Since December 2019, the outbreak of infection caused by 2019 novel coronavirus (2019-nCoV) has become a major public health emergency worldwide. Approximately 40% of the confirmed cases of 2019-nCoV infection (judged by positivity of viral RNA testing) were initially asymptomatic but later developed overt clinical symptoms of coronavirus disease-2019 (COVID-19).[1] A presumed asymptomatic carrier of 2019-nCoV was also reported.[2] But her infection of 2019-nCoV was not fully confirmed. As far as we know, confirmed 2019-nCoV carriers but without overt clinical symptoms have been not yet reported.
As part of the Epidemiological Study of 2019-nCoV Infection in Anhui, we collected data of all confirmed cases of 2019-nCoV infection in the Anqing city onto case report forms adapted from International Severe Acute Respiratory and Emerging Infection Consortium/World Health Organization Clinical Characterization Protocol for severe emerging infections. Briefly, information on symptoms and disease onset, epidemiology investigation, visit(s) to healthcare facilities, hospitalization, treatment, pathogen and laboratory tests, and clinical outcomes were collected.
Trained investigators collected information from the medical record system in Anqing Hospital Affiliated to Anhui Medical University (Anqing Municipal Hospital), where all suspected and confirmed cases were hospitalized. Then data were uploaded to the REDCap® data capture system by one investigator. Another investigator verified these records. Then a third investigator validated the data by crosschecking with the data in the medical record system and communication with the physicians attending the individuals. Two independent licensed radiologists reviewed the original images of the chest computed tomography (CT). We adopted the results which had consistent interpretation.
We confirmed 2019-nCoV infection according to guidelines released by the National Health Commission of the People's Republic of China: positive results in throat swabs or respiratory specimens of real-time reverse transcription polymerase chain reaction (RT-PCR) assay repeated twice using two types of 2019-nCoV nucleic acid detection kits.
In Anhui province, China, to improve the quality of the detection, a two-step confirmation strategy was adopted. Samples of an individual were first tested in the laboratory of municipal Centers for Disease Control and Prevention (CDC) with two different detection kits. The municipal CDC laboratory crosschecked the results of the two kits to report a positive case. Then these positive samples were sent to the laboratory Anhui Provincial CDC using the same procedure to test for the virus. If the positivity could be repeated in the provincial CDC laboratory, this case was finally confirmed as positive. All suspected and confirmed cases in the Anhui Province, including our investigated individuals, were required to go through such a two-step confirmation. Good laboratory practice has been also made to avoid false positivity in RT-PCR assays.
By February 21, 2020, among the 83 cases of confirmed 2019-nCoV infection in Anqing, we found eight cases asymptomatic at confirmation. Among them, seven cases developed symptoms afterward, during hospitalization. One confirmed 2019-nCoV carrier patient A, who was from a familial cluster of 2019-nCoV infection did not develop COVID-19 symptoms even after hospitalization for 17 days. This study was approved by the Institutional Board of the First Affiliated Hospital of University of Science and Technology of China (No. 2020-XG(H)-009). The investigated individuals all agreed to participate in the study and provided written informed consent.
Patient A was a 50-year-old woman, who lived with her husband (patient B) in the city of Anqing. She and her husband did not travel to Wuhan (Hubei, China) or adjacent areas, and reported no exposure to wild animals recently. Patient B visited his brother (patient D) on January 23, 24, and 26, 2020. Patient D lived with his wife, patient B's sister-in-law (patient C). Patients C and D did not travel to Wuhan or adjacent areas either and reported no exposure to wild animals recently. But patient D worked on January 21 with patient E who travelled from Wuhan to Anqing before meeting patient D and was later confirmed to have 2019-nCoV infection.
