Abstract
Background
Excessive inflammatory responses play a critical role in the development of severe acute pancreatitis (SAP), and controlling such inflammation is vital for managing this often fatal disease. Dexmedetomidine has been reported to possess protective properties in inflammatory diseases. Therefore, this study aimed to investigate whether dexmedetomidine pre-treatment exerts an anti-inflammatory effect in rats with SAP induced by sodium taurocholate, and if so, to determine the potential mechanism.
Methods
SAP was induced with sodium taurocholate. Rats received an intraperitoneal injection of dexmedetomidine 30 min before sodium taurocholate administration. α-bungarotoxin, a selective alpha-7 nicotinic acetylcholine receptor (α7nAchR) antagonist, was injected intra-peritoneally 30 min before dexmedetomidine administration. The role of the vagus nerve was evaluated by performing unilateral cervical vagotomy before the administration of dexmedetomidine. Efferent discharge of the vagal nerve was recorded by the BL-420F Data Acquisition & Analysis System. Six hours after onset, serum pro-inflammatory cytokine (tumor necrosis factor α [TNF-α] and interleukin 6 [IL-6]) levels and amylase levels were determined using an enzyme-linked immunosorbent assay and an automated biochemical analyzer, respectively. Histopathological changes in the pancreas were observed after hematoxylin and eosin staining and scored according to Schmidt criteria.
Results
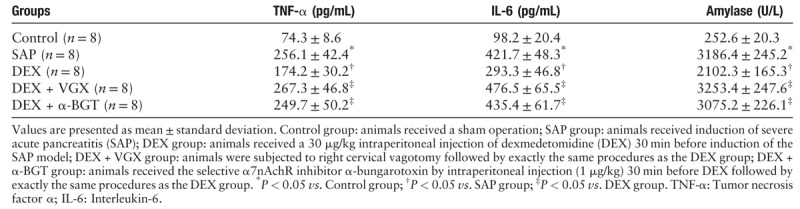
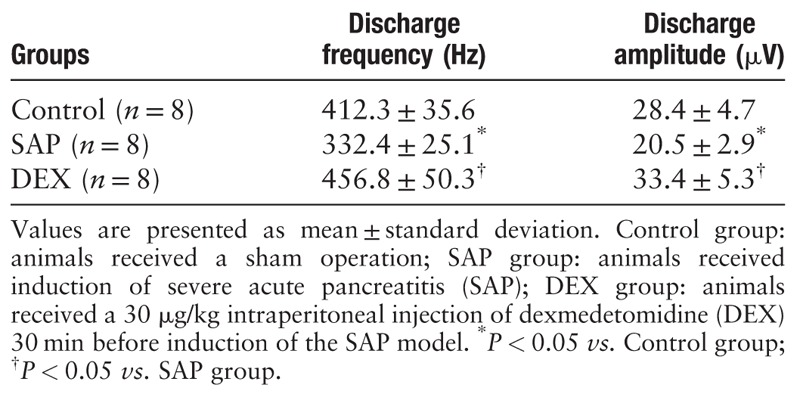
Pre-treatment with dexmedetomidine significantly decreased serum levels of TNF-α, IL-6, and amylase, strongly alleviating pathological pancreatic injury in the rat model of SAP (TNF-α: 174.2 ± 30.2 vs. 256.1±42.4 pg/ml; IL-6: 293.3 ± 46.8 vs. 421.7 ± 48.3 pg/ml; amylase: 2102.3 ± 165.3 vs. 3186.4 ± 245.2 U/L). However, the anti-inflammatory and pancreatic protective effects were abolished after vagotomy or pre-administration of α-bungarotoxin. Dexmedetomidine also significantly increased the discharge frequency and amplitude of the cervical vagus nerve in the SAP rat model (discharge frequency: 456.8 ± 50.3 vs. 332.4 ± 25.1 Hz; discharge amplitude: 33.4 ± 5.3 vs. 20.5 ± 2.9 μV).
