Abstract
OBJECTIVE:
We aimed to evaluate the association between platelet (PLT) count and the risk and progression of hand, foot, and mouth disease (HFMD).
METHODS:
In total, 122 HFMD patients and 40 healthy controls were enrolled in the study. The differences between variables among the different subgroups were compared. Logistic regression analyses were performed to assess the relationship between various parameters and HFMD risk/progression. Sensitivity analysis was conducted by detecting the trend of the association between PLT count quartiles and HFMD risk/progression. A generalized additive model was used to identify the nonlinear relationship between PLT count and HFMD risk/progression. The relationship between gender and PLT count as well as the risk/progression of HFMD was detected using a stratified logistic regression model.
RESULTS:
Significant differences were observed in terms of age, male/female ratio, white blood cell (WBC) count, and PLT count between patients with stage I-II, III-IV HFMD and healthy controls. Moreover, the alanine aminotransferase and magnesium levels between patients with stage I-II and III-IV HFMD significantly differed. Moreover, a significant difference was noted in the male/female ratio among the different PLT groups. The group with a low PLT count had a lower risk of HFMD progression than the group with a high PLT count (Q4) (p=0.039). Lower age, male gender, and WBC count were found to be associated with HFMD risk. Meanwhile, PLT count was correlated to HFMD progression. The sensitivity analysis yielded a similar result using the minimally adjusted model (p for trend=0.037), and minimal changes were observed using the crude and fully adjusted model (p for trend=0.054; 0.090). A significant nonlinear relationship was observed between PLT count and HFMD progression after adjusting for age, gender, and WBC (p=0.039).
CONCLUSIONS:
PLT was independently associated with HFMD progression in a nonlinear manner.
Keywords: Platelet, Risk, Progression, Hand, Foot, Mouth Disease
INTRODUCTION
Hand, foot, and mouth disease (HFMD) is a common syndrome mainly caused by intestinal viruses of the picornaviridae family 1. HFMD usually affects children younger than 5 years 2. The most common clinical symptoms are ulcers and rashes on certain body parts 3. Most patients with mild HFMD can recover spontaneously. However, about 1.1% of individuals in China develop severe HFMD and present with serious clinical complications, such as encephalitis, aseptic meningitis, and even death, during progression 4,5. Antiviral therapy and vaccination against certain enterovirus have been the primary methods used for treating and preventing HFMD 6,7. However, in severe cases, patients are resistant to the traditional therapy and had a poor prognosis. Due to its potential danger, HFMD has become an important health concern among children worldwide. Thus, early prevention and therapy of HFMD may be imperative.
Recently, several studies 8-10 have focused on the risk factors of severe HFMD. Some pathogens, including enterovirus 71, were found to be responsible for the progression of HFMD 11. Moreover, inflammation was implicated in the development of HFMD 12. Meteorological and geographical factors were closely associated with the incidence of HFMD 13. However, there are some limitations in the management of such condition. For example, several laboratory parameter tests are not available in primary medical institutions. On the other hand, a high number of children present with herpangina and HFMD in suburban districts during the epidemic season. Therefore, the identification of low-cost and easily available alternative biomarkers for HFMD risk and progression is of great clinical significance.
Platelet (PLT) is an important component of the blood, and it plays an important role in several physiological and pathological processes, such as coagulation, thrombosis, and inflammation 14. PLT count is associated with inflammatory diseases. Inflammation correlated with endothelial dysfunction is a cause of organ failure and is associated with PLT activation and consumption 15. In addition, a relationship was observed between changes in PLT count and the prognosis of critically ill patients 16-18. Inflammatory and thrombotic conditions may cause changes in PLT size 19. Larger PLTs are more reactive than smaller ones as they can more easily release chemical mediators in response to various stimuli. Hence, alterations in PLT count are associated with morbidity and mortality in patients with various diseases. Moreover, PLT is easily available as it is a parameter of complete blood count test, which is performed using the automatic analyzer. Thus, it can be generalized and applied in primary medical institutions.
To identify the possible role of PLT in HFMD, we evaluated the association between PLT and HFMD risk and progression. HFMD was classified into four clinical stages based on the involvement of injured organs and disease severity. Meanwhile, the relationship between other clinical/laboratory parameters and HFMD as well as the role of other variables on the association between PLT and HFMD were analyzed.
