Abstract
We report an 86-year-old woman who was diagnosed with multiple myeloma (MM) and was receiving chemotherapy since the age of 82. A high echoic mass attached to the mitral valve was observed on transthoracic echocardiography 4 years after the treatment. The possibility of malignancy could not be ruled out, and hence, the mass was excised surgically. Pathologically, most of the mass consisted of calcified lesion without tumour tissue, and these findings were not inconsistent with calcified amorphous tumour (CAT). This case suggests that CAT may be associated with MM and has been reported after a thorough literature review.
Keywords: cardiovascular medicine, haematology (incl blood transfusion)
Background
Calcified amorphous tumour (CAT) is a non-neoplastic mass first described by Reynolds et al in 1997.1 On examination of cases in which the clinical presentation was in the form of a tumour and subsequently excised, they found examples of altered blood components and chronic inflammation in pathological tissue, as well as calcification nodules of varying size on a background of fibrin-like material. This condition is described as CAT and is comparatively more frequent in older adults and those with chronic kidney failure. Furthermore, this condition is defined as not including calcified tumours.1 Reports of CAT are rare, and a clear cause leading to CAT remains elusive. To our knowledge, this is the first case of CAT associated with multiple myeloma (MM), making it a valuable learning opportunity for clinicians and researchers in the field.
Case presentation
We present a case of an 86-year-old woman who had been on amlodipine 5 mg and mitiglinide 10 mg since she was 70 years of age for the treatment of hypertension and type 2 diabetes mellitus (DM).
At the age of 82, the patient was diagnosed with IgG k type MM with International Staging System Phase I (albumin 3.7 g/dL and serum β2 microglobulin 2.7 mg/dL).
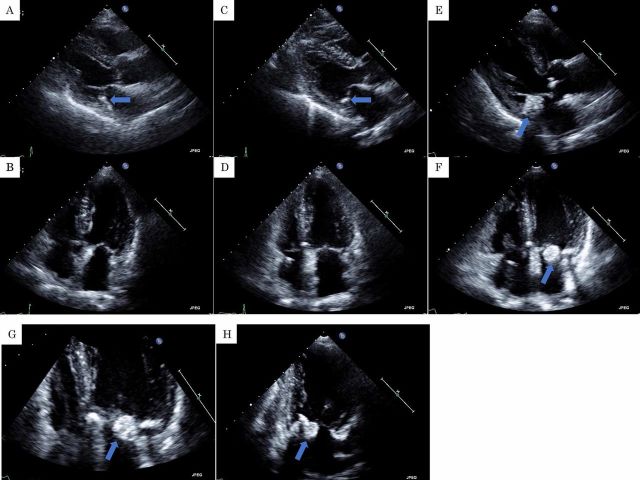
At that time, the serum creatinine was 0.61 mg/dL, estimated glomerular filtration rate of 69.75 mL/min and stage 2 chronic kidney disease (CKD). Haemoglobin A1c (HbA1c) was at 7.4% and a plain X-ray of skull did not reveal the presence of bone abnormalities (figure 1A, B). Furthermore, a transthoracic echocardiography (TTE) performed prior to chemotherapy revealed left ventricular ejection fraction (LVEF) of 60%. Mitral annular calcification (MAC) and trivial mitral regurgitation (MR) were observed but mitral stenosis (MS) was not. There were no apparent tumorous lesions on the mitral valve (MV) or other sites in the heart (figure 2A, B).
Figure 1.
(A, B) A plain X-ray of skull did not reveal the presence of bone abnormalities.
Figure 2.
(A, B) A transthoracic echocardiography (TTE) prior to chemotherapy revealed mitral annular calcification (MAC) (arrow). There were not any apparent tumorous lesions on the mitral valve or other sites. (C, D) A TTE approximately 31 months after MPB therapy and prior to treatment with lenalidomide, dexamethasone and darbepoetin alfa showed MAC (arrow). There were not any obvious tumorous lesions on the mitral valve or other sites. (E, F, G and H) A TTE approximately 18 months after treatment with lenalidomide, dexamethasone and darbepoetin alfa revealed a 20 mm high echoic mass on the mitral valve (arrow). (A, C, E) parasternal long axis view; (B, F, G) apical four-chamber view; (H) apical two-chamber view.
Three courses of melphalan, prednisolone and bortezomib (MPB) therapy (melphalan (MEL) 8 mg (days 1–4), prednisolone (PSL) 25 mg (days 1, 8, 22), bortezomib (BOR) 1.3 mg (1 mg/m2) (days 1–4)) were administered, and then seven courses of BOR 1.7 mg (1.3 mg/m2) (days 1, 8, 15, 22) and dexamethasone (DEX) 12 mg (day 1, 8, 15, 22) (once weekly for 4 weeks, then discontinued for 5 weeks) were conducted. Afterwards, an International Myeloma Working Group uniform response criteria of a stable disease (SD) was maintained.
