Abstract
PURPOSE
Whether dosimetric advantages of proton beam therapy (PBT) translate to improved clinical outcomes compared with intensity-modulated radiation therapy (IMRT) remains unclear. This randomized trial compared total toxicity burden (TTB) and progression-free survival (PFS) between these modalities for esophageal cancer.
METHODS
This phase IIB trial randomly assigned patients to PBT or IMRT (50.4 Gy), stratified for histology, resectability, induction chemotherapy, and stage. The prespecified coprimary end points were TTB and PFS. TTB, a composite score of 11 distinct adverse events (AEs), including common toxicities as well as postoperative complications (POCs) in operated patients, quantified the extent of AE severity experienced over the duration of 1 year following treatment. The trial was conducted using Bayesian group sequential design with three planned interim analyses at 33%, 50%, and 67% of expected accrual (adjusted for follow-up).
RESULTS
This trial (commenced April 2012) was approved for closure and analysis upon activation of NRG-GI006 in March 2019, which occurred immediately prior to the planned 67% interim analysis. Altogether, 145 patients were randomly assigned (72 IMRT, 73 PBT), and 107 patients (61 IMRT, 46 PBT) were evaluable. Median follow-up was 44.1 months. Fifty-one patients (30 IMRT, 21 PBT) underwent esophagectomy; 80% of PBT was passive scattering. The posterior mean TTB was 2.3 times higher for IMRT (39.9; 95% highest posterior density interval, 26.2-54.9) than PBT (17.4; 10.5-25.0). The mean POC score was 7.6 times higher for IMRT (19.1; 7.3-32.3) versus PBT (2.5; 0.3-5.2). The posterior probability that mean TTB was lower for PBT compared with IMRT was 0.9989, which exceeded the trial’s stopping boundary of 0.9942 at the 67% interim analysis. The 3-year PFS rate (50.8% v 51.2%) and 3-year overall survival rates (44.5% v 44.5%) were similar.
CONCLUSION
For locally advanced esophageal cancer, PBT reduced the risk and severity of AEs compared with IMRT while maintaining similar PFS.
INTRODUCTION
Multimodality therapy for locally advanced esophageal cancer (EC) can incur significant morbidities, such as cardiopulmonary events that manifest as postoperative complications (POCs) or as late toxicities after concurrent chemoradiotherapy (CRT). Although three-dimensional conformal RT (3D CRT) is the standard RT technique for EC, newer technologies reduce radiation dose exposure to nearby organs at risk. Intensity-modulated RT (IMRT) reduces doses to normal tissues1,2; on the basis of single-institutional and population-based data showing that IMRT may significantly reduce cardiopulmonary morbidity or mortality compared with 3D CRT,3,4 IMRT is the standard at many institutions.
Whereas 3D CRT and IMRT are photon based, proton beam therapy (PBT) is a more advanced modality that exploits physical properties inherent to heavier particles.5 Numerous dosimetric studies have illustrated superior cardiopulmonary dose sparing with PBT compared with both 3D CRT and IMRT.6-11 This may result in lower toxicities, fewer POCs, and/or improved outcomes based on retrospective data.12-14 However, PBT is more expensive than photon-based RT,15,16 and to date, an insufficient level of evidence has demonstrated that the dosimetric superiority of PBT translates to clinically and economically meaningful benefits. This randomized phase IIB trial compared PBT with IMRT for locally advanced EC on the basis of progression-free survival (PFS) for efficacy and a composite toxicity index, the total toxicity burden (TTB), for safety.
METHODS
Design
This study was a phase IIB, single-institutional, open-label, nonblinded, randomized trial of patients with locally advanced EC. The protocol of this trial, approved by the MD Anderson institutional review board and ethics committee, is presented in the Data Supplement (online only). An external data safety monitoring board (DSMB) oversaw the study and evaluated safety and efficacy end points at prespecified intervals.
Patients ≥ 18 years old with a Karnofsky performance score ≥ 60 (or Zubrod performance status of 0-2) were eligible if they had newly diagnosed, histologically documented squamous cell or adenocarcinoma of the cervical/thoracic esophagus, gastroesophageal junction, or gastric cardia. Both unresectable and potentially resectable cases were allowed, as was prior endoscopic mucosal resection provided that the disease was stage II-III and eligible to receive concurrent CRT. Prior thoracic irradiation was acceptable only with minimal/no overlap with the treatment area.
Patients were excluded if RT/chemotherapy alone was indicated, although induction chemotherapy before concurrent CRT was allowed at physician discretion. In addition, patients who were undergoing systemic therapies for other cancers and/or with active metastatic disease (from any neoplasm) also were ineligible. Patients also were required to have no clinically significant uncontrolled cardiorespiratory, hepatorenal, gastrointestinal, or hematologic diseases. These were not limited to the following: no myocardial infarction within 3 months of registration; symptomatic congestive heart failure, unstable angina, or cardiac dysrhythmia (uncontrolled by implantable electronic devices); and any active uncontrolled infection. Before enrollment, patients were evaluated with a history/physical examination, serum laboratory values, positron emission tomography/computed tomography (PET/CT) imaging, pulmonary function testing, and esophagogastroduodenoscopy (EGD) and/or endoscopic ultrasound (EUS).
Random Assignment
Patients were randomly assigned (1:1) without masking by the Department of Biostatistics at MD Anderson Cancer Center using the Pocock-Simon method17 to balance for four factors: induction chemotherapy (yes v no), potential resectability (yes v no), stage (I-II v III), and histology (squamous cell v adenocarcinoma). Of note, age (< 65 v ≥ 65 years) was included initially as a stratification factor but was removed in October 2016 after enrolling 102 patients, which stemmed from patterns of insurance denial in the PBT arm.
