Abstract
PURPOSE
AREN0321 evaluated the activity of vincristine and irinotecan (VI) in patients with newly diagnosed diffuse anaplastic Wilms tumor (DAWT) and whether a regimen containing carboplatin (regimen UH1) in addition to regimen I agents used in the National Wilms Tumor Study 5 (NWTS-5; vincristine, doxorubicin, cyclophosphamide, and etoposide plus radiotherapy) would improve patient outcomes.
PATIENTS AND METHODS
Patients with stage II to IV DAWT without measurable disease received regimen UH1. Patients with stage IV measurable disease were eligible to receive VI (vincristine, 1.5 mg/m2 per day intravenously on days 1 and 8; irinotecan, 20 mg/m2 per day intravenously on days 1-5 and 8-12 of a 21-day cycle) in an upfront window; those with complete (CR) or partial response (PR) had VI incorporated into regimen UH1 (regimen UH2). The study was designed to detect improvement in outcomes of patients with stage II to IV DAWT compared with historical controls treated with regimen I.
RESULTS
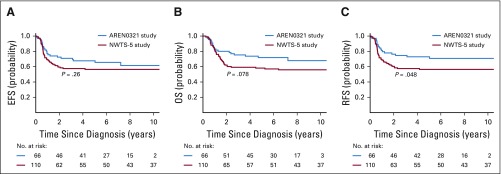
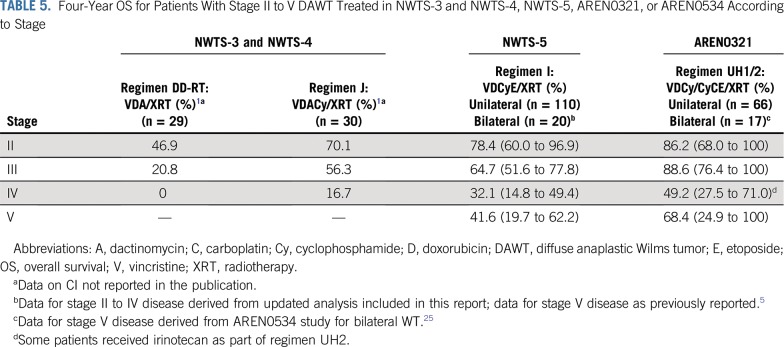
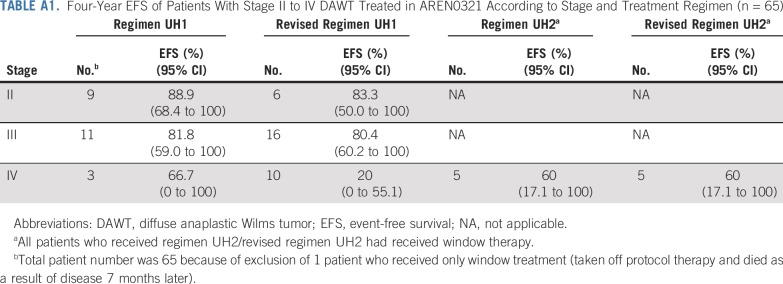
Sixty-six eligible patients were enrolled. Of 14 patients with stage IV measurable disease who received VI, 11 (79%) achieved CR (n = 1) or PR (n = 10) after 2 cycles. Doses of doxorubicin, cyclophosphamide, and etoposide were reduced midstudy because of nonhematologic toxicity. Four patients (6%) died as a result of toxicity. Four-year event-free survival, relapse-free survival, and overall survival rates were 67.7% (95% CI, 55.9% to 79.4%), 72.9% (95% CI, 61.5% to 84.4%), and 73.7% (95% CI, 62.7% to 84.8%), respectively, compared with 57.5% (95% CI, 47.6% to 67.4%; P = .26), 57.5% (95% CI, 47.6% to 67.4%; P = .048), and 59.2% (95% CI, 49.4% to 69.0%; P = .08), respectively, in NWTS-5.
CONCLUSION
VI produced a high response rate in patients with metastatic DAWT. AREN0321 treatment seemed to improve outcomes for patients with stage II to IV DAWT compared with NWTS-5, but with increased toxicity. The UH2 regimen warrants further investigation with modifications to reduce toxicity.
INTRODUCTION
Anaplasia is present in 7.5% of Wilms tumors (WTs) and carries an adverse prognosis.1-4 In the National Wilms Tumor Study 3 (NWTS-3) and NWTS-4, patients with stage II to IV diffuse anaplastic histology WT (DAWT) were treated with vincristine, dactinomycin, and doxorubicin with or without cyclophosphamide. The 4‐year relapse-free survival (RFS) estimates for those with stage II, III, or IV DAWT treated without cyclophosphamide were 40.0%, 33.3%, and 0%, respectively, compared with 71.6%, 58.7%, and 16.7%, respectively, with cyclophosphamide.1 In NWTS-5, therapy was further increased using a regimen of vincristine, doxorubicin, and cyclophosphamide alternating with cyclophosphamide plus etoposide for 24 weeks plus radiotherapy (regimen I), resulting in additional improvement in patient outcomes.5
The progressive improvement in outcomes for patients with DAWT with additional chemotherapy agents suggested that additional gains may be achieved with continued augmentation of therapy. Previous phase II studies of carboplatin as a single agent and in combination with etoposide for relapsed or refractory WT showed objective response rates of 53% and 73%, respectively.6,7 The combination of ifosfamide, carboplatin, and etoposide has also demonstrated significant activity against WT.8-10
Preclinical and early-phase clinical data showed promising antitumor activity of topotecan in WT.11,12 Irinotecan had similar activity to topotecan in patient-derived xenograft models and has less hematologic toxicity. The combination of camptothecins with vincristine resulted in greater-than-additive antitumor effect.13
On the basis of this rationale, the Children’s Oncology Group (COG) AREN0321 study evaluated the antitumor activity of the vincristine and irinotecan (VI) combination in a phase II window in patients with newly diagnosed stage IV DAWT and whether an intensive regimen containing carboplatin in addition to the regimen I agents would improve event-free survival (EFS) or overall survival (OS) for patients with stage II to IV DAWT. AREN0321 also used a higher radiation dose to the flank for local stage III DAWT, because 46% of the recurrences in NWTS-5 were local.5 Here, we report the results of the new therapeutic strategy for stage II to IV DAWT.