Conclusions
Dexmedetomidine administration attenuated the systemic inflammatory response and local pancreatic injury caused by SAP in rats through the cholinergic anti-inflammatory pathway involving vagus- and α7nAChR-dependent mechanisms.
Keywords: Dexmedetomidine, Severe acute pancreatitis, Cholinergic anti-inflammatory pathway, Inflammation, Vagus nerve, α7nAChR
Introduction
Severe acute pancreatitis (SAP) develops in 20% of patients with acute pancreatitis (AP), and is associated with significant mortality rates between 30% and 60%.[1–3] In AP, the release of inflammatory mediators initiates systemic inflammatory response syndrome (SIRS), which may cause cardiovascular, pulmonary, and renal dysfunction, with or without secondary necrosis, infection, and possibly death.[4,5] In theory, anti-inflammatory treatment may overcompensate and inhibit the inflammatory response, rendering the host at risk of systemic infection. Therefore, excessive inflammatory responses play a central role in determining the severity of AP, and initial management of it should be based on supportive measures aimed to minimizing the extent of SIRS.[6,7] Unfortunately, various promising experimental approaches aimed at interfering with the inhibition of inflammatory mediators have failed to show beneficial effects in the clinical setting.[8] Thus, new effective anti-inflammatory drugs and treatments are needed to improve outcomes for patients with AP.
Research in recent years shows that the autonomic nervous system plays an essential role in sensing and controlling inflammation and in tuning immune responses.[9,10] The best-described autonomic nerve-immune system interaction occurs through the cholinergic anti-inflammatory pathway, which modulates the innate immune cellular response. The cholinergic anti-inflammatory pathway, which includes the efferent vagus nerve, acetylcholine and the alpha-7 nicotinic acetylcholine receptor (α7nAchR), has been reported to inhibit pro-inflammatory cytokine release and protect against systemic inflammation in experimental sepsis,[11,12] surgical operations[13] and ischemia-reperfusion injury.[14,15] Activated by inflammation, the central nervous cholinergic neurons deliver information to efferent vagus nerves, thereby releasing acetylcholine, which can inhibit the production of pro-inflammatory factors by interacting with α7nAchR on immune cells. The study of neuro-immune interactions has greatly advanced our understanding of immunity and opened up new therapeutic possibilities for inflammatory diseases. Previous studies have shown that electrical stimulation of the vagal nerve and cholinergic agonists, such as nicotine and GTS-21, can reduce the release of pro-inflammatory mediators in experimental pancreatitis,[16] whereas drug blocking of α7nAchR or unilateral vagotomy was found to exert the opposite effect,[17] suggesting that the cholinergic anti-inflammatory pathway is a potential pharmacotherapeutic target for SAP.
Dexmedetomidine (DEX), a highly selective alpha-2 adrenergic receptor agonist, is widely used in intensive care units for its sedative effects and can reduce sympathetic nerve activity, resulting in a balance shift towards the parasympathetic nervous system, including the vagus nerve.[18] Recently, DEX has been shown to be effective in reducing mortality and circulating cytokine levels in different experimental inflammatory diseases. Subsequent studies have indicated that DEX might suppress systemic inflammation via the cholinergic anti-inflammatory pathway involved in vagal- and α7nAChR-dependent mechanisms.[19,20] However, the effect of DEX on the inflammatory response in SAP has rarely been reported,[21] and the potential mechanisms underlying its action are unknown. In the current study, we evaluated whether DEX might have anti-inflammatory effects in experimental SAP and, if so, elucidate its possible mechanisms of action.
Methods
Animals
Male Sprague-Dawley rats (body weight, 280–350 g, free from specified pathogens) were purchased from the Animal Core Facility of Nanjing Medical University, China. All animals were housed in an environmentally controlled room at a temperature between 20 and 24°C with a 12-h:12-h light-dark cycle, and free access to food and water. The study was performed under the approval of the Institutional Animal Care and Use Committee of Nanjing Medical University, China (No. 1802012), and was conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (1996).
