Abstract
PURPOSE
To compare the outcomes of patients with Hodgkin or non-Hodgkin lymphoma undergoing nonmyeloablative haploidentical or unrelated cord blood (UCB) hematopoietic cell transplantation.
PATIENTS AND METHODS
We retrospectively studied 740 patients with Hodgkin lymphoma (n = 283, 38%) and non-Hodgkin lymphoma (n = 457, 62%) age 18-75 years who received transplantations from 2009 to 2016. Data were reported to the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation, Eurocord, or Center for International Blood and Marrow Transplant Research. Of the 526 patients who received haploidentical transplantation, 68% received bone marrow and 32% received peripheral blood. All patients received a uniform transplantation conditioning regimen (2 Gy of total-body irradiation, cyclophosphamide, and fludarabine) and graft-versus-host disease prophylaxis (calcineurin inhibitor and mycophenolate). In addition, patients who received a haploidentical transplantation received posttransplantation cyclophosphamide.
RESULTS
Compared with haploidentical bone marrow and peripheral-blood transplantations and adjusted for age, lymphoma subtype, and disease status, survival was lower after UCB transplantation (hazard ratio [HR], 1.55; P = .001; and HR, 1.59; P = .005, respectively). Similarly, progression-free survival was lower after UCB transplantations compared with haploidentical bone marrow and peripheral-blood transplantations (HR, 1.44; P = .002; and HR, 1.86; P < .0001), respectively. The 4-year overall and progression-free survival rates after UCB transplantation were 49% and 36%, respectively, compared with 58% and 46% after haploidentical bone marrow transplantation and 59% and 52% after peripheral-blood transplantation, respectively. Lower survival was attributed to higher transplantation-related mortality after UCB transplantation compared with haploidentical bone marrow and peripheral-blood transplantation (HR, 1.91; P = .0001; and HR, 2.27; P = .0002, respectively).
CONCLUSION
When considering HLA-mismatched transplantation for Hodgkin or non-Hodgkin lymphoma, the data support haploidentical related donor transplantation over UCB transplantation.
INTRODUCTION
Despite recent advances in the treatment of Hodgkin and non-Hodgkin lymphoma, such as targeted therapies and chimeric antigen receptor T-cell therapy, allogeneic hematopoietic cell transplantation offers long-term cure. Approximately one fourth of whites and most ethnic minorities do not have an available HLA-matched related or unrelated donor.1 For these patients, unrelated cord blood (UCB) or a haploidentical relative are donor options for allogeneic hematopoietic cell transplantation. Both donor sources are readily available, tolerance has been demonstrated for donor-recipient HLA disparity, and reports suggest comparable outcomes between the 2 donor types for hematologic malignancy.2-11 Donor selection (UCB or haploidentical relative) is often based on transplantation center experience, donor availability, and the cost associated with transplantation, although there are no comparative economic analyses to date. However, several reports included heterogenous conditioning regimens and graft-versus-host disease (GVHD) prophylaxis. Therefore, the aim of the current analysis was to compare outcomes after UCB and haploidentical transplantations in a relatively homogenous population (Hodgkin or non-Hodgkin lymphoma [diffuse large B-cell, follicular, mantle cell, or T-cell subtypes]) with low-intensity conditioning regimens.
PATIENTS AND METHODS
Patients and Donors
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR), the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (LWP-EBMT), and Eurocord. Participating centers reported consecutive transplantations, and patients were followed longitudinally. Data were collected on standardized reporting forms. One hundred twenty-six centers contributed patients (Appendix Fig A1, online only). Of these, 111 centers contributed < 10 patients; 11 centers, 10-20 patients; 3 centers, 31-40 patients; and 1 center, 138 patients. Patients age 18-75 years with Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, or T-cell lymphoma receiving their first allogeneic hematopoietic cell transplantation from 2009 to 2016 were included in the study. Recipients of haploidentical donor transplantation received bone marrow or peripheral-blood grafts. Recipients of UCB transplantation received 1 or 2 units of UCB to achieve a minimum total nucleated cell (TNC) dose of 3.0 × 107/kg of recipient body weight. All patients received conditioning regimens with cyclophosphamide, fludarabine, and 2 Gy of total-body irradiation (TBI). UCB recipients received cyclophosphamide 50 mg/kg and fludarabine 200 mg/m2. Haploidentical recipients received cyclophosphamide 29 mg/kg and fludarabine 150 mg/m2. GVHD prophylaxis included calcineurin inhibitor with mycophenolate for UCB transplantation and posttransplantation cyclophosphamide (100 mg/kg), calcineurin inhibitor, and mycophenolate for haploidentical transplantation. Recipients of in vivo T-cell depletion or ex vivo graft manipulation including expanded UCB units were excluded. Patients provided written informed consent for research. The Institutional Review Board of the National Marrow Donor Program approved the study.
