Abstract
Background:
Lipid-based nutrient supplements (LNS) have been effective in the treatment of acute malnutrition among children. We evaluated the use of LNS supplementation for improving the micronutrient status of young children.
Methods:
A 12-month randomized controlled trial was conducted among children aged 6–18 month living in Intibucá, Honduras. Communities (n=18) were randomized into clusters matched by poverty indicators (9 intervention, n=160 and 9 controls, n=140). Intervention participants received LNS. All children received food vouchers and nutrition education. Primary outcomes included measures of micronutrient status: at baseline, 6 and 12 month blood was collected for assessment of folate, iron, zinc, riboflavin and vitamin B12 status; hemoglobin was measured every 3 months; and dietary and anthropometry collected monthly. Longitudinal analyses were based on intent-to treat and LNS adherence. Generalized estimating equations were used in the estimation of generalized linear regression models specified for the data.
Results:
At 6 months follow-up, children in the intervention group had a lower proportion classified as deficient for B12 (43.6%) compared to the control (67.7%; P=0.03). The intervention group had a higher mean concentration for folate at 6 months (P=0.06), and improvements continued through 12 months for folate (P=0.002) and vitamin A deficiency (P=0.03). This pattern of results, with improved significance, remained in sub-analysis based on LNS adherence.
Conclusion:
These data demonstrate that LNS improved select micronutrient status in young non-malnourished Honduran children.
Keywords: lipid-based nutrient supplements, child undernutrition, prevention, micronutrients, Honduras, randomized-controlled trial
About 171 million children aged 0–5 years suffer from chronic undernutrition, mainly in developing countries undergoing economic transitions.1 In Latin America this represents a total of about 7 million children, where stunting is now much more common than underweight and wasting. 1 Large disparities in economic growth in Latin America have led to higher levels of undernourishment, food insecurity and poor access to quality foods for those living in the rural areas. 2 A diet low in animal-source foods, fruits and vegetables, and high in phytates (beans, maize) may result in micronutrient deficiencies that lead to anemia, poor growth, developmental delays and increased morbidity.3,4 Efforts to improve micronutrient intake should target children during the first two years of life when the irreversible outcomes of malnutrition may be prevented.1,3
Food-based interventions targeting children under 2 have shown some improvements in growth outcomes and micronutrient status.3 A review of these strategies indicated that interventions pairing supplementation of energy dense products with education have better results compared to educational interventions or food fortification strategies alone.3
Lipid-based nutrient supplements (LNS) are energy dense products that provide essential fatty acids and micronutrients. LNS may be advantageous over other types of food-based efforts because they allow more nutrients to be consumed in a serving, do not require major diet behavior changes and include lipids that improve the absorption of fat-soluble vitamins;5 evidence suggests that LNS products are effective in treating severe child malnutrition,6–9 and are tolerated and consumed by infants.5,10,11
LNS studies in Sub-Saharan Africa, targeting prevention of chronic malnutrition, have reported improvements in weight gain and a reduced incidence of wasting and stunting. 12–15 These ranged from 3–12 month-long-interventions for children 6 to 60 months old using varying doses of LNS. Only one study has examined micronutrients (i.e. zinc and selenium) but no improvements were reported.12 Given the lack of evidence on the effect of LNS on micronutrient status of children, this study used a cluster-randomized trial to test if LNS supplementation can improve the micronutrient status of children 6–18 months old living in a rural poor region of Central America.
Methods
Setting
The study was conducted in three municipalities (Santa Lucia, Magdalena and San Antonio) in the southwestern part of Intibucá, Honduras, bordering El Salvador. Recruitment for the 12-month intervention was conducted during the dry season (March-April 2009) with follow-up conducted until April 2010. All study participants experienced 6 months each of the dry and rainy season.
Study Design
This was a cluster randomized controlled trial. 18 communities were paired by region and matched on several poverty indicators; percent of houses with dirt floors, no toilet, 4 or more people per room, and total poverty score (the sum of all the indicators). Clusters were geographically separated to avoid potential cross contamination. One cluster within each pair was then randomized to intervention (n=9) or control group (n=9). Thereafter the mother-infant pairs were enrolled in the study groups according to their cluster randomization.
Our research staff made initial contact with mothers living in the selected communities at community centers, health centers and schools. Recruitment was conducted at each site over a one-month period with one recruitment day per village. Eligible caregiver-child pairs were enrolled from their site of recruitment or from the health clinic on their date of recruitment, making this a convenience sample.
The primary outcomes include micronutrient biomarkers (folate, iron, zinc, vitamin B12, vitamin A, and riboflavin). Secondary outcomes include growth, dietary intake and food insecurity. The study was blinded to study group allocation at the data entry level and at the biomarker analysis level. Given the difficulties of working in this rural setting, delivery of the intervention was not blinded for project staff conducting the assessments.
The Institutional Review Board of the University of North Carolina at Chapel Hill and the Honduran IRB committee approved the study protocol. The Intibucá Ministry of Health endorsed the study’s objectives and collaborated in its implementation. An advisory committee monitored the incidence of any adverse effects. Mothers/caretakers gave informed consent for the participation of their child. Participants were referred to a health clinic for iron supplementation if hemoglobin values were <100 g/L.