Thirty months after the beginning of chemotherapy, complete atrioventricular block was observed. Therefore, dual chamber pacemaker implantation surgery was performed. A TTE at that time was not much different from that of prior to chemotherapy (figure 2C, D).
Afterwards, chemotherapy for MM was discontinued and patient progress was observed carefully. However, 5 months later, the condition transitioned into a state of progressive disease, so a regimen of lenalidomide (Ld) 5 mg (days 1–21 (every other day)+DEX 8 mg (days 8, 22) was started. Additionally, 100 mg of aspirin was initiated to prevent thrombosis. Twelve courses were administered. Later, the patient complained of acetabulofemoral joint inflammation. DEX was suspected to be the cause. Therefore, we changed the regimen to 5 mg dose of Ld alone and further six courses were conducted. Progress later improved to SD. Eleven subcutaneous injections of darbepoetin alfa 30 µg were administered after the start of Ld treatment for anaemia that developed in conjunction with MM. Haemoglobin later stabilised to around 100–110 g/L.
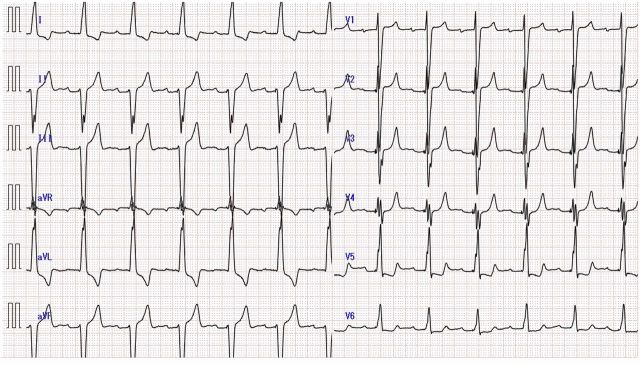
A TTE conducted approximately 18 months after treatment with Ld, DEX and darbepoetin alfa showed a 20 mm high echoic immobile mass on the MAC not observed at the time of pacemaker implantation (figure 2E–H) (video 1). MR was trivial and MS was not observed. LVEF was preserved at 60%. CAT was strongly suspected, but a cardiac neoplasm could not be ruled out. At that time, the subject was conscious and lucid. Shortness of breath and other notable symptoms were not evident. Blood pressure was 130/80 mm Hg, pulse rate was 70 bpm, body temperature was 36.5℃ and transcutaneous oxygen saturation was 99% in room air. There were no obvious abnormal findings on the body surface. No heart murmur was observed, and there were no abnormal neurological findings such as paralysis. The 12-lead ECG showed atrial response and ventricular stimulation rhythm from a pacemaker (figure 3).
Video 1.
Figure 3.
The 12-lead ECG showed atrial response and ventricular stimulation rhythm from a pacemaker.
At the time, type I collagen cross-linked N-telopeptide (NTX) was 35.1 nmol bone collagen equivalent (BCE)/L (normal range: 10.7–24.0 nmol BCE/L), tartrate-resistant acid phosphatase 5b (TRACP-5b) was 423 mU/dL (normal: 120–420 mU/dL) and total type I procollagen N-terminal propeptide (TOTAL P1NP) was 90.7 ng/mL (normal: 26.4–98.2 ng/mL). Abnormal signal intensity indicating embolism was not observed in a diffusion-weighted MRI (figure 4A, B). During this time, the corrected calcium value was within normal range. However, an MRI of the spine showed several lesions with low signal in T1 contrast images and high signal with short TI inversion recovery (STIR) (figure 4C–F). The patient remained within the range of CKD stage 2-3b given serum creatinine values of about 0.6–0.8 mg/dL throughout the period.
Figure 4.