Outcomes
Overall survival was the primary end point. Death from any cause was an event, and surviving patients were censored at last follow-up. Progression-free survival was defined as survival without relapse or progression. Progression or relapse was defined as progressive disease or recurrence after a complete remission; death without relapse or progression was the competing risk. Transplantation-related mortality was defined as death from any cause without relapse or progression; relapse or progression was the competing risk. Acute grade 2-4 GVHD and chronic GVHD were assigned and graded using standard criteria.12,13
Statistical Analysis
The incidence of acute and chronic GVHD, relapse or progression, and transplantation-related mortality was calculated using the cumulative incidence estimator to accommodate competing risks.14 Multivariable analyses were performed using Cox proportional hazards models for overall and progression-free survival, acute and chronic GVHD, relapse or progression, and transplantation-related mortality to examine the effect of donor type on transplantation outcomes.15 A stepwise model-building approach was adopted, and variables that attained a P ≤ .05 were retained in the final model. The variables tested were donor-graft type (haploidentical bone marrow v haploidentical peripheral blood v UCB), age, sex, performance score, recipient cytomegalovirus serostatus, lymphoma subtype (Hodgkin v diffuse large B-cell v follicular v mantle cell v T-cell non-Hodgkin lymphoma), disease status, prior autologous transplantation, and transplantation period (Table 1). All variables met the assumption of proportional hazards, and there were no first-order interactions between the variables held in final Cox models. The effect of acute and chronic GVHD on survival was examined by fitting acute and chronic GVHD as time-dependent variables in the final Cox model for overall survival. The probabilities of overall and progression-free survival were calculated from the final Cox model. Transplantation center effect on survival and transplantation-related mortality was tested using the frailty model16 and sensitivity analysis using Cox proportional hazards regression.5 All P values are 2-sided, and all analyses were done using SAS version 9.4 (SAS Institute, Cary, NC).
TABLE 1.
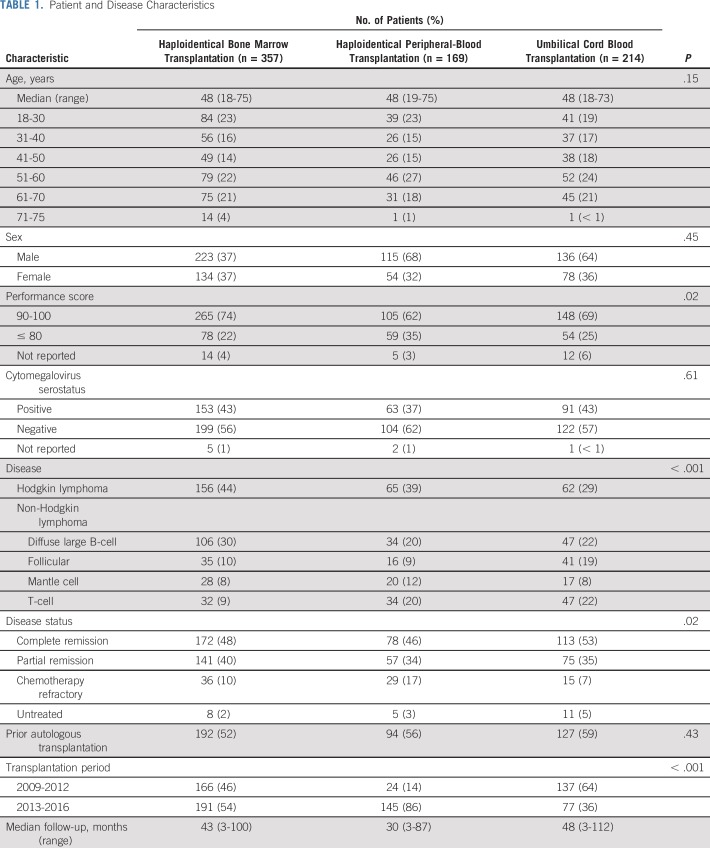
Patient and Disease Characteristics
RESULTS
Patient, Disease, and Transplantation Characteristics
The characteristics of the patients and their disease by donor-graft type are listed in Table 1. Haploidentical bone marrow transplantations were more common for Hodgkin lymphoma and less likely for T-cell lymphoma compared with haploidentical peripheral-blood and UCB transplantations. UCB transplantations were more common for follicular lymphoma. Although most transplantations occurred during complete or partial remission, haploidentical peripheral blood recipients were more likely to have chemotherapy-refractory disease. Bone marrow was the predominant graft for haploidentical transplantations. Most UCB transplantations (66%) were mismatched at 2 HLA loci considering low-resolution typing at A and B and high-resolution typing at DRB1. The remaining UCB transplantations were mismatched at 1 HLA locus (30%) or matched (3%). There were 22 single-unit UCB transplantations with a median TNC dose of 4 × 107/kg (interquartile range [IQR], 3.6-4.5 × 107/kg), and there were 192 double-unit UCB transplantations with a median TNC dose of 5 × 107/kg (IQR, 4.4-5.9 × 107/kg). The frequency of UCB transplantations decreased over time, whereas the opposite was observed for haploidentical peripheral-blood transplantations. The median follow-up time of recipients of haploidentical peripheral-blood transplantations was 30 months, compared with 43 months for haploidentical bone marrow transplantations and 48 months for UCB transplantations.