MATERIAL AND METHODS
Patient population
The patients diagnosed with HFMD at First People's Hospital of Lianyungang between January 2016 and April 2018 were screened. Nearly 600 patients visit the outpatient department daily. Patients who were diagnosed with HFMD at presentation were enrolled in the current study. HFMD was diagnosed according to the World Health Organization criteria for HFMD 20. The patients with HFMD were divided into four groups based on clinical stages: clinical stage I (no involvement of other organs), stage II (involvement of the nervous system), stage III (early cardiopulmonary failure), and stage IV (cardiopulmonary failure). However, patients with signs and symptoms not consistent with the diagnostic criteria and those who were immunosuppressed were excluded. This retrospective study was conducted according to the Declaration of Helsinki. Moreover, informed consent was obtained from the guardians of all participants.
Data collection
We obtained data about the clinical and laboratory parameters of the patients from the medical records. Demographic and clinical data, such as age and gender, were reviewed respectively. Laboratory data included white blood cell (WBC) count and hemoglobulin (HB), PLT, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), serum creatinine (Scr), calcium (Ca), phosphorus (P), and magnesium (Mg) levels. All parameters were assessed during the first day after the admission of the patients to the hospital. The data of the controls, including age, gender, WBC count, and PLT count, were obtained. PLT count was also expressed in quartiles.
Statistical analysis
Continuous variables were expressed as mean/standard deviation of the normal distribution or median/quartile in the skewed distribution. Meanwhile, categorical variables were presented as frequency. One-way analysis of variance and the Kruskal–Wallis H test were used to detect the differences in continuous variables. The chi-square test was utilized to identify the differences in categorical variables. Logistic regression analyses were used to assess the relationship between various clinical and laboratory parameters and HFMD risk/progression. The association between PLT count and HFMD risk/progression was assessed via a logistic regression analysis using the crude, minimally adjusted, and fully adjusted models. Moreover, a sensitivity analysis was performed by detecting the trend of the association between PLT count quartiles and HFMD risk/progression. A generalized additive model was used to identify the nonlinear relationship between PLT count and HFMD risk/progression. A subgroup analysis in terms of sex of the patient was conducted using the stratified logistic regression model. The likelihood ratio test was used to detect the interaction between the subgroups. Statistical analyses were conducted using the software R and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A p value <5% was considered statistically significant.
RESULTS
Baseline characteristics of the participants
A total of 122 HFMD patients and 40 controls were enrolled in our study (Table 1). There were 104 and 18 patients with stage I-II (n=40, stage I; n=64, stage II) and III-IV HFMD, respectively. The median ages of the patients with stage I-II HMFD, those with stage III-IV HFMD, and controls were 1.83, 1.87, and 6, respectively. Significant differences were observed in terms of age, male/female ratio, WBC count, and PLT count among patients with stage I-II and III-IV HFMD and controls. Moreover, the ALT and Mg levels between patients with stage I-II and those with III-IV HFMD significantly differed. No statistically significant differences were observed in terms of age, WBC count, and HB, ALT, AST, Scr, Ca, P, and Mg levels among the different PLT groups (Table 2). The male/female ratio significantly differed among the PLT groups (Table 2). The group with a low PLT count (Q1) had a lower risk of HFMD progression than the group with a high PLT count (Q4) (p=0.039) (Table 2).
Table 1.
Baseline characteristics of the participants in terms of disease state.
| Patients with stage I-II HFMD (n=104) | Patients with stage III-IV HFMD (n=18) | Controls (n=40) | p-value | |
|---|---|---|---|---|
| Age (years) | 1.83 (1.31, 2) | 1.87 (1.21, 2.75) | 6 (4, 8.25) | <10-4 |
| Gender (male/female) | 76/28 | 15/3 | 20/20 | 0.01 |
| WBC count (109/L) | 11.29 (9.43, 14.57) | 12.95 (10.71, 17.92) | 8.75 (6.95, 10.98) | 0.001 |
| HB level (g/L) | 119.5 (114, 123) | 118 (109, 125) | -- | 0.86 |
| PLT count (109/L) | 261.23±77.02 | 309.06±74.49 | 283.75±55.86 | 0.044 |
| ALT level (u/L) | 16 (12, 21) | 14.5 (12, 19.5) | -- | 0.046 |
| AST level (u/L) | 31 (26, 41) | 27 (25, 38) | -- | 0.14 |
| ALB level (g/L) | 45.6 (43.2, 47.7) | 45.75 (43.58, 48.83) | -- | 0.99 |
| Scr level (umol/L) | 21.45 (17.38, 24.9) | 20 (17.8, 22.8) | -- | 0.53 |
| Ca level (mmol/L) | 2.48±0.10 | 2.46±0.13 | -- | 0.39 |
| P level (mmol/L) | 1.76±0.21 | 1.75±0.22 | -- | 0.79 |
| Mg level (mmol/L) | 0.90±0.09 | 0.85±0.08 | -- | 0.024 |
HFMD: hand, foot, and mouth disease, WBC: white blood cell, HB: hemoglobulin, PLT: platelet, ALT: alanine aminotransferase, AST: aspartate aminotransferase, ALB: albumin, Scr: serum creatinine, Ca: calcium, P: phosphorus, Mg: magnesium.
