Abstract
Mixed adenocarcinoma with neuroendocrine tumour of pancreas has been reported infrequently and consists of both epithelial and neuroendocrine component. We encountered an 81-year-old male patient who presented with clinical features of painful progressive jaundice for 1 month. Contrast-enhanced CT abdomen reported a mass in the pancreatic head with dilated common bile duct and pancreatic duct. He underwent pancreatoduodenectomy and histopathological examination revealed two different tumours: ductal adenocarcinoma admixed with neuroendocrine tumour of pancreas. He received adjuvant chemotherapy, and at the end of 1-year follow-up, he has no recurrence. Here, we reported this rare malignancy of pancreas for which pancreatoduodenectomy was done and diagnosed on histopathology with immunohistochemistry.
Keywords: adenocarcinoma, neuroendocrine, mixed, pancreas, pancreatoduodenectomy
Background
Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) has been redefined by WHO in 2017 as the association of two morphologically different neoplastic components, that is neuroendocrine and non-neuroendocrine tumour (NET).1 It comprises of adenocarcinoma either acinar or ductal with NET, and either element should account for at least 30% of the tumour.1 MiNEN usually involves various gastrointestinal organs, however, it is very infrequent in pancreas.2 3 It is the histopathology (HP) with immunohistochemistry that diagnoses MiNEN and grades the malignancy.
Due to its low incidence and gloomy prognosis, it is important to share all the individual experience-based information of such rare malignancy. Herein, we report a case of MiNEN in the head of the pancreas who was managed with pancreatoduodenectomy, received adjuvant therapy and doing well after 1 year.
Case presentation
An 81-year-old man without any comorbidity presented with progressively worsening pain in right upper abdomen since 3 months, not associated with vomiting, relieved with analgesics to recur again. He also noticed jaundice with cholestatic features for 15 days, which was also associated with anorexia and weight loss. On examination, he was icteric, and on abdominal examination, gall bladder was palpable. Liver function test revealed: bilirubin—3.8 mg/dL; aspartate transaminase—76 U/L; alanine transaminase—163 U/L; alkaline phosphatase—826 IU/L, while carbohydrate antigen 19-9 was 46.9 U/mL.
Investigations
Ultrasound of abdomen showed hypoechoic mass lesion in the head of the pancreas with double-duct sign positivity. Contrast-enhanced computed tomography (CECT) of abdomen reported an ill-defined hypodense mass in head of the pancreas (~2.1×1.6 cm) with abrupt cut-off of common bile duct (CBD) and main pancreatic duct with their upstream dilatation. Distal gastroduodenal artery was encased by the lesion, and area of contact with superior mesenteric vein (SMV) was less than 180°, however, no contour irregularity or intraluminal filling defect was noted. With the working diagnosis of carcinoma head of pancreas, he was planned for pancreatoduodenectomy.
Treatment
Diagnostic laparoscopy showed no distant metastasis. At laparotomy,~3×3 cm mass present in head of pancreas extending into uncinate process. CBD was dilated and firm pancreas with pancreatic duct was ~5 mm. Common hepatic artery was retroportal with distal gastroduodenal artery encased and SMV adherent to tumour (<180°). Pancreaticojejunostomy was performed by modified Blumgart’s technique followed by hepaticojejunostomy and antecolic gastrojejunostomy.
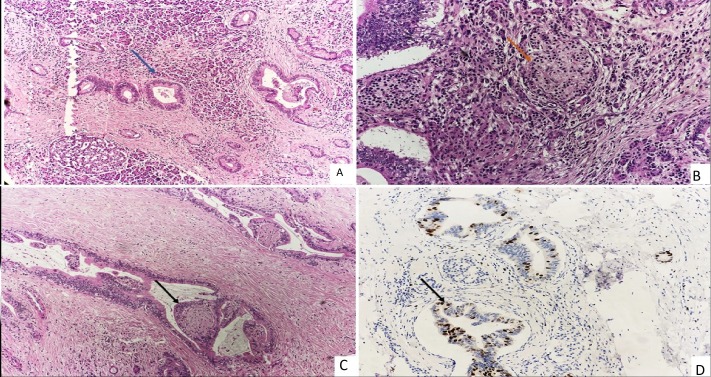
HP revealed two types of tumour cell populations in pancreas. The major component was well to moderately differentiated adenocarcinoma with irregular, medium-sized cells arranged in duct-like and tubular structures of variable shape, embedded in desmoplastic stroma. Non-adenocarcinoma cells have round nuclei, scant cytoplasm and coarse chromatin and composed of >30% of total tumour volume and intermixed with adenocarcinoma cells. Many atypical ductal structures were invading perineural spaces. The Ki 67 for non-NET cells was 40% and 2% in neuroendocrine component (figure 1A–D). The tumour cells were reaching till submucosa of duodenum and wall of CBD and all retrieved eight lymph nodes were negative for malignancy.