Hematopoietic Recovery
The median times to neutrophil recovery after UCB, haploidentical bone marrow, and peripheral-blood transplantations were 20, 19, and 17 days, respectively. The day 28 neutrophil recovery rate was lower after UCB transplantation (69%; 95% CI, 58% to 78%) compared with haploidentical bone marrow and peripheral blood transplantations (85% [95% CI, 79% to 91%] and 92% [95% CI, 86% to 97%], respectively; P < .0001). The day 100 platelet recovery rate was also lower after UCB transplantation (67%; 95% CI, 57% to 77%) compared with haploidentical bone marrow and peripheral-blood transplantations (91% [95% CI, 85% to 96%] and 90% [95% CI, 83% to 96%], respectively; P < .0001).
GVHD
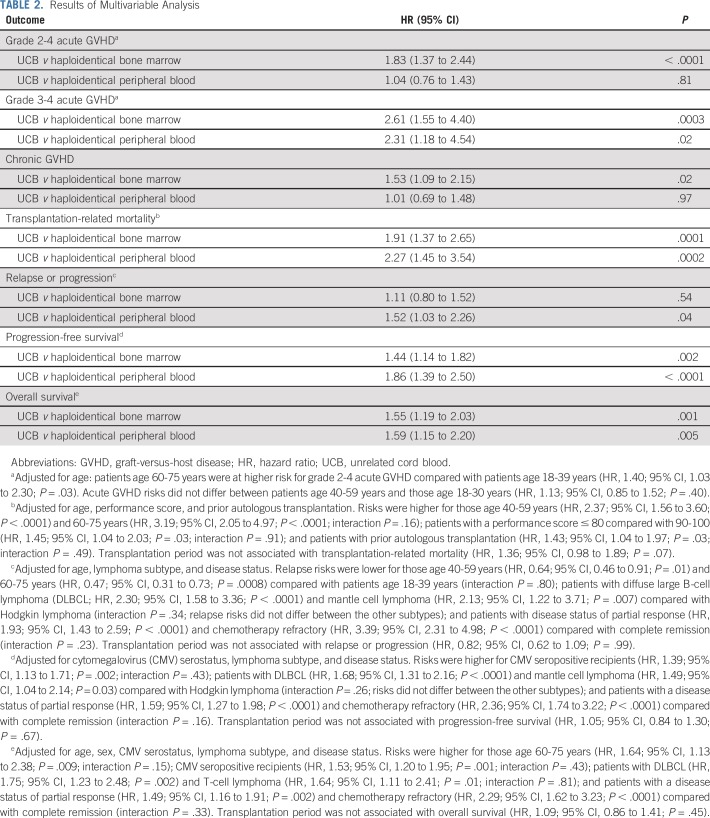
The day 100 grade 2-4 acute GVHD rate was higher after UCB transplantation compared with haploidentical bone marrow transplantation (43% [95% CI, 36% to 50%] v 20% [95% CI, 16% to 25%], respectively; P < .0001). Acute grade 2-4 GVHD did not differ after UCB and haploidentical peripheral-blood transplantation (35%; 95% CI, 28% to 43%; P = .17). Multivariable analysis confirmed a higher risk of grade 2-4 GVHD after UCB transplantation compared with haploidentical bone marrow transplantation after adjusting for age, the only variable that was significantly associated with acute grade 2-4 GVHD (Table 2). The incidence of grade 3-4 acute GVHD was higher after UCB transplantation compared with haploidentical transplantation either with bone marrow or with peripheral blood (Table 2). The day 100 grade 3-4 acute GVHD rates were 18% (95% CI, 13% to 24%) after UCB, 5% (95% CI, 3% to 8%) after haploidentical bone marrow, and 6% (95% CI, 3% to 11%) after peripheral-blood transplantations (P < .0001). The median time to onset of chronic GVHD was 5 months (IQR, 4-8 months) after UCB transplantation compared with 6 months (IQR, 4-11 months) and 6 months (IQR, 4-9 months) after haploidentical bone marrow and peripheral-blood transplantations. The 6-month incidence of chronic GVHD was higher after UCB transplantation (17%; 95% CI, 12% to 23%) compared with haploidentical bone marrow transplantation (11%; 95% CI, 8% to 14%; P = .04), but not after haploidentical peripheral-blood transplantation (18%; 95% CI, 12% to 24%; P = .91); the corresponding 4-year chronic GVHD rates were 28% (95% CI, 22% to 34%), 24% (95% CI, 19% to 29%; P = .31), and 32% (95% CI, 25% to 40%; P = .42). The 2-year incidence of severe chronic GVHD was higher after UCB (14%; 95% CI, 10% to 20%) and haploidentical peripheral-blood transplantation (16%; 95% CI, 10% to 22%) compared with haploidentical bone marrow transplantation (8%; 95% CI, 5% to 12%; P = .02). Multivariable analysis showed a higher risk of chronic GVHD after UCB transplantation compared with haploidentical bone marrow transplantation but not haploidentical peripheral-blood transplantation (Table 2). No other factors were associated with chronic GVHD.