Anesthesia and surgical isolation of the cervical vagus nerve
All rats were fasted for 12 h with free access to water before initiating the surgical procedure. Intraperitoneal injection of urethane (Sigma-Aldrich, St. Louis, MO, USA, 700 mg/kg) was used to anesthetize each rat before operation. The back and the neck of each rat were shaved. To access the right vagus nerve at the cervical level, we made an incision approximately 2.0 cm in length on the mid-line ventral side of the neck. The sternohyoid and sternomastoid muscles were separated longitudinally with small forceps, and the vagus nerve was visualized. The vagus nerve was then carefully isolated from the surrounding tissue until a length of nerve sufficient for electrode placement was exposed.
Cervical vagus nerve activity recordings
A pair of bipolar stainless steel electrodes was used to record vagus nerve activity. The nerve was gently placed into the bend of each electrode pole. Once the nerve was within each bend, the poles were gently tightened to ensure good electrical contact with the nerve [Figure 1A]. Thirty min after the model was established, the activity of the cervical vagus nerve was continuously recorded using the BL-420F Data Acquisition & Analysis System (Tai Meng Software Co. Ltd., Chengdu, China) for 1 min [Figure 1B].
Figure 1.
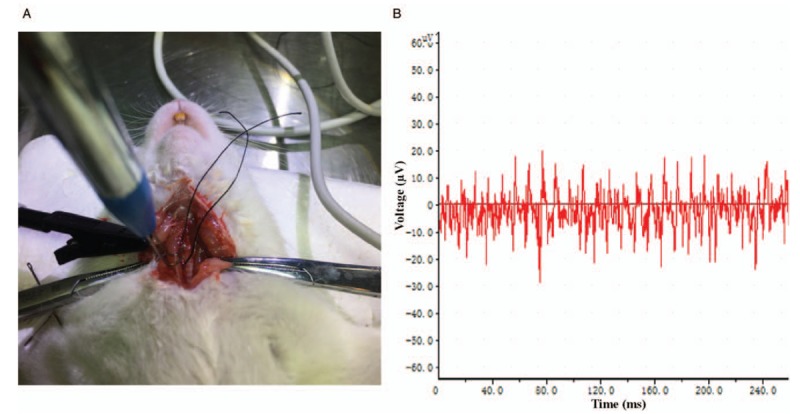
Cervical vagus nerve discharge activity monitoring. (A) The cervical vagus nerve was softly placed into the bend of each electrode pole after it was carefully isolated from the surrounding tissues. The pole was gently tightened to maintain good electrical contact with the nerve. (B) The activity of the cervical vagus nerve was sampled and stored in the computer via the BL-420F Data Acquisition & Analysis System.
Experimental model
The SAP model was implemented by retrograde infusion of 3.5% sodium taurocholate (Sigma-Aldrich) into the cholangiopancreatic duct as described previously,[22] and a typical dose of 1 mL/kg was used. After infusion, the part of the cholangiopancreatic duct that enters the duodenum was clipped for 5 min. The vascular clip was removed, and the incision was closed with 4-0-silk suture after the pancreatic tissue became dark purple, swollen, and bleeding locally. All animals received a saline infusion of 5 mL·kg–1·h–1 via their tail veins.
Experimental groups
Forty animals were randomly assigned to the following five groups, with eight animals per group. (1) Control group animals each received a sham operation, and the cholangiopancreatic duct was exposed but no infusion was administered; (2) SAP group animals each received induction of SAP as described previously; (3) DEX group animals each received a 30 μg/kg intraperitoneal injection of DEX (Xin Chen Pharmaceutical Company, Jiangsu, China) 30 min before induction of the SAP model; (4) DEX + VGX group animals were subjected to right cervical vagotomy (VGX) followed by exactly the same procedures as the DEX group; and (5) DEX + α-BGT group animals received the selective α7nAchR inhibitor α-bungarotoxin (α-BGT, Sigma-Aldrich) by intraperitoneal injection (1 μg/kg) 30 min before DEX followed by exactly the same procedures as the DEX group.