Procedures
After random assignment, all patients received concurrent chemotherapy (agents/schedules at medical oncologist discretion) and RT (50.4 Gy in 28 daily fractions). Restaging with PET/CT imaging and EGD/EUS was performed 4-6 weeks after CRT completion; patients eligible for esophagectomy then received resection 8-10 weeks after completion of CRT, or when deemed clinically recovered from CRT-related toxicities. After the completion of definitive CRT/esophagectomy, no additional therapy was planned, and patients followed up every 3-4 months with serum laboratory tests and PET/CT or contrast-enhanced CT imaging. Subsequent therapy for disease recurrence/progression was at the discretion of the treating oncologist.
RT details are in the study protocol (Data Supplement). Briefly, three- or four-dimensional volumetric imaging was used for RT planning. PBT was delivered as passive scattered or scanning beam techniques and IMRT as static beam or volumetric arc techniques. The target consisted of the gross tumor volume (GTV) with a 4-cm craniocaudal and 1-cm radial margin; in addition to grossly involved lymphatics, elective nodal irradiation was performed on the basis of primary tumor location. Treatment planning was individualized, including beam orientations, such that the cardiopulmonary system and liver would receive the lowest doses possible while maintaining tolerance limits to the spinal cord. Image-guided RT was implemented, comparing daily orthogonal kilovoltage x-ray bony alignment with the original simulation radiograph. For IMRT, a once-weekly cone-beam CT scan also assisted with setup verification, a capability that was not available for the patients receiving PBT. Weekly toxicity assessments were done for all patients while receiving CRT.
Outcomes
The prespecified coprimary end points were TTB and PFS. The latter was defined as the time from enrollment to the date of tumor recurrence (any location) or death, whichever occurred first. The TTB end point (Data Supplement) synthesizes the cumulative severity of multiple adverse events (AEs) that patients with EC may experience after CRT with or without surgery (including both CRT-related events and POCs).18 Specifically, TTB was defined by 13 possible instances of 11 distinct AEs (7 POCs measured up to 30 days postoperatively and 6 potentially recurrent toxicities measured up to 12 months after random assignment). The severity levels of these events were defined by Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0), type of intervention required, or binary occurrence, depending on the particular toxicity. For quantification purposes, a numerical weight (0-100) was assigned to the severity (or occurrence) of each grade of each type of toxicity. These weights were elicited prior to trial commencement by a multidisciplinary group of radiation, medical, and surgical oncologists; the numerical values enumerate the physician-perceived relative extent of medical harm that a patient endures upon experiencing each toxicity (and severity level thereof) recorded in the trial. The TTB for each patient was calculated as a composite score of all toxicities experienced. The statistical analysis methodology, trial design, and severity weight elicitation process have been described in detail elsewhere.18
Prespecified secondary objectives included a description of physician-assigned toxicities, as defined by CTCAE v4.0. Patient-reported quality of life (QOL) was evaluated using the European Quality of Life Five Dimension Five Level (EQ-5D-5L) questionnaire, including the EuroQol algorithm index and visual analog scale (VAS). QOL was measured at baseline, weekly during RT, and at follow-up time points; a score of 0 represented death (or worst perceived health), and 1 referred to perfect (best) health. Other secondary end points were overall survival (OS), defined as the time from enrollment to death as a result of any cause, and the pathologic response for operated patients.19 Additionally, to evaluate the degree of radiation-induced immunosuppression, lymphopenia was assessed by comparing absolute lymphocyte counts at the time of registration (baseline), weekly during CRT, 1 month following CRT completion, and every 3-4 months thereafter. Lastly, a post-hoc dosimetric analysis between cohorts also was performed to better evaluate cardiopulmonary doses received in each arm.
Statistical Analysis
This trial was conducted using a Bayesian group sequential design, which had larger statistical power and required smaller sample sizes than a conventional bivariate group sequential design.18 The design provided a higher probability of correctly concluding that one modality had superior TTB/PFS than the other, hence being able to stop accrual more promptly because of ethical concerns of continuing enrollment in the presence of a significant TTB/PFS difference.18 As part of this design, three interim analyses were to be conducted when 33%, 50%, and 67% of the expected clinical information (patient accrual, as adjusted for expected follow-up) was available (with a fourth analysis 1 year after final patient enrollment, if applicable). At each interim analysis, the posterior probability that the mean TTB/PFS of one modality was superior to the other was calculated and compared with a predetermined interim stopping boundary. Accrual was to be stopped if the former was greater than the latter. In the presence of indeterminate results for both end points, enrollment continued until the next planned analysis.
The design of this trial provided at least 83% power to detect a 50% reduction in mean TTB while controlling its false-positive rate at ≤ 2% with a mean sample size of 146 patients. The design also had 89% power to detect a PFS hazard ratio of 2.0 while controlling its false-positive rate at ≤ 4% with a mean sample size of 160 patients. Sequential stopping boundaries were selected to control familywise type I error at < 7%.