PATIENTS AND METHODS
The AREN0321 study (ClinicalTrials.gov identifier: NCT00335556) was open to enrollment from June 2006 to December 2012. The study was temporarily closed to enrollment twice, for approximately 22 months, to assess toxicity and amend the treatment regimens. AREN0321 was permanently closed to accrual in November 2013 after data release by the data safety monitoring committee. The study was approved by the National Cancer Institute Pediatric Central Institutional Review Board (IRB) and local IRBs according to institutional policy. All participants or their legally authorized guardians provided consent. Central review of pathology slides, surgical summaries, and computed tomography (CT) scans was performed through mandatory prior enrollment in the COG AREN03B2 biology and classification protocol.
Patients
Eligible patients were age < 30 years with normal organ function. Patients must not have received prior systemic chemotherapy or radiotherapy unless they were enrolled in the COG AREN0532 or AREN0533 study, wherein the discovery of diffuse anaplasia at delayed nephrectomy allowed entry into this study.14,15
Treatment
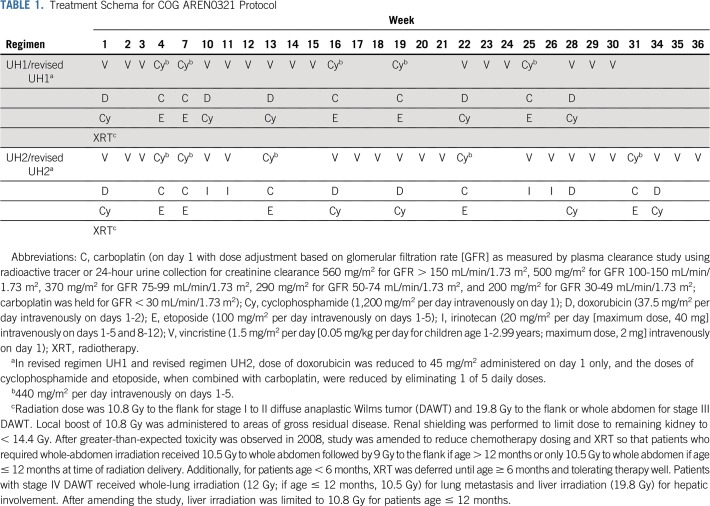
The COG/NWTS group standard surgical procedure was unilateral radical nephroureterectomy with lymph node sampling.16,17 Biopsy or nephrectomy was required before initiation of protocol therapy, which began no later than day 14 after the diagnostic procedure. Patients with unresectable tumors received chemotherapy for 6 to 12 weeks before nephrectomy. Patients with stage II to IV nonmeasurable DAWT received a chemotherapy regimen consisting of vincristine, doxorubicin, cyclophosphamide, carboplatin, and etoposide plus radiotherapy (regimen UH1; Table 1). Patients with stage IV measurable DAWT had the option to receive an upfront window of vincristine (1.5 mg/m2 per day) intravenously on days 1 and 8 and irinotecan (20 mg/m2 per day) intravenously on days 1 to 5 and 8 to 12 of a 21-day cycle. In the absence of progressive disease (PD), a second cycle was administered. Patients with partial response (PR) to the VI window had VI incorporated into regimen UH1 (regimen UH2; Table 1). Patients with stable disease or PD after the VI window received regimen UH1 without additional VI cycles. To keep the cumulative doxorubicin dose ≤ 225 mg/m2, the week 28 doxorubicin was omitted in patients who started treatment in AREN0532 or AREN0533 and received preoperative chemotherapy with doxorubicin. Dexrazoxane use was permitted but not required or recommended.
TABLE 1.
Treatment Schema for COG AREN0321 Protocol
In October 2008, study enrollment was suspended because of a frequency of nonhematologic toxicities that exceeded predefined stopping rules. Regimens UH1 and UH2 were modified (called revised regimens UH1 and UH2) to reduce the cumulative doses of doxorubicin, cyclophosphamide, and etoposide to levels similar to those used in regimen I. The doxorubicin dose was reduced by 40% (to 45 mg/m2, administered on day 1 only) to a total cumulative dose of 225 mg/m2, and the doses of cyclophosphamide and etoposide when combined with carboplatin were reduced by 20% (by eliminating 1 of the 5 daily doses).
Radiotherapy was started with the initiation of chemotherapy, no later than 2 weeks after nephrectomy, except for patients treated in the VI window, who started radiotherapy at the beginning of regimen UH1 or UH2. At the time of study amendment for chemotherapy reduction, whole-abdomen radiotherapy doses were limited to 10.5 Gy; however, patients with stage III disease continued to receive 19.8 Gy to the flank (Table 1). Supportive care, radiotherapy guidelines, and required evaluations are provided in the Appendix (online only).
Statistical Considerations
The study was designed to detect improvement in EFS for patients with stage II to IV DAWT compared with historical controls. A baseline 4-year RFS estimate of 55% was assumed based on the superior treatment arm of NWTS-3 and NWTS-4 (regimen J) and NWTS-5 regimen I.1 A sample size of 75 provided approximately 90% power to detect an improvement in 4-year EFS to 70%. The total accrual was set at 295 patients, including other study strata for clear cell sarcoma, focal anaplastic WT, renal cell carcinoma, and rhabdoid tumor; this report is restricted to patients with stage II to IV DAWT. A 2-stage design was used to evaluate the activity of VI in the window with the aim to accrue 10 patients in the first stage and continue accrual if ≥ 4 tumor responses were observed. The window study closed after other strata of AREN0321 reached their accrual targets.