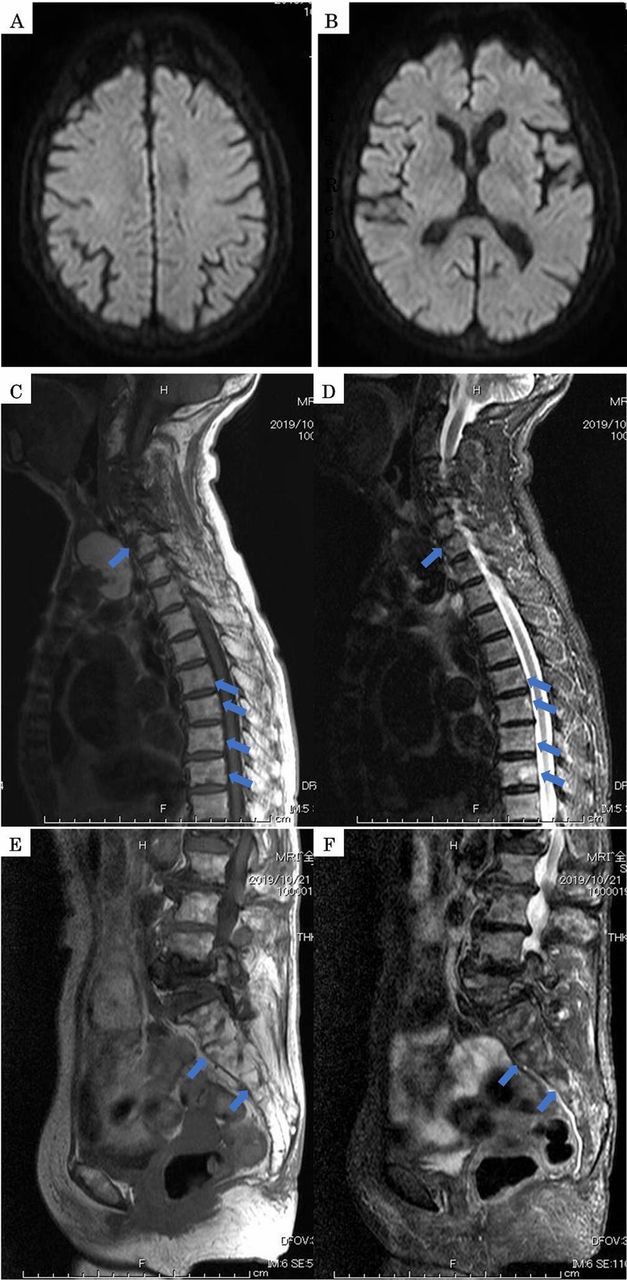
(A, B) Abnormal signal intensity was not observed in a diffusion-weighted MRI. (C, D, E and F) An MRI of the spine showed several lesions with low signal in T1 contrast images and high signal with short TI inversion recovery (arrow).
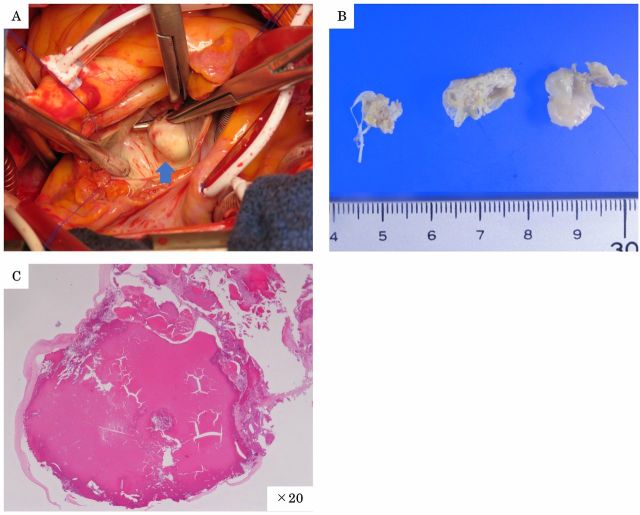
There was no clear growth during the subsequent 2-month period, but the intracardiac mass seemed to have grown rapidly, and we were concerned of increasing risk of valve dysfunction, embolism or malignancy. Therefore, open-heart surgery was performed 2 months after the mass lesion was found, at which time the mass was excised, and the MV was replaced with magna mitral EASE 23 mm (Edwards Lifesciences, Irvine, California, USA). Findings during surgery included visible mass formed on the calcified portions of the MV spanning P2-P3 from valve cusp to valve annulus (figure 5A). No signs of invasion by the intracardiac mass into the myocardium were observed. Yellowish-white tumour was excised (figure 5B). Pathologically, most of the mass consisted of calcified lesion without tumour tissue (figure 5C), and these findings were not inconsistent with CAT.
Figure 5.
(A) Findings during surgery included visible mass formed on the calcified portions of the MV spanning P2-P3 from valve cusp to valve annulus (A) (arrow). (B) Photograph of the excised tumour. Yellowish-white tumour was excised. (C) Pathologically, tumour tissue was not found in the mass. Most of the mass was a calcified lesion, consistent with calcified amorphous tumour. (C) H&E; ×20.
Differential diagnosis
Although CAT was strongly suspected as the sonographic factors suggest high echoic mass, we could not rule out a benign cardiac tumour such as myxoma, lipoma, papillary fibroelastoma, rhabdomyoma, fibroma, haemangioma and malignant tumours such as sarcomas, lymphoma, pericardial mesothelioma. Therefore, open-heart surgery was performed, and the mass was excised. Histologically, most of the mass was consisted with a calcified lesion without tumour tissue (figure 5C), and these findings were not inconsistent with CAT.
Outcome and follow-up
Apparent complications were absent in the perioperative period and the recovery was favourable. Postoperatively, overall, MR and MS were not observed in TTE images, and LVEF was maintained at a level of 60%. MM treatment was discontinued, and the patient was followed up for 4 months. No obvious findings indicating recurrence of MM or CAT were observed.