TTB is reported using standard summary statistics (mean, median, standard deviation, and range) as well as the incidence of each specific AE and POC. Comparison of TTB between arms was performed by posterior computation using Markov Chain Monte Carlo implemented with OpenBUGS v3 using the BRugs package.20 Two-sample t tests of mean TTB and mean POC severity were added as unplanned analyses. PFS/OS were estimated by the Kaplan-Meier method21 and compared between cohorts by the stratified log-rank test. Secondary analyses included Cox proportional hazard regression to assess the association and concordance between TTB and PFS and account for baseline prognostic characteristics, as well as logistic regression to evaluate the pathologic response rate as a function of treatment arm.22 To analyze QOL, EQ-5D-5L indices and VAS scores were compared between arms at baseline (before RT), during RT, from the end of RT to 1 month thereafter, from 1 to 3 months after RT, and ≥ 3 months after RT using generalized linear mixed models for each interval with multiple EQ-5D-5L scores nested within each patient (absolute scores and the change from baseline).23 Additional adjusted models included age and comorbidity as covariates and stratified by receipt of surgery. Sensitivity analyses also tested longitudinal mixed effects growth curve models comparing randomization arms.
RESULTS
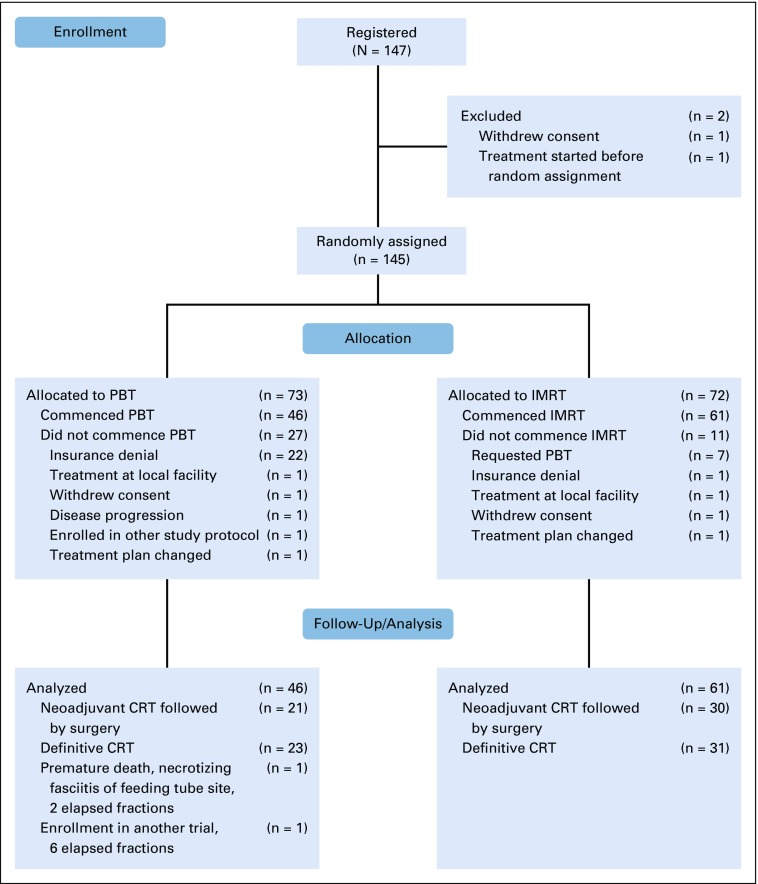
This trial, commenced on April 3, 2012, was approved for closure and analysis by the DSMB before activation of the NRG-GI006 trial on March 11, 2019, immediately before the planned 67% interim analysis. At the time of closure and data lock on May 27, 2019, 145 patients had been randomly assigned (72 IMRT, 73 PBT), of whom 107 (61 IMRT, 46 PBT) were evaluable (Fig 1). For the unevaluable PBT patients, the majority (22 of 27; 81%) were unevaluable because of insurance denial and were treated off protocol with IMRT. For the IMRT group, the majority of nonevaluability (7 of 11; 63%) was due to patients who requested PBT. Table 1 lists the baseline characteristics of the study population, with the Data Supplement showing details for all randomly assigned patients and by operative status.
FIG 1.
Trial profile. CRT, chemoradiotherapy; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy.
TABLE 1.
Patient, Tumor, and Treatment Characteristics
All but 2 patients (both in the PBT arm) completed concurrent CRT. One patient died after 2 fractions as a result of necrotizing fasciitis; the other patient had received 6 fractions without AEs but was taken off study because of enrollment in another clinical trial. Of the remaining patients, 7 received < 50.4 Gy (range, 41.4-48.7 Gy; 4 IMRT, 3 PBT). Eighty percent of the PBT cohort was treated with passive scattering. The median number of chemotherapy cycles was 5 in both arms (range, 2-6 cycles for IMRT and 3-6 cycles for PBT). Most patients received fluorouracil and capecitabine with a taxane (59; 55%), carboplatin with a taxane (23; 22%), or fluorouracil with oxaliplatin (20; 19%).
Fifty-one patients (30 IMRT, 21 PBT) underwent resection at a median of 70 (IMRT) and 76 (PBT) days after CRT completion (P = .55). Surgical outcomes are listed in the Data Supplement. The most commonly used surgical technique was Ivor-Lewis esophagectomy, and negative surgical margins were achieved in all but 2 patients (1 in each arm). All but 1 patient (in the IMRT arm) were potentially resectable at diagnosis (P = .99), whereas 44 potentially resectable patients at diagnosis did not receive surgery (27 IMRT, 17 PBT; P = .55). There were no differences in pathologic complete response rate (29%-30% in both groups; P = .60). The median length of postoperative hospitalization was 8 days in both arms (P = .48), but the mean length was 13 days for IMRT and 8 days for PBT (P = .06).