Tumor responses to the VI window were centrally reviewed and assessed using the RECIST (version 1.1) criteria in addition to volumetric criteria for tumor size. For the volumetric criteria, the volume of up to the 3 largest lesions was calculated using the following formula: (length × width × depth)/2. Bone lesions were considered nonmeasurable.
Adverse events (AEs) were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (versions 3.0 and 4.0 [after October 1, 2011]). The study included toxicity monitoring rules for grade 4 cardiac toxicity, grade 4 sinusoidal obstruction syndrome, and treatment-related death and other grade ≥ 3 nonhematologic toxicity, with the exception of grade 3 anorexia, weight loss, nausea, fatigue, mucositis, diarrhea, fever, electrolytes/metabolic abnormalities, and infection.
The clinical characteristics of patients treated in AREN0321 and NWTS-5 were compared using the analysis of variance test for age, Fisher’s exact test for race/ethnicity and treatment-related mortality rate, and χ2 test for sex and stage. EFS, RFS, and OS were calculated from the time of diagnosis. For EFS, an event was defined as relapse/progression, development of metachronous disease, second malignant neoplasm, or death resulting from any cause, whichever occurred first. For RFS, an event was defined as relapse/progression or development of metachronous disease. Local failure was defined as relapse in the operative bed, abdomen outside the operative bed, or pelvis; tumor spread to the liver was considered distant metastasis. The 4-year EFS, RFS, and OS rates were estimated using the Kaplan-Meier method,18 with CIs estimated by the Peto-Peto method,19 and were compared between groups using the log-rank test.20 The median follow-up time was calculated based on the Kaplan-Meier estimates.21 Patient follow-up was current through September 30, 2017. We compared the local relapse rate between AREN0321 and NWTS-5 using the χ2 test.
RESULTS
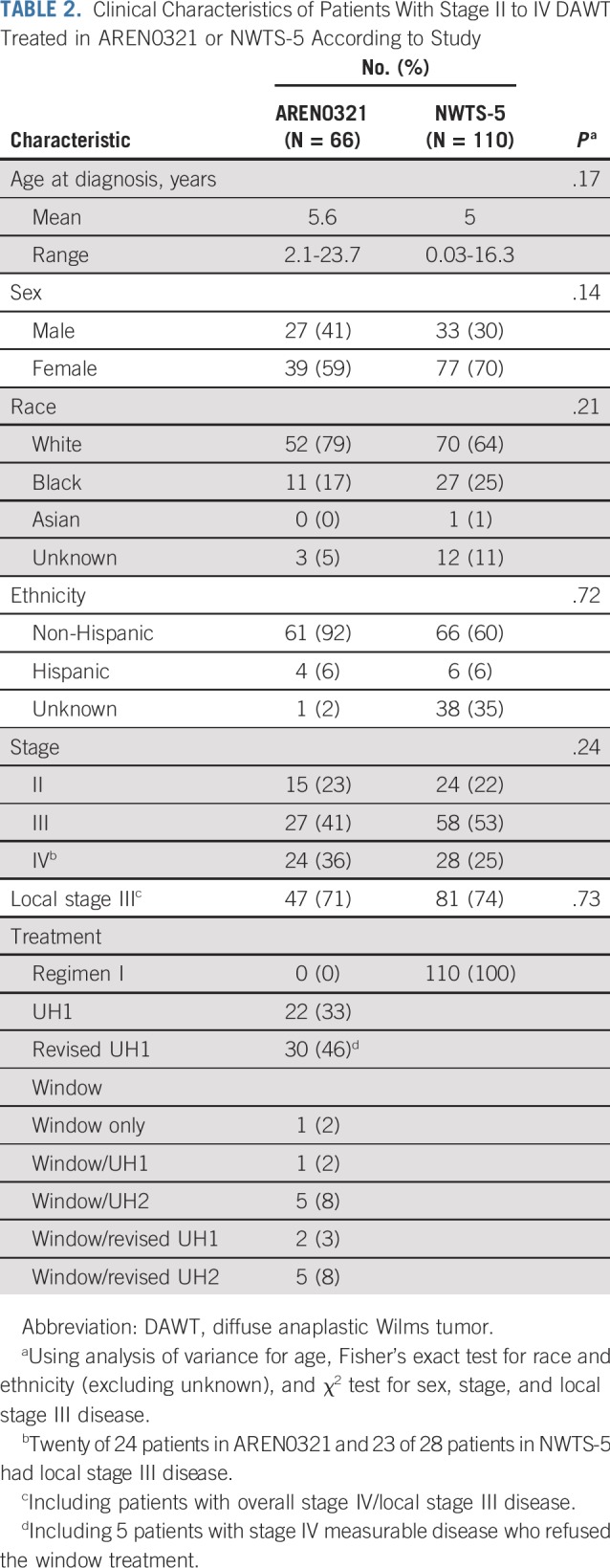
Eligible patients with stage II to IV DAWT were enrolled at 187 institutions over approximately 44 months. The data safety monitoring committee recommended study closure and data release when the total study accrual was 291 patients (nearing the target accrual of 295) and accrual to the stage II to IV DAWT arm was 66 of the planned 75. The clinical characteristics of the 66 patients treated in AREN0321 were similar to those of 110 historical control patients with stage II to IV DAWT treated in NWTS-5 (Table 2). Fifteen patients (stage III, n = 5; stage IV, n = 10) had delayed nephrectomy after preoperative chemotherapy.
TABLE 2.