Study Population
Infants and caretakers (mothers/caretakers >16 years of age) were eligible for the study if the infants were 5–18 months of age at time of recruitment, not participating in a child health brigade that provided vitamin A supplementation, residing within the three study municipalities, had no plans to move outside of the study region in the next 2 months, and had no medical conditions. Infants with congenital anomalies, mental retardation, severe physical handicap, under-nutrition caused by medical conditions, and allergy to peanuts (determined with an allergy reaction test using 5–15 grams of LNS) were ineligible. Infant with a weight for height z-score ≤ −2 standard deviations below the norm were not eligible and were referred to a qualified health care provider.
Sample Size
We attempted to recruit 150 participants per study group, based on funding as well as on a cluster randomization power analysis (Software Power and Sample Size (PASS), NCSS LLC; http://www.ncss.com. See online Supplementary Material for details.
Intervention
Participants in the intervention group received a monthly supply of a LNS product (Plumpy’doz by Nutriset, Malaunay, France) throughout the 12-month study period. Plumpy’doz provides 247 kcal, 5.9 g protein, 16.0 g fat, 400 μg vitamin A, 0.9 μg vitamin B12, 9 mg iron and 9 mg zinc in a 46.3 g dose. Caretakers in the intervention group were counseled to feed 3 tsp, 3 times per day (a total of 46.3 g) of LNS to infants 6–12 months and 4.5 tsp. 3 times per day (a total of 70 g) to children 13–30 months. These doses were informed by consultation with experts, recognizing that the product had never been used for this purpose and in this setting. Infants enrolled at 5 months were gradually transitioned to the 46.3 gm dose when solid foods were introduced. Caregivers were advised on correct spoon size and were allowed to mix the product with other foods. The Project Staff counseled mothers to continue breast feeding as normal and not to force feed LNS to the children.
Given the high levels of food insecurity in this region, our study provided food vouchers to all participants, as well as monthly nutritional education sessions. The food vouchers were also intended to offset sharing of the LNS product with other children in the intervention families. Food vouchers were granted based on household size: <4 = L 200 per month, 5–8 = L 300 per month, >=8 = L 400 per month (L= Lempiras; 18L = US $1 during the study period). These were redeemable at local stores for rice, beans, corn, vegetables and fruits which provided only a minimal percentage of the household’s food supplies. Use of the food vouchers by study participants and store owners was monitored. Ten culturally- tailored, age-appropriate educational sessions were delivered on nutritional and health topics.
Data and Blood Sample Collection
Non-fasting blood samples were collected at baseline, 6 months and at the end of the 12 month study period. Venous blood samples were drawn by a nurse using trace element free BD Vacutainer tubes (1.5 mL), 2 hour post-prandial (See online Supplementary Material for details). Hemoglobin was measured at baseline and every three months thereafter from a finger prick using a StatSite-MHgb (Stanbio Laboratory, Boerne, Texas) and recorded to the nearest g/L. The StatSite-MHgb was calibrated three times during the study.
Age-specific cut points for deficiency or suboptimal states were used for hemoglobin16 and B12.17 For biomarkers without age-specific cut-points, generally-accepted cut-points were used: <3 ng/ml for folate18, >8.3 ug/ml for soluble transferrin19, <65 ug/dl for zinc20, <0.70 μmol/L of retinol for Vitamin A deficiency21,22 and <170 nmol/L for riboflavin.23 For CRP, >3 mg/L indicated acute inflammation. 24
Sociodemographic, Anthropometric, Diet and Food Security Measures
Baseline demographic information was collected using culturally-appropriate questionnaires. Infant weight and length were measured monthly, without clothing or wet diapers, using an electronic weighing scale to the nearest 10 g for weight, and with an infantometer to the nearest 0.10 cm for length. Growth parameters were assessed using WHO 2006 Child Growth Standards.25 Monthly 24-hour recalls were conducted by two trained local staff using the multiple-pass approach with the aid of measurement models (i.e. cups, plates) and the Minnesota Nutrition Data System for Research food guide.26 Additionally, a validated food security questionnaire was implemented at baseline, 6 months and end of study27. The results for these secondary outcomes are not included in this analysis.
Statistical Methods
A concern about the potential effect of LNS on breastfeeding led us to determine the proportion of women who self-reported any breast feeding (yes/no) from the 24-hour recall at baseline, 3, 6, 9, and 12 months. Based on the intent-to-treat assumption and LNS adherence, generalized linear regression models were estimated using generalized estimation equations (GEE) to compare treatment and control groups with randomization at the village (group) level. The analyses were performed using SAS procedure GENMOD.