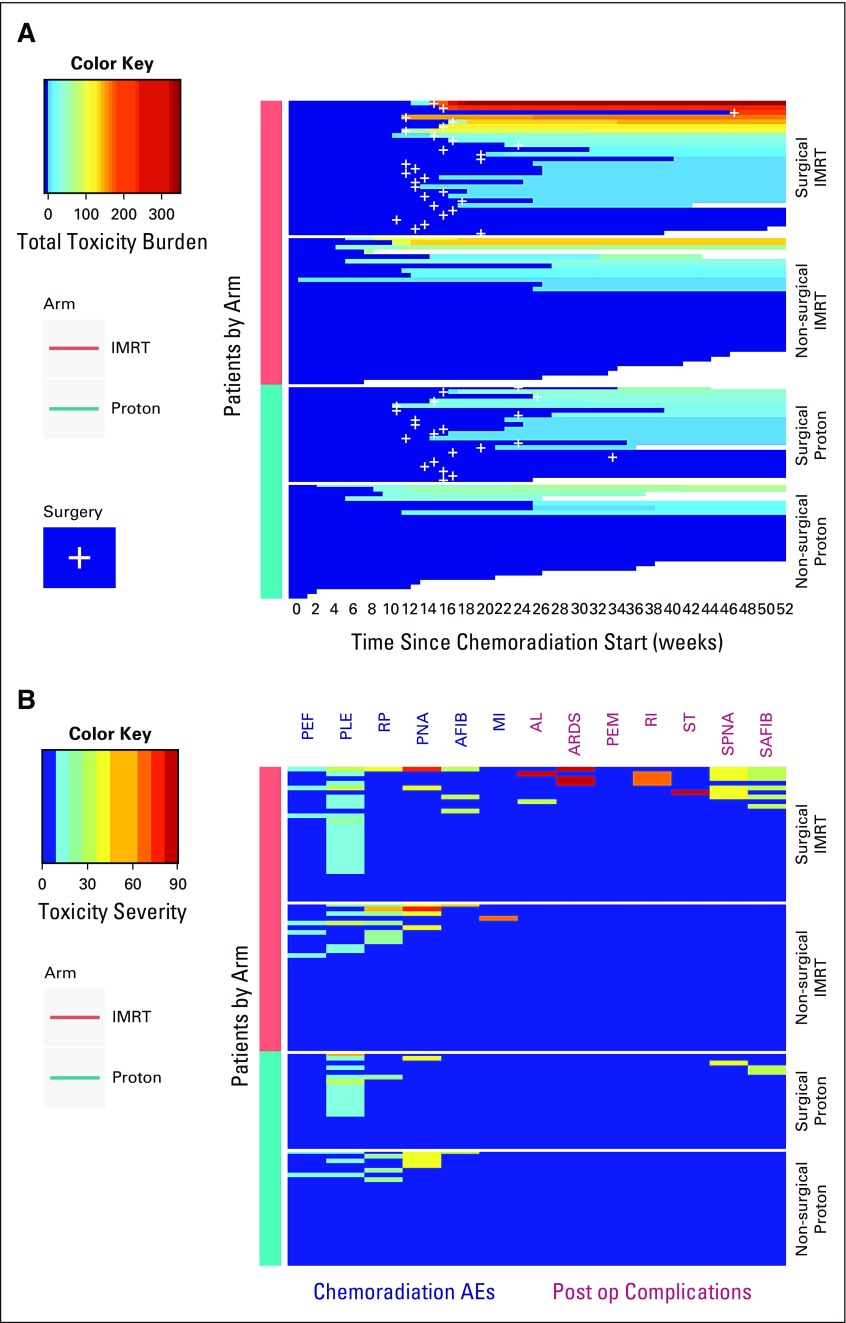
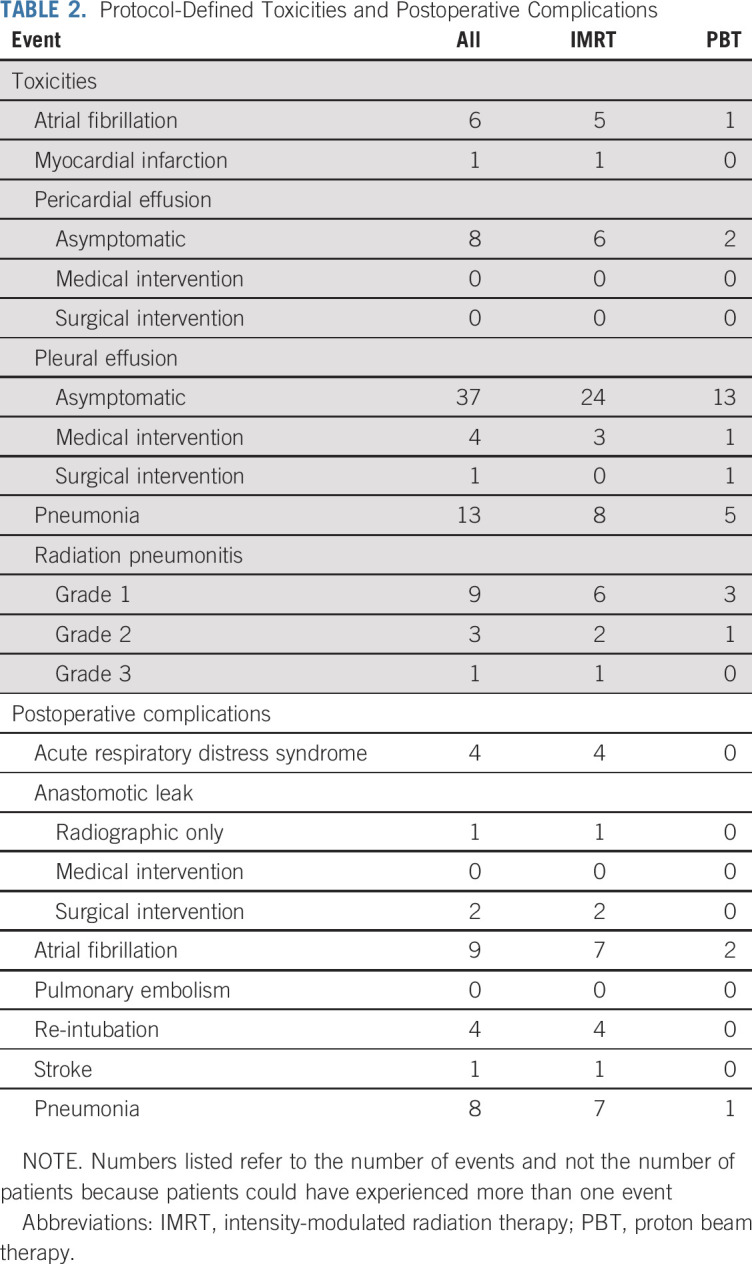
For the primary analysis of the TTB at the time of data lock, the posterior probability that the mean TTB was lower for PBT compared with IMRT was 0.9989, which exceeded the trial design’s stopping boundary of 0.9942 at the 67% interim analysis (Data Supplement). Figure 2A graphically illustrates the primary results stratified by treatment arm and surgery, along with cumulative TTB for each patient over the 52-week monitoring period. Figure 2B shows the severity of the 13 individual toxicities acquired by each patient for both arms. The posterior mean TTB was 2.3 times higher for IMRT (39.9; 95% highest posterior density interval, 26.2-54.9) compared with PBT (17.4; 10.5-25.0; Data Supplement). When evaluating the surgical population, the mean POC score was 7.6 times higher for IMRT (19.1; 7.3-32.3) versus PBT (2.5; 0.3-5.2; Data Supplement). Unplanned frequentist tests comparing TTB and POC scores between arms yielded P values of .018 and .02, respectively. The Data Supplement lists the patient-level TTB summary for both arms. Protocol-defined toxicities and POCs experienced by both cohorts are listed in Table 2. The PBT arm experienced numerically fewer cardiopulmonary toxicities and POCs. The most pronounced numeric differences were atrial fibrillation, asymptomatic effusions, lower-grade radiation pneumonitis, acute respiratory distress syndrome (ARDS), and re-intubation. For patients who completed CRT, there were three grade 5 events, all in the IMRT group. One patient died 47 days after surgery as a result of ARDS, pneumonia, and sustained atrial fibrillation. Two patients who did not undergo surgery died 12 and 15 days after completing CRT as a result of pulmonary embolism and myocardial infarction, respectively. The Data Supplement lists the toxicities; likely because of the relatively lower event rates of any single toxicity, there were no statistically apparent differences for individual AEs.