Table 2.
Baseline characteristics of the participants in terms of PLT quartiles.
| Q1 (40) | Q2 (38) | Q3 (40) | Q4 (40) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 2.0 (1.33, 3) | 2.5 (1.83, 5.75) | 2 (1.33, 4.0) | 2 (1.65, 4.0) | 0.80 |
| Gender (male/female) | 28/12 | 33/5 | 20/20 | 26/14 | 0.006 |
| WBC count (109/L) | 10.41 (9.24, 12.78) | 10.77 (8.09, 12.67) | 10.32 (8.02, 14.62) | 12.2 (10.26, 14.80) | 0.27 |
| HB level (g/L) | 118.5 (111.75, 123.5) | 119.5 (113.5, 125.5) | 119 (111, 122) | 119 (116, 122.25) | 0.78 |
| ALT level (u/L) | 14 (11, 21) | 16 (13, 20.0) | 16 (13, 22.2) | 16 (12, 20.2) | 0.729 |
| AST level (u/L) | 31 (26, 39) | 30 (27, 39) | 31.5 (25.9, 40.2) | 31 (25, 41) | 0.923 |
| ALB level (g/L) | 43.9 (42.6, 46.1) | 45.4 (43.9, 47.3) | 46.3 (44.2, 48) | 45.7 (43.4, 48.3) | 0.133 |
| Scr level (umol/L) | 22.4 (16.9, 23.8) | 22.8 (19.3, 25.7) | 21.1 (16.9, 24.3) | 20.5 (17.8, 24.8) | 0.621 |
| Ca level (mmol/L) | 2.46±0.11 | 2.46±0.09 | 2.48±0.12 | 2.50±0.11 | 0.419 |
| P level (mmol/L) | 1.72±0.20 | 1.71±0.17 | 1.79±0.21 | 1.78±0.23 | 0.202 |
| Mg level (mmol/L) | 0.89±0.10 | 0.89±0.09 | 0.91±0.10 | 0.88±0.09 | 0.647 |
| HFMD | Q4 vs Q1 | 0.084 | |||
| None | 6 | 12 | 11 | 11 | |
| Stage I-II | 32 | 22 | 24 | 22 | |
| HFMD | Q4 vs Q1 | 0.039 | |||
| Stage I-II | 32 | 22 | 24 | 22 | |
| Stage III-IV | 2 | 4 | 5 | 7 |
HFMD: hand, foot, and mouth disease, WBC: white blood cell, HB: hemoglobulin, PLT: platelet, ALT: alanine aminotransferase, AST: aspartate aminotransferase, ALB: albumin, Scr: serum creatinine, Ca: calcium, P: phosphorus, Mg: magnesium.
Univariate logistic regression analysis
The results of the univariate logistic regression analyses are shown in Table 3. WBC count was positively associated with HFMD risk (95% CI: 1.035-1.346, p=0.014), indicating that higher level of WBC count increased the risk of HFMD susceptibility. Lower age and male gender were also found to be associated with HFMD risk. However, HB, PLT, ALB, ALT, AST, Scr, Ca, P, and Mg levels were not associated with HFMD risk, and a relationship was not observed between age, male gender, WBC count, and HB, ALB, ALT, AST, Scr, Ca, and p levels as well as HFMD progression.
Table 3.