Blood and pancreatic tissue preparation
Rats in all the five groups were sacrificed by taking their blood via intra-cardiac puncture at 6 h post-treatment. The separate blood samples were collected, conserved at room temperature for 30 min, and centrifuged at 3000 r/min for 15 min. The serum was preserved at −20°C for subsequent amylase and cytokine assays. A section of each pancreatic tissue sample was immediately cut into a 1 mm3 size and then fixed in 4% paraformaldehyde for morphological examination.
Biochemical analysis
Serum amylase levels were determined using an automated biochemical analyzer (Toshiba-40FR, Tokyo, Japan). The serum concentrations of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) were measured via a commercially available enzyme-linked immunosorbent assay, according to the manufacturer's recommendations (Servicebio, Wuhan, China).
Morphological examination and histopathological scoring
Pancreas samples were fixed in 4% paraformaldehyde and embedded in paraffin. The tissues were each sectioned at 5μm thicknesses with a microtome and stained with hematoxylin and eosin (H&E). All specimens were evaluated by two pathologists who were unaware of the origin of the samples. The histopathological score of each pancreas was assessed for edema, acinar necrosis, hemorrhage, inflammation, and perivascular infiltrate, according to the scoring system described by Schmidt et al.[23]
Statistical analysis
Results are presented herein as the mean ± standard deviation. Following a one-way analysis of variance, post-hoc comparisons were made using Bonferroni multiple comparison tests when the P value was significant (P < 0.05). All data analyses were performed with SPSS software Version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
Effect of DEX on serum pro-inflammatory cytokines
TNF-α and IL-6 cytokine levels were measured to further investigate the anti-inflammatory effects of DEX. As shown in Table 1, the concentrations of both pro-inflammatory cytokines showed significant increases in the SAP group compared with the control group (P < 0.05). DEX, administered by intraperitoneal injection before SAP induction, lowered the serum concentrations of TNF-α and IL-6 (P < 0.05). However, the anti-inflammatory effect of DEX was abolished by unilateral cervical vagotomy (P < 0.05), or by pre-intraperitoneal injection of α-bungarotoxin (P < 0.05). No statistically significant difference was observed in TNF-α and IL-6 levels among the SAP, DEX + VGX, and DEX + α-BGT groups (P > 0.05).
Table 1.
Effect of dexmedetomidine on the serum pro-inflammatory cytokines.
Effect of DEX on serum amylase
As illustrated in Table 1, the serum amylase level was significantly higher in the SAP group as compared with the control group (P < 0.05). Pre-treatment with DEX before SAP induction dramatically decreased the serum amylase level (P < 0.05). However, when compared with the DEX group, amylase in the DEX + VGX and DEX + α-BGT groups was much higher (both P < 0.05). The difference in serum amylase was not significant in the SAP, DEX + VGX, and DEX + α-BGT groups (P > 0.05).
Effect of DEX on microscopic pathological changes and the Schmidt score of the pancreas
Pancreatic tissues stained with H&E are shown in Figure 2A–E. The control group showed the normal acinar architecture, with no fat necrosis or hemorrhage. Contrastingly, in the SAP group, edema, hemorrhage, necrosis, and neutrophil infiltration were detected sporadically in the pancreatic tissues, with the disappearance of the structures of acinus and lobule. In the DEX group, the degree of pathological injury was clearly alleviated when compared with the SAP group, suggesting that DEX pre-treatment can mitigate the pathological injury present in SAP. However, the severity of pancreatic injury was significantly greater in the DEX + VGX and DEX + α-BGT groups when compared with the DEX group. The morphologic characteristics were similar among the SAP, DEX + VGX, and DEX + α-BGT groups.
Figure 2.