TABLE 2.
Results of Multivariable Analysis
Transplantation-Related Mortality and Relapse or Progression
Transplantation-related mortality was higher after UCB transplantation compared with haploidentical transplantation with bone marrow or peripheral blood, adjusted for age, performance score, and prior autologous transplantation (Table 2 and Fig 1A). After adjusting for age, lymphoma subtype, and disease status, the risk of relapse or progression did not differ between UCB and haploidentical bone marrow transplantations (Table 2 and Fig 1B). However, risks were lower after haploidentical peripheral-blood transplantation compared with UCB transplantation.
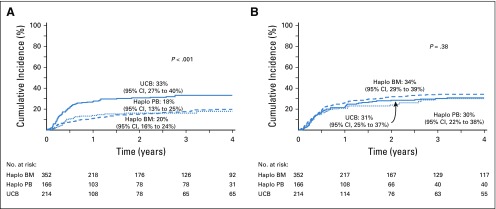
FIG 1.
(A) Transplantation-related mortality. The 4-year cumulative incidence of transplantation-related mortality was 20% (95% CI, 16% to 24%) for haploidentical bone marrow (BM) transplantations, 18% (95% CI, 12% to 25%) for haploidentical peripheral-blood (PB) transplantations, and 33% (95% CI, 27% to 40%) for unrelated cord blood (UCB) transplantations. (B) Relapse or progression. The 4-year cumulative incidence of relapse or progression was 34% (95% CI, 29% to 39%) for haploidentical BM transplantations, 30% (95% CI, 22% to 38%) for haploidentical PB transplantations, and 31% (95% CI, 25% to 37%) for UCB transplantations. Haplo, haploidentical.
Overall and Progression-Free Survival
Overall and progression-free survival outcomes were lower after UCB transplantation compared with haploidentical bone marrow and peripheral-blood transplantation after adjusting for age, type of lymphoma. and disease status at transplantation (Table 2 and Figs 2A and 2B). The effect of grade 3-4 acute GVHD on survival confirmed higher mortality after UCB transplantation compared with haploidentical bone marrow (hazard ratio [HR] 1.64; 95% CI, 1.23 to 2.18; P = .0008) and peripheral-blood transplantations (HR, 1.65; 95% CI, 1.17 to 2.33; P = .005). Similarly, chronic GVHD was also associated with a higher mortality after UCB transplantation compared with haploidentical bone marrow (HR, 1.73; 95% CI, 1.13 to 2.30; P = .0002) and peripheral-blood transplantations (HR, 1.76; 95% CI, 1.25 to 2.47; P = .001). Ninety-eight (51%) of 193 recipients of UCB are dead. One hundred fifteen (37%) of 313 recipients of haploidentical bone marrow and 58 (37%) of 159 recipients of haploidentical peripheral blood are dead. Recurrent disease was the most common cause of death in all treatment groups but did not differ between the groups (P = .17). Infection was the second most common cause of death and did not differ between treatment groups (P = .34). The proportion of deaths attributed to GVHD was higher after UCB transplantation (18%) compared with after haploidentical bone marrow and peripheral-blood transplantations (6% and 9%, respectively; P = .01). Other causes of death, including interstitial pneumonitis and organ failure, did not differ between the donor groups (data not shown).
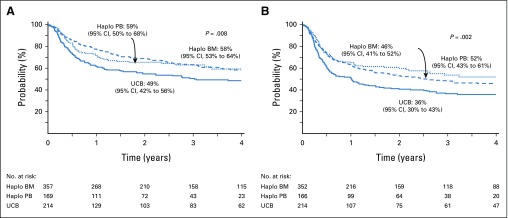
FIG 2.
(A) Overall survival. The 4-year probability of overall survival was 58% (95% CI, 53% to 64%) for haploidentical bone marrow (BM) transplantations, 59% (95% CI, 50% to 68%) for haploidentical peripheral-blood (PB) transplantations, and 49% (95% CI, 42% to 56%) for unrelated cord blood (UCB) transplantations. (B) Progression-free survival. The 4-year probability of progression-free survival was 46% (95% CI, 41% to 52%) for haploidentical BM transplantations, 52% (95% CI, 43% to 61%) for haploidentical PB transplantations, and 36% (95% CI, 30% to 43%) for UCB transplantations. Haplo, haploidentical.