Clinical Characteristics of Patients With Stage II to IV DAWT Treated in AREN0321 or NWTS-5 According to Study
Tumor Response and AEs During VI Window
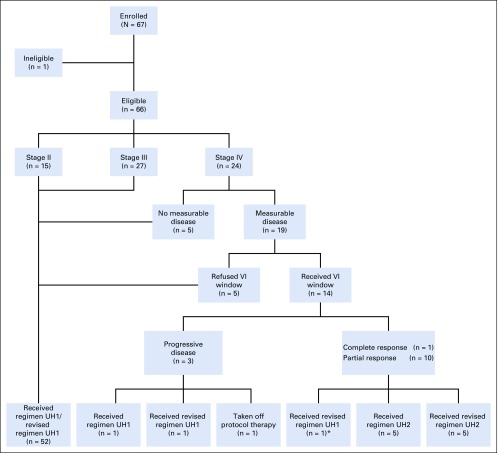
Of the 24 patients with stage IV DAWT, 19 had measurable disease and 5 had lung nodules that were too small to be considered measurable. Fourteen of the 19 patients with measurable disease participated in the VI window (Fig 1). Of these 14 patients, 1 achieved complete response (CR), 10 PR, and none stable disease, resulting in a 79% CR plus PR rate. Three patients had PD: 1 subsequently had PD on regimen UH1, 1 had CR on regimen UH1, and 1 was taken off protocol therapy and died as a result of disease 7 months later.
FIG 1.
Study flow diagram. Patients enrolled in AREN0321 by stage and response to treatment with vincristine and irinotecan (VI) window. (*)Although a partial response was achieved, the treating physician elected to treat with revised regimen UH1.
During the VI window, 1 patient had a grade 4 AE (hypokalemia) and 2 patients had grade 3 AEs (diarrhea and increased AST). In addition, each of the following grade 3 AEs occurred in 1 patient: anorexia, increased bilirubin, colonic hemorrhage, hyperglycemia, hypertension, hypoalbuminemia, hypoxia, thromboembolic event, and wound infection. There were no deaths during the window treatment.
Patient Outcomes
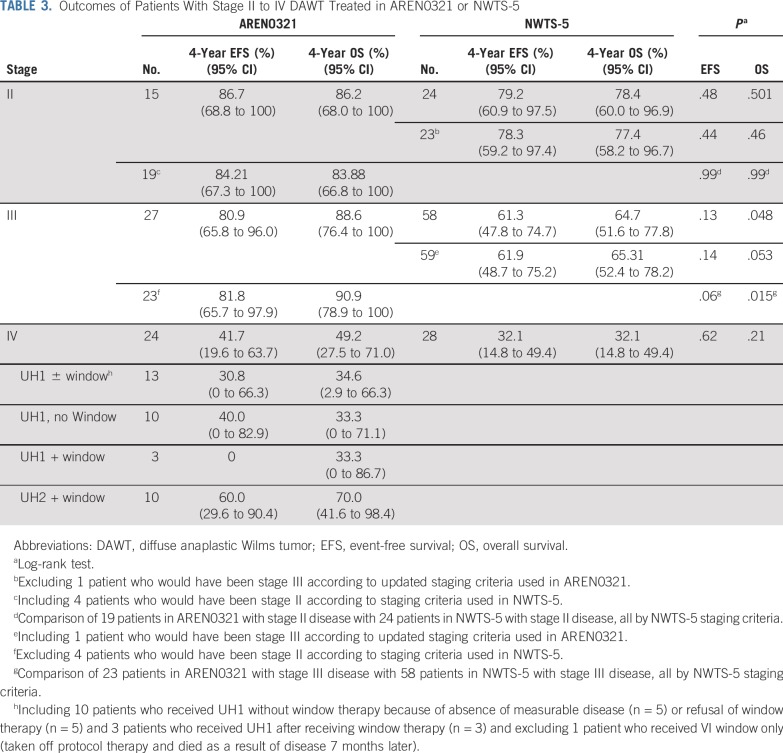
The median follow-up for survivors in AREN0321 was 6.95 years. The 4-year EFS and OS rates for the 66 patients with stage II to IV DAWT treated in AREN0321 were 67.7% (95% CI, 55.9% to 79.4%) and 73.7% (95% CI, 62.7% to 84.8%), respectively. Outcomes were similar for patients with stage II and III disease and inferior for those with stage IV disease (Table 3; Appendix Fig A1, online only). No difference in EFS was seen when comparing the original and revised regimens UH1/UH2, with a possible exception in patients with stage IV disease treated with regimen UH1 versus revised UH1, but the number of patients treated with UH1 (n = 3) was too small to draw a conclusion (Appendix Table A1, online only).
TABLE 3.
Outcomes of Patients With Stage II to IV DAWT Treated in AREN0321 or NWTS-5
Seventeen of the 66 patients had disease relapse/progression as a first event in the lungs only (n = 11), lungs and liver (n = 1), liver only (n = 1), buttock (n = 1), abdomen only (n = 1), abdomen plus liver (n = 1), and abdomen plus lungs and distant lymph nodes (n = 1). One patient had a tumor that developed in the contralateral kidney. One patient with stage III disease had a second malignant neoplasm. For four patients, death was the first event.
Among the 15 patients with delayed nephrectomy, blastemal histology was found in 1 with stage III disease and 5 with stage IV disease. Relapse occurred in 5 of 6 patients with blastemal histology, compared with 3 of 9 patients without blastemal histology. Five patients with stage IV disease who received the window therapy had delayed nephrectomy. Four patients, including 2 of 2 patients with blastemal histology, achieved PR and 1 patient without blastemal histology had PD.