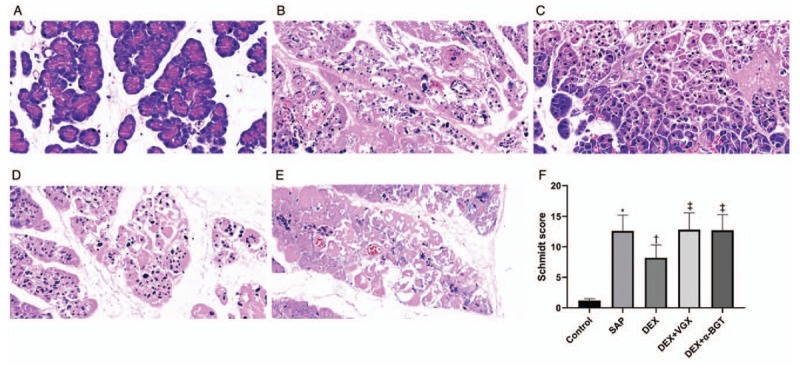
Effects of dexmedetomidine on pancreatic histopathological changes. Pancreatic tissue sections were stained with hematoxylin and eosin (Original magnification ×400) at 6 h (A–E). (A) Control group; (B) SAP group; (C) DEX group; (D) DEX + VGX group; (E) DEX + α-BGT group. (F) Histopathologic severity scores of pancreatic injury. ∗P < 0.05 vs. Control group; †P < 0.05 vs. SAP group; ‡P < 0.05 vs. DEX group. The data presented are the mean ± standard deviation (n = 8). Control group: animals received a sham operation; SAP group: animals received induction of severe acute pancreatitis (SAP); DEX group: animals received a 30 μg/kg intraperitoneal injection of dexmedetomidine (DEX) 30 min before induction of the SAP model; DEX + VGX group: animals were subjected to right cervical vagotomy followed by exactly the same procedures as the DEX group; DEX + α-BGT group: animals received the selective α7nAchR inhibitor α-bungarotoxin by intraperitoneal injection (1 μg/kg) 30 min before DEX followed by exactly the same procedures as the DEX group.
The Schmidt scoring criteria, a quantitative evaluation tool, was used to determine the degree of pancreatic histopathologic injury. As illustrated in Figure 2F, there was a significantly higher Schmidt score in the SAP group compared with the control group (P < 0.05). The Schmidt score was significantly lower in the DEX group than in the SAP group (P < 0.05). Nevertheless, the Schmidt score was considerably higher in the DEX + VGX and DEX + α-BGT groups than in the DEX group (both P < 0.05). There were no significant differences in the Schmidt score among the SAP, DEX + VGX, and DEX + α-BGT groups (P > 0.05).
Effect of DEX on the discharge pattern of the vagus nerve
As Table 2 shows, the discharge frequency and amplitude of the afferent vagus nerves from the SAP rats were markedly lower than those of the control group (P < 0.05). However, DEX increased the frequency and amplitude discharge of the cervical vagus nerve in the rats with SAP (P < 0.05).
Table 2.
Effect of dexmedetomidine on the discharge pattern of the vagus nerve.
Discussion
SAP is a fatal disease, with mortality rates exceeding 30%. Regardless of various promising experimental studies, there is still no specific anti-inflammatory approach or randomized clinical trial for this disease.[24] Accordingly, we focused on a drug commonly used in the intensive care setting with potential anti-inflammatory properties for SAP. Using a rat model of SAP, we investigated the anti-inflammatory effect of DEX and elucidated its potential mechanism via vagotomy and exogenous administration of α7nAChR antagonists. The above-mentioned results indicate that pre-treatment with DEX markedly protects against morphologic injury in experimental SAP. The effect seems to be mediated by reducing pro-inflammatory mediator release, as suggested by reduced TNF-α and IL-6 serum levels. However, the anti-inflammatory and pancreas-protective effects of DEX were not abrogated by unilateral vagotomy or pre-administration of the α7nAchR antagonist, α-bungarotoxin. Furthermore, our data showed that the activity of the cervical vagus nerve was severely restricted in the pancreatitis model; however, pre-emptively administering DEX significantly increased the activity of the cervical vagus nerve. These findings imply that as a central alpha-2 agonist, DEX suppresses systemic inflammation via the cholinergic anti-inflammatory pathway involved in both a vagus- and α7nAChR-dependent mechanism.