Patient D developed a fever on January 26, and patient C on January 30. They were first admitted to the Second People's Hospital of Anqing and had their throat swabs tested. On February 5, their samples were confirmed 2019-nCoV positive. Soon after on the same day, the couple of patients A and B received a throat swab test and chest CT scan. Their throat swabs were both confirmed positive of 2019-nCoV on February 6. By this day, both patients A and B did not present with any symptoms, including fever, coughing, apnea, or diarrhea. However, the chest CT scan of patient B revealed lesions of ground-glass opacity in the lower right lung suggesting viral infection. Meanwhile, no significant abnormalities were found in patient A's chest CT scan. Patients A and B were then admitted to Anqing Municipal Hospital to be treated and observed on February 6. Epidemiology information is depicted in Figure 1.
Figure 1.
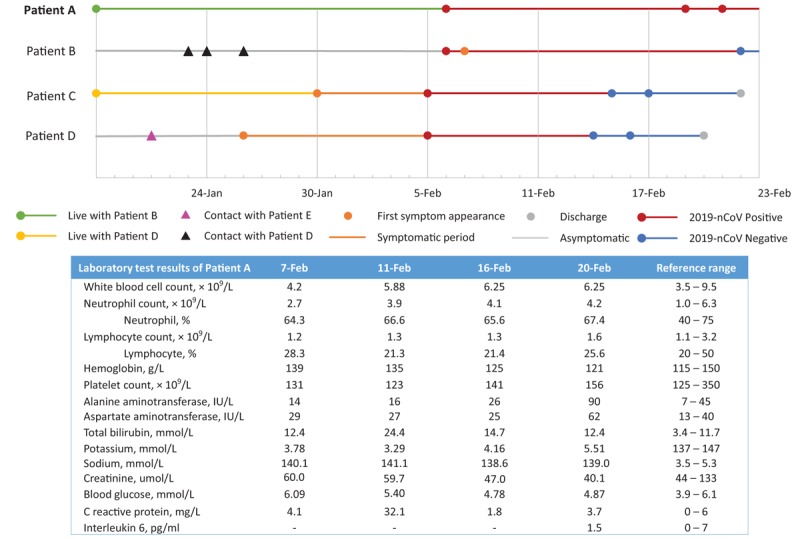
Epidemiology information and inhospital laboratory test results of Patient A, the asymptomatic carrier of 2019 novel coronavirus (2019 nCov).
Patient A was otherwise healthy, with no significant medical history including hypertension, diabetes, chronic liver disease, or chronic kidney disease. On admission, her vital signs were normal, with body temperature at 36.6°C, the pulse rate at 88 beats/min, respiratory rate at 20 breaths/min, blood pressure of 136/90 mmHg, and subcutaneous oxygen saturation (SpO2) of 99%. During hospitalization, patient A did not report elevated temperature measures, fatigue, pain, or any gastrointestinal and respiratory symptoms, including coughing, sore throat, diarrhea, or vomiting. Nor were such symptoms observed by nurses or physicians. Her body temperature stayed below 36.8°C, and SpO2 remained above 97% during hospitalization.
Following the National Recommendations for Diagnosis and Treatment of Pneumonia Caused by the 2019-nCoV (the 5th edition), patient A was treated with aerosolized interferon (IFN) α2β, and two lopinavir/ritonavir tablets (200 mg/50 mg) twice a day for 10 days between February 6 and 16. Then lopinavir/ritonavir was discontinued, and intravenous ribavirin 0.5 g every 12 h were administered. By February 16, her laboratory test results were largely normal [Figure 1]. On February 20, a mild liver enzyme elevation was observed. Still, she did not report any change in her feelings, and no changes in signs or symptoms were observed by nurses and physicians. Hepaprotective agent glycyrrhizinate was added to her treatment. Chest CT scans were repeated on February 11 and 20. The results were both negative for signs of viral pneumonia.
Notably, the second set of throat swabs and anal swabs of hers were sent to test for 2019-nCoV on February 19, and the third set of samples were tested on February 21. Viral RT-PCR test results remained positive in the throat and anal swabs. Patient A was still hospitalized and treated by February 23 when we first submitted this article.