Discussion
Reports of CAT are rare, and our investigation only revealed 63 cases1–44 (table 1).
Table 1.
Literature review of cardiac calcified amorphous tumour cases (n=63)
| Variables | Values |
| Age | 58±17 (16–85) |
| Male:Female | 24:39 |
| Tumour site | |
| Left heart | 45 (71) |
| MV (multiple possible) | 30 (48) |
| LV (multiple possible) | 16 (25) |
| LA | 5 (8) |
| PM (multiple possible) | 1 (2) |
| Right heart | 18 (29) |
| RV (multiple possible) | 8 (14) |
| TV (multiple possible) | 2 (3) |
| RA (multiple possible) | 9 (14) |
| SVC (multiple possible) | 1 (2) |
| Comorbidities | |
| ESRD | 23 (37) |
| HD | 21 (23) |
| Non-HD | 1 (2) |
| Peritoneal dialysis | 1 (2) |
| MAC | 19 (32) |
| DM | 15 (24) |
| Embolism | 21 (33) |
| Arterial system | 15 (24) |
| CI | 9 (14) |
| TIA | 1 (2) |
| Retinal artery | 3 (5) |
| ALI | 1 (2) |
| MI | 1 (2) |
| Venous circulation | 6 (10) |
| PE | 6 (10) |
| Treatment | |
| Surgical excision | 56 (89) |
| Aspirin | 1 (2) |
Values are given as mean±SD, no. (%) or <range>.
ALI, acute limb ischaemia; CI, cerebellar infarction; ESRD, end-stage renal disease; HD, haemodialysis; LA, left atrial; LV, left ventricular; MAC, mitral annular calcification; MI, myocardial infarction; MV, mitral valve; PE, pulmonary embolism; PM, papillary muscle; RA, right atrial; RV, right ventricular; SVC, superior vena cava; TIA, transient ischaemic attack; TV, tricuspid valve.
Among these cases, the mean age was 58±17 (mean±SD) (range: 16–85 years) and the male:female ratio was 24:39. The majority of attachment sites were on either the MV in 30 cases (48%)1–3 5–9 15 19 24 27 29 30 32–34 36–44 or the left ventricle (LV) in 16 cases (25%).1–5 7 19 20 27 28 31 44 Attachment occurred on the left heart (LV, left atrium, papillary muscle (PM), MV) in 45 cases (71%), the right heart (superior vena cava (SVC), right atrium (RA), right ventricle (RV) and tricuspid valve (TV)) in 18 cases (31%).1 10–14 16–18 22 25–27 Some cases involved multiple attachment sites, such as six cases of LV+MV,1–3 5 7 24 one case of LV+PM,7 one case of RV+TV1 and one case of RA+SVC.1 Combined presence in the left and right heart was not found. The most commonly reported comorbidities are end-stage renal disease (ESRD)1 6–9 15 16 29 30 33 34 37–39 41 43 44 with 23 cases (37%). The cases of combined with MAC and ESRD were found in 14 cases (22%).6–9 15 29 30 33 34 37 38 41 Among the ESRD cases, there was one each involving absence of dialysis29 and presence of peritoneal dialysis.29 After ESRD, there were many cases of MAC6–9 17 23 29–34 37 38 40–42 and DM.1 2 7 8 15 27 29 30 33 35 37 39 41–43 Symptoms of embolisms were found in 21 cases (33%),1 2 4 11–13 17 19 25 30 33–36 38 40 44 which is considered a relatively high percentage. Specifically, nine cases of cerebellar infarctions (14%),1 19 33–35 38 44 one case of a transient ischaemic attack (2%),36 three cases of retinal artery occlusion (5%),1 2 4 one case of acute limb ischaemia (2%),30 one case of myocardial infarction (2%)40 and six cases of pulmonary embolisms (10%).11–13 17 25 Also, of the 15 arterial embolism cases, 33% (13/45) involving CAT formation in the left heart is a considerably high rate. Similar investigation of the right heart revealed a rate of 33% (6/18), or a risk of embolism approximately the same as with the left heart. These findings suggest that both the left and the right heart share an approximate 30% risk of CAT embolism, warranting thorough consideration. There is only one reported case of regression under an aspirin regimen,34 and most cases (56 cases, 89%) involve surgical removal.1 2 4 6–27 29–33 35–44
The mechanism by which CAT occurs is not clear, but examination of the selected literature suggests two purported mechanisms.