Treatment Toxicity
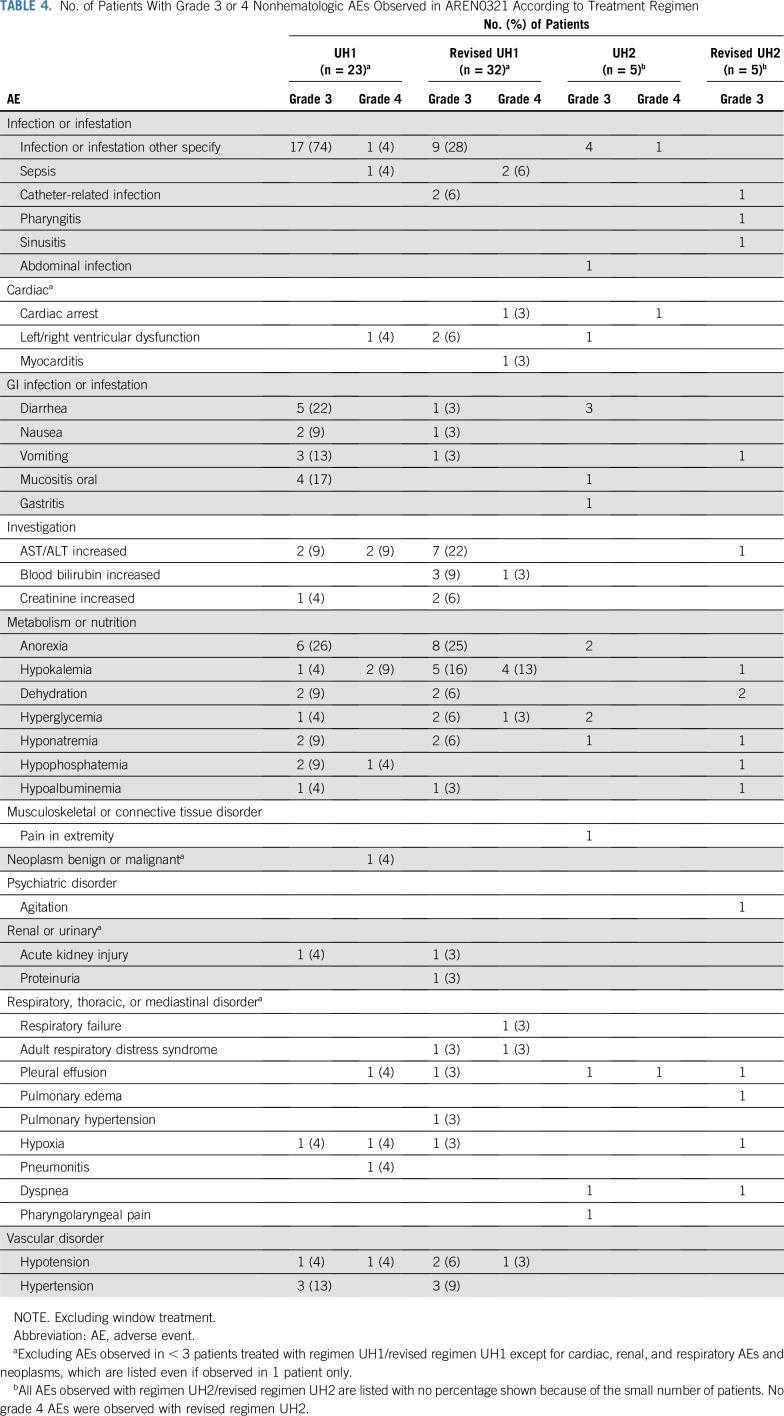
Table 4 summarizes all grade 3 and 4 nonhematologic AEs observed in AREN0321, excluding the window treatment. No grade 4 or 5 AEs were observed with revised UH2. Three patients age 3 to 5 years (regimen UH1, n = 1; revised regiment UH1, n = 2) died as a result of toxicity during therapy: cardiomyopathy (n = 1; cumulative doxorubicin dose, 225 mg/m2), congestive heart failure (n = 1; cumulative doxorubicin dose, 45 mg/m2), and pulmonary edema (n = 1). One additional patient with stage III DAWT treated with regimen UH1 died 6.5 years after the end of therapy as a result of cardiac failure in the setting of hypertension because of a new benign renal neoplasm (cumulative doxorubicin dose, 345 mg/m2). Details regarding grade 5 toxicities are summarized in Appendix Table A2 (online only). The treatment-related mortality rate was 6%, compared with 1.3% for contemporaneous patients with clear cell sarcoma of the kidney treated with regimen I in AREN0321 (P = .18).
TABLE 4.
No. of Patients With Grade 3 or 4 Nonhematologic AEs Observed in AREN0321 According to Treatment Regimen
Comparison of Patient Outcomes With Those in NWTS-5
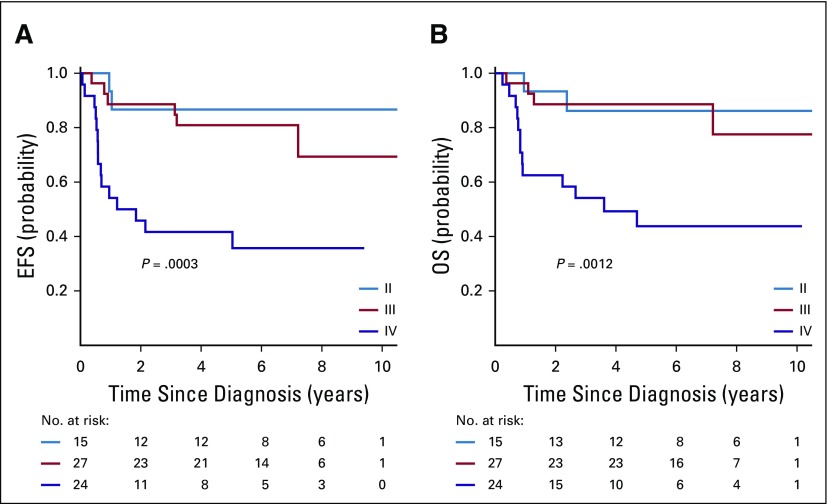
The median follow-up for survivors in NWTS-5 was 10.85 years. Four-year EFS, RFS, and OS rates in AREN0321 were 67.7% (95% CI, 55.9% to 79.4%), 72.9% (95% CI, 61.5% to 84.4%), and 73.7% (95% CI, 62.7% to 84.8%), respectively, compared with 57.5% (95% CI, 47.6% to 67.4%; P = .26), 57.5% (95% CI, 47.6% to 67.4%; P = .048), and 59.2% (95% CI, 49.4% to 69.0%; P = .08), respectively, for 110 patients treated in NWTS-5 (RFS was identical to EFS; Fig 2). Table 3 lists the outcomes of patients treated in AREN0321 versus NWTS-5 according to stage. Because local spill, including percutaneous needle biopsy, was considered a criterion for stage III disease in AREN0321 but was considered stage II disease in NWTS-5, we repeated the analysis excluding the patients who would have had a different stage depending on the study and observed no differences.