FIG 2.
Total toxicity burden in the proton beam therapy and intensity-modulated radiation therapy arms as (A) stratified for surgical status and (B) the severity of individual toxicities in both arms. AE, adverse event; AFIB, atrial fibrillation; AL, anastomotic leak; ARDS, acute respiratory distress syndrome; MI, myocardial infarction; PEF, pericardial effusion; PEM, pulmonary embolism; PLE, pleural effusion; PNA, pneumonia; Post op, postoperative; RI, respiratory insufficiency; RP, radiation pneumonitis; SAFIB, surgical atrial fibrillation; SPNA, surgical pneumonia; ST, stroke.
TABLE 2.
Protocol-Defined Toxicities and Postoperative Complications
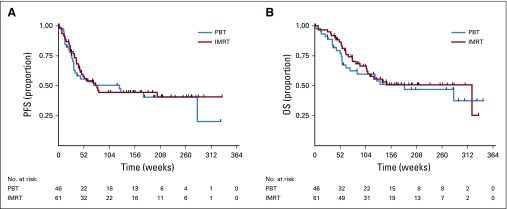
At a median follow-up of 44.1 months, the 3-year PFS rate was 44.5% (95% CI, 31.3% to 56.9%) for IMRT and 44.5% (95% CI, 28.8% to 59.1%) for PBT. Respective median values were 18.1 months (95% CI, 10.0 months to not reached) v 28.5 months (95% CI, 7.1 months to not reached; P = .70; Fig 3A; Data Supplement). The posterior probability that PBT reduced the rate of PFS compared with IMRT was 0.58, which failed to achieve statistical significance. The median OS was 73.6 months (95% CI, 24.4 months to not reached) and 42.1 months (95% CI, 14.5 months to not reached) for IMRT and PBT, respectively; the 3-year OS rates were 50.8% (95% CI, 36.2% to 63.6%) v 51.2% (95% CI, 34.8% to 65.4%; P = .60; Fig 3B), respectively. The nonsignificant differences persisted when stratifying for resection and a sensitivity analysis (Data Supplement).
FIG 3.
Kaplan-Meier (A) progression-free survival (PFS) and (B) overall survival (OS) curves between the proton beam therapy (PBT) and intensity-modulated radiation therapy (IMRT) arms.
