase-2 trial. Hematol Oncol. 2019;37:110–111. [Google Scholar]
- 31.Kreitman RJ, Dearden C, Zinzani PL, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]