The cholinergic anti-inflammatory pathway, which comprises the efferent vagus nerve, acetylcholine, and α7nAchR, has been reported to attenuate excessive inflammatory responses in experimental models of inflammatory disease. In this circuit, acetylcholine released from the vagus nerve binds to α7nAChR and triggers a signaling cascade that results in inhibition of pro-inflammatory cytokines synthesis in macrophages in the circulation and in the spleen.[25,26] It was reported that vagus nerve stimulation failed to reduce serum pro-inflammatory cytokines in α7nAChR knockout mice, indicating that α7nAChR is required for the functional integrity of the cholinergic anti-inflammatory pathway.[27,28] Furthermore, administration of nicotine, an α7nAChR agonist,[17] or GTS-21,[16] resulted in an increase in survival and reduction in serum pro-inflammatory cytokines in SAP; conversely, administration of mecamylamine, an α7nAChR antagonist increased the severity of pancreatitis.[16] Consistent with previous studies, we found that the anti-inflammatory effect of DEX was abrogated by α-bungarotoxin injection before DEX in rats with sodium taurocholate-induced SAP, indicating pre-administration of DEX was insufficient to reduce the pro-inflammatory cytokines measured in rats with SAP. This finding suggests that DEX exerts an anti-inflammatory effect in rats with SAP via a mechanism dependent on α7nAChR.
The parasympathetic neurotransmitter is the effector transmitter of the vagus nerve and forms part of the cholinergic anti-inflammatory pathway. Shutting down this system by vagotomy may increase mortality and pro-inflammatory factor levels in experimental sepsis and other models of inflammatory disease. In this study, the protective effects of DEX were also abolished by surgical unilateral cervical vagotomy, suggesting that DEX had an anti-inflammatory effect based on the unimpaired vagus nerve. This result accords with previous studies showing that surgical vagotomy can enhance pro-inflammatory cytokine production and accelerate the development of SAP[16,17] in other experimental inflammatory models,[29,30] whereas significant electrical stimulation of the vagus nerve was able to reduce systemic inflammation and improve survival in such diseases.[31,32]
As mentioned previously, DEX-induced reduction of pro-inflammatory cytokine levels to alleviate the extent of pancreatic damage in SAP depends on an intact vagus nerve and α7nAChR, which are the two most important component parts of the cholinergic anti-inflammatory pathway. Nevertheless, these findings are still unable to provide direct evidence that the effect of DEX is regulated and controlled by cholinergic anti-inflammatory pathway activation. Previous studies have suggested that the probable mechanism responsible for the anti-inflammatory effects of DEX may involve the downregulation of cytokine production by activating the parasympathetic nervous system.[19,20] However, these studies did not provide direct evidence substantiating that DEX can increase the activity of the efferent vagus nerve. Here, we revealed that pre-administration of DEX not only decreased the serum concentrations of pro-inflammatory mediators such as IL-6 and TNF-α, but also increased the discharge frequency and amplitude of the cervical vagus nerve in experimental pancreatitis in rats. This study provided direct evidence that DEX can activate the vagus nerve in experimental animals with pancreatitis. Thus, our data suggest that the anti-inflammatory and protective mechanism of DEX in SAP may be regulated by increasing vagus nerve activation through the efferent vagus nerve-based cholinergic anti-inflammatory pathway.
DEX, an alpha-2 adrenoceptor agonist with sedative, analgesic, sympatholytic, and anxiolytic properties, is widely used in intensive care units. It is interesting that our study explored a new protective effect on the pancreas through modulation of the cholinergic anti-inflammatory pathway, as a complement to DEX. Because the drug is already routinely used by intensive care clinicians and as adjunct sedative medication as well, the clinical usability of these drugs on SAP may be easier to implement than developing new drugs, and; therefore, warrants evaluation in clinical trials. Our results show that DEX is a potential therapeutic agent for SAP.