Figure 1.
(A) Unremarkable acini admixed with atypical duct-like structure (blue arrow) (H&E, 10×). (B) Clusters of neuroendocrine cells (orange arrow) along with acini (H&E, 20×). (C) Invasion of perineural space (black arrow) (H&E, 10×). (D) Ki 67 is 40% in malignant ductal structures (black arrow) and less than 2% in neuroendocrine cells (Immunohistochemistry, 20×).
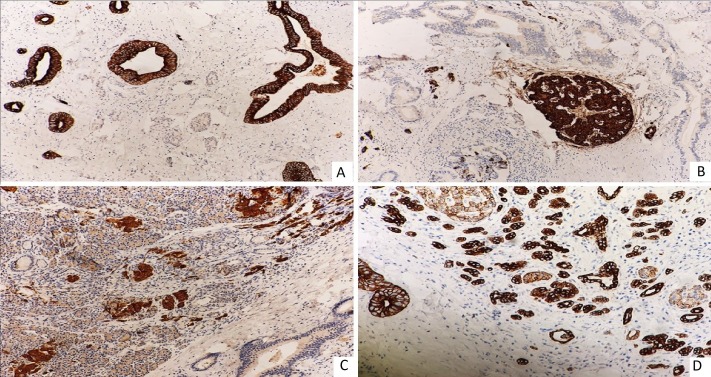
On immunohistochemistry, the atypical ductal structures were positive for CK-7, and nests of neuroendocrine cells were immunoreactive to chromogranin A (CgA) and synaptophysin (Syn). E-cadherin showed strong membranous immunoreactivity in ductal structure and weak positivity in neuroendocrine cells (figure 2A–D). These findings were consistent with MiNEN of pancreas.
Figure 2.
(A) CK7 membranous immunoreactivity in duct-like structure (IHC, 20×). (B) Synaptophysin immunoreactivity in neuroendocrine cells (IHC, 20×). (C) Chromogranin immunoreactivity in neuroendocrine cells (IHC, 10×). (D) E-cadherin showing strong membranous immunoreactivity in ductal structure and weak in neuroendocrine cells (IHC, 10×). IHC, Immmunohistochemistry.
Outcome and follow-up
His feeding jejunostomy trial feed was given on second postoperative day (POD), which he tolerated well and gradually his feed was increased. He was allowed orally on fourth POD, which he tolerated well. His abdominal drain was removed on fifth POD and discharged on seventh POD in stable condition. He received four cycles of adjuvant chemotherapy (gemcitabine with cisplatin). At the end of 1 year follow-up, surveillance of CECT abdomen showed no evidence of recurrence and he is doing well.
Discussion
MiNEN of pancreas is characterised by admixture of two malignant components, adenocarcinoma and NET, with one constituent involves at least one-third of the tumour.1 In MiNEN, acinar adenocarcinoma as one component has been reported frequently in the literature,4 5 but ductal adenocarcinoma as one constituent has been stated rarely.2 6–8 In context to the neuroendocrine component, MiNEN can have either NET (grade 1 or 2) or neuroendocrine carcinoma (NEC) (grade 3), which was previously termed as mixed adeno-NEC (MANEC).1 Usually, MiNEN contains NEC (poorly differentiated, Ki67 >20%),2 5 8 however, NET (well differentiated, low Ki67)6 7 can also be present, as in our case.
MiNEN is further subcategorised morphologically into collision tumour in which two different types of cancer are separated by stroma with no mixture area in transition zone.6 7 9 This pattern consists of both neoplastic ductal and neuroendocrine cells forming glandular, cribriform, solid or trabecular structures and often showing intracellular or extracellular mucin accumulation. Another variant that has intermingled component of both malignancies with no separate zone, actually formed the true mixed variety of MANEC. This type of true mixed variant has been reported only three times in the past,2 8 10 and our case added the fourth one in this category.
MiNEN usually presents in elderly (median age ~60 years) man with median size of the tumours ~2.1 cm (2.0–2.5 cm) and located variably in all parts of pancreas.4 9 The clinical picture is also flexible with patients presenting with pain in abdomen, jaundice or sometimes diagnosed incidentally. We also encountered this in an elderly male patient with tumour located in head of pancreas measuring about 3 cm and presented with painful progressive jaundice.
CECT abdomen usually fails to diagnose MiNEN preoperatively as this is primarily a pathological diagnosis. Adenocarcinomas are usually hypovascular lesions and NETs are well circumscribed enhancing lesions on CECT. Imaging features will depend on the ratios of adenocarcinoma and NEC cells and hence, diagnosis by radiological imaging is difficult.
Endoscopic ultrasound-guided fine needle aspiration cytology is an adjunct tool for preoperative diagnosis.4 However, it is not used in all cases due to its high negative predictive value and second it will not be able to access whole tumour to diagnose MiNEN especially.