FIG 2.
(A) Event-free survival (EFS), (B) overall survival (OS), and (C) relapse-free survival (RFS) of patients with stage II to IV diffuse anaplastic Wilms tumor according to study.
Three of the 66 AREN0321 patients (4.5%) had local relapse, including 2 who had concurrent distant failure. All 3 patients had local stage III disease (overall stage III, n = 1; overall stage IV, n = 2). One patient received treatment before and 2 patients after the amendment to reduce chemotherapy and radiation doses. In comparison, 12 of the 110 NWTS-5 patients (10.9%) had local relapse; all 12 patients had local stage III disease (overall stage III, n = 8; overall stage IV, n = 4). The local relapse rate among those with local stage III disease (Table 2) in AREN0321 (3 [6.4%] of 47) seemed lower than that for NWTS-5 (12 [14.8%] of 81), but the difference was not statistically significant (P = .15).
DISCUSSION
AREN0321 identified a new combination of chemotherapy that is highly active in patients with high-risk WT. The VI combination produced a high response rate (79%) in patients with metastatic DAWT and was well tolerated. VI offers a good treatment option because of its limited potential acute and long-term toxicity and will be incorporated into future COG studies.
The outcomes of patients treated in AREN0321 were overall improved compared with those treated in NWTS-5, but only the difference in RFS achieved statistical significance. This reflects that the augmented chemotherapy regimens in AREN0321 provided better disease control, which was counterbalanced by events related to toxicity. The improvement in disease-specific outcomes continues a pattern of incremental advances with therapy augmentation (Table 5) and may be related to the use of carboplatin, VI, a higher radiation dose for stage III patients, or a combination of these factors. Patients with stage IV DAWT who received UH2/revised UH2 seemed to fare better than patients who received UH1/revised UH1, suggesting a positive contribution of the VI combination. However, the number of patients was small, and the effectiveness of VI requires confirmation. The grade 5 toxicities were related to cardiac and pulmonary dysfunction. The upcoming COG study will incorporate VI in addition to the agents used in AREN0321 for the treatment of DAWT, with strategies to mitigate toxicity, such as using dexrazoxane as a cardioprotectant and avoiding the administration of doxorubicin and carboplatin, potent radiosensitizers, close to the timing of radiotherapy.22-24
TABLE 5.
Four-Year OS for Patients With Stage II to V DAWT Treated in NWTS-3 and NWTS-4, NWTS-5, AREN0321, or AREN0534 According to Stage
All local relapses in both studies occurred in patients with local stage III disease, suggesting that 10.8 Gy provides excellent control for local stage I or II DAWT. In addition, the local relapse rate for local stage III disease in AREN0321 (6.4%) seemed lower than that in NWTS-5 (14.8%), although not statistically significant. Although the higher dose of flank/abdomen radiation (19.8 Gy) used for local stage III DAWT in AREN0321 was perhaps more effective than the 10.8-Gy dose used in NWTS-5, it is unlikely to be the only reason for the improved local control seen in AREN0321.5 In NWTS-3 and NWTS-4, 11 (18.6%) of 59 patients with stage II to IV DAWT had local recurrence as a first event, and a majority of patients had radiation doses > 18 Gy,1 suggesting that a higher dose of radiation in itself is insufficient to explain the better local control rate in AREN0321. The current data support the use of 10.8 Gy to the flank for stage I to II DAWT and 19.8 Gy to the flank or 10.5 Gy to the whole abdomen followed by 9 Gy to the flank for stage III DAWT.
The AREN0321 study had several important limitations. First, the study design was a comparison with historical controls rather than a randomized controlled trial, which may have introduced biases related to changes in imaging resolution and supportive care and stage migration, all of which could have contributed to improved outcomes. With regard to stage migration, patients with local intraoperative tumor spillage or percutaneous biopsy were designated as having stage II disease in NWTS-5 and stage III disease in AREN0321. This change may have improved survival rates for both stage II and III disease, although only 4 patients in AREN0321 were so affected (Table 3). Another potential for stage migration is that the increased use and sensitivity of chest CT led to a greater number of patients with detectable subcentimeter lung nodules. It is encouraging that the stage distribution for DAWT in AREN0321 was similar to that in NWTS-5 (Table 2); however, there were 5 patients with tiny lung nodules who did not qualify for the window therapy. The patients who received UH2/revised UH2 had outcomes at least as good as those who did not receive this therapy (Appendix Table A1), even though patients who received VI had to have measurable disease. This suggests that the apparent improvement in outcomes was not solely a result of the inclusion of patients with small lung nodules. A second limitation of the study is that the actual accrual fell short of the target because of toxicity-related interruptions in accrual (66 actual v 75 target enrollments of patients with stage II-IV DAWT). It is possible that full enrollment may have modified survival in either direction. Moreover, the therapy modifications that were made midstudy resulted in 2 differently treated groups. Comparison of those groups revealed no difference in outcomes, but it is possible that the higher-dose regimen may have benefitted patients with stage IV disease (Appendix Table A1). Finally, given the rarity of DAWT, the study was designed to detect improvement in outcomes for stage II to IV disease collectively, not for each individual stage. On the basis of these limitations and the observed toxicities, we have determined that additional study of a modified UH2 regimen is warranted.