Univariate analysis results (HFMD stage I-II versus control; *HFMD III-IV versus HFMD stage I-II).
| Index | Effect size (β) | 95% CI | p-value | |||
|---|---|---|---|---|---|---|
| Age | 0.233 | *1.234 | 0.140-0.388 | *0.829-1.835 | <10-4 | *0.299 |
| Gender | ||||||
| Female | Reference: 1 | − | − | |||
| Male | 2.714 | *1.842 | 1.274-5.782 | *0.495-6.848 | 0.0096 | *0.362 |
| WBC count | 1.180 | *1.1093 | 1.035-1.346 | *0.995-1.237 | 0.014 | *0.062 |
| HB level | −0.001 | *0.995 | −0.001-0.001 | *0.943-1.049 | 0.999 | *0.855 |
| PLT count | 0.996 | *1.008 | 0.990-1.0008 | *1.001-1.015 | 0.097 | *0.020 |
| ALB level | 0.999 | *0.999 | 0.00001-1.0001 | *0.868-1.149 | 0.999 | *0.985 |
| ALT level | 0.001 | *0.973 | −0.001-0.001 | *0.914-1.036 | 0.999 | *0.392 |
| AST level | −0.001 | *0.976 | −0.001-0.001 | *0.933-1.021 | 0.999 | *0.289 |
| Scr level | 0.001 | *0.978 | −0.001-0.001 | *0.9001-1.062 | 0.999 | *0.597 |
| Ca level | −0.001 | *0.126 | −0.001-0.001 | *0.0012-13.103 | 0.999 | *0.382 |
| P lebel | 0.001 | *0.725 | −0.001-0.001 | *0.067-7.842 | 0.999 | *0.791 |
| Mg level | −0.001 | *0.002 | −0.001-0.001 | *0.00001-0.505 | 0.998 | *0.028 |
HFMD: hand, foot, and mouth disease, WBC: white blood cell, HB: hemoglobulin, PLT: platelet, ALB: albumin, ALT: alanine aminotransferase, AST: aspartate aminotransferase, Scr: serum creatinine, Ca: calcium, P: phosphorus, Mg: magnesium.
Relationship between PLT count and HFMD risk and progression
We used the univariate logistic regression model to evaluate the association between PLT count and HFMD. The non-adjusted and adjusted models are shown in Table 4.
Table 4.
Relationship between PLT and HFMD risk and progression in the different models.
| Index | Crude model | Minimally adjusted model | Fully adjusted model |
|---|---|---|---|
| PLT count | #0.996 (0.990-1.001), 0.097 | #0.993 (0.984-1.003), 0.172 | #0.999 (0.998-1.0003), 0.999 |
| *1.008 (1.001-1.015), 0.02 | *1.008 (1.002-1.015), 0.016 | *1.009 (1.001-1.018), 0.036 | |
| PLT quartile | |||
| Q1 | #Reference | #Reference | #Reference |
| *Reference | *Reference | *Reference | |
| Q2 | #0.345 (0.112-1.054), 0.062 | #0.672 (0.071-6.347), 0.729 | #0.999 (0.00001-1.0004), 0.999 |
| *2.909 (0.489-17.287), 0.240 | *2.585 (0.429-15.590), 0.300 | *3.021 (0.393-23.213), 0.288 | |
| Q3 | #0.409 (0.133-1.262), 0.119 | #0.247 (0.029-2.094), 0.199 | #0.999 (0.00001-1.0003), 0.999 |
| *3.333 (0.595-18.674), 0.171 | *4.019 (0.691-23.391), 0.122 | *2.728 (0.317-23.456), 0.361 | |
| Q4 | #0.375 (0.121-1.165), 0.105 | #0.237 (0.029-1.956), 0.181 | #0.999 (0.00001-1.0005), 0.999 |
| *5.091(0.966-26.844), 0.055 | *5.337 (1.002-28.419), 0.049 | *6.585 (0.822-52.724), 0.076 | |
| P for trend | #0.136 | #0.122 | #0.999 |
| *0.054 | *0.037 | *0.090 |
HFMD: hand, foot, and mouth disease, PLT: platelet, crude model: adjusted for none, minimally adjusted model: adjusted for age and gender, fully adjusted model: adjusted for age, gender, WBC count and HB, ALB, ALT, AST, Scr, Ca, P, and Mg levels;
relationship between PLT count and HFMD risk
relationship between PLT count and HFMD progression.
In the crude model, PLT count was not associated with HFMD risk. Moreover, similar results were obtained using the minimally adjusted and fully adjusted models. In the sensitivity analysis, PLT was considered a categorical variable (quartile), and the same trend was observed. PLT count was found to be associated with HFMD progression regardless whether the crude, minimally, and fully adjusted model was used. Sensitivity analysis yielded a similar result using the minimally adjusted model, and only minimal changes were observed using the crude and fully adjusted models.
