To The Editor:
In December 2010, results from three randomized, phase 3 trials of treatments for multiple myeloma showed an excess of hematologic cancers among patients with multiple myeloma who received lenalidomide maintenance therapy (see Table 1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Researchers have been aware of second cancers after multiple myeloma — particularly acute myeloid leukemia and myelodysplastic syndromes — for more than four decades (Table 1 in the Supplementary Appendix). In 1979, Bergsagel et al. (see the Supplementary Appendix) found an increased risk of all forms of acute leukemia among 364 patients with multiple myeloma treated with low-dose melphalan (Table 1 in the Supplementary Appendix). Studies conducted after the introduction of high-dose melphalan followed by autologous stem-cell transplantation suggested that conventional chemotherapy before autologous stem-cell transplantation is a more likely contributing factor to myelodysplastic syndromes and acute myeloid leukemia than pre-transplantation myeloablative therapy (Table 1 in the Supplementary Appendix).
With the introduction of new drugs for multiple myeloma, patients are living longer.1 Estimates extrapolated from data on other cancers (e.g., Hodgkin’s lymphoma)2 and improvements in survival among patients with multiple myeloma suggest that the incidence of second cancers may increase in the future.
Although the underlying biologic mechanisms of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma are unknown, treatment-related factors are presumed to be responsible for the elevated risk of these conditions. In some studies, but not others, the risk of acute myeloid leukemia increases with the increasing cumulative dose of melphalan, the duration of melphalan therapy, or both (Table 1 in the Supplementary Appendix). Quantifying the risks of second tumors in relation to a given therapy is complex because we do not know the baseline incidence of second cancers in untreated patients with multiple myeloma.
To test the hypothesis that factors unrelated to treatment may play a role in subsequent cancers after plasma-cell dyscrasias, we estimated the risk of subsequent cancers among 5652 patients with monoclonal gammopathy of undetermined significance (MGUS) and compared risk patterns in relation to the general population.3 Interestingly, we found an increased risk of acute myeloid leukemia and myelodysplastic syndromes among patients with MGUS and IgG and IgA (but not IgM); the risk among patients with MGUS with monoclonal protein (M component) concentrations of 1.5 g per deciliter or higher was higher than among those with concentrations of less than 1.5 g per deciliter. These findings provide support for the role of disease-related factors in second cancers.3 Furthermore, polymorphisms in germline genes may contribute to a person’s susceptibility to subsequent cancers.4,5 The potential influence of environmental and behavioral factors remains poorly understood.
On a clinical note, for most patients, multiple myeloma remains an incurable cancer and, on average, the risk of dying from multiple myeloma is considerably higher than the risk of the development of a second cancer (Fig. 1). Despite the lack of clear answers, clinicians need to discuss treatment risks and benefits with patients and stay updated as more knowledge becomes available.
Figure 1. Cumulative Probability of the Development of a Second Cancer and of Death from All Other Causes (Excluding Second Cancers).
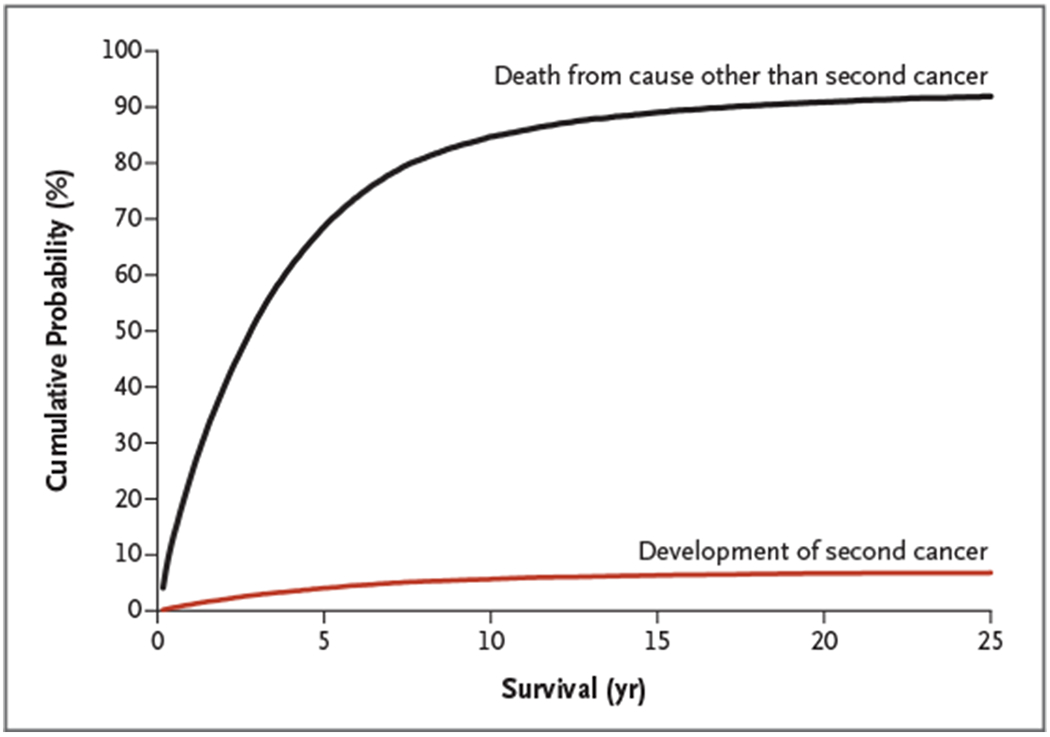
Data, which are based on 33,229 patients who received a diagnosis of multiple myeloma between 1973 and 2008 in the United States, are from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol 2010; 28:830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol 2000;18:498–509. [DOI] [PubMed] [Google Scholar]
- 3.Mailankody S, Pfeiffer RM, Kristinsson SY, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011; 118:4086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren O, Ma W, Kyle RA, Rajkumar SV, Korde N, Albitar M. Polymorphism of the erythropoietin gene promotor and the development of myelodysplastic syndromes subsequent to multiple myeloma. Leukemia 2011. September 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 2005;5:943–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