Retinol analyses included CRP levels as a covariate. Micronutrient biomarkers in their continuous form were log-transformed and complete case analysis was used for each outcome variable. Binary outcome variables were created for biomarker analyses based on deficiency cut points described above. Hemoglobin values were adjusted for altitude using the correction by Dirren et al.28
Sub-analyses were done with regard to adherence to the LNS consumption protocol assessed from mother’s report of child’s dietary intake collected by 24 hour recalls at 3, 6, 9 and 12 months. Two variables described adherence, one based on the study’s protocol called “protocol” adherence (at least 46 g/d for children <12 months and at least 70 g/d for those ≥12 months), and the other based on “any consumption” of the product. These variables were coded dichotomously, with adherence coded as 1. We computed the average amount of LNS consumed only among those children coded as adherent.
Missing data occurred primarily due to participants’ missed visits (see figure 1). The amount of missing data was similar between treatment groups at each time point though missed visits were more frequent toward the end of the study. Multiple imputation was used to examine the influence of missing data using the MI procedure in SAS with no difference in results between the imputed and intent-to-treat analyses (See online Supplementary Materials for details).29–33 Therefore, only the intent-to-treat and subanalyses results are presented in this paper. SAS (version, 9.3; SAS Institute, Cary, NC) was used for all data analyses.
Figure 1.
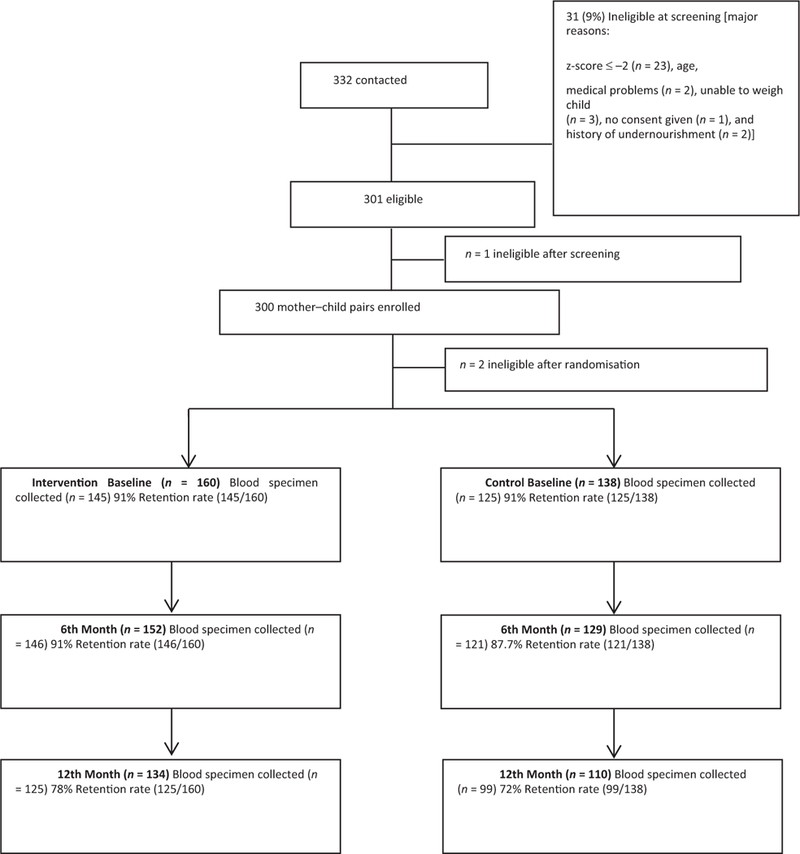
Attrition rates based on completed blood specimen collection.
Results
Of the 300 children enrolled in the study, 160 were in the intervention group and 140 in the control group. Sample collection rates at baseline, 6 months, and 12 months are shown in Figure 1. After verification of eligibility criteria our final sample size was 298. Table 1 details the socio- demographic and selected maternal characteristics of the participants. Because the randomization occurred at the village, and not at the participant, level, we would not expect the treatment groups to have similar characteristics. Mean (±SD) length-for-age, weight-for-age z, and weight-for-length z scores were −0.56 ± 1.05, −0.35 ± 0.93, and −0.08 ± 0.99 for the control children respectively and −0.72 ± 1.06, −0.53 ± 0.96, and −0.19 ± 0.91for the intervention children; these were not statistically different (data not shown). The mean food insecurity score for the population at baseline was 33 ± 5.4 (range 19 to 42) which indicates moderate food insecurity.34
Table 1.