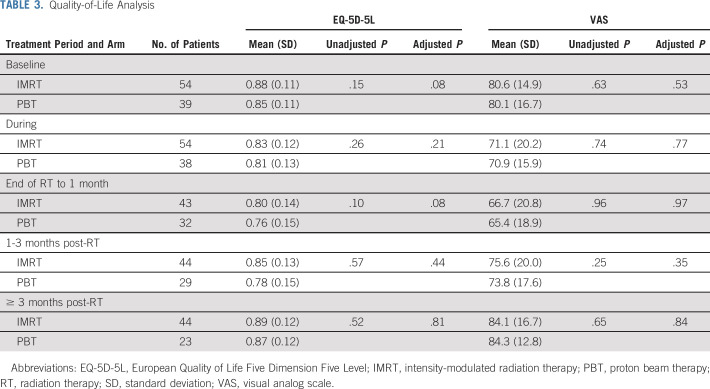
Results of the QOL evaluation are listed in Table 3. EQ-5D-5L index and VAS scores did not differ between arms, overall, or at intervals during/after RT (or after adjustment as listed in the Data Supplement). They also did not differ after adjusting for age/comorbidities, stratifying by surgery, or modeling longitudinally using mixed effects growth curve models. There were no differences between arms when analyzing the degree of QOL decline from baseline (Data Supplement). There were also no significant QOL differences when stratifying for receipt of surgery (Data Supplement).
TABLE 3.
Quality-of-Life Analysis
Evaluation of lymphopenia (Data Supplement) showed that absolute lymphocyte counts were similar at baseline (1,620 IMRT v 1,580 PBT; P = .67) and after induction chemotherapy (n = 10). During CRT, however, the counts declined to a significantly greater degree in the IMRT arm (P < .05 for all time points between 2 and 5 weeks), which corresponded with a higher rate of grade 4 lymphopenia (33% v 14% at the 4th week, 52% v 27% at the 5th week; P < .05 for both).
A post hoc dosimetric analysis (Data Supplement) revealed that the GTVs (46.1 v 44.8 cm3; P = .95) and planning target volumes (PTVs; 556.2 v 579.1 cm3; P = .68) were equivalent between the IMRT and PBT arms, respectively, but PBT delivered significantly higher mean doses to the GTV (52.6 v 52.3 Gy; P = .006) and PTV (52.4 v 52.1 Gy; P = .02). PBT yielded significantly lower doses to total lung parameters (V5, 41.4% v 19.7%; V20, 13.6% v 8.4%; mean lung dose, 8.4 v 4.8 Gy; P < .001 for all) as well as mean doses to the heart (19.8 v 11.3 Gy; P < .001) and liver (12.1 v 2.4 Gy; P < .001) but similar maximum spinal cord doses (38.4 v 38.3 Gy; P = .47).
DISCUSSION
Up to this point, whether the dosimetric advantages of PBT produce clinical benefits for patients with locally advanced EC has been unclear. On the basis of this randomized trial, PBT yielded a markedly lower TTB than IMRT but similar PFS and QOL. This trial provides the first known randomized evidence supporting the utility of PBT for oncologic management, and suggests that PBT is a clinically safer modality than IMRT with a similar PFS for the EC population.
Differences in TTB between arms seemed to be driven by both toxicities and POCs but were likely influenced to a greater extent by the latter. This notion supports retrospective data12,13 demonstrating that the lower integral dose delivered by PBT may result in fewer wound and cardiopulmonary events after esophagectomy. The first of these studies illustrated that improved pulmonary complications with PBT/IMRT compared with 3D CRT may be driven by more favorable lung dosimetry using more advanced radiation techniques.12 In the second,13 a reduction in the number and severity of postoperative complications translated to a reduced length of postoperative hospitalization with PBT. Although surgical approaches were at the discretion of surgical oncologists, this reflects clinical practice and allows for a relatively broad application of the results of this trial. Moreover, differences in POCs between cohorts are especially noteworthy given that this trial was conducted at a high-volume, tertiary care surgical center; thus, POC differences could be even more pronounced at lower-volume institutions.
TTB is a unique endpoint that has special utility for EC patients. Whereas in most trials it is more common to evaluate the tabular rate of individual CTCAE-defined toxicities with equal weights assigned to each grade of toxicity, without regard to recurring events over time, TTB was specifically created to evaluate the total patient experience throughout the cancer journey, accounting for the cumulative adverse events that can occur over a 52-week window with weighted measures that reflect the severity or grade of toxicities. TTB made the trial applicable to both operated and nonoperated patients, thus potentially broadening the utility of PBT as a toxicity-sparing approach for a diverse EC population. Additionally, TTB is an endpoint with economic implications, aimed to address PBT’s cost-effectiveness concerns by payers and economic systems.14-15 Because management of adverse events leads to higher healthcare costs, it is expected that reductions in TTB may enhance the cost-effectiveness profile of PBT in this setting. This is particularly important because 22 PBT patients could not continue on study owing to insurance denial, representing 30% of the population initially randomized to PBT (n = 73) and 81% of the PBT population who did not commence protocol therapy (n = 27).
Although QOL is another important outcome impacting cost-effectiveness,23 the similar QOL profiles of the two arms (despite differences in TTB and POCs) may be explained by several reasons. First, the EQ-5D-5L tool pertains to general functioning rather than specific to the site/symptomatology of interest, hence, more disease-specific QOL cannot be ascertained from these data. Second, there was a nonsignificant trend towards higher baseline QOL in the IMRT arm following adjustment for age and comorbidities. Third, this trial was not powered to detect QOL differences between groups. Fourth, the baseline QOL response rate was relatively low. These results imply that, although QOL remained equivocal between cohorts herein, it cannot rule out finer differences in site-specific QOL that could have gone undetected.
