Supplemental Digital Content is available in the text.
Background.
Endothelium-enriched microRNAs (miRs) are involved in the development of cardiac allograft vasculopathy (CAV). Recently, serum-derived miR-126-3p and -5p, known endothelial microRNAs with a crucial function in angiogenesis and re-endothelialization, provided additional predictive power for cardiac allograft vasculopathy in addition to clinical predictors. However, their myocardial expression in and relationship with CAV are still unknown. Our study aim was to investigate the expression of endomyocardial microRNA-126-3p and microRNA-126-5p levels in heart transplant recipients and their relationship with allograft vasculopathy.
Methods.
We studied 39 heart transplant recipients, 21 with proven allograft vasculopathy (CAV+) and 18 without allograft vasculopathy (CAV−) with serial coronary angiograms. Additionally, 8 patients with end-stage native coronary artery disease (CAD) were added to the study to investigate disease specificity of the microRNA signature. The mRNA levels of miR-126-3p and miR-126-5p were determined by qRT-PCR in the right ventricular endomyocardial biopsies obtained at baseline and during routine follow-up.
Results.
MiR-126-3p levels were significantly lower in the CAV+ group compared to the CAV− group at follow-up, while miR-126-5p levels were unaltered. This was in stark contrast to native CAD patients in whom miR-126-3p and -5p levels were significantly higher. qPCR levels of miR-126 targets are differentially regulated in CAV versus ischemic cardiomyopathy and are influenced by the administration of immunosuppressive agents in endothelial cells.
Conclusions.
Our data provide evidence for a distinct microRNA signature in heart transplantation patients with allograft vasculopathy. In contrast to CAD patients, lower miR-126-3p levels coincide with the development of cardiac allograft vasculopathy. Further studies in a larger patient population are warranted to determine if the serial measurement of myocardial microRNA-126 products could help in risk assessment and early detection of CAV.
Cardiac allograft vasculopathy (CAV) remains Achilles’ heel of long-term survival after heart transplantation (HTx).1 One in 3 patients develop CAV in the first 5 years posttransplantation, and 1 in 8 deaths beyond the first year are due to CAV.2 In contrast to the focal, eccentric, and proximal epicardial lesions in atherosclerosis, CAV affects both epicardial and intramural vessels. These events occur as a result of coronary endothelial inflammation, injury, and dysfunction1 and are triggered and maintained by immune as well as nonimmune insults ensuing in progressive narrowing of the lumen. The disease that gently starts within the first year following HTx has a biphasic response, initially involving intimal thickening with expansion of the external elastic membrane and relative preservation of luminal area, followed by constrictive remodeling and luminal narrowing.3,4 Over time, plaque composition changes from early fibrous and fibrofatty tissue to a late atheromatous necrotic core with excessive calcifications.
The surveillance methods for detecting CAV, used in daily clinical practice, have significant limitations and are suboptimal for diagnosing early disease, which is nevertheless quintessential to treat or prevent further deterioration. Nowadays, the severity and extent of CAV is still graded with standard coronary angiography according to the guidelines of the ISHLT.1 It has to be emphasized, however, that more recent imaging techniques, especially intravascular ultrasound (IVUS), are more sensitive to detect early disease,4 but these techniques are not readily available in clinical practice, yet merely for research purposes. Although the search for biomarkers to predict CAV is ongoing and evolving fast, it remains difficult to prove an added value of these biomarkers on top of established clinical risk factors. The triglycerides-to-high-density lipoprotein cholesterol ratio, plasma insulin level, C-reactive protein, vascular cell adhesion molecule 1, circulating C-X-C motif chemokine 12 levels, donor-specific antihuman leucocyte antibodies, antibodies against heterogeneous nuclear ribonucleoprotein K, and angiogenesis-related proteins, for example, vascular endothelial growth factor (VEGF)-A, VEGF-C, and platelet factor-4, all have been associated with allograft vasculopathy.5