Analyses of nonlinear relationships
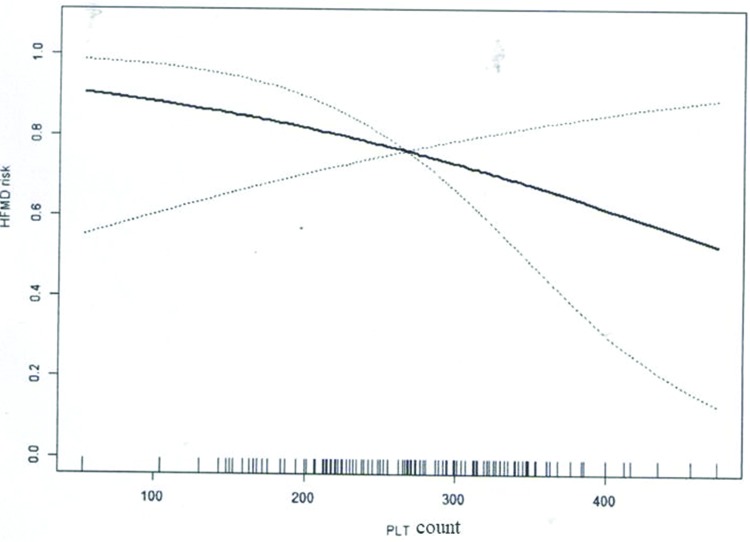
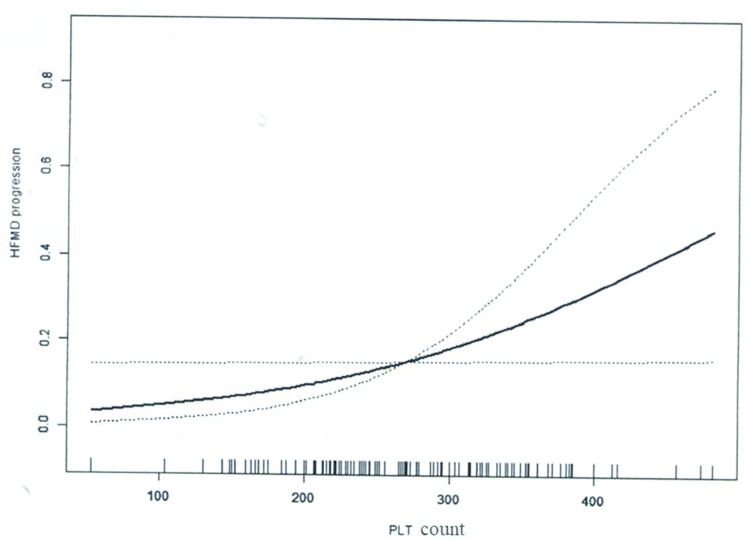
With the use of PLT as a continuous variable, the nonlinear relationship between PLT count and HFMD risk/progression was assessed. Results showed that PLT count was not associated with HFMD risk after adjusting for age, gender, and WBC count based on the nonlinear relationship analysis (Table 5, Figure 1). Notably, a significant nonlinear relationship was noted between PLT count and HFMD progression after adjusting for age, gender, and WBC (Table 5, Figure 2).
Table 5.
Nonlinear association between PLT count and HFMD risk/progression.
| Index | Effect size (β) | 95% CI | p-value |
|---|---|---|---|
| HFMD risk | 961.27 | 19.33-47802.25 | 0.296 |
| HFMD progression | 0.016 | 0.0016-0.163 | 0.039 |
HFMD: hand, foot, and mouth disease, PLT: platelet.
Figure 1.
Nonlinear association between PLT count and HFMD risk.
Figure 2.
Nonlinear association between PLT count and HFMD progression.
Results of the subgroup analyses
As shown in Table 6, the relationship between PLT count/quartiles and gender as well as HFMD risk and progression was not significant.
Table 6.
Role of gender on the association between PLT count and HFMD risk/progression.
| Index | Effect size (β) | 95% CI | p for interaction |
|---|---|---|---|
| #HFMD risk | |||
| Male | 0.997 | 0.987-1.007 | 0.089 |
| Female | 0.973 | 0.947-0.999 | |
| *HFMD risk | |||
| Male | 0.996 | 0.981-1.012 | 0.290 |
| Female | 0.982 | 0.958-1.006 | |
| #HFMD progression | |||
| Male | 1.007 | 0.999-1.014 | 0.688 |
| Female | 1.011 | 0.993-1.029 | |
| *HFMD progression | |||
| Male | 1.010 | 1.001-1.021 | 0.198 |
| Female | 0.994 | 0.973-1.016 |
HFMD: hand, foot, and mouth disease, PLT: platelet
PLT count and HFMD
PLT Q4 and HFMD.