One hypothesis to explain this is the tendency to form a thrombus.1 12 45 In fact, Multiple Myeloma is identified by the onset of thrombotic events.46 This mechanism involves defective fibrin structure and fibrinolysis due to increased immunoglobulin levels. The presence of antibodies such as lupus anticoagulant have shown increased rates of acquired activated protein C resistance, and synthesis of other inflammatory markers such as interleukin 6.47 48
Although reports indicate an increase in thromboembolic events with Ld and steroid therapies49–51 and MEL and PSL therapy, the underlying mechanism remains to be elucidated.52 53 In addition, when large doses of erythropoietin are used during Ld or DEX therapy, thromboembolic events increase, compared with when erythropoietin is not used.51 54 55
Considering International Myeloma Working Group guidelines,56 low molecular weight heparin (LMWH) or vitamin K antagonist is recommended as an antithrombotic drug for reducing thromboembolic events in this case. Alternatively, there are reports that aspirin has been appropriate prophylaxis in patients who received Ld in combination with DEX, MEL, doxorubicin or erythropoietin, reducing the incidence of venous thromboembolism (VTE),50 51 57 58 and LMWH was associated with a significant reduction in the risk of symptomatic VTE compared with vitamin K antagonists, although the difference between LMWH and aspirin was not statistically significant.59 For these reasons, we have used aspirin to prevent the thromboembolic event.
These considerations also indicate that the CAT in this case may have been formed due to increased thrombotic tendency as a side effect of MM treatment. However, neither clots nor fibrin-like structures were observed during pathological analysis. Moreover, the use of aspirin did not inhibit CAT. Therefore, we cannot deny the possibility that a prothrombotic environment contributed to the onset of CAT.
Another hypothesis is that CAT resulted from calcium metabolism abnormality.6 8 12 28 30 39 41 43 45 60 Patients with MM, such as the individual described herein, much like patients undergoing dialysis, suffer from severe disease-causing osteolytic lesions via the inhibition of bone formation regardless of increased bone resorption.61 This, in turn, is known to present as irregular calcium metabolism, manifesting in bone metabolism as an elevation in bone resorption markers due to MM. Conversely, bone formation markers are shown to be relatively low.61 62 These bone metabolism markers are thought to be useful in the early diagnosis of bone disease, predicting the onset of bone-related phenomena, and evaluating the efficacy of treatment.63 Patients undergoing dialysis are also thought to be more receptive to MAC given their abnormal metabolite of calcium and phosphor. Reduction of the progression of calcification is linked to the inhibition of MAC and CAT, and the importance of maintaining balanced calcium and phosphorus concentrations is considered key.39 41 In cases of advanced ectopic calcification, particularly in patients undergoing chronic dialysis therapy, MAC is more likely to progress towards CAT.44 CAT has also been reported in patients with normal calcium values like this case.30 43
In this case, NTX 35.1 nmol BCE/L and TRACP-5b at 423 mU/dL showed increased bone absorption, while TOTAL P1NP at 90.7 ng/mL not only failed to increase bone formation but also presented bone metabolic abnormality.
From these laboratory findings, bone metabolic abnormalities due to physiological osteoporosis were equated; however, the lesions showed as low signal in T1 contrast images, and high signal with STIR in the MRI of the spine,61 64 suggesting that the lesion was deemed to be MM. Together, these factors may have led to abnormal calcium metabolism as a result of MM. From this, in the case in our study, it is thought that abnormal calcium metabolism caused by MM combined with MAC, DM and advanced age, led to CAT formation. There have been no previous case reports of comorbidity with MM, therefore, our case is considered to be rare.
In addition, we evaluated X-rays of the skull before initiating MM treatment and could not observe bone lesions. Still, it is said that MRI is more useful and appropriate for detecting early bone lesions.65
In conclusion, there is still much uncertainty regarding physiopathology of CAT; however, we suggested that the CAT was developed in association with MM of such a clinical course. We found references to concurrent risk of embolism in the literature and, therefore, careful follow-up by TTE or another method is recommended to mitigate such a risk. To our knowledge, this is the first case of CAT associated with MM, and further studies involving more patients are needed.
Learning points.
Although clear cause leading to calcified amorphous tumour (CAT) remains elusive, according to the reports so far, the tendency to form a thrombus and/or calcium metabolism abnormalities is presumed as the cause.
Pathological evaluation is necessary for definitive diagnosis of CAT.
We should recognise the possibility of CAT to develop in association with multiple myeloma and CAT poses an approximately 30% risk of embolism, which is not low.
Acknowledgments
We would like to show our greatest appreciation to Dr Koutarou Miyata, Dr Yoshimaro Ichinohe, Dr Masanori Ono and Dr Mitsuko Iiyama. Without their persistent help, this paper would not have been possible.