MicroRNAs (miRs) regulate gene expression in a wide range of biological processes, including cardiac biology and are able to modulate distinct immunological pathways. Interestingly, recent human data have demonstrated that plasma or serum levels of endothelial cell-enriched microRNAs, among which miR-126, have a diagnostic potential for the detection of CAV. Using a univariate model, it was shown that they have diagnostic ability for detecting CAV beyond clinical predictors or other endothelial biomarkers.5 In line with these observations, experimental data have shown that microRNA-126, the most abundant microRNA in endothelial cells, promotes the replicative regeneration of atherosclerotic lesion formation by regulating endothelial cell turnover.6 The premicroRNA-126 is split into 2 functional strands, a guide strand (miR-126-3p) and a passenger strand (miR-126-5p).6 Some of their targets (VCAM-1, PIK3R2, SPRED1 for miR-126-3p, and Dlk1 for miR-126-5p) play a role in atherosclerosis formation by regulating inflammation, endothelial cell repair, and proliferation.7 Recently, also serum miR-628-5p was recently reported to be a CAV-specific biomarker.8
What is, however, unclear until now, are the individual contributors to the miR-126 signal that is observed in the serum of heart transplant patients. Because right ventricular endomyocardial biopsies (EMB) are still taken routinely during follow-up of heart transplant patients albeit with a significant amount of sampling bias to detect allograft vasculopathy, we investigated the expression of endomyocardial microRNA-126-3p and -5p in EMBs of HTx recipients with (CAV+) and without CAV (CAV−), to see if the heart—where CAV eventually causes problems—is the source of the miR-126 signal, and we evaluated whether these cardiac endothelial microRNAs might play a role in the development of CAV and help to predict cardiac allograft vasculopathy.
MATERIALS AND METHODS
Study Population
Thirty-nine HTx patients who were referred for routine annual elective diagnostic left–right heart catheterization were included in the study (Table 1). In all HTx recipients, serial right ventricular EMBs (baseline versus follow-up) were procured at the end of a routine right heart catheterization. The baseline biopsy was always taken in the first or second week after transplantation. Patients with histologically proven graft rejection according to the ISHLT criteria were excluded. The severity of cardiac graft vasculopathy was graded according to the ISHLT working formulation of a standardized nomenclature for CAV.1 Patients with CAV1, CAV2, and CAV3 were pooled and constituted the CAV-positive group (CAV+). The CAV-negative group comprised patients with CAV0, defined as no detectable angiographic lesions. To investigate whether the results were disease-specific and to distinguish CAV from ischemic cardiomyopathy, right ventricular EMBs of 8 patients with end-stage ischemic cardiomyopathy due to native coronary artery disease (CAD), obtained at time of explantation of the heart before heart transplantation, were included in the study. All cardiac biopsies were immediately snap-frozen in liquid nitrogen for RNA analysis.
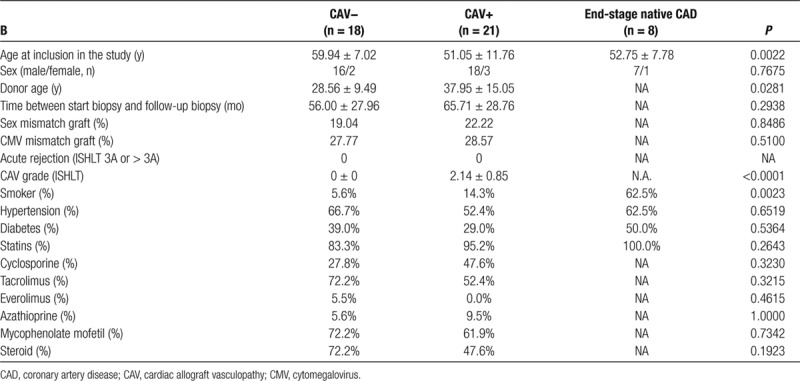
TABLE 1.
Clinical and biochemical characteristics, and immunosuppressive therapy of patients without CAV (CAV−), patients with CAV (CAV+), and of patients with end-stage native CAD
Ethics
Patients provided written informed consent for the procurement of serial biopsies. The protocol was reviewed and approved by the local ethical committee of OLV Hospital Aalst, Belgium. The research was performed with respect for all applicable laws and guidelines, for example, the Declaration of Helsinki and the Global Directive for Privacy Regulations.