A purported PFS/OS benefit of PBT relates to the lower integral dose delivered by PBT reducing grade 4 lymphopenia better than IMRT, which delivers a substantially higher volume of low-radiation dose throughout the thoracic cavity.24-26 Although there was a higher rate of grade 4 lymphopenia nadir at the end of RT in the IMRT group, and retrospective evidence has correlated grade 4 lymphopenia with OS,27 this has not yet generated a difference in survival outcomes at this point. Although this may seem contrary to retrospective evidence that shows higher PFS and OS with PBT compared with photon-based techniques,14 no conclusions with regard to survival end points can be made herein thus far. Additional follow-up of patients on this trial, as well as analyzing this relationship in the larger cohort of patients from NRG-GI006, will be needed to further ascertain the relationship between radiation-induced immunosuppression and disease-specific outcomes.
When the initial generation of PBT facilities were constructed worldwide, the only available technique was passive scattered PBT; more contemporary centers now utilize intensity-modulated PBT (IMPT), which offers enhanced dose conformality than passive scattered PBT, analogous to the relationship between photon IMRT and 3DCRT. Additionally, despite the vast majority of patients herein having undergone passive scattered PBT, TTB differences between arms were still apparent. The differences could be even more pronounced with the use of IMPT, and early reports of the use of IMPT in EC have been promising.28,29
There are limitations of this trial that merit discussion. First, despite the robust statistical assessment herein, the relatively smaller sample sizes and accrual over a longer time at a single institution may require further validation from larger cooperative group studies, such as NRG-GI006. Second, TTB is not a validated end point; despite intergroup differences, the similar QOL (including low baseline response rate) could overestimate its clinical importance. However, unlike more validated unilateral end points of toxicity, TTB uniquely encompasses the severity of multiple events simultaneously. Potential causes of the equipoise in QOL are also mentioned above. Third, as mentioned above, these results may not necessarily extrapolate to lower-volume and/or community facilities; the degree of PBT-associated toxicity reduction likely relates to the baseline level of toxicities incurred by a given patient at a given institution. Fourth, although these findings may insinuate that 3D CRT should no longer be the standard technique for EC, the lack of a 3D CRT arm herein makes this contention difficult. Finally, selection biases because of insurance denial of PBT or patients’ request to receive PBT cannot be entirely excluded. However, those may have created a negative bias against PBT (eg, statistically better Zubrod performance status in the IMRT arm); in other words, older patients who may be expected to have worse outcomes were also more likely to receive PBT. NRG-GI006 may help to further address these issues by requiring insurance approval before random assignment.
In summary, to our knowledge, this trial provides the first known randomized evidence supporting the utility of PBT for oncologic management. PBT for neoadjuvant or definitive treatment of locally advanced EC produced a lower toxicity profile and fewer POCs, thus leading to a lower TTB, but similar PFS, compared to IMRT.
ACKNOWLEDGMENT
We thank the members of the DSMB as well as the trial coordinators for logistical and technical support. We also appreciate the participation of all patients in this trial.
PRIOR PRESENTATION
Presented at the 2019 Annual Meeting of the American Society of Radiation Oncology, Chicago, IL, September 15-18, 2019.
SUPPORT
Supported by National Cancer Institute Cancer Center Support (Core) grant CA016672 to the University of Texas MD Anderson Cancer Center and National Cancer Institute grant U19CA021239 to Massachusetts General Hospital and the University of Texas MD Anderson Cancer Center.
AUTHOR CONTRIBUTIONS
Conception and design: Steven H. Lin, Brian P. Hobbs, Ritsuko U. Komaki, Joe Y. Chang, Melenda D. Jeter, Reza J. Mehran, Wayne L. Hofstetter, Thomas F. Delaney, Zhongxing Liao, Radhe Mohan
Financial support: Steven H. Lin, Thomas F. Delaney
Administrative support: Steven H. Lin, Albert C. Koong
Provision of study material or patients: Steven H. Lin, Isabel Mok, Stephen G. Chun, Melenda D. Jeter, Jaffer A. Ajani, Ara A. Vaporciyan, Reza J. Mehran, Wayne L. Hofstetter, Zhongxing Liao
Collection and assembly of data: Steven H. Lin, Rebecca S. Tidwell, Erin M. Corsini, Isabel Mok, Xiong Wei, Luyang Yao, Xin Wang, Stephen G. Chun, Melenda D. Jeter, Stephen G. Swisher, Mariela Blum-Murphy, Reza J. Mehran, Saumil J. Gandhi, Wayne L. Hofstetter
Data analysis and interpretation: Steven H. Lin, Brian P. Hobbs, Vivek Verma, Rebecca S. Tidwell, Grace L. Smith, Xiudong Lei, Xin Wang, Joe Y. Chang, Jaffer A. Ajani, Ara A. Vaporciyan, Albert C. Koong, Theodore S. Hong, Thomas F. Delaney, Radhe Mohan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steven H. Lin
Honoraria: AstraZeneca, MedImmune
Recipient: You
Speakers’ Bureau: Varian Medical Systems
Research Funding: STCube Pharmaceuticals, Genentech, Roche, Hitachi Chemical, New River Labs
Brian P. Hobbs
Stock and Other Ownership Interests: Presagia
Consulting or Advisory Role: SimulStat
Research Funding: Amgen
Uncompensated Relationships: Presagia
Rebecca S. Tidwell
Research Funding: Galera Therapeutics (Inst)
Grace L. Smith
Research Funding: Varian Medical Systems (I)
Joe Y. Chang