In summary, the VI combination is highly active in DAWT. Its incorporation into future treatment regimens for WT may potentially improve patient outcomes. The AREN0321 treatment approach improved RFS compared with NWTS-5, but with increased toxicity. Strategies to alleviate toxicity will be implemented in the next COG trial.
ACKNOWLEDGMENT
We thank the parents and children who enrolled in this study and the many research coordinators, pediatric oncologists, pathologists, surgeons, radiation oncologists, radiologists, and other health professionals who cared for the children entered in the study.
Appendix
Vincristine was held until peristalsis was established after nephrectomy. Mesna was used with cyclophosphamide for uroprotection. Absolute neutrophil count ≥ 750/µL and platelet count ≥ 75,000/µL were required to start chemotherapy cycles. Myeloid growth factor support was recommended after myelosuppressive chemotherapy but not after irinotecan. In patients who experienced grade ≥ 3 GI toxicity during the first cycle of the vincristine/irinotecan (VI) window, cefixime was used in combination with VI during the second cycle of window therapy. When used, the antibiotic was started at least 1 day (preferably 5 days) before irinotecan and continued for 1 week after the last irinotecan dose.
All treatment volumes were treated concurrently in patients who required whole-lung irradiation in addition to flank or whole-abdomen irradiation. Radiotherapy was delivered at Children’s Oncology Group–approved centers, and all radiation doses, treatment plans, and fields were reviewed retrospectively at the Imaging and Radiation Oncology Core, Lincoln, Rhode Island.
Laboratory testing, computed tomography (CT) of the chest, abdomen, and pelvis, and echocardiography were performed at baseline and end of therapy, and electrocardiography was performed at baseline. An echocardiogram was repeated before the third, fourth, and fifth cycles containing doxorubicin. Glomerular filtration rate was assessed before every other carboplatin-containing cycle by a plasma clearance study using a radioactive tracer or by a 24-hour urine creatinine clearance. CT of the chest, abdomen, and pelvis was repeated after 2, 4, and 8 cycles of chemotherapy, but during the VI window, it was obtained before each cycle of VI and after completion of the second cycle.
After completion of therapy, patients had follow-up visits for disease surveillance every 3 months during the first 2 years, every 6 months during the third and fourth years, and every 12 months during the fifth year. CT of the chest, abdomen, and pelvis was obtained at every visit during the first 2 years and switched to chest radiography and abdominal ultrasonography during the subsequent years.
TABLE A1.
Four-Year EFS of Patients With Stage II to IV DAWT Treated in AREN0321 According to Stage and Treatment Regimen (n = 65)
TABLE A2.
Summary of Grade 5 Toxicity for Patients With DAWT Treated in AREN0321
FIG A1.
(A) Event-free survival (EFS) and (B) overall survival (OS) of 66 patients with stage II to IV diffuse anaplastic Wilms tumor treated in AREN0321 according to disease stage.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, Chicago, IL, May 30-June 3, 2014; the 46th Congress of the International Society of Paediatric Oncology, Toronto, Ontario, Canada, October 22-25, 2014; and the 49th Congress of the International Society of Paediatric Oncology, Washington, DC, October 12-15, 2017.
Supported by St Baldrick’s Foundation and grants from the National Institutes of Health to the Children’s Oncology Group (U10CA180886, U10CA180899, U10CA098543, U10CA098413, and U24CA114766). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Najat C. Daw, John A. Kalapurakal, James I. Geller, Peter F. Ehrlich, Elizabeth J. Perlman, Elizabeth A. Mullen, Anne B. Warwick, Paul E. Grundy, Deborah Ward, James R. Anderson, Conrad V. Fernandez, Jeffrey S. Dome
Provision of study material or patients: Najat C. Daw, Elizabeth A. Mullen, Jeffrey S. Dome
Collection and assembly of data: Najat C. Daw, Yueh-Yun Chi, John A. Kalapurakal, Fredric A. Hoffer, James I. Geller, Elizabeth J. Perlman, Peter F. Ehrlich, Elizabeth A. Mullen, Anne B. Warwick, Eric Gratias, James R. Anderson, Geetika Khanna, Brett Tornwall, Jeffrey S. Dome
Data analysis and interpretation: Najat C. Daw, John A. Kalapurakal, Yeonil Kim, James I. Geller, Elizabeth J. Perlman, Elizabeth A. Mullen, Anne B. Warwick, Paul E. Grundy, Arnold C. Paulino, James R. Anderson, Brett Tornwall, Conrad V. Fernandez, Jeffrey S. Dome
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Activity of Vincristine and Irinotecan in Diffuse Anaplastic Wilms Tumor and Therapy Outcomes of Stage II to IV Disease: Results of the Children’s Oncology Group AREN0321 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Najat C. Daw
Consulting or Advisory Role: Incyte, Bayer
Yeonil Kim
Employment: Merck
Arnold C. Paulino
Employment: MD Anderson Cancer Center
Patents, Royalties, Other Intellectual Property: Royalty from Elsevier for book on PET/CT in radiotherapy treatment planning
Travel, Accommodations, Expenses: Henry Ford Hospital, University of Southern California
Eric Gratias
Employment: MedSolutions, eviCore healthcare
