Abstract
High-dose chemotherapy and whole brain radiotherapy (WBRT) can prolong survival in primary CNS lymphoma (PCNSL) patients, but is often associated with clinically significant cognitive decline. In this study we assessed neuropsychological functioning prospectively in newly diagnosed PCNSL patients treated with induction chemotherapy followed by reduced-dose WBRT. Twelve patients underwent neuropsychological evaluations at diagnosis, after induction chemotherapy, and 6 and 12 months after WBRT. Nine patients completed additional cognitive evaluations 18 and 24 months post-treatment. At diagnosis, patients had impairments in Executive Functions, Verbal Memory, and Motor Speed. There was a significant improvement in Executive Functions (P < 0.01) and Verbal Memory (P < 0.05) following induction chemotherapy, and scores remained relatively stable up to 12 months post-treatment. Among the nine patients who completed a 2-year follow-up, there was a significant improvement in the Executive domain (P < 0.05) and a trend toward a decline in the Verbal Memory domain. Executive and Verbal Memory functions improved following induction chemotherapy, likely due to decreased tumor burden and discontinuation of corticosteroid and anticonvulsant medications. There was no significant cognitive decline up to 24 months post-chemotherapy and reduced-dose WBRT in this group of PCNSL patients, however, difficulties in Verbal Memory and Motor speed persisted over the follow-up period.
Keywords: Neuropsychological assessment, Cognitive, Primary CNS lymphoma, Chemotherapy, Radiotherapy
Introduction
Treatment of primary central nervous system lymphoma (PCNSL) typically consists of high-dose methotrexate (MTX)-based chemotherapy with or without whole brain radiotherapy (WBRT). Although this treatment prolongs survival [1], there is a risk of neurotoxicity that increases with advanced age at treatment, and in patients with pro-longed disease free survival [2]. MTX and WBRT may each cause CNS damage, but there is a synergistic toxic effect when these two modalities are combined [3]. The adverse effects of chemotherapy and radiotherapy are not well understood, but have been hypothesized to involve demyelination, inflammatory response, and microvascular injury [4], and the prevalence increases with age and radiation dose. Recent evidence also suggests that, chemotherapy and radiotherapy disrupt neurogenesis in the dentate gyrus of the hippocampus [5–7], and can interfere with memory functions [8].
PCNSL patients often experience cognitive difficulties that can interfere with their ability to function at premorbid levels despite adequate disease control. However, cognitive outcome has been included in a relatively limited number of clinical trials. A review of the literature [9] indicated that treatment with WBRT and high-dose chemotherapy was associated with cognitive impairment in the majority of PCNSL survivors, but the retrospective designs made it difficult to determine the specific contributions of tumor and treatment. Prospective studies involving patients treated with high-dose chemotherapy alone, reported cognitive impairment at baseline and no significant decline over time; however, most of these studies had methodological problems such as variable follow-up intervals and inclusion of patients with tumor progression [9].
In this study we assessed neuropsychological functions prospectively, as per published guidelines [9], in a subset of newly diagnosed PCNSL patients treated with a modified regimen aimed at improving efficacy and decreasing neurotoxicity. Briefly, in a phase II clinical trial, Rituximab, a chimeric monoclonal antibody, was incorporated into the standard MTX-based chemotherapy treatment, and a lower dose of WBRT was administered to patients who achieved a complete response after chemotherapy [10].
Patients and methods
Patient characteristics
About 12 of 19 immunocompetent patients with newly diagnosed histologically confirmed B-cell PCNSL who underwent induction chemotherapy followed by reduced-dose WBRT and consolidation chemotherapy at Memorial Sloan Kettering Cancer Center (MSKCC) completed serial neuropsychological evaluations. All patients signed written informed consent prior to participation in a phase II clinical trial designed to assess the efficacy and safety of this new treatment regimen [10], and the study protocol was approved by the Institutional Review Board. Induction chemotherapy consisted of five to seven cycles of rituximab, methotrexate, procarbazine and vincristine (R-MPV); one patient was treated with intra-Ommaya MTX. All 12 patients attained a complete response after R-MPV and received WBRT to a total dose of 23.4 Gy (1.8 Gy per fraction × 13 daily). After completion of WBRT, all but one patient received two cycles of high-dose cytarabine (ARA-C) consolidation chemotherapy.