Transplantation center effect.
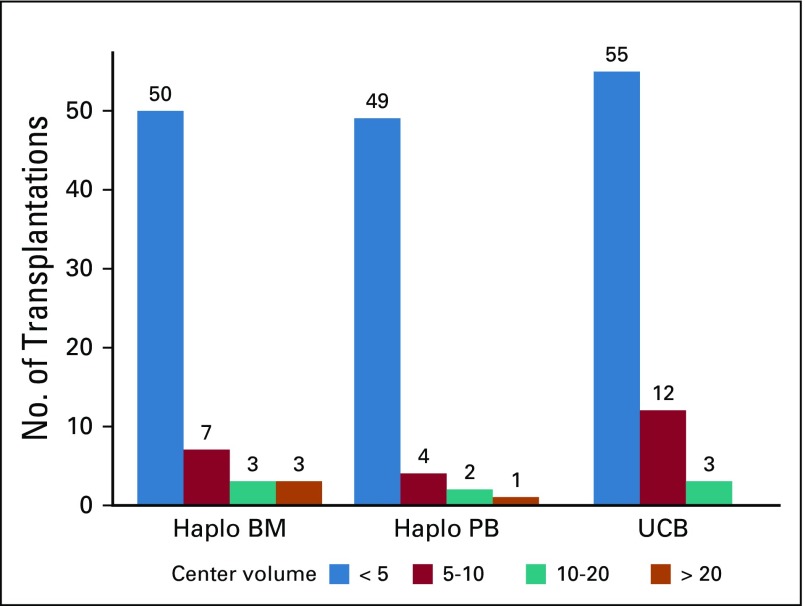
We tested for an effect of transplantation center on survival (P = .25) and transplantation-related mortality (P = .17) using the frailty model and found no effect. Several sensitivity analyses were performed to address transplantation center effects. We first compared risks for overall mortality (1−survival) and transplantation-related mortality at the center that contributed 134 haploidentical transplantations to the risks at the other centers that performed haploidentical bone marrow transplantations and did not find a significant difference (HR, 1.20; 95% CI, 0.81 to 1.79; P = 0.37; and HR, 1.47; 95% CI, 0.84 to 2.57; P = .18, respectively). A second analysis comparing the 6 high-volume haploidentical bone marrow transplantation centers to the other centers that performed haploidentical bone marrow transplantation did not identify differences in overall mortality (HR, 1.20; 95% CI, 0.86 to 1.72; P = .24), transplantation-related mortality (HR, 1.21; 95% CI, 0.69 to 2.12; P = .51), relapse or progression (HR, 1.00; 95% CI, 0.68 to 1.48; P = .99), or progression-free survival (HR, 1.06; 95% CI, 0.78 to 1.44; P = .72). A third analysis compared the 3 treatment groups without the 6 high-volume centers that performed haploidentical bone marrow transplantations (Data Supplement), and the effect of donor type on transplantation outcomes is consistent with the main analysis (Table 2). A fourth analysis compared the 3 treatment groups with transplantation center volume held in the final Cox model (Data Supplement). Centers were considered as those that contributed ≤ 10 patients versus each center that contributed > 10 patients. As with the other sensitivity analyses, the effects of donor type on transplantation outcomes were consistent with the main analysis (Table 2).
DISCUSSION
Multicenter retrospective studies have compared outcomes after UCB and haploidentical donor transplantation with mixed results, although these studies have been largely for leukemia and myelodysplastic syndrome.17-20 In unselected patients with acute leukemia, survival did not differ after UCB transplantation compared with haploidentical transplantation.17,18 However, when patients with acute myeloid leukemia received a uniform myeloablative conditioning regimen (thiotepa, busulfan, and fludarabine) or unselected patients with myelodysplastic syndrome, overall and leukemia-free survival were higher after haploidentical transplantation.19,20 The current analysis, which includes only patients with lymphoma, showed lower overall and progression-free survival after UCB transplantation compared with haploidentical donor transplantation, which to our knowledge has not been previously reported. Differences in overall and progression-free survival were a result of higher transplantation-related mortality and not relapse or progression after UCB transplantation. Lower progression-free and overall survival after UCB transplantation compared with haploidentical donor transplantation was independent of other factors associated with these outcomes including disease type and disease status at transplantation. Compared with Hodgkin lymphoma, transplantation-related mortality and relapse or progression rates were higher and progression-free and overall survival lower in patients with diffuse large B-cell and mantle cell lymphoma but not in patients with other non-Hodgkin lymphoma subtypes. Similarly, relapse or progression was higher for patients who received transplantation in partial remission or who had chemotherapy-refractory disease compared with those in remission. Our analysis confirmed that the effects of disease subtype and disease status are independent of donor type (Table 2). Secondary graft failure was 7% after UCB, compared with 5% and 4% after haploidentical bone marrow and peripheral-blood transplantations, respectively, but its assessment was confounded by relapse and progressive disease. We examined for an effect of transplantation center volume on transplantation outcomes and did not record differences in the effect of donor type on outcomes, leading us to conclude that better outcomes after haploidentical transplantation in the current analyses cannot be explained by transplantation center volume. Our analyses considered a relatively recent period, and many centers may have overcome their learning curve in regard to the donor groups being studied.