MicroRNA Isolation, RNA Isolation, and Quantitative RT-PCR
Total RNA was isolated from right ventricular EMBs using the miRVANA isolation kit (Ambion, Warrington, United Kingdom) according to the manufacturer’s instructions and without enrichment for small RNAs. Homogenization of the tissue took place just before RNA extraction. Potential genomic DNA contamination was removed using the DNA-free kit (Ambion). To identify miRNA, cDNA was generated using the TaqMan MicroRNA Reverse Transcription Kit followed by quantitative real-time PCR using TaqMan MicroRNA-Assays. For real-time PCR analysis of miRNA target genes, RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit and quantified using TaqMan Gene Expression Assays in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). A complete and detailed list of used kits and materials is provided in Tables S1 and S2, SDC, http://links.lww.com/TXD/A250, respectively. All microRNAs were normalized to U6 snRNA. Normal mRNAs were normalized to GAPDH as a standard housekeeping gene.
In Vitro Experiments
Human cardiac microvascular endothelial cells were used to perform the experiments with immunosuppressive agents. The cells were cultured on 12-well plates until 90% confluency. The following immunosuppressive agents were added onto the cells separately: methylprednisolone ad 1 µg/mL, tacrolimus (FK506) ad 0.05 ng/mL, ciclosporin ad 1 ng/mL, and mycophenolate mofetil ad 1 µg/mL, dissolved in DMSO 20× and subsequently in cell medium. After 24 hours, the medium was removed, the cells were washed 3 times with PBS medium and cells were retrieved in (micro)RNA lysis buffer as to be able to proceed to qPCR according to the above-mentioned protocol.
Statistics
Data represent mean ± SEM unless otherwise stated. Statistical significance was calculated by ANOVA with Bonferroni post-hoc testing. For comparisons between 2 groups, a standard Student’s t-test was used for normally distributed data, and a Mann–Whitney test was used for not normally distributed data. Normality testing was performed with the Kolmogorov–Smirnov and Shapiro–Wilk normality tests. For analysis of categorical variables, Fisher’s exact test or a Chi-square test (χ) was used. All data were analyzed statistically with the GraphPad Prism v5.0d interface. A 2-sided P value of <0.05 was considered as statistically significant.
RESULTS
Baseline Characteristics in CAV−, CAV+, and CAD Patients
Baseline patient characteristics are summarized in Table 1. Of the 39 heart transplant recipients, 21 developed CAV within 5 years, whereas 18 did not. The age of the recipients at the time of transplantation was younger in the CAV+ group; the age of the allograft donors was older in the CAV+ group as compared with the CAV− group. No significant difference in the time lapse between start and follow-up biopsies was noted between both groups. Gender distribution and cytomegalovirus mismatch was similar between the CAV− and CAV+ groups. Finally, grades 1, 2 and 3 cardiac allograft vasculopathy were distributed fairly equally (6, 6, and 9 patient samples, respectively).
Endomyocardial microRNA-126-3p and -5p Levels in CAV+, CAV−, and CAD Patients
Although no differences in microRNA-126-3p and -5p were observed in CAV+ and CAV− patients immediately post-HTx, miRNA-126-3p levels at follow-up were significantly lower in CAV+ patients as compared with CAV− patients and CAD (Figure 1A). MiR-126-3p levels tended to increase in CAV− patients over time. Surprisingly, miR-126-3p levels were significantly higher in CAD patients compared to both CAV− and CAV+ patients. MiR-126-5p levels did not significantly change over time in both CAV− and CAV+ patients, and were similar in CAV+ and CAD patients (Figure 1B). Finally, there were no significant differences in target mRNA levels between CAV− and CAV+ patients; however, there were important differences between CAV+ and CAD patients, pointing toward the completely different pathophysiologic circumstances (Figure 1C–F). As shown in Figure 2A, the receiver operator characteristics curve of microRNA-126-3p for detecting CAV shows an area under the curve of 0.7857 (P = 0.002) for CAV− versus CAV+ patients. The value of microRNA-126-3p with the optimal predictive accuracy was <1.188. When this cutoff value was used, all but 9 patients were correctly categorized, with sensitivity, specificity, and accuracy of 76%, 73%, and 73%, respectively (Figure 2B). Furthermore, at least in biopsy material, mir-126-3p had better diagnostic accuracy than miR-628-5p (Figure 2C and D) and miR-92a-3p (Figure 2E and F).