Footnotes
Contributors: TY wrote the initial draft of the manuscript. TY, TF, TU and ST contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. The final version of the manuscript was approved by all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Reynolds C, Tazelaar HD, Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT). Hum Pathol 1997;28:601–6. 10.1016/S0046-8177(97)90083-6 [DOI] [PubMed] [Google Scholar]
- 2.Vlasseros I, Katsi V, Tousoulis D, et al. Visual loss due to cardiac calcified amorphous tumor: a case report and brief review of the literature. Int J Cardiol 2011;152:e56–7. 10.1016/j.ijcard.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Ho HH, Min JK, Lin F, et al. Images in cardiovascular medicine. Calcified amorphous tumor of the heart. Circulation 2008;117:171–2. 10.1161/CIRCULATIONAHA.107.730838 [DOI] [PubMed] [Google Scholar]
- 4.Nazli Y, Colak N, Atar IA, et al. Sudden unilateral vision loss arising from calcified amorphous tumor of the left ventricle. Tex Heart Inst J 2013;40:453–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Habib A, Friedman PA, Cooper LT, et al. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol 2010;29:175–8. 10.1007/s10840-009-9418-3 [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara M, Watanabe H, Iino T, et al. Two cases of calcified amorphous tumor mimicking mitral valve vegetation. Circulation 2012;125:432–4. 10.1161/CIRCULATIONAHA.111.072793 [DOI] [PubMed] [Google Scholar]
- 7.Kubota H, Fujioka Y, Yoshino H, et al. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg 2010;90:1692–4. 10.1016/j.athoracsur.2010.04.097 [DOI] [PubMed] [Google Scholar]
- 8.Kawata T, Konishi H, Amano A, et al. Wavering calcified amorphous tumour of the heart in a haemodialysis patient. Interact Cardiovasc Thorac Surg 2013;16:219–20. 10.1093/icvts/ivs430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamedali B, Tatooles A, Zelinger A. Calcified amorphous tumor of the left ventricular outflow tract. Ann Thorac Surg 2014;97:1053–5. 10.1016/j.athoracsur.2013.06.115 [DOI] [PubMed] [Google Scholar]
- 10.Lewin M, Nazarian S, Marine JE, et al. Fatal outcome of a calcified amorphous tumor of the heart (cardiac cat). Cardiovasc Pathol 2006;15:299–302. 10.1016/j.carpath.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 11.Vaideeswar P, Karunamurthy A, Patwardhan AM, et al. Cardiac calcified amorphous tumor. J Card Surg 2010;25:32–5. 10.1111/j.1540-8191.2009.00943.x [DOI] [PubMed] [Google Scholar]
- 12.Fealey ME, Edwards WD, Reynolds CA, et al. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol 2007;16:115–8. 10.1016/j.carpath.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Chaowalit N, Dearani JA, Edwards WD, et al. Calcified right ventricular mass and pulmonary embolism in a previously healthy young woman. J Am Soc Echocardiogr 2005;18:275–7. 10.1016/j.echo.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Khulbey S, Ramana KV, Kumar S, et al. Amorphous cardiac tumor of right atrium late after atrial septal defect closure. Indian J Thorac Cardiovasc Surg 2008;24:129–31. 10.1007/s12055-008-0018-0 [DOI] [Google Scholar]
- 15.Inamdar V, Wanat FE, Nanda NC, et al. Amorphous calcific tumor of the mitral annulus echocardiographically mimicking a vegetation. Echocardiography 2008;25:537–9. 10.1111/j.1540-8175.2008.00638.x [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-Barrios A, Muriel-Cueto P, Lancho-Novillo C, et al. Calcified amorphous tumor of the heart. Rev Esp Cardiol 2008;61:892–3. 10.1016/S1885-5857(08)60239-X [DOI] [PubMed] [Google Scholar]
- 17.Flynn A, Mukherjee G. Calcified amorphous tumor of the heart. Indian J Pathol Microbiol 2009;52:444–6. 10.4103/0377-4929.55026 [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Hote M, Ray R. Calcified amorphous tumor of the heart in an adult female: a case report. J Med Case Rep 2010;4:278. 10.1186/1752-1947-4-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greaney L, Chaubey S, Pomplun S, et al. Calcified amorphous tumour of the heart: presentation of a rare case operated using minimal access cardiac surgery. BMJ Case Rep 2011;2011. 10.1136/bcr.02.2011.3882. [Epub ahead of print: 03 Jun 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananthakrishna R, Nanjappa MC, Kamalapurkar G, et al. Cardiac tumour in a patient with rheumatic heart disease. BMJ Case Rep 2011;2011:bcr0420114146. 10.1136/bcr.04.2011.4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y-C, Tsai Y-T, Tsai C-S. Calcified amorphous tumor of left atrium. J Thorac Cardiovasc Surg 2011;142:1575–6. 10.1016/j.jtcvs.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 22.Sousa JSde, Tanamati C, Marcial MB, et al. Calcified amorphous tumor of the heart: case report. Rev Bras Cir Cardiovasc 2011;26:500–3. 10.5935/1678-9741.20110031 [DOI] [PubMed] [Google Scholar]