A phase III randomized trial designed based on the findings of 2 parallel phase II trials3 that used conditioning regimens and GVHD prophylaxis similar to transplantations in the current analysis is expected to enroll 205 patients to each arm of the trial (UCB and haploidentical bone marrow transplantation; ClinicalTrials.gov identifier: NCT01597778). Given the enrollment to the phase II trials, we anticipate that approximately 140 patients with lymphoma will be enrolled in the trial. In contrast, the current analysis with 214 UCB and 526 haploidentical transplantation recipients has allowed us to study the effect of donor-graft type in a larger population with lymphoma and is a strength of the current analysis. We are also able to address outcomes through 4 years after transplantation, unlike the phase III trial, which is designed to detect a 15% difference in 2-year progression-free survival. We found 2-year progression-free survival rates of 40% (95% CI, 33% to 47%) and 53% (95% CI, 48% to 59%) after UCB and haploidentical bone marrow transplantations (P < .001), respectively, an absolute difference of 13%.
The recorded high incidence of transplantation-related mortality after UCB transplantation is consistent with other reports.3-5,10 Several factors may have contributed to the higher transplantation-related mortality, including higher grade 3-4 acute and chronic GVHD, higher incidence of bacterial and viral infections,21 and HLA disparity.22 Most UCB transplantations were mismatched at 2 HLA loci considering lower resolution HLA match and, therefore, likely to be mismatched at ≥ 3 HLA loci considering allele-level match.22 Although haploidentical transplantations are mismatched at ≥ 2 HLA loci, differences in survival have not been reported when compared with HLA-matched sibling transplantations.8,23 Although slower neutrophil recovery may have increased risk of systemic infection(s) and in part contributed to higher early mortality, the proportion of deaths attributed to infection did not differ between the 3 donor groups. It is plausible that differences in chemotherapy dosing (ie, conditioning and GVHD prophylaxis) could have contributed to the higher transplantation-related mortality in the UCB group. Despite the same dose of TBI (2 Gy), there were differences in the total dose of fludarabine (200 mg/m2 in the UCB group v 150 mg/m2 in the haploidentical group) and cyclophosphamide (50 mg/kg in the UCB group v 129 mg/kg in the haploidentical group). Nevertheless, as mentioned earlier, the proportion of deaths as a result of infection and organ failure was not significantly different among the 3 donor groups, making this hypothesis less likely. Another factor known to mitigate early mortality is delivery of adequate TNC dose. The total TNC dose for all UCB transplantations in the current analyses met the minimum required dose of 3 × 107/kg. In fact, the median TNC dose was 4.8 × 107/kg (IQR, 4.0-5.6 × 107/kg).3,22,24 A recent phase II trial of expanded UCB transplantation (median TNC dose, 4.9 × 107/kg) for leukemia reported lower transplantation-related mortality and higher relapse without a survival advantage compared with a contemporary cohort of nonexpanded UCB transplantations.25 Thus, a minimum TNC dose is desired for UCB transplantation but perhaps without an added advantage to infusing higher than the desired TNC. Strategies to lower GVHD after UCB transplantation should also be investigated, such as the use of posttransplantation cyclophosphamide.26
Our study has several limitations, the first being a retrospective cohort analysis in which treatment choices were physician dependent and/or institutional preference and the second being our inability to adjust for unknown or unmeasured factors, including consideration of hematopoietic cell transplantation comorbidities (not collected for cord blood recipients reported to Eurocord) known to be associated with survival.27 We considered prefreeze TNC as that was available for all UCB units and other reports have identified CD34 count of the unit as a better predictor for hematopoietic recovery.28 Haploidentical transplantations were more recent, and these patients may have received newer therapies before transplantation. However, lower progression-free and overall survival rates after UCB transplantation were a result of differences in mortality and not relapse or progression. We also did not record differences in outcome by transplantation period. Fewer UCB transplantations after 2012 coincide with publication of results of the parallel phase II trials of UCB and haploidentical bone marrow transplantation, which may have prompted increased use of haploidentical donors.3 A strength of the current analysis is the inclusion of > 700 patients with Hodgkin and non-Hodgkin lymphoma, which allowed us to compare the effect of graft type for haploidentical transplantations (bone marrow and peripheral blood) compared with UCB transplantations. Accruing 700 patients for randomized clinical trials in transplantation can be lengthy and the costs prohibitive. Thus, these data are informative for clinical decision making. Consistent with an earlier report that included primarily patients with acute myeloid leukemia,29 we did not record an advantage for progression-free survival (HR, 1.28; 95% CI, 0.96 to 1.72; P = .10) or overall survival (HR, 1.00; 95% CI, 0.72 to 1.39; P = .97) with peripheral blood compared with bone marrow for haploidentical transplantation. Therefore, when considering HLA-mismatched donor transplantation, our data support using a haploidentical relative, with UCB reserved when such a donor is not available.30,31
Appendix
FIG A1.