FIGURE 1.
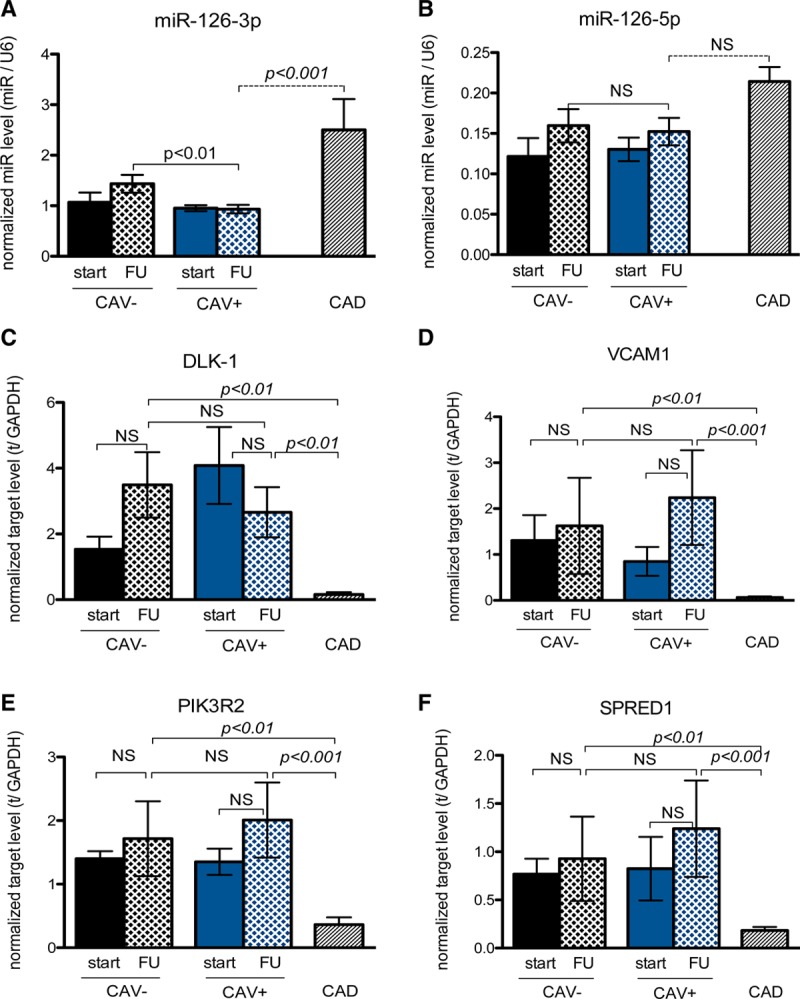
MicroRNA-126-3p is significantly decreased in CAV-positive patients and discriminates CAV from ischemic cardiomyopathy. A, MicroRNA-126-3p levels are significantly lower in CAV+ patients than in CAV− patients (P <0.01), but microRNA-126a-3p levels in the end-stage native CAD patients are significantly higher than in CAV+ patients (P < 0.001). B, There is no significant difference in microRNA-126-5p levels in all groups; however, there is a trend toward higher miR-126-5p levels in native CAD patients. C–F, The miR-126 targets (respectively, DLK-1, VCAM1, PIK3R2, and SPRED1) are differentially regulated on a mRNA level in CAV+ patients compared to native CAD patients. CAD, coronary artery disease; CAV, cardiac allograft vasculopathy; miR, microRNA.
FIGURE 2.