Twelve patients completed neuropsychological assessments at diagnosis, after induction chemotherapy and prior to reduced-dose WBRT and ARA-C, and approximately 6 and 12 months after completion of WBRT and ARA-C. Among the seven patients who did not complete cognitive evaluations, two had disease relapse, two were not fluent in English, two were treated at outside institutions, and one declined cognitive testing. Nine of the 12 patients completed additional cognitive evaluations approximately 18 and 24 months post-treatment. Among the three patients who did not complete the long-term follow-up, one had disease relapse, one declined cognitive testing, and one could not travel to MSKCC for additional follow-ups.
Measures
Neuropsychological assessment
Patients completed a battery of standardized neuropsychological tests and self-report scales of mood and quality of life as previously described in a study of PCNSL survivors [11]. Given the small number of patients in this study, the neuropsychological tests found to be most sensitive to detect cognitive dysfunction in the prior study were selected to examine changes over time (i.e., executive, verbal memory, and motor tests). The test battery was administered by a trained psychometrist under the direct supervision of a board certified neuropsychologist (DDC). Alternate test forms of the Hopkins Verbal Learning Test-Revised (4 alternate versions) and the Trail Making Test, part B (1 alternate version) were used at the follow-ups.
Raw cognitive test scores were compared with published normative values according to age and education, and converted into z-scores to characterize the presence and severity of cognitive difficulties. Cognitive dysfunction was defined as z-score ≥1.5 standard deviations below the mean of the normative sample. Given the large number of cognitive tests and the small number of patients, composite scores were calculated by adding the z-scores for each test within a cognitive domain and dividing the sum by the number of tests. Three cognitive domains were calculated: Executive (Trail Making Test Prts A and B; Brief Test of Attention— BTA), Verbal Memory (Hopkins Verbal Learning Test-Revised—HVLT-R: Total Learning and Delayed Recall), and Motor Speed (Grooved Pegboard Test, Dominant and Non-Dominant hand). Patients also completed the Beck Depression Inventory (BDI) to assess the possible contribution of mood to cognitive test performance, and the Functional Assessment of Cancer Therapy-Brain (FACT-BR) questionnaire to assess quality of life.
Neuroimaging
MRI scans of the brain, performed within a maximum of 3 months of the cognitive evaluation, were rated for the presence of white matter disease by two neurologists (LEA, FI) who were blind to the cognitive test results. Radiographic endpoints were measured using a modified Fazekas scale [12] and included no white matter change (Grade 0); minimal patchy white matter foci (Grade 1); start of fluent areas (Grade 3); confluence with cortical and subcortical involvement (Grade 4); leukoencephalopathy (Grade 5); and possible radiation necrosis (Grade 6).
Statistical analyses
Spearman correlation was used to confirm that the tests that comprised each cognitive domain were correlated, and to assess the relation between cognitive test scores and quality of life, mood, and white matter ratings. Spearman correlation was also used to assess inter-reliability for the MRI ratings performed independently by two raters. Given the small sample size, paired t-tests were performed to compare cognitive test performance between baseline and each follow-up evaluation. Corrections for multiple statistical testing were not made given the small sample size and attrition, and because the analyses were in part exploratory; P < 0.05 was considered statistically significant. SPSS 12.0 was used for the statistical analyses.
Results
Baseline, post-R-MVP, 6- and 12-month follow-ups (n = 12)
The median age of the twelve patients at diagnosis was 57.5 years (range = 47–76), and 6 were men (Table 1). Table 1 presents demographic information separately for all patients and for the 9 patients who completed the 24-month evaluation. Mean verbal IQ was estimated based on the North American Adult Reading Test [13] or Barona Index [14]. Prior to diagnosis, eight patients were employed, two were retired and two were not in the work force. One-year post-treatment, six of the eight employed patients had resumed their work activities, but two were working at a lower capacity than prior to diagnosis.
Table 1.
Patient demographic characteristics (means and standard deviations)
| All patients | 9 Patientsa | |
|---|---|---|
| Sex (M/F) | 6/6 | 5/4 |
| Handedness (R/L) | 10/2 | 7/2 |
| Age at baseline (years) | ||