Center volume by donor group. BM, bone marrow; Haplo, haploidentical; PB, peripheral blood; UCB, unrelated cord blood.
SUPPORT
The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service Grant/Cooperative Agreement No. 5U24-CA076518 (M.E.) from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; Grant No. 5U10HL069294 from NHLBI and NCI; Contract No. HHSH250201200016C with the Health Resources and Services Administration (Department of Health and Human Services); and Grants No. N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration or any other agency of the US Government.
See accompanying Editorial on page 1501
AUTHOR CONTRIBUTIONS
Conception and design: Giancarlo Fatobene, Vanderson Rocha, Andrew St. Martin, Mei-Jie Zhang, Silvia Montoto, Mary Eapen
Financial support: Mary Eapen
Administrative support: Andrew St. Martin, Hervé Finel, Fernanda Volt
Provision of study materials or patients: Vanderson Rocha, Asad Bashey, Claudio Brunstein, Luca Castagna, Alida Dominetto, Yves Chalandon, Mohamed Kharfan-Dabaja, Hélène Labussiere-Wallet, Jose M. Moraleda, Rocco Pastano, Miguel-Angel Perales, Ibrahim Yakoub-Agha, Anna Sureda, Eliane Gluckman, Silvia Montoto, Mary Eapen
Collection and assembly of data: Andrew St. Martin, Hervé Finel, Chantal Kenzey, Fernanda Volt, Silvia Montoto, Mary Eapen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nonmyeloablative Alternative Donor Transplantation for Hodgkin and Non-Hodgkin Lymphoma: From the LWP-EBMT, Eurocord, and CIBMTR
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Giancarlo Fatobene
Consulting or Advisory Role: Janssen-Cilag
Travel, Accommodations, Expenses: AstraZeneca, Amgen
Vanderson Rocha
Honoraria: Takeda, Novartis, Roche
Consulting or Advisory Role: Takeda, Agios, Zodiac Pharma, Novartis, AbbVie
Speakers' Bureau: Bristol-Myers Squibb, Takeda, Amgen, Agios, Takeda, Pfizer, Janssen
Mehdi Hamadani
Honoraria: Celgene
Consulting or Advisory Role: MedImmune, Cellerant Therapeutics, Janssen Research & Development, Incyte Corporation, Pharmacyclics, ADC Therapeutics, Puma Biotechnology (I), Verastem
Speakers' Bureau: Genzyme, Celgene, AstraZeneca
Research Funding: Takeda, Spectrum Pharmaceuticals, Otsuka US, Astellas Pharma, Genzyme
Stephen Robinson
Honoraria: Genentech, Takeda, Kite/Gilead, Novartis
Consulting or Advisory Role: Takeda, Kite/Gilead, Genentech
Claudio Brunstein
Consulting or Advisory Role: AlloVir
Research Funding: Magenta (Inst), Gamida Cell (Inst), Astex (Inst)
Yves Chalandon
Honoraria: Incyte (Inst)
Consulting or Advisory Role: Bristol-Myers Squibb (Inst), Novartis (Inst), Incyte (Inst), Pfizer (Inst), MSD Oncology (Inst), AbbVie (Inst), Roche (Inst), Jazz Pharmaceuticals (Inst), Gilead (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Amgen (Inst), Roche (Inst), Celgene (Inst), Gilead Sciences (Inst), AbbVie (Inst), MSD (Inst), Novartis (Inst), Jazz Pharmaceuticals (Inst), Janssen-Cilag (Inst)
Mohamed Kharfan-Dabaja
Honoraria: Pharmacyclics, Daiichi Sankyo
Consulting or Advisory Role: Pharmacyclics, Daiichi Sankyo
Jose M. Moraleda
Consulting or Advisory Role: Gilead Sciences, Celgene
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Gilead
Miguel-Angel Perales
Stock and Other Ownership Interests: NexImmune
Consulting or Advisory Role: Seattle Genetics, Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed, Medigene, Takeda, Nektar, AbbVie
Research Funding: Incyte (Inst), Miltenyi Biotec (Inst)
Anna Sureda
Honoraria: Takeda, Bristol-Myers Squibb, Merck Sharp & Dohme, Celgene, Janssen, Sanofi, Roche, Novartis, Gilead Sciences
Consulting or Advisory Role: Takeda, Bristol-Myers Squibb, Gilead Sciences, Celgene, Janssen, Novartis
Speakers' Bureau: Takeda
Other Relationship: Sanofi, Takeda, Roche, Celgene, Gilead Sciences
Ibrahim Yakoub-Agha
Honoraria: Celgene, Novartis, Gilead, Biotest, Janssen, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Gilead
Silvia Montoto
Consulting or Advisory Role: Bayer
Travel, Accommodations, Expenses: Gilead
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues CA, Rocha V, Dreger P, et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: Similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica. 2014;99:370–377. doi: 10.3324/haematol.2013.088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachanova V, Burns LJ, Wang T, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50:197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez C, Gayoso J, Canals C, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: A registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplant. J Clin Oncol. 2017;35:3425–3432. doi: 10.1200/JCO.2017.72.6869. [DOI] [PubMed] [Google Scholar]