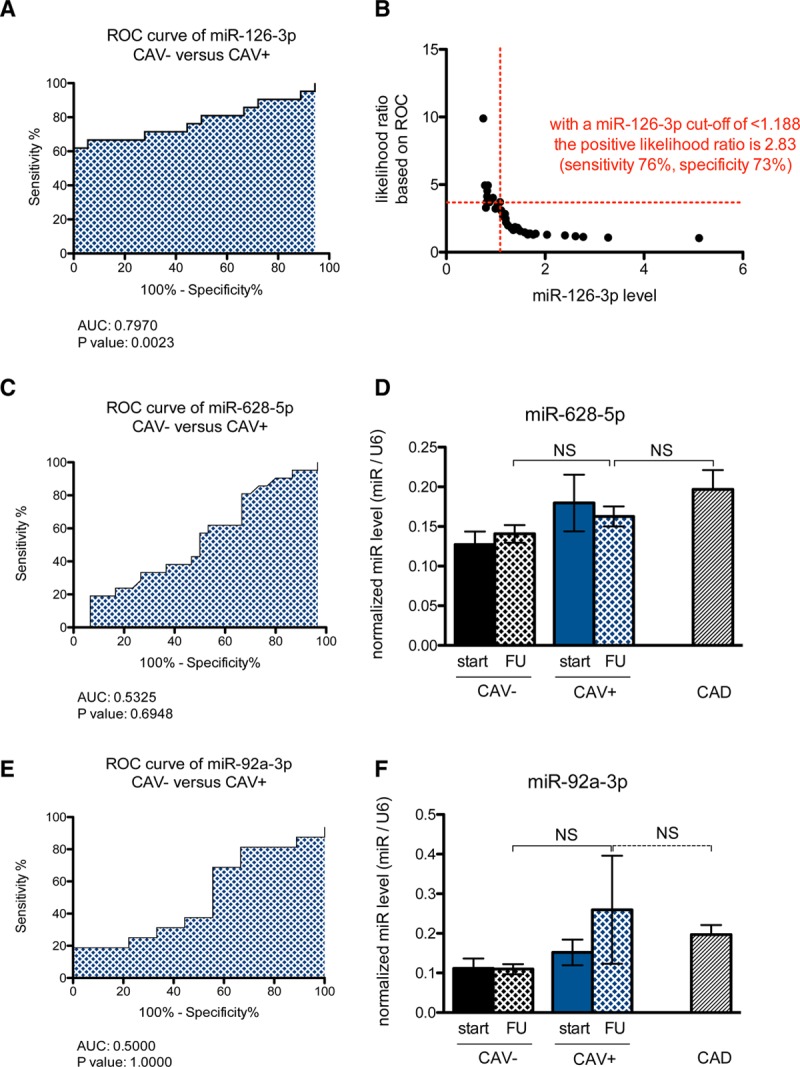
Receiver operator curve and cutoff value for miR-126 as a diagnostic tool for the detection of CAV. A, The receiver-operator characteristic (ROC) curve for microRNA-126-3p between CAV− and CAV+ patients shows an area under the curve (AUC) of 0.7970 with a P value of 0.0023. B, Based on the ROC curve, with a miR-126-3p level below 1.188, the positive likelihood ratio for CAV in HTx is 2.83 with a sensitivity of 76% and a specificity of 73%. C, The ROC curve for miR-628-5p shows a low AUC (0.5325) with a nonsignificant P value. D, There were no significant differences in miR-628-5p levels in CAV−, CAV+, or CAD patients. E, The ROC curve for miR-92a-3p shows a low AUC (0.5000) with a nonsignificant P value. F, There were no significant differences in miR-92a-3p levels in CAV−, CAV+, or CAD patients. CAD, coronary artery disease; CAV, cardiac allograft vasculopathy; HTx, heart transplantation; miR, microRNA.
Effect of Immunosuppressive Agents on miR-126 and Its Targets
When human microvascular cardiac endothelial cells were treated with immunosuppressive agents for 24 hours, PIK3R2 levels were significantly lower in the treated cells versus control (P < 0.05). Regarding SPRED1, levels only decreased in the methylprednisolone-treated cells. Furthermore, the short treatment with immunosuppressive agents led to a significant increase in miR-126-3p (not -5p) only in the mycophenolate-treated cells (Figure 3). The others targets (VCAM1 and Dlk1) were undetectable in this cell type, and therefore, these data are not shown.