Pancreatoduodenectomy is the standard procedure for carcinoma head of pancreas with R0 resection. Histopathological examination with immunohistochemistry of surgically resected specimen is the gold standard to confirm MiNEN. Various hypotheses have been proposed for the development of MiNEN. It ranges from origin of both ductal and endocrine components from similar totipotent pancreatic stem cells (common precursor cells) to dysfunction of numerous tumour–suppressor genes resulting in different types of malignancy (random association).8 10 The diagnosis is clinched with immunohistochemistry in which periodic acid–Schiff reactivity and positivity for carcinoembryonic antigen (CEA) and mucin-1 (MUC1) indicate ductal differentiation and positivity for Syn and CgA indicates neuroendocrine differentiation.3 The tumour cells may contain mucin and neurosecretory granules. MiNEN must be distinguished from ductal adenocarcinomas showing cords and nests of non-neoplastic neuroendocrine cells intimately attached to well-differentiated neoplastic duct-like glands.
Due to its low incidence, the clinical and biological behaviour is unclear. Adjuvant chemotherapy is usually considered after R0 resection, however, it is not clear whether to deal with both tumours or the one with major component with adjuvant treatment. La Rosa et al suggested that management should focus on the dominant cell type because the outcome of such mixed tumour follows that of a more aggressive cell type.3 In our case, the ductal adenocarcinoma component was predominant and neuroendocrine component was non-functional, hence the first component was dealt in adjuvant settings. The overall prognosis is poor for MiNENs,2 3 5 however, due to its paucity and scarcity of follow-up data, the actual prognosis is yet to be defined.
Hence, to conclude MiNEN of the pancreas is extremely rare, hence it is important to report such anecdotal cases to get knowledge about their clinical and biological behaviour of such tumour and standardise optimal therapy.
Learning points.
Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) of pancreas is a rare pathology and difficult to diagnose preoperatively.
Resectable carcinoma head of pancreas in elderly should be considered for R0 resection, as it can offer a disease-free interval with improved quality of life.
Histopathology with immunohistochemistry should be mandatory for all pancreatoduodenectomy specimen to diagnose such rare malignancy.
Long-term follow-up is required to get knowledge about the clinical and biological behaviour of all MiNENs.
Footnotes
Contributors: VKV was involved as the treating surgeon and in patient management. BV, JNB and TY were involved in the diagnosis of disease. BV, JNB and VKV were involved in writing the initial draft of the manuscript. All authors contributed to refining the study and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.de Mestier L, Cros J, Neuzillet C, et al. . Digestive system mixed Neuroendocrine-Non-Neuroendocrine neoplasms. Neuroendocrinology 2017;105:412–25. 10.1159/000475527 [DOI] [PubMed] [Google Scholar]
- 2.Düzköylü Y, Aras O, Bostancı EB, et al. . Mixed Adeno-Neuroendocrine carcinoma; case series of ten patients with review of the literature. Balkan Med J 2018;35:263–7. 10.4274/balkanmedj.2017.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Rosa S, Sessa F, Uccella S, et al. . Mixed Neuroendocrine-Nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol 2016;27:284–311. 10.1007/s12022-016-9432-9 [DOI] [PubMed] [Google Scholar]
- 4.Strait AM, Sharma N, Tsapakos MJ, et al. . Pancreatic mixed acinar-neuroendocrine carcinoma, a unique diagnostic challenge on FNA cytology: a small series of two cases with literature review. Diagn Cytopathol 2018;46:971–6. 10.1002/dc.23981 [DOI] [PubMed] [Google Scholar]
- 5.Ogbonna OH, Garcon MC, Syrigos KN, et al. . Mixed acinar-neuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med 2013;2013:1–3. 10.1155/2013/705092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Gandhi S, Basu A, et al. . Pancreatic collision tumor of ductal adenocarcinoma and neuroendocrine tumor. ACG Case Rep J 2018;5:e39. 10.14309/crj.2018.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serafini S, Da Dalt G, Pozza G, et al. . Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J Surg Oncol 2017;15:93. 10.1186/s12957-017-1157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata M, Takahashi H, Yamada M, et al. . A case of mixed adenoneuroendocrine carcinoma of the pancreas: immunohistochemical analysis for histogenesis. Medicine 2017;96:e6225. 10.1097/MD.0000000000006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imaoka K, Fukuda S, Tazawa H, et al. . A mixed adenoneuroendocrine carcinoma of the pancreas: a case report. Surg Case Rep 2016;2:133. 10.1186/s40792-016-0263-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xenaki S, Lasithiotakis K, Andreou A, et al. . A rare case of mixed neuroendocrine tumor and adenocarcinoma of the pancreas. Case Rep Surg 2016;2016:1–4. 10.1155/2016/3240569 [DOI] [PMC free article] [PubMed] [Google Scholar]