- 7.Raiola A, Dominietto A, Varaldo R, et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphoma. Bone Marrow Transplant. 2014;49:190–194. doi: 10.1038/bmt.2013.166. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich S, Finel H, Martinez C, et al. Post-transplant cyclophosphamide-based haplo-identical transplantation as alternative to matched sibling or unrelated donor transplantation for non-Hodgkin lymphoma: A registry study by the European Society for Blood and Marrow Transplantation. Leukemia. 2016;30:2086–2089. doi: 10.1038/leu.2016.125. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier J, Castagna L, Garnier F, et al. Reduced-intensity and non-myeloablative allogeneic stem cell transplantation from alternative HLA-mismatched donors for Hodgkin lymphoma: A study by the French Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant. 2017;52:689–696. doi: 10.1038/bmt.2016.349. [DOI] [PubMed] [Google Scholar]
- 10.Paviglianiti A, Tozatto Maio K, Rocha V, et al. Outcomes of advanced Hodgkin lymphoma after umbilical cord blood transplantation: A Eurocord and EBMT Lymphoma and Cellular Therapy & Immunobiology Working Party Study. Biol Blood Marrow Transplant. 2018;24:2265–2270. doi: 10.1016/j.bbmt.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Mariotti J, Devillier R, Bramanti S, et al. T cell-replete haploidentical transplantation with post-transplantation cyclophosphamide for Hodgkin lymphoma relapsed after autologous transplantation: Reduced incidence of relapse and of chronic graft-versus-host disease compared with HLA-identical related donors. Biol Blood Marrow Transplant. 2018;24:627–632. doi: 10.1016/j.bbmt.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 13.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 16.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: A Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 18.Ruggeri A, Labopin M, Savani B, et al. Hematopoietic stem cell transplantation with unrelated cord blood or haploidentical donor grafts in adult patients with secondary acute myeloid leukemia, a comparative study from Eurocord and the ALWP EBMT. Bone Marrow Transplant. 2019;54:1987–1994. doi: 10.1038/s41409-019-0582-5. [DOI] [PubMed] [Google Scholar]
- 19.Giannotti F, Labopin M, Shouval R, et al. Haploidentical transplantation is associated with better overall survival when compared to single cord blood transplantation: An EBMT-Eurocord study of acute leukemia patients conditioned with thiotepa, busulfan, and fludarabine. J Hematol Oncol. 2018;11:110. doi: 10.1186/s13045-018-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robin M, Porcher R, Ruggeri A, et al. HLA-mismatched donors in patients when myelodysplastic syndrome: An EBMT registry analysis. Biol Blood Marrow Transplant. 2019;25:114–120. doi: 10.1016/j.bbmt.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Ballen K, Woo Ahn K, Chen M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1636–1645. doi: 10.1016/j.bbmt.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurdy SR, Kasamon YL, Kanakry CG, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 2017;102:391–400. doi: 10.3324/haematol.2016.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaradavou A, Brunstein CG, Eapen M, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz ME, Wease S, Blackwell B, et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019;37:367–374. doi: 10.1200/JCO.18.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacigalupo A, Sica S, Laurenti L, et al. Unrelated cord blood transplantation and post-transplant cyclophosphamide. Haematologica. 2019;104:e77–e78. doi: 10.3324/haematol.2018.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: A Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2015;21:1479–1487. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124:2905–2912. doi: 10.1182/blood-2014-03-566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosuri S, Wolff T, Devlin SM, et al. Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: Practical implications for donor selection. Biol Blood Marrow Transplant. 2017;23:965–970. doi: 10.1016/j.bbmt.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker JN, Boughan K, Dahi PB, et al. Racial disparities in access to HLA-matched unrelated donor transplants: A prospective 1312-patient analysis. Blood Adv. 2019;3:939–944. doi: 10.1182/bloodadvances.2018028662. [DOI] [PMC free article] [PubMed] [Google Scholar]