Stock and Other Ownership Interests: eviCore healthcare, Express Scripts, Cigna
Travel, Accommodations, Expenses: eviCore healthcare
James R. Anderson
Employment: Merck
Stock and Other Ownership Interests: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Jeffrey S. Dome
Patents, Royalties, Other Intellectual Property: Rockland Immunochemicals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1994;12:2126–2131. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 2.Vujanić GM, Harms D, Sandstedt B, et al. New definitions of focal and diffuse anaplasia in Wilms tumor: The International Society of Paediatric Oncology (SIOP) experience. Med Pediatr Oncol. 1999;32:317–323. doi: 10.1002/(sici)1096-911x(199905)32:5<317::aid-mpo1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Weirich A, Ludwig R, Graf N, et al. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol. 2004;15:808–820. doi: 10.1093/annonc/mdh171. [DOI] [PubMed] [Google Scholar]
- 4.Verschuur A, Van Tinteren H, Graf N, et al. Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol. 2012;30:3533–3539. doi: 10.1200/JCO.2011.35.8747. [DOI] [PubMed] [Google Scholar]
- 5.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: Results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 6.de Camargo B, Melaragno R, Saba e Silva N, et al. Phase II study of carboplatin as a single drug for relapsed Wilms’ tumor: Experience of the Brazilian Wilms’ Tumor Study Group. Med Pediatr Oncol. 1994;22:258–260. doi: 10.1002/mpo.2950220409. [DOI] [PubMed] [Google Scholar]
- 7.Pein F, Tournade MF, Zucker JM, et al. Etoposide and carboplatin: A highly effective combination in relapsed or refractory Wilms’ tumor—A phase II study by the French Society of Pediatric Oncology. J Clin Oncol. 1994;12:931–936. doi: 10.1200/JCO.1994.12.5.931. [DOI] [PubMed] [Google Scholar]
- 8.Marina NM, Wilimas JA, Meyer WH, et al. Refining therapeutic strategies for patients with resistant Wilm’s tumor. Am J Pediatr Hematol Oncol. 1994;16:296–300. [PubMed] [Google Scholar]
- 9.Abu-Ghosh AM, Krailo MD, Goldman SC, et al. Ifosfamide, carboplatin and etoposide in children with poor-risk relapsed Wilms’ tumor: A Children’s Cancer Group report. Ann Oncol. 2002;13:460–469. doi: 10.1093/annonc/mdf028. [DOI] [PubMed] [Google Scholar]
- 10.Daw NC, Gregornik D, Rodman J, et al. Renal function after ifosfamide, carboplatin and etoposide (ICE) chemotherapy, nephrectomy and radiotherapy in children with Wilms tumour. Eur J Cancer. 2009;45:99–106. doi: 10.1016/j.ejca.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dome J, Neale G, Hill D. Anti-tumor activity of topotecan against Wilms tumor: Translation of a xenograft model to a phase II study. Pediatr Blood Cancer. 2005;45:432–433. [Google Scholar]
- 12.Metzger ML, Stewart CF, Freeman BB, III, et al. Topotecan is active against Wilms’ tumor: Results of a multi-institutional phase II study. J Clin Oncol. 2007;25:3130–3136. doi: 10.1200/JCO.2007.10.9298. [DOI] [PubMed] [Google Scholar]
- 13.Thompson J, George EO, Poquette CA, et al. Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res. 1999;5:3617–3631. [PubMed] [Google Scholar]
- 14.Fernandez CV, Mullen EA, Chi YY, et al. Outcome and prognostic factors in stage III favorable-histology Wilms tumor: A report from the Children’s Oncology Group Study AREN0532. J Clin Oncol. 2018;36:254–261. doi: 10.1200/JCO.2017.73.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dix DB, Seibel NL, Chi YY, et al. Treatment of stage IV favorable histology Wilms tumor with lung metastases: A report from the Children’s Oncology Group AREN0533 Study. J Clin Oncol. 2018;36:1564–1570. doi: 10.1200/JCO.2017.77.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez CV, Geller JI, Ehrlich PF, et al: Renal tumors, in Pizzo PA, Poplack DG (eds): Principles and Practice of Pediatric Oncology (ed 7). Alphen aan den Rijn, the Netherlands, Wolters Kluwer, 2016, pp 753-771. [Google Scholar]
- 17.Ehrlich PF, Ritchey ML, Hamilton TE, et al. Quality assessment for Wilms’ tumor: A report from the National Wilms’ Tumor Study-5. J Pediatr Surg. 2005;40:208–212, discussion 212-213. doi: 10.1016/j.jpedsurg.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19. Peto R, Peto J: Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A 135:184-206, 1972. [Google Scholar]
- 20.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 22.Sepe DM, Ginsberg JP, Balis FM. Dexrazoxane as a cardioprotectant in children receiving anthracyclines. Oncologist. 2010;15:1220–1226. doi: 10.1634/theoncologist.2010-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Douple EB, O’Hara JA, et al. Enhanced radiation-induced cell killing by carboplatin in cells of repair-proficient and repair-deficient cell lines. Radiat Res. 1995;144:230–236. [PubMed] [Google Scholar]
- 24.Yang LX, Douple EB, O’Hara JA, et al. Carboplatin enhances the production and persistence of radiation-induced DNA single-strand breaks. Radiat Res. 1995;143:302–308. [PubMed] [Google Scholar]
- 25.Ehrlich P, Chi YY, Chintagumpala MM, et al. Results of the first prospective multi-institutional treatment study in children with bilateral Wilms tumor (AREN0534): A report from the Children’s Oncology Group. Ann Surg. 2017;266:470–478. doi: 10.1097/SLA.0000000000002356. [DOI] [PMC free article] [PubMed] [Google Scholar]