DISCUSSION
Increasingly attention has been paid to the prevention and monitoring of HFMD. Outbreaks of HFMD commonly occur worldwide 21. The identification of a low-cost, easily available predictor for HFMD risk and progression is effective for the prevention of HFMD. This study first investigated the association between PLT count and the risk and progression of HFMD. Our investigation showed that a significant, independent, and nonlinear relationship existed between PLT count and HFMD progression. Moreover, PLT can be an alternative, low-cost biomarker for HFMD progression.
Several mechanisms may account for our findings. First, platelets promote the transfer of progenitor cells and leukocytes to the inflammation sites and release of several substances that mediate inflammation 22. Inflammation plays an important role in the development of HFMD. Systemic inflammatory response may play a role in the pathogenesis of HFMD 23. Enterovirus, which causes HFMD, significantly increases the release of circulating inflammatory mediators 23. Interleukin (IL)-27 is associated with early cardiopulmonary failure in enterovirus-induced HFMD 24. Cytokines and chemokines play a role in the development of HFMD 25. Moreover, patients with mild HFMD but without nervous system complications had increased levels of serum inflammatory cytokines 25. The abovementioned data support the notion that PLT may increase the susceptibility to HFMD by promoting inflammation. Studies have shown that platelets contribute to the risk of sepsis and acute lung injury 26. Moreover, they play an important role in trans-cellular metabolism, leading to the production of pro-inflammatory and anti-inflammatory molecules 27. An inflamed endothelium was found to be associated with leukocytes, which can roll on the template of adherent platelets and can migrate through the platelets 28. Second, platelet size can affect these functions. Mean PLT volume is the most commonly used marker in evaluating the changes in platelet function and activation. Platelets levels increase in high-grade inflammation, resulting in the decreased level of mean PLT volume due to the migration of large reactive platelets to inflammatory sites and the consumption of these platelets 29. Finally, HFMD is commonly caused by multiple viruses, including enterovirus 30, which may affect the PLT count. Severe HFMD is often complicated by organ damage, which may exacerbate inflammatory response, and this phenomenon is associated with platelet activation. Increased PLT count may be an early warning sign of severe HFMD, thereby indicating that a more aggressive treatment may be required.
Our findings also give rise to two questions. First, the in-depth mechanism behind the role of PLT in the development of HFMD must be further investigated. In addition to inflammation, other factors may also be involved in the pathogenesis of HFMD. Second, multiple pathogens can induce the onset of HFMD. Whether a close relationship exists between PLT count and the infectious pathogens of HFMD remains unclear. Previous studies have investigated the risk factors for HFMD progression. Chen et al. 23 have shown that IL-6, IL-10, and IL-13 were associated with the development of HFMD in children. David Lee et al. 13 have presented that weather conditions and geographic location play a role in the occurrence of HFMD epidemics. Liu et al. 31 have revealed that a moderately warm environment promotes the transmission of HFMD viruses, whereas particularly cold and hot climate conditions inhibit their transmission. Liu et al. 32 have reported that blisters and constipation are potential warming signs, and front-line clinicians must manage the sudden increase in the number of children diagnosed with mild HFMD during a pandemic. Previous findings indicated that the incidence and progression of HFMD are associated with environmental factors, which can affect the risk of inflammation. PLT may affect HFMD progression by regulating inflammation. Our findings were consistent with the abovementioned data, and PLT was found to be correlated the severity of HFMD. Interestingly, PLT was not associated with the risk of stage I-II HFMD, indicating that PLT was not involved in the development of early-stage HFMD. Notably, in the epidemic of HFMD or in susceptible cases, the changes in PLT count could not reflect the risk of HFMD. Meanwhile, in HFMD cases, PLT count reflects the severity of HFMD.
This study had several limitations that should be considered. First, its retrospective study design might have led to recall bias. However, we obtained comparatively reliable data. Second, the correlation analysis between PLT count and prognosis was not assessed due to the lack of prognostic data. Finally, the limited number of participants reduced the statistical power. Hence, studies with a larger sample size should be conducted in the future.
In conclusion, PLT count was independently associated with HFMD progression in a nonlinear manner. Thus, it can be used as an alternative, low-cost biomarker for HFMD progression.