Sociodemographic characteristics of the study population at baseline per study group
| Demographic Characteristic | Treatment (n=160) | Control (n=138) | ||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| Interviewee is the mother of participant | ||||
| No | 14 | (9) | 4 | (3) |
| Yes | 146 | (91) | 134 | (97) |
| Interviewee is the child’s primary caregiver | ||||
| No | 1 | (1) | 0 | (0) |
| Yes | 159 | (99) | 138 | (100) |
| Age of primary caregiver (years) | ||||
| ≤22 | 47 | (29) | 46 | (33) |
| 23–30 | 61 | (38) | 54 | (39) |
| ≥31 | 52 | (33) | 38 | (28) |
| Maximum education level of primary caregiver | ||||
| Did not attend school | 13 | (8) | 6 | (4) |
| Primary school (1st−6th grade) | 131 | (82) | 112 | (81) |
| Secondary school (7th−9th grade) | 6 | (4) | 7 | (5) |
| Career/diversified | 10 | (6) | 12 | (9) |
| University | 0 | (0) | 1 | (1) |
| Marital status of primary caregiver | ||||
| Cohabiting | 63 | (39) | 70 | (51) |
| Married | 42 | (26) | 42 | (30) |
| Single/Divorced/Widowed/Separated | 21 | (13) | 26 | (19) |
| Age at first birth of primary caregiver/mother (years) | ||||
| ≤22 | 132 | (84) | 113 | (82) |
| 23–30 | 24 | (15) | 21 | (15) |
| ≥31 | 1 | (1) | 3 | (2) |
| Missing | 3 | 1 | ||
| Total number of pregnancies | ||||
| 1 | 45 | (28) | 44 | (32) |
| 2 | 29 | (18) | 32 | (23) |
| ≥3 | 86 | (54) | 62 | (45) |
| Total number of live children | ||||
| 1 | 49 | (31) | 50 | (36) |
| 2 | 31 | (19) | 33 | (24) |
| ≥3 | 79 | (50) | 54 | (39) |
| Missing | 1 | 1 | ||
| Number of people living in home | ||||
| ≤4 | 33 | (21) | 26 | (19) |
| 5–8 | 82 | (51) | 74 | (54) |
| ≥9 | 45 | (28) | 38 | (28) |
| Number of adults living in home | ||||
| ≤2 | 86 | (54) | 64 | (46) |
| 3–5 | 62 | (39) | 62 | (45) |
| ≥6 | 12 | (8) | 12 | (9) |
| Other people’s children live/eat in interviewee’s home | ||||
| No | 116 | (73) | 87 | (63) |
| Yes | 44 | (27) | 51 | (37) |
| Primary caregiver was employed at study entry | ||||
| No | 143 | (90) | 125 | (91) |
| Yes | 16 | (10) | 13 | (9) |
| Missing | 1 | 0 | ||
| Age of Child | ||||
| ≤12 months | 86 | (54) | 72 | (52) |
| > 12 months | 74 | (46) | 66 | (48) |
Adherence to the intervention
Overall, there was low adherence to the study protocol of consuming Plumpy’ doz. At study month 3, protocol adherence was 5% (Table 2). Adherence increased to 9% at study month 6 and then declined. The mean daily intake of LNS by children with protocol adherence ranged from 82 g to 105 g. However, approximately 73% of children reported any LNS consumption from study months 3 to 9 and this decreased to 69% at month 12. The average amount consumed by children in this category ranged from 34 g to 50 g, achieving stability after month 6 (Table 2). Given the low adherence to the suggested LNS dose, the sub-analysis was limited to the main study outcomes of the ‘any’ dose category.
Table 2.
Adherence and grams of Plumpy’doz consumed daily at four time points among intervention children.
| Time Point (Sample size) |
Protocol dose adherenceb |
Any Plumpy’doz consumption |
||
|---|---|---|---|---|
| % | Mean intakea (SD) | % | Mean intakea (SD) | |
| Month 3 (149) | 4.7 | 88.3 (42.4) | 72.5 | 34.7 (23.8) |
| Month 6 (150) | 8.7 | 102.2 (40.5) | 72.7 | 50.5 (28.3) |
| Month 9 (128) | 5.5 | 105.3 (43.6) | 72.7 | 48.5 (26.9) |
| Month 12 (129) | 1.6 | 81.6 (10.5) | 69.0 | 50.0 (16.7) |
Mean intake in grams, SD=standard deviations among consumers only
Protocol adherence: 46.3+ daily grams for children aged ≤12 months; 70+ daily grams for children aged >12 months
The pattern of self-reported breastfeeding was different between the study groups (See Supplemental Table #1 for details). At baseline and at three months, there were no differences between study groups in the proportion of caregivers who reported any breastfeeding. However, beginning at month 6, a greater reduction in breastfeeding was observed in the intervention group compared to the controls (64% control versus 56% in the intervention group) and this continued throughout the remaining intervention period.
Micronutrient status
There were no subjects identified as folate deficient in either group at any time during the study. At baseline there were significantly lower concentrations of retinol and thus a higher frequency of vitamin A deficiency in the control group compared to the intervention group (Table 3). Additionally we observed higher levels of CRP in the intervention group compared to the control. No other significant differences between intervention and control groups were noted.
Table 3.