- 23.Nishigawa K, Takiuchi H, Kubo Y, et al. Calcified amorphous tumor: three-dimensional transesophageal echocardiography. Asian Cardiovasc Thorac Ann 2012;20:355. 10.1177/0218492311423071 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Nishimori H, Wariishi S, et al. Cardiac calcified amorphous tumor stuck in the aortic valve that mimicked a chameleon's tongue: report of a case. Surg Today 2014;44:1751–3. 10.1007/s00595-013-0698-y [DOI] [PubMed] [Google Scholar]
- 25.Rehman A, Heng EE, Cheema FH. Calcified amorphous tumour of right ventricle. Lancet 2014;383:815. 10.1016/S0140-6736(13)60997-6 [DOI] [PubMed] [Google Scholar]
- 26.Choi EK, Ro JY, Ayala AG. Calcified amorphous tumor of the heart: case report and review of the literature. Methodist Debakey Cardiovasc J 2014;10:38–40. 10.14797/mdcj-10-1-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain N, Rahman N, Rehman A. Calcified amorphous tumors (CATs) of the heart. Cardiovasc Pathol 2014;23:369–71. 10.1016/j.carpath.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Mitsunaga M, Uchida H, Nagao T, et al. A case of hemodialysis patient who had subcutaneous tumoral calcinosis of the upper extremities and calcified amorphous tumor of the heart. Nihon Toseki Igakkai Zasshi 2001;34:1427–33. (in Japanese) (Abstract in English) 10.4009/jsdt.34.1427 [DOI] [Google Scholar]
- 29.Takahashi S, Kikuta T, Hashiwada S, et al. Left ventricular outflow tract mobile mass echo in the follow up mitral annulus calcification: a case report. Jpn J Med Ultrasound Technol 2016;41:400–6. (in Japanese). [Google Scholar]
- 30.Nakashima Y, Terauchi Y, Noguchi T, et al. A case of cardiac calcified amorphous tumor (cardiac CAT) causing acute embolism in right common iliac artery. J Cardiol Cases 2015;11:81–4. 10.1016/j.jccase.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao K, Shigehisa Y. Suddenly appearing swinging calcified amorphous tumor in the left ventricular outflow tract in a patient with end-stage renal failure. Jpn J Cardiovasc Surg 2017;46:226–30. (in Japanese) (Abstract in English) 10.4326/jjcvs.46.226 [DOI] [Google Scholar]
- 32.Honda S, Kawasaki T, Yamano M, et al. A case of calcified amorphous tumor with caseous calcification of the mitral annulus. Choonpa Igaku 2016;43:577–80. (in Japanese) (Abstract in English) 10.3179/jjmu.JJMU.A.60 [DOI] [Google Scholar]
- 33.Kimura N, Haruta H, Tamaki T, et al. A case of calcified amorphous tumor found with cerebral infarction. Shinzo 2017;49:502–7. (in Japanese). [Google Scholar]
- 34.Saito K, Doi M, Karikusa M, et al. Cerebral infarction in right pons during the course of mobile mitral annular calcification-related calcified amorphous tumor during a long time hemodialysis. Rinsho Shinkeigaku 2016;56:580–3. (in Japanese) (Abstract in English). 10.5692/clinicalneurol.cn-000895 [DOI] [PubMed] [Google Scholar]
- 35.Suh JH, Kwon JB, Park K, et al. Calcified amorphous tumor in left atrium presenting with cerebral infarction. J Thorac Dis 2014;6:1311–4. 10.3978/j.issn.2072-1439.2014.07.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasi Teshnizi M, Ghorbanzadeh A, Zirak N, et al. Cardiac calcified amorphous tumor of the mitral valve presenting as transient ischemic attack. Case Rep Cardiol 2017;2017:2376096:1–3. 10.1155/2017/2376096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shikata N, Muro T, Nakajima H, et al. A case of rapidly progressing calcified amorphous tumor on the mitral valve. J Cardiol Jpn Ed 2011;6:77–80. (in Japanese). [Google Scholar]
- 38.Osawa H, Yamaguchi T, Sato H, et al. [Surgical removal of left atrial tumor originating from mitral annular calcification in a chronic hemodialysis patient: a case report]. Kyobu Geka 2000;53:1119–21. (in Japanese) (Abstract in English). [PubMed] [Google Scholar]
- 39.Morishima A, Sasahashi N, Ueyama K. [Calcified amorphous tumors with excision in hemodialysis patients: report of 2 cases]. Kyobu Geka 2006;59:851–4. (in Japanese) (Abstract in English). [PubMed] [Google Scholar]
- 40.Kim A, Kotani S, Kure Y, et al. A case of calcified amorphous tumor developed rapidly after acute myocardial infarction. Shinzo 2018;50:305–10. (in Japanese). [Google Scholar]
- 41.Satou F, Yamauchi T, Terasawa F, et al. A case of calcified amorphous tumor presenting rapid growth in a hemodialysis patent. Shinzo 2017;49:141–5. (in Japanese). [Google Scholar]