AUTHOR CONTRIBUTIONS
Miao L and Liu Y performed the data collection. Liu J and Lu S designed the study. Mao S and Luo P were responsible for the data analyses and manuscript writing.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant number: 81600578), Weak Discipline Construction of Shanghai Health Family Planning Commission (grant number: 2016ZB0102-03), China Hospital Development Institute of Shanghai Jiao Tong University (grant number: CHDI-2018-B-06), the First People's Hospital of Lianyungang (grant number: BS1901), and the Young Talents Hausen Fund of the First People's Hospital of Lianyungang (grant number: QN121001).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Chen F, Liu T, Li J, Xing Z, Huang S, Wen G. MRI characteristics and follow-up findings in patients with neurological complications of enterovirus 71-related hand, foot, and mouth disease. Int J Clin Exp Med. 2014;7((9)):2696–704. [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Hu W, Jiao K, Ren C, Jiang B, Ma W. The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010-2013: a multicity study. BMC Infect Dis. 2019;19((1)):969. doi: 10.1186/s12879-019-4594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang G, Zhao J, He L, Yan S, Zhuo Z, Zheng H, et al. Reduning injection for fever, rash, and ulcers in children with mild hand, foot, and mouth disease: a randomized controlled clinical study. J Tradit Chin Med. 2013;33((6)):733–42. doi: 10.1016/S0254-6272(14)60005-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, Li R, Zhang W, Li G, Ma Z, Chen X, et al. A Case-control Study on Risk Factors for Severe Hand, Foot and Mouth Disease. Sci Rep. 2017;7:40282. doi: 10.1038/srep40282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9((11)):1097–105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 6.Cai K, Wang Y, Guo Z, Yu H, Li H, Zhang L, et al. Clinical characteristics and managements of severe hand, foot and mouth disease caused by enterovirus A71 and coxsackievirus A16 in Shanghai, China. BMC Infect Dis. 2019;19((1)):285. doi: 10.1186/s12879-019-3878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L, Wang J, Zhang C, He W, Mo J, Zeng J, et al. Effectiveness of enterovirus A71 vaccine in severe hand, foot, and mouth disease cases in Guangxi, China. Vaccine. 2020;38((7)):1804–9. doi: 10.1016/j.vaccine.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Lin S, Marks T, Zhang W, Deng T, Yu S, et al. Weather effects on hand, foot, and mouth disease at individual level: a case-crossover study. BMC Infect Dis. 2019;19((1)):1029. doi: 10.1186/s12879-019-4645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YP, Wang MQ, Deng HL, Li M, Zhang X, Dang SS, et al. Association of polymorphisms in the vitamin D receptor gene with susceptibility to and severity of hand, foot, and mouth disease caused by coxsackievirus A16. J Med Virol. 2020;92((3)):271–8. doi: 10.1002/jmv.25603. [DOI] [PubMed] [Google Scholar]
- 10.Qin L, Dang D, Wang X, Zhang R, Feng H, Ren J, et al. Identification of immune and metabolic predictors of severe hand-foot-mouth disease. PLoS One. 2019;14((5)):e0216993. doi: 10.1371/journal.pone.0216993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauleekoonphairoj J, Vongpunsawad S, Puenpa J, Korkong S, Poovorawan Y. Complete genome sequence analysis of enterovirus 71 isolated from children with hand, foot, and mouth disease in Thailand, 2012-2014. Virus Genes. 2015;51((2)):290–3. doi: 10.1007/s11262-015-1239-0. [DOI] [PubMed] [Google Scholar]
- 12.Yu P, Gao Z, Zong Y, Bao L, Xu L, Deng W, et al. Distribution of enterovirus 71 RNA in inflammatory cells infiltrating different tissues in fatal cases of hand, foot, and mouth disease. Arch Virol. 2015;160((1)):81–90. doi: 10.1007/s00705-014-2233-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, Tang JH, Hwang JS, Shigematsu M, Chan TC. Effect of Meteorological and Geographical Factors on the Epidemics of Hand, Foot, and Mouth Disease in Island-Type Territory, East Asia. Biomed Res Int. 2015;2015:805039. doi: 10.1155/2015/805039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elia E, Montecucco F, Portincasa P, Sahebkar A, Mollazadeh H, Carbone F. Update on pathological platelet activation in coronary thrombosis. J Cell Physiol. 2019;234((3)):2121–33. doi: 10.1002/jcp.27575. [DOI] [PubMed] [Google Scholar]