As for patient B, he had an intermittent low fever during February 7 to 13, with the highest body temperature of 37.5°C, but otherwise asymptomatic. He also received aerosolized IFN α2β, lopinavir/ritonavir tablets, and low-dose intravenous methylprednisolone following the National Recommendations. His throat and anal swabs were collected on February 22 and the 2019-nCoV RT-PCR test results had turned negative.
In this study, we identified a confirmed case of asymptomatic 2019-nCoV infection, patient A, and reported her clinical follow-ups. Despite largely normal laboratory and chest CT findings, her persistent positivity of the virus nucleic acid in her throat swabs and anal swabs for at least 17 days suggested that she was very likely a healthy carrier. Notably, the detection of nucleic acids in her anal swabs also added to the importance of testing anal swabs or stool samples for the virus, even after respiratory specimens turn negative, in case that COVID-19 is transmittable via a fecal-oral route.[3]
In addition, from the epidemiological information, it can be deduced that her husband, patient B transmitted the viral infection to patient A. Although his CT scan had mild abnormality, he did not present with any symptoms until February 7, after his infection was confirmed. This provided evidence that the disease was transmittable during the asymptomatic phase, as was suggested previously.[2] Without epidemiological investigation and close monitoring, it was challenging to identify asymptomatic carriers such as patient A, and patients during their asymptomatic phase such as patient B. The couples were initially unaware of the infection and did not isolate themselves. During the period of 12 days (January 26–February 6), they might have been transmitting the disease to others before they were isolated. To prevent the disease from spreading in this case, close monitoring of asymptomatic individuals would be an option, but this would be costly. A better solution may be the development of a protective vaccine.
Although patient A was treated with IFN α2β and anti-virus agents, there is not enough evidence indicating such treatment is associated with her asymptomatic status. Also, despite the treatment of lopinavir/ritonavir and ribavirin during admission, her persistent positive findings of the virus nucleic acid suggested that such anti-virus agents seem not effective, as was indicated in a recent trial.[4] Besides, the elevation of liver enzymes observed after the treatment could be associated with lopinavir/ritonavir, of which liver impairment is a common side effect.[5] Therefore, for asymptomatic patients or patients with mild symptoms, isolation and close observation may be more suitable.
Collectively, we identified a confirmed case of an asymptomatic carrier of 2019-nCoV. We also provided evidence that COVID-19 was transmittable during the asymptomatic phase. As such, preventive strategies of 2019-nCoV spread should be changed. Besides close monitoring of asymptomatic individuals, the development of a protective vaccine should be urged.
Funding
This work was supported by grants from the Fundamental Research Funds for the Central Universities “Emergency response to new coronavirus infection scientific and technological project” (Nos. YD9110004001, YD9110002002), and Anhui Provincial Department of Science and Technology, Anhui Provincial Health Commission Emergency Research Project “Epidemiological and clinical characteristics of new coronavirus pneumonia” (No. 202004a07020002).
Conflicts of interest
None.
Footnotes
How to cite this article: Luo SH, Liu W, Liu ZJ, Zheng XY, Hong CX, Liu ZR, Liu J, Weng JP. A confirmed asymptomatic carrier of 2019 novel coronavirus. Chin Med J 2020;133:1123–1125. doi: 10.1097/CM9.0000000000000798
Si-Hui Luo and Wei Liu contributed equally to the work.
References
- 1.Chen N, Zhou M, Dong X, Qu JM, Gong FY, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; published ahead of print. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling Y, Bao SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J 2020; 133:1039–1043. published ahead of print. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Ling Y, Xi XH, Liu P, Li F, Li T, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia [in Chinese]. Chin J Infect Dis 2020; published ahead of print. doi: 10.3760/cma.j.cn311365-20200210-00050. [Google Scholar]
- 5.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs 2003; 63:769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]