Stock and Other Ownership Interests: Global Oncology One
Honoraria: Varian Medical Systems
Consulting or Advisory Role: AstraZeneca
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Varian Medical Systems
Stephen G. Chun
Consulting or Advisory Role: AstraZeneca
Stephen G. Swisher
Travel, Accommodations, Expenses: Peter MacCallum
Uncompensated Relationships: Ethicon
Jaffer A. Ajani
Honoraria: Eli Lilly, Bristol-Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech, Zymeworks, Astellas Pharma
Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol-Myers Squibb
Research Funding: Novartis, Bristol-Myers Squibb, Taiho Pharmaceutical, Roche, Genentech, Amgen, Eli Lilly, ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda Pharmaceuticals, Prolinx, Zymeworks, Daiichi Sankyo
Patents, Royalties, Other Intellectual Property: Research funding from Genentech, Roche, Bristol-Myers Squibb, Taiho Pharmaceutical, MedImmune, Merck, Amgen, Eli Lilly
Mariela Blum-Murphy
Honoraria: EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Roche (Inst)
Albert C. Koong
Stock and Other Ownership Interests: Aravive
Saumil J. Gandhi
Consulting or Advisory Role: Novocure
Research Funding: AstraZeneca, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Novocure
Theodore S. Hong
Consulting or Advisory Role: Clinical Genomics, EMD Serono, Merck
Research Funding: Novartis (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Intrao (Inst), Tesaro (Inst), Bristol-Myers Squibb (Inst)
Thomas F. DeLaney
Honoraria: UpToDate, Wolters Kluwer, Oakstone Publishing
Open Payments Link: https://openpaymentsdata.cms.gov/physician/648786
Zhongxing Liao
Honoraria: Varian Medical Systems
Speakers’ Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
Radhe Mohan
Stock and Other Ownership Interests: General Electric
No other potential conflicts of interest were reported.
REFERENCES
- 1.Nutting CM, Bedford JL, Cosgrove VP, et al. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiother Oncol. 2001;61:157–163. doi: 10.1016/s0167-8140(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 2.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122:917–928. doi: 10.1002/cncr.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1177/15330346070060S403. Patyal B: Dosimetry aspects of proton therapy. Technol Cancer Res Treat 6:17-23, 2007 (suppl) [DOI] [PubMed] [Google Scholar]
- 6.Isacsson U, Lennernäs B, Grusell E, et al. Comparative treatment planning between proton and x-ray therapy in esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;41:441–450. doi: 10.1016/s0360-3016(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2008;72:278–287. doi: 10.1016/j.ijrobp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81:1336–1342. doi: 10.1016/j.ijrobp.2010.07.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Palmer M, Bilton SD, et al. Comparing proton beam to intensity modulated radiation therapy planning in esophageal cancer. Int J Part Ther. 2015;1:866–877. [Google Scholar]
- 10.Shiraishi Y, Xu C, Yang J, et al. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or intensity-modulated radiation therapy. Radiother Oncol. 2017;125:48–54. doi: 10.1016/j.radonc.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Hirano Y, Onozawa M, Hojo H, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2018;13:23. doi: 10.1186/s13014-018-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2013;86:885–891. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123:376–381. doi: 10.1016/j.radonc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Xi M, Xu C, Liao Z, et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: A retrospective, single-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99:667–676. doi: 10.1016/j.ijrobp.2017.06.2450. [DOI] [PubMed] [Google Scholar]
- 15.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483–1501. doi: 10.1002/cncr.29882. [DOI] [PubMed] [Google Scholar]
- 16.Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95:488–497. doi: 10.1016/j.ijrobp.2015.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115; 1975. [PubMed] [Google Scholar]
- 18.Hobbs BP, Thall PF, Lin SH. Bayesian group sequential clinical trial design using total toxicity burden and progression-free survival. J R Stat Soc Ser C Appl Stat. 2016;65:273–297. doi: 10.1111/rssc.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 20.Thomas A, O’Hara B, Ligges U, et al. Making BUGS Open. R News. 2006;6:12–17. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Harrell FE. Regression Modeling Strategies. New York, NY: Springer; 2001. [Google Scholar]
- 23.Verma V, Simone CB, II, Mishra MV. Quality of life and patient-reported outcomes following proton radiation therapy: A systematic review. J Natl Cancer Inst. 2018;110:djx208. doi: 10.1093/jnci/djx208. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–160. doi: 10.1016/j.radonc.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther. 2018;4:23–32. doi: 10.14338/IJPT-17-00033.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routman DM, Garant A, Lester SC, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol. 2019;4:63–69. doi: 10.1016/j.adro.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Zeng YC, Vyas S, Dang Q, et al. Proton therapy posterior beam approach with pencil beam scanning for esophageal cancer: Clinical outcome, dosimetry, and feasibility. Strahlenther Onkol. 2016;192:913–921. doi: 10.1007/s00066-016-1034-4. [DOI] [PubMed] [Google Scholar]
- 29.Prayongrat A, Xu C, Li H, et al. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: A single institutional experience. Adv Radiat Oncol. 2017;2:301–307. doi: 10.1016/j.adro.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]