FIGURE 3.
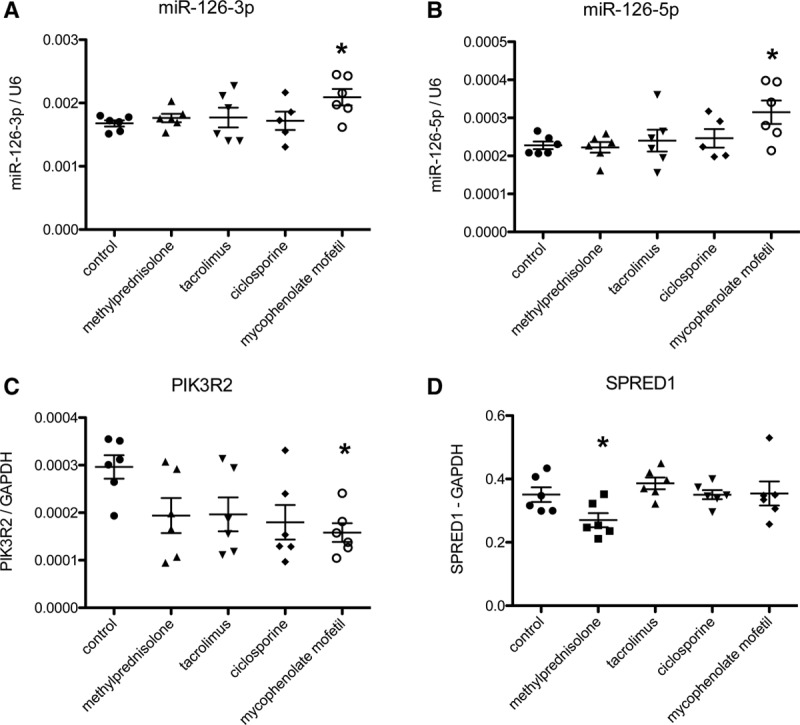
MiR-126 and target levels in HMVCECs treated with immunosuppressive agents. A, MiR-126-3p levels are not influenced by the administration of immunosuppressive agents (P = 0.112 for trend, n = 5–6 per group) although there is a trend toward higher levels of miR-126-3p in mycophenolate-treated cells. B, miR-126-5p are altered by the administration of immunosuppressants (P = 0.054 for trend, n = 5–6 per group) again with higher levels in mycophenolate-treated cells. C, PIK3R2 levels are lower in all groups treated with immunosuppressive agents, especially in the mycophenolate group (P = 0.044 for trend, n = 5–6 per group); D, SPRED1 levels are especially lower in methylprednisolone-treated cells and not in the other groups (P = 0.053 for trend, n = 5–6 per group). *P < 0.05 vs control, ANOVA with post-hoc correction. HMVCECs, human microvascular cardiac endothelial cells; miR, microRNA.
DISCUSSION
Our pilot study is the first to demonstrate that EMB-derived microRNA-126-3p levels have diagnostic potential for the detection of allograft vasculopathy in heart transplant recipients. CAV+ patients are characterized by significantly lower miRNA-126-3p levels as compared with CAV− patients. Several miRNAs play a role in atherosclerosis by interfering with the regulation of function, proliferation, and growth of vascular endothelial cells. One of the most extensively characterized endothelial miRNAs is miR-126, highly preserved in lung and heart vasculature. The precursor miR-126 generates 2 mature miR-strands: microRNA-126-3p (guide strand) and microRNA-126-5p (passenger strand) which both are functional in endothelial cell biology and play a role in atherosclerosis.6,9 Although the mechanisms and targets of each microRNA strand are different, overall both mature strands have protective vascular function. MiR-126-5p promotes endothelial cell proliferation and thereby improves re-endothelialization following vascular injury by targeting Dlk1. MiR-126-3p does not affect re-endothelialization, but promotes angiogenic growth factor signaling during endothelial repair through targeting Spred1, VCAM-1, and PIK3R2.