- 15.Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and Multi-Organ Failure in Sepsis. Int J Mol Sci. 2017;18((10)):E2200. doi: 10.3390/ijms18102200. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu H, Zhang Y. [Evaluation of platelet function in critically ill patients and its clinical significance] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30((3)):284–8. doi: 10.3760/cma.j.issn.2095-4352.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Wang G, Zhou H, Lei G, Yang L, Fang Z, et al. Preoperative platelet count, preoperative hemoglobin concentration and deep hypothermic circulatory arrest duration are risk factors for acute kidney injury after pulmonary endarterectomy: a retrospective cohort study. J Cardiothorac Surg. 2019;14((1)):220. doi: 10.1186/s13019-019-1026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Q, Wu Z, Xia T, Liu D, Yang Y, Tang H. Pre-treatment thrombocytosis predicts prognosis of endometrial cancer: A meta-analysis of 11 studies. Exp Ther Med. 2020;19((1)):359–366. doi: 10.3892/etm.2019.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17((1)):47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) A guide to clinical management and public health response for hand, foot and mouth disease (HFMD) 2011. Available from: http://www.wpro.who.int/publications docs/Guidance for the clinical management of HFMD.pdf. [Google Scholar]
- 21.Lai FF, Yan Q, Ge SX, Tang X, Chen RJ, Xu HM. Epidemiologic and etiologic characteristics of hand, foot, and mouth disease in Chongqing, China between 2010 and 2013. J Med Virol. 2016;88((3)):408–16. doi: 10.1002/jmv.24349. [DOI] [PubMed] [Google Scholar]
- 22.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7((11)):1759–66. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Li R, Xie Z, Huang G, Yuan Q, Zeng J. IL-6, IL-10 and IL-13 are associated with pathogenesis in children with Enterovirus 71 infection. Int J Clin Exp Med. 2014;7((9)):2718–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Huang M, Du W, Liu J, Zhang H, Cao L, Yang W, et al. Interleukin-27 as a Novel Biomarker for Early Cardiopulmonary Failure in Enterovirus 71-Infected Children with Central Nervous System Involvement. Mediators Inflamm. 2016;2016:4025167. doi: 10.1155/2016/4025167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao P, Wu X, Li H, Wu Z, Yang Z, Yao H. Clinical significance of inflammatory cytokine and chemokine expression in hand, foot and mouth disease. Mol Med Rep. 2017;15((5)):2859–66. doi: 10.3892/mmr.2017.6324. [DOI] [PubMed] [Google Scholar]
- 26.Powers ME, Becker RE, Sailer A, Turner JR, Bubeck Wardenburg J. Synergistic Action of Staphylococcus aureus α-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell Host Microbe. 2015;17((6)):775–87. doi: 10.1016/j.chom.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahler J, Woeller C, Spinelli S, Blumberg N, Phipps R. A novel method for overexpression of peroxisome proliferator-activated receptor-γ in megakaryocyte and platelet microparticles achieves transcellular signaling. J Thromb Haemost. 2012;10((12)):2563–72. doi: 10.1111/jth.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak J, Vangansewinkel T, Gervois P, Merckx G, Hilkens P, Quirynen M, et al. Angiogenic Properties of 'Leukocyte- and Platelet-Rich Fibrin'. Sci Rep. 2018;8((1)):14632. doi: 10.1038/s41598-018-32936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Li X, Zhang M, Huang X, Bai J, Pan Z, et al. The role of Mean Platelet Volume/platelet count Ratio and Neutrophil to Lymphocyte Ratio on the risk of Febrile Seizure. Sci Rep. 2018;8((1)):15123. doi: 10.1038/s41598-018-33373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu H, Liu Z, Zhang L, Chen Y, Yang S, Zhang W, et al. [The etiological and clinical characteristics of hospitalized children with hand, foot and mouth disease in Beijing in 2013] Zhonghua Er Ke Za Zhi. 2015;53((6)):459–63. [PubMed] [Google Scholar]
- 31.Liu W, Ji H, Shan J, Bao J, Sun Y, Li J, et al. Spatiotemporal Dynamics of Hand- Foot- Mouth Disease and Its Relationship with Meteorological Factors in Jiangsu Province, China. PLoS One. 2015;10((6)):e0131311. doi: 10.1371/journal.pone.0131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Luo L, Yan S, Wen T, Bai W, Li H, et al. Clinical Features for Mild Hand, Foot and Mouth Disease in China. PLoS One. 2015;10((8)):e0135503. doi: 10.1371/journal.pone.0135503. [DOI] [PMC free article] [PubMed] [Google Scholar]