- 42.Ijuin S, Sonoda M, Tanoue K, et al. A case of calcified amorphous tumor on the mitral valve in patient with non-Hemodialysis. Shinzo 2014;46:1261–6. (in Japanese). [Google Scholar]
- 43.Yoshimura S, Kawano H, Minami T, et al. Cardiac calcified amorphous tumors in a patient with hemodialysis for diabetic nephropathy. Intern Med 2017;56:3057–60. 10.2169/internalmedicine.9057-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchihashi K, Nozawa A, Marusaki S, et al. Mobile intracardiac calcinosis: a new risk of thromboembolism in patients with haemodialysed end stage renal disease. Heart 1999;82:638–40. 10.1136/hrt.82.5.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Hemptinne Q, de Cannière D, Vandenbossche J-L, et al. Cardiac calcified amorphous tumor: a systematic review of the literature. Int J Cardiol Heart Vasc 2015;7:1–5. 10.1016/j.ijcha.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catovsky D, Ikoku NB, Pitney WR, et al. Thromboembolic complications in myelomatosis. Br Med J 1970;3:438–9. 10.1136/bmj.3.5720.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elice F, Fink L, Tricot G, et al. Acquired resistance to activated protein C (aAPCR) in multiple myeloma is a transitory abnormality associated with an increased risk of venous thromboembolism. Br J Haematol 2006;134:399–405. 10.1111/j.1365-2141.2006.06208.x [DOI] [PubMed] [Google Scholar]
- 48.Zangari M, Saghafifar F, Mehta P, et al. The blood coagulation mechanism in multiple myeloma. Semin Thromb Hemost 2003;29:275–82. 10.1055/s-2003-40965 [DOI] [PubMed] [Google Scholar]
- 49.Rajkumar SV, Jacobus S, Callander N, et al. A randomized phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group [abstract]. ASH Annu Meet Abstr 2006;108:799. [Google Scholar]
- 50.Menon SP, Rajkumar SV, Lacy M, et al. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer 2008;112:1522–8. 10.1002/cncr.23336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med 2006;354:2079–80. 10.1056/NEJMc053530 [DOI] [PubMed] [Google Scholar]
- 52.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007;370:1209–18. 10.1016/S0140-6736(07)61537-2 [DOI] [PubMed] [Google Scholar]
- 53.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006;367:825–31. 10.1016/S0140-6736(06)68338-4 [DOI] [PubMed] [Google Scholar]
- 54.Dimopoulos MA, Spencer A, Attal M, et al. Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of a phase 3 study (MM-010). ASH Annu Meet Abstr 2005;6:106. [Google Scholar]
- 55.Weber D, Wang M, Chen C, et al. Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): results of 2 phase III studies (MM-009, MM-010) and subgroup analysis of patients with impaired renal function. ASH Annu Meet 2006;3547:108. [Google Scholar]
- 56.Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–81. 10.1016/S1470-2045(19)30336-5 [DOI] [PubMed] [Google Scholar]
- 57.Kristinsson SY. Thrombosis in multiple myeloma. Hematology Am Soc Hematol Educ Program 2010;2010:437–44. 10.1182/asheducation-2010.1.437 [DOI] [PubMed] [Google Scholar]
- 58.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22:414–23. 10.1038/sj.leu.2405062 [DOI] [PubMed] [Google Scholar]
- 59.Di Nisio M, Porreca E, Candeloro M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2016;12:CD008500. 10.1002/14651858.CD008500.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuura M, Tsugawa Y, Sato T, et al. Submitral nodular calcinosis in a patient on maintenance hemodialysis. J Jpn Soc Dial Ther 1992;25:113–8. (in Japanese)(Abstract in English) 10.4009/jsdt1985.25.113 [DOI] [Google Scholar]
- 61.Udaka K, Ozaki S. [Multiple Myeloma: from Diagnosis to the Up-to-date Treatment. Topics: III. Bone Disease in Multiple Myeloma]. Nihon Naika Gakkai Zasshi 2016;105:1216–23. (in Japanese). [PubMed] [Google Scholar]
- 62.Hashimoto T, Abe M, Oshima T, et al. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma. Br J Haematol 2004;125:38–41. 10.1111/j.1365-2141.2004.04864.x [DOI] [PubMed] [Google Scholar]
- 63.Terpos E, Dimopoulos MA, Sezer O, et al. The use of biochemical markers of bone remodeling in multiple myeloma: a report of the International myeloma Working group. Leukemia 2010;24:1700–12. 10.1038/leu.2010.173 [DOI] [PubMed] [Google Scholar]
- 64.Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 2015;33:657–64. 10.1200/JCO.2014.57.9961 [DOI] [PubMed] [Google Scholar]
- 65.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma Working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]