There are some limitations to the current study. First, this is only a preliminary animal study to provide some initial evidence to support the anti-inflammatory effect of DEX. The clinical significance of its usage to relieve the severity of SAP in patients should be further evaluated. Second, in the current study, DEX was prophylactically used, so its therapeutic effect on rats with SAP needs further study. Third, our study is not related to the downstream signaling pathway of α7nAChRs detection, and further experiments are required to clarify the downstream mechanisms.
In conclusion, we concluded that DEX, a medicine commonly used in intensive care units and an alpha-2 agonist acting on the central nervous system, alleviates the systematic inflammatory response and pancreatic injury in experimental SAP. The therapeutic effects of DEX may be related to its parasympathetic effects via the cholinergic anti-inflammatory pathway. It should be pointed out that because DEX is widely used as a sedative in intensive care units, this may also limit its use as an anti-inflammatory medicine. Hence, the clinical significance of the current study might be that (1) the wide usage of DEX may be part of the reason that SAP patients have a better outcome when treated in intensive care units, and (2) for SAP patients, once a sedative is considered by a clinician, DEX might be the first choice. However, further studies focused on evaluating the clinical evidence to support the use of DEX as an anti-inflammatory adjuvant in patients with pancreatitis are essential.
Funding
This work was supported by a grant from the National Natural Sciences Foundation of China (No. 81672449).
Conflicts of interest
None.
Footnotes
How to cite this article: Huang DY, Li Q, Shi CY, Hou CQ, Miao Y, Shen HB. Dexmedetomidine attenuates inflammation and pancreatic injury in a rat model of experimental severe acute pancreatitis via cholinergic anti-inflammatory pathway. Chin Med J 2020;133:1073–1079. doi: 10.1097/CM9.0000000000000766
References
- 1.Campion EW, Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med 2016; 375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 2.Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: an evidence-based approach. Gastroenterology 2019; 156:1994–2007. doi: 10.1053/j.gastro.2019.01.269. [DOI] [PubMed] [Google Scholar]
- 3.Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 2019; 68:1044–1051. doi: 10.1136/gutjnl-2017-314657. [DOI] [PubMed] [Google Scholar]
- 4.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology 2019; 156:2008–2023. doi: 10.1053/j.gastro.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider L, Jabrailova B, Strobel O, Hackert T, Werner J. Inflammatory profiling of early experimental necrotizing pancreatitis. Life Sci 2015; 126:76–80. doi: 10.1016/j.lfs.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Sarr MG. Minimal access necrosectomy: the newest advance of many in the treatment of necrotising pancreatitis. Gut 2018; 67:599–600. doi: 10.1136/gutjnl-2017-314660. [DOI] [PubMed] [Google Scholar]
- 7.Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology 2019; 156:1941–1950. doi: 10.1053/j.gastro.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uc A, Andersen DK, Borowitz D, Glesby MJ, Mayerle J, Sutton R, et al. Accelerating the drug delivery pipeline for acute and chronic pancreatitis-knowledge gaps and research opportunities: overview summary of a National Institute of Diabetes and Digestive and Kidney Diseases workshop. Pancreas 2018; 47:1180–1184. doi: 10.1097/MPA.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarroch EE. Autonomic nervous system and neuroimmune interactions: new insights and clinical implications. Neurology 2019; 92:377–385. doi: 10.1212/WNL.0000000000006942. [DOI] [PubMed] [Google Scholar]
- 10.Badke CM, Marsillio LE, Weese-Mayer DE, Sanchez-Pinto LN. Autonomic nervous system dysfunction in pediatric sepsis. Front Pediatr 2018; 6:280.doi: 10.3389/fped.2018.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capcha J, Rodrigues CE, Moreira RS, Silveira MD, Dourado PM, Dos SF, et al. Wharton's jelly-derived mesenchymal stem cells attenuate sepsis-induced organ injury partially via cholinergic anti-inflammatory pathway activation. Am J Physiol Regul Integr Comp Physiol 2019; 318:R135–R147. doi: 10.1152/ajpregu.00098.2018. [DOI] [PubMed] [Google Scholar]