Intent to Treat, Comparison of biomarkers at three time points, per study group
| Biomarker | Controla [n] | Interventionb [n] | P-valuec |
|---|---|---|---|
| Vitamin B12 (pg/mL) | |||
| Baseline | 177.3±2.3 [93] | 226.5±2.1 [105] | 0.14 |
| Month 6 | 189.1±2.0 [97] | 248.5±2.0 [110] | 0.03 |
| Month 12 | 227.0±2.2 [99] | 285.3±2.0 [122] | 0.08 |
| Vitamin B12 deficiencyd (%) | |||
| Baseline | 58.1 [93] | 47.6 [105] | 0.25 |
| Month 6 | 67.0 [93] | 43.6 [105] | 0.01 |
| Month 12 | 61.6 [99] | 48.3 [122] | 0.09 |
| CRP (mg/L) | |||
| Baseline | 0.75±4.2 [97] | 1.35±5.2 [115] | 0.03 |
| Month 6 | 0.89±4.7 [96] | 0.78±4.4 [114] | 0.60 |
| Month 12 | 0.74±6.2 [99] | 0.73±6.8 [121] | 0.96 |
| CRP >3 mg/L (%) | |||
| Baseline | 18.6 [97] | 33.0 [115] | 0.02 |
| Month 6 | 21.9 [96] | 16.7 [114] | 0.43 |
| Month 12 | 23.2 [99] | 20.7 [121] | 0.53 |
| Folate (ng/mL)e | |||
| Baseline | 17.36±1.4 [93] | 17.66±1.5 [110] | 0.76 |
| Month 6 | 16.65±1.4 [97] | 18.48±1.5 [115] | 0.06 |
| Month 12 | 16.25±1.4 [99] | 18.73±1.4 [122] | 0.002 |
| Hemoglobin (g/L)f | |||
| Baseline | 101.8±1.2 [134] | 106.7±1.1 [144] | 0.10 |
| Month 6 | 102.5±1.2 [128] | 106.6±1.2 [144] | 0.17 |
| Month 12 | 105.7±1.1 [109] | 108.7±1.1 [134] | 0.14 |
| Anemia (%)f | |||
| Baseline | 50.0 [134] | 39.6 [144] | 0.18 |
| Month 6 | 65.6 [128] | 50.7 [144] | 0.07 |
| Month 12 | 55.1 [109] | 51.5 [134] | 0.60 |
| Transferrin receptors (μg/mL) | |||
| Baseline | 8.11±1.6 [97] | 8.70±1.5 [115] | 0.26 |
| Month 6 | 7.81±1.7 [96] | 8.20±1.6 [112] | 0.55 |
| Month 12 | 7.62±1.5 [98] | 7.11±1.8 [122] | 0.63 |
| Transferrin >8.3 μg/mL (%) | |||
| Baseline | 49.5 [97] | 52.2 [115] | 0.68 |
| Month 6 | 45.8 [96] | 49.1 [112] | 0.67 |
| Month 12 | 28.6 [98] | 26.2 [122] | 0.86 |
| Zinc (μg/dL) | |||
| Baseline | 62.4±1.6 [109] | 66.2±1.4 [128] | 0.35 |
| Month 6 | 65.6±1.3 [91] | 64.9±1.3 [111] | 0.84 |
| Month 12 | 63.4±1.2 [77] | 65.1±1.2 [99] | 0.55 |
| Zinc <65 μg/dL (%) | |||
| Baseline | 47.7 [109] | 37.5 [128] | 0.42 |
| Month 6 | 51.7 [91] | 54.1 [111] | 0.83 |
| Month 12 | 62.3 [77] | 55.6 [99] | 0.48 |
| Retinol (μmol/L)f | |||
| Baseline | 0.75±1.3 [82] | 0.80±1.3 [97] | 0.006 |
| Month 6 | 0.82±1.3 [89] | 0.92±1.2 [102] | 0.03 |
| Month 12 | 0.82±1.4 [75] | 0.88±1.3 [96] | 0.34 |
| Vitamin A Deficiency (Retinol <0.70 μmol/L) (%)g | |||
| Baseline | 45.1 [82] | 26.8 [97] | <0.0001 |
| Month 6 | 25.8 [89] | 13.7 [102] | 0.19 |
| Month 12 | 33.3 [75] | 17.7 [96] | 0.03 |
| Riboflavin (nmol/L) | |||
| Baseline | 215.9±1.7 [92] | 254.4±1.4 [134] | 0.11 |
| Month 6 | 273.5±1.4 [78] | 277.3±1.4 [52] | 0.89 |
| Month 12 | 261.4±1.3 [86] | 265.1±1.3 [117] | 0.78 |
| Riboflavin <170 nmol/L (%) | |||
| Baseline | 20.7 [92] | 8.2 [134] | 0.18 |
| Month 6 | 6.4 [78] | 11.5 [52] | 0.31 |
| Month 12 | 4.7 [86] | 3.4 [117] | 0.70 |
Geometric means and geometric standard deviation.
P-values based on GEE analysis using PROC GENMOD, taking into account randomization at village level.
Cut points were <167.9 pg/ml for 5–11 mo old, <266.79 pg/ml for 11–24 mo olds, and <319.6 for >24 mo olds.
There were no cases of folate deficiency defined as <3 ng/mL at any time point.
Hemoglobin values adjusted for altitude. Cut points were <100 g/L for <12 mo olds, <110 g/L for 12 to 24 mo olds, and <111 g/l for 2 to 5 yr olds.
P-values for retinol and vitamin A deficiency based on GEE analysis using PROC GENMOD, taking into account randomization at village level and adjusted for baseline value and time point-specific measured CRP level.