In our study, we demonstrate that in contrast to patients with end-stage typical coronary atherosclerosis, CAV+ patients are characterized by significantly lower endomyocardial miR-126-3p levels. The exact mechanism underlying this observation is unclear and remains the subject of further investigation, but it at least points to the fact that CAV differs significantly from ischemic heart disease and most likely needs a completely different therapeutic approach. The relatively higher endomyocardial miR-126 levels observed in coronary atherosclerosis patients are in accordance with previous observations demonstrating downregulation of miR-126 only in lesions of carotid artery, not in those from coronary atherosclerosis.10,11
In our study, miR-126-3p levels gradually decrease according to the extent of CAV, although the differences were not statistically significant due to limited patient numbers. This observation possibly indicates an altered balance between angiogenic growth versus endothelial repair capacity. This hypothesis is corroborated by recent findings of reduced circulating levels of miR-126-3p12-14 in patients with an increased risk of cardiovascular disease. Likewise, in a MiR-126−/− mice model, delivery of exogenous miR-126-3p by endothelial microparticles promoted endothelial repair specifically at predilection sites with inadequate replicative capacity of endothelial cells under conditions of hyperlipidemic stress.15,16 The in vitro experiments show that—even after only 24 hour treatment—immunosuppressive agents can influence the levels of miR-126 and its targets in endothelial cells, probably meaning that miR-126 might have a pathophysiological role in CAV, more than being just a bystander.
In our study, we demonstrate that in contrast to patients with coronary atherosclerosis, CAV+ patients were characterized by significant lower endomyocardial miR-126-3p. Although the exact mechanism remains unclear, the more diffuse involvement of both macrocirculation and microcirculation due to the graft vasculopathy may account for this observation. Of note, EMBs of HTx recipients show endoluminal narrowing and stenotic thickening not only from the major but also from the microvessels, whereas in CAD, evidence points toward more focal stenosis in the main epicardial vessels. Because miR-126-3p plays an antiatherogenic role, its significantly lower expression in HTx may result in an impaired endothelial repair following injury thereby making these patients prone to the development of graft vasculopathy. Finally, Singh et al5 also pointed toward a clear distinction in endothelial biology between CAV and CAD, as evidenced by serum miRNA-92 levels between patients with CAV versus patients with stable CAD. Using multivariate analysis age, creatinine, serum miR-126-5p, and serum miR92a-3p as covariables conferred good discrimination between patients with and without CAV. Taken together, all these observations demonstrate a distinct miRNA signature of CAV versus CAD.
Our study has 2 important limitations. First, due to the lack of protein extracts from the investigated biopsies, we were unable to perform Western blots to study the influence of altered miR-126 levels on its targets at a protein level. Second, the fact that we do not have the serum of a large proportion of these patients (at the time of biopsy procurement), we were unable to study miR-126-5p and -3p levels in this same patient cohort. Third, the size of the patient cohort (n = 39) is rather small, which warrants further investigation in a larger patient group to corroborate our findings.
CONCLUSIONS AND CLINICAL IMPLICATIONS
Because the management of CAV is focused on primary prevention, surveillance imaging and early treatment,1 there is an urgent need for more sensitive and specific biomarkers to detect CAV at an early stage. Our observations of the differential expression of miR-126 in EMBs supports its potential role as a tissue specific biomarker of graft vasculopathy, although we still do not know if a lower miR-126-3p also precedes clinically relevant CAV. The advantage of using miR-126-3p, or another biomarker, in EMBs on top of histological analysis, might be that sampling bias probably is of less importance in microRNA measurements compared to histological analysis. Above all, further understanding of miR-126 regulation and its downstream role in the vascular stress responses can be a new therapeutic target favorably modifying coronary atherosclerosis. Further prospective studies, not only about the use of miR-126 as a biomarker but also as a potential therapeutic in CAV, are therefore warranted.