- 12.Kanashiro A, Sonego F, Ferreira RG, Castanheira FV, Leite CA, Borges VF, et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol Res 2017; 117:1–8. doi: 10.1016/j.phrs.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Hou L, Yang H, Ge J, Wang S, Tian W, et al. Electroacupuncture pretreatment attenuates acute lung injury through alpha7 nicotinic acetylcholine receptor-mediated inhibition of HMGB1 release in rats after cardiopulmonary bypass. Shock 2018; 50:351–359. doi: 10.1097/SHK.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Chen Q, Li J, Zhao H, Mi E, Chen Y, et al. Dexmedetomidine-mediated prevention of renal ischemia-reperfusion injury depends in part on cholinergic anti-inflammatory mechanisms. Anesth Analg 2018; doi: 10.1213/ANE.0000000000003820. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Abe C, Kohro T, Tanaka S, Huang L, Yao J, et al. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int 2019; 95:563–576. doi: 10.1016/j.kint.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 2006; 130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Ma P, Yu K, Yu J, Wang W, Ding Y, Chen C, et al. Effects of nicotine and vagus nerve in severe acute pancreatitis-associated lung injury in rats. Pancreas 2016; 45:552–560. doi: 10.1097/MPA.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Akiyama T, Kawada T, Sata Y, Mizuno M, Kamiya A, et al. Medetomidine, an alpha(2)-adrenergic agonist, activates cardiac vagal nerve through modulation of baroreflex control. Circ J 2012; 76:152–159. doi: 10.1253/circj.cj-11-0574. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Li ST. Dexmedetomidine may produce extra protective effects on sepsis-induced diaphragm injury. Chin Med J 2015; 128:1407–1411. doi: 10.4103/0366-6999.156808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dardalas I, Stamoula E, Rigopoulos P, Malliou F, Tsaousi G, Aidoni Z, et al. Dexmedetomidine effects in different experimental sepsis in vivo models. Eur J Pharmacol 2019; 856:172401.doi: 10.1016/j.ejphar.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Schneider L, Jabrailova B, Salem M, Kilk K, Hofer S, Brenner T, et al. Stimulation of central alpha2 receptors attenuates experimental necrotizing pancreatitis. Pancreas 2016; 45:260–264. doi: 10.1097/MPA.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 22.Laukkarinen JM, Van Acker GJ, Weiss ER, Steer ML, Perides G. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut 2007; 56:1590–1598. doi: 10.1136/gut.2007.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg 1992; 215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev 2017; 4:D11384.doi: 10.1002/14651858.CD011384.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 26.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 27.Koopman FA, van Maanen MA, Vervoordeldonk MJ, Tak PP. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med 2017; 282:64–75. doi: 10.1111/joim.12626. [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Inaba Y, Watanabe H, Matsukawa T, Matsumoto M, Inoue H. Nicotinic alpha-7 acetylcholine receptor deficiency exacerbates hepatic inflammation and fibrosis in a mouse model of non-alcoholic steatohepatitis. J Diabetes Investig 2019; 10:659–666. doi: 10.1111/jdi.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ri M, Fukatsu K, Miyakuni T, Yanagawa M, Murakoshi S, Yasuhara H, et al. Influences of vagotomy on gut ischemia-reperfusion injury in mice. Shock 2017; 47:646–652. doi: 10.1097/SHK.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 30.Li-Sha G, Xing-Xing C, Lian-Pin W, De-Pu Z, Xiao-Wei L, Jia-Feng L, et al. Right cervical vagotomy aggravates viral myocarditis in mice via the cholinergic anti-inflammatory pathway. Front Pharmacol 2017; 8:25.doi: 10.3389/fphar.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komegae EN, Farmer D, Brooks VL, McKinley MJ, McAllen RM, Martelli D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun 2018; 73:441–449. doi: 10.1016/j.bbi.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caravaca AS, Gallina AL, Tarnawski L, Tracey KJ, Pavlov VA, Levine YA, et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front Neurosci 2019; 13:877.doi: 10.3389/fnins.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]