At the 6th month time point, a significant difference between groups was found for the mean concentration of vitamin B12 and retinol; the latter was adjusted for baseline level and time-specific CRP levels (Table 3). Additionally, the intervention group had a significantly lower proportion of children classified as deficient for B12. The intervention group had a higher mean concentration for folate compared to controls (P=0.06).
At the 12th month time point, mean folate concentration continued to be higher in the intervention than the control group (Table 3). The trend towards higher levels of B12 in the intervention group (285.3±2.04), compared to the control group (227±2.16) continued (P=0.08). Children in the intervention group continued to have lower risk of vitamin B12 and A deficiency.
The subanalysis taking into account the adherence reflected those found with the intent-to-treat analysis (See online Supplementary Materials for details).
Adverse effects
Throughout the 12 month study duration, no participants experienced any clinician-documented adverse effect and none experienced a serious adverse effect.
Comment
This trial of an LNS product conducted in Intibucá Honduras for children 6–18 months resulted in improved mean vitamin B12, folate and retinol concentrations after 6 months of the intervention. After one year of the intervention, a significant difference between the intervention and control groups continued for folate concentrations and the intervention group had a significantly lower prevalence of vitamin A deficiency not attributed to baseline values or CRP levels. Furthermore, the trend of improvement for B12 continued. Subanalysis based on adherence with the LNS product mirrored the results found in the intent-to-treat analysis, with improved significance.
This study shows that the LNS supplement was able to improve mean folate concentration for the children; mean plasma folate concentration ranged from 16 to19 ng/mL and normal folate concentration for children usually ranges between 2.7 to 17 ng/mL.35 Folate deficiency was not detected at any time point in either group. We speculate that the control group may have had increased consumption of folic acid fortified wheat (as a result of increases in corn prices) or high intake of folate rich foods such as beans due to the vouchers. In Honduras more than 80% of wheat flour is fortified with iron, folic acid and vitamins B1, B2 and niacin as established by a national mandate implemented in 2002.36
Our study population had a baseline prevalence of anemia (50.0% control and 39.6% intervention) of severe public health significance as defined by WHO.37 In contrast to studies conducted in Africa among moderately malnourished children, results from this study did not show improvements in iron status as measured by hemoglobin or transferrin receptors.5,10 This difference in the study populations, moderately malnourished versus non-malnourished children, may explain our lack of findings for iron status improvement.
Vitamin A (retinol) deficiency also met criteria for severe public health significance38 and was higher in the control group at baseline (45% control vs. 27% intervention). This intervention reduced vitamin A deficiency to moderate levels (≥10% to <20%) among children in the intervention group, values comparable to those reported in a Honduran national survey in 1996 .38 Serum retinol concentration was significantly improved in the intervention group at the 6 month time point and to a lesser degree at the 12 months after controlling for baseline values and CRP. The ability to detect larger differences between groups could have been masked by concomitant vitamin A supplementation by the health clinic prevention programs. Although the study guidelines instructed clinical staff not to provide additional vitamin A supplementation to children participating in the study, it was difficult to monitor adherence to these instructions. To the best of our knowledge, this is the first LNS trial to measure and report significant increases in retinol among intervention participants.
The risk of zinc deficiency in this population is high (37.5–62.3% at baseline).39 This trial aimed to supplement children with 9 to 14 mg of zinc daily, the upper level of suggested intake.20,40,41 High consumption of phytates in their dietary staples (i.e. beans, corn), and low adherence to LNS may explain the consistently high prevalence of zinc deficiency throughout the study. Studies using food fortification have also shown no reduction in the prevalence of low serum zinc concentrations42–44, which may be explained by poor absorption, or interference by the iron fortificant.45 A lack of effect on plasma zinc levels was also reported in other studies.10,12
The lower than expected levels of adherence to the LNS protocol partly may be explained by measurement error or sharing of the product within the household. Adherence was measured using one 24-hour recall collected each month which did capture true consumption over the entire month if mothers ran out of LNS the day before. However, the very low proportion of participants reporting protocol adherence suggests this is not the only explanation. Furthermore, qualitative process evaluations showed that sharing or improper use of the product (i.e. spoon sizes) was present only among a small subgroup of households. Nevertheless, approximately 73% of participants reported consuming an average dose of 50 g/d with positive effects on vitamin B12, folate and retinol. Adherence results indicated that a higher dose of 70 g/d was not achievable by the Honduran children 12–36 months living in a rural area.
This study reported a reduction in breast milk consumption among intervention participants, specifically after study month 6. These findings contrast those reported in Malawi and the Democratic Republic of Congo which reported no displacement of breast milk by complementary foods.46,47 However, both of these studies used short follow up periods with small sample sizes and comparison groups that provided an equivalent source of energy; our control participants did not receive any other equivalent source of energy dense foods, therefore providing a more realistic scenario for comparison. This reduction of breast milk consumption among intervention participants is expected for children increasing the amount of complementary foods in their diet.48
A limitation of the study design was the integration of food vouchers and complementary feeding education to both groups. This most likely contributed to the improvement in food security we saw over time (data not shown), and limited our ability to detect larger changes. A second limitation was the inability to truly match the villages on all socio-economic indicators. The sampling clusters differed in some socio-economic characteristics which may influence adherence to the trial and health outcomes. Finally, although we were unable to mask mothers to the intervention group and they were able to travel from an intervention to control village, data entry and laboratory personnel were masked to group allocation.