ACKNOWLEDGMENTS
We wish to thank the nurses from the OLV Aalst Heart Failure, Transplantation and Perfusion Unit for their help in exploring the heart transplant database. W.H. wants to gratefully mention the Frans Van de Werf Fund for Cardiovascular Research, Leuven Research and Development, KU Leuven, Leuven, Belgium and the VZW Cardiovascular Research Aalst, Moorselbaan 164, B-9300 Aalst, Belgium as well as the Young Talent Programme of the CardioVascular Onderzoek Nederland (CVON), The Netherlands, for financial support.
Supplementary Material
Footnotes
Published online 15 April, 2020.
The authors declare no funding or conflicts of interest.
W.A.H. made substantial contributions to the conception and design of the work, and the acquisition and interpretation of the data. L.D. and K.D. made important contributions to the acquisition of the data. R.D., S.V., M.G., J.B., and M.V. made important contributions to the interpretation of the data for the paper. All authors contributed to drafting the work, and critical revision for intellectual content, and provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Chih S, Chong AY, Mielniczuk LM, et al. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol. 2016; 68:80–91 [DOI] [PubMed] [Google Scholar]
- 2.Tona F, Marra MP, Fedrigo M, et al. Recent developments on coronary microvasculopathy after heart transplantation: a new target in the therapy of cardiac allograft vasculopathy. Curr Vasc Pharmacol. 2012; 10:206–215 [DOI] [PubMed] [Google Scholar]
- 3.Guddeti RR, Matsuo Y, Matsuzawa Y, et al. Clinical implications of intracoronary imaging in cardiac allograft vasculopathy. Circ Cardiovasc Imaging. 2015; 8:e002636. [DOI] [PubMed] [Google Scholar]
- 4.Okada K, Fearon WF, Luikart H, et al. Attenuated-signal plaque progression predicts long-term mortality after heart transplantation: IVUS assessment of cardiac allograft vasculopathy. J Am Coll Cardisol. 2016; 68:382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Heggermont W, Fieuws S, et al. Endothelium-enriched microRNAs as diagnostic biomarkers for cardiac allograft vasculopathy. J Heart Lung Transplant. 2015; 34:1376–1384 [DOI] [PubMed] [Google Scholar]
- 6.Schober A, Nazari-Jahantigh M, Wei Y, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014; 20:368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013; 304:H1050–H1059 [DOI] [PubMed] [Google Scholar]
- 8.Neumann A, Napp LC, Kleeberger JA, et al. MicroRNA 628-5p as a novel biomarker for cardiac allograft vasculopathy. Transplantation. 2017; 101:e26–e33 [DOI] [PubMed] [Google Scholar]
- 9.Boon RA, Dimmeler S. MicroRNA-126 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2014; 34:e15–e16 [DOI] [PubMed] [Google Scholar]
- 10.Raitoharju E, Oksala N, Lehtimäki T. MicroRNAs in the atherosclerotic plaque. Clin Chem. 2013; 59:1708–1721 [DOI] [PubMed] [Google Scholar]
- 11.Raitoharju E, Lyytikäinen LP, Levula M, et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011; 219:211–217 [DOI] [PubMed] [Google Scholar]
- 12.Leistner DM, Boeckel JN, Reis SM, et al. Transcoronary gradients of vascular miRNAs and coronary atherosclerotic plaque characteristics. Eur Heart J. 2016; 37:1738–1749 [DOI] [PubMed] [Google Scholar]
- 13.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011; 31:2383–2390 [DOI] [PubMed] [Google Scholar]
- 14.De Rosa S, Fichtlscherer S, Lehmann R, et al. Transcoronary concentration gradients of circulating microRNAs. Circulation. 2011; 124:1936–1944 [DOI] [PubMed] [Google Scholar]
- 15.Cao WJ, Rosenblat JD, Roth NC, et al. Therapeutic angiogenesis by ultrasound-mediated microRNA-126-3p delivery. Arterioscler Thromb Vasc Biol. 2015; 35:2401–2411 [DOI] [PubMed] [Google Scholar]
- 16.Wei Y, Nazari-Jahantigh M, Neth P, et al. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013; 33:449–454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