In summary, this study is the first LNS efficacy trial to be reported in Latin America. Caregivers were highly engaged in the process and had low attrition rates throughout the study period. Acceptability of the product was good and overall consumption of LNS by children that averaged close to 50 g per day is reasonable given the uniqueness of the product. Clearly, our expectation of 70 g for older children was too high. These results add to the current literature on the feasibility and efficacy of LNS strategies using integrated approaches to prevent malnutrition. Results from this study could inform future development of effectiveness LNS trials for hard-to-reach rural populations in Latin America.
Supplementary Material
Acknowledgements
This work was supported by the Mathile Institute for the Advancement of Human Nutrition and The Department of Nutrition Obesity Research Center (Grant #DK56350). The authors would like to acknowledge the work of the US Project Director, Hayley Holland, data analyst Howard Chen who contributed to the initial data analysis procedures, Dr. Ruben Martinez who facilitated implementation of the intervention within the community, the community members of this region and the women and children of the study.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.de Onis M, Blossner M, Borghi E. Prevalence and trends of stunting among pre-school children, 1990–2020. Public health nutrition. January 2012;15(1):142–148. [DOI] [PubMed] [Google Scholar]
- 2.Millenium development goals report 2010, United Nations. [computer program]. New York: United Nations; 2010. [Google Scholar]
- 3.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & child nutrition. April 2008;4 Suppl 1:24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. The Journal of nutrition. May 2009;139(5):1022–1030. [DOI] [PubMed] [Google Scholar]
- 5.Kuusipalo H, Maleta K, Briend A, Manary M, Ashorn P. Growth and change in blood haemoglobin concentration among underweight Malawian infants receiving fortified spreads for 12 weeks: a preliminary trial. Journal of pediatric gastroenterology and nutrition October 2006;43(4):525–532. [DOI] [PubMed] [Google Scholar]
- 6.Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden MH. Ready-to-use therapeutic food for treatment of marasmus. Lancet. May 22 1999;353(9166):1767–1768. [DOI] [PubMed] [Google Scholar]
- 7.Briend A Highly nutrient-dense spreads: a new approach to delivering multiple micronutrients to high-risk groups. The British journal of nutrition. May 2001;85 Suppl 2:S175–179. [PubMed] [Google Scholar]
- 8.Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Archives of disease in childhood. June 2004;89(6):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandige H, Ndekha MJ, Briend A, Ashorn P, Manary MJ. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. Journal of pediatric gastroenterology and nutrition. August 2004;39(2):141–146. [DOI] [PubMed] [Google Scholar]
- 10.Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. The American journal of clinical nutrition. April 2008;87(4):929–938. [DOI] [PubMed] [Google Scholar]
- 11.Flax VL, Thakwalakwa C, Phuka J, et al. Malawian mothers’ attitudes towards the use of two supplementary foods for moderately malnourished children. Appetite. October 2009;53(2):195–202. [DOI] [PubMed] [Google Scholar]
- 12.Lin CA, Manary MJ, Maleta K, Briend A, Ashorn P. An energy-dense complementary food is associated with a modest increase in weight gain when compared with a fortified porridge in Malawian children aged 6–18 months. The Journal of nutrition. March 2008;138(3):593–598. [DOI] [PubMed] [Google Scholar]
- 13.Phuka JC, Maleta K, Thakwalakwa C, et al. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Archives of pediatrics & adolescent medicine. July 2008;162(7):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isanaka S, Nombela N, Djibo A, et al. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA : the journal of the American Medical Association. January 21 2009;301(3):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defourny I MA, Harczi G, Doyon S, Shepherd S, Tectonidis M. et al. A large-scale distribution of milk based fortified spreads: Evidence for a new approach in regions with high burden of acute malnutrition. . PLoS ONE. 2009;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay G, Johnston C, Whitelaw A, Trygg K, Refsum H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. The American journal of clinical nutrition. July 2008;88(1):105–114. [DOI] [PubMed] [Google Scholar]
- 17.Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control; April 3 1998;47(Rr-3):1–29. [PubMed] [Google Scholar]
- 18.Herbert V Making sense of laboratory tests of folate status: folate requirements to sustain normality. American journal of hematology. October 1987;26(2):199–207. [DOI] [PubMed] [Google Scholar]
- 19.Grant FK, Martorell R, Flores-Ayala R, et al. Comparison of indicators of iron deficiency in Kenyan children. The American journal of clinical nutrition. May 2012;95(5):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown KH, Rivera JA, Bhutta Z, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and nutrition bulletin. March 2004;25(1 Suppl 2):S99–203. [PubMed] [Google Scholar]
- 21.H F. Frequency distributions of serum vitamin A levels in cross-sectional surveys and in surveys before and after vitamin In: Underwood BA OJ, ed. A brief guide to current methods of assessing vitamin A status. A report of the international vitamin A consultative group (IVACG). Washington, DC: The Nutrition Foundation; 1993: 9–11. [Google Scholar]
- 22.Underwood BA. Hypovitaminosis A: international programmatic issues. The Journal of nutrition. August 1994;124(8 Suppl):1467s–1472s. [DOI] [PubMed] [Google Scholar]
- 23.Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clinical chemistry. November 2005;51(11):2162–2165. [DOI] [PubMed] [Google Scholar]
- 24.Schlebusch H LN, Kalina E, Klein C. High sensitive CRP and creatinine: Reference intervals from infancy to childhood. . LaboratoriumsMedizin. 2002;26((5–6)):341–346. [Google Scholar]
- 25.WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica (Oslo, Norway : 1992). Supplement. April 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 26.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Computer methods and programs in biomedicine. September 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez W, Jimenez A, Madrigal G, Munoz LM, Frongillo EA. Development and validation of measure of household food insecurity in urban Costa Rica confirms proposed generic questionnaire. The Journal of nutrition. March 2008;138(3):587–592. [DOI] [PubMed] [Google Scholar]
- 28.Dirren H, Logman MH, Barclay DV, Freire WB. Altitude correction for hemoglobin. European journal of clinical nutrition. September 1994;48(9):625–632. [PubMed] [Google Scholar]
- 29.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. American journal of epidemiology. December 15 1995;142(12):1255–1264. [DOI] [PubMed] [Google Scholar]
- 30.PD A Missing data: Quantitative applications in the social sciences. British Journal of Mathematical and Statistical Psychology. 2002;55(1):193–196. [Google Scholar]
- 31.Lee KJ, Galati JC, Simpson JA, Carlin JB. Comparison of methods for imputing ordinal data using multivariate normal imputation: a case study of non-linear effects in a large cohort study. Statistics in medicine. December 30 2012;31(30):4164–4174. [DOI] [PubMed] [Google Scholar]
- 32.J S. Analysis of incomplete multivariate data.: Chapman & Hall/CRC Imprint; 1997. [Google Scholar]
- 33.SAS/STAT(R) 9.2 user’s guide 2nd ed Cary, NC, USA: SAS Institute Inc; 2010. [Google Scholar]
- 34.Ben-Davies ME, Kinlaw A, Estrada Del Campo Y, Bentley ME, Siega-Riz AM. Risk factors associated with the presence and severity of food insecurity in rural Honduras. Public health nutrition. August 5 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evert AZD. Folic acid test. Updated 2011; http://www.nlm.nih.gov/medlineplus/ency/article/003686.htm. Accessed 04/29, 2013.
- 36.Initiative. TM. Latin America and Caribbean region food industry assessment.: Micronutrient Initiative;2007. [Google Scholar]
- 37.(WHO) WHO. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. . Geneva, Switzerland: : WHO;2008. [Google Scholar]
- 38.(WHO) WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. . Geneva, Switzerland: WHO;2009. [Google Scholar]
- 39.de Benoist B, Darnton-Hill I, Davidsson L, Fontaine O, Hotz C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food and nutrition bulletin. September 2007;28(3 Suppl):S480–484. [DOI] [PubMed] [Google Scholar]
- 40.(IOM) IoM. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: IOM;2001. [PubMed] [Google Scholar]
- 41.(WHO) WHO. Vitamin and mineral requirements in human nutrition. . Geneva, Switzerland: WHO;2004. [Google Scholar]
- 42.Gibson RS, Kafwembe E, Mwanza S, et al. A micronutrient-fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc. The Journal of nutrition. May 2011;141(5):935–943. [DOI] [PubMed] [Google Scholar]
- 43.Brown KH, Lopez de Romana D, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. The American journal of clinical nutrition. February 2007;85(2):538–547. [DOI] [PubMed] [Google Scholar]
- 44.Hess SY, Brown KH. Impact of zinc fortification on zinc nutrition. Food and nutrition bulletin. March 2009;30(1 Suppl):S79–107. [DOI] [PubMed] [Google Scholar]
- 45.Dewey KZY, Boy E. Systematic review and meta-analysis of home fortification of complementary foods. . Maternal and Child Nutrition. 2009;5(4):283–321. [Google Scholar]
- 46.Galpin L, Thakwalakwa C, Phuka J, et al. Breast milk intake is not reduced more by the introduction of energy dense complementary food than by typical infant porridge. The Journal of nutrition. July 2007;137(7):1828–1833. [DOI] [PubMed] [Google Scholar]
- 47.Owino VO, Bahwere P, Bisimwa G, Mwangi CM, Collins S. Breast-milk intake of 9–10-mo-old rural infants given a ready-to-use complementary food in South Kivu, Democratic Republic of Congo. The American journal of clinical nutrition. June 2011;93(6):1300–1304. [DOI] [PubMed] [Google Scholar]
- 48.Islam MM, Khatun M, Peerson JM, et al. Effects of energy density and feeding frequency of complementary foods on total daily energy intakes and consumption of breast milk by healthy breastfed Bangladeshi children. The American journal of clinical nutrition. July 2008;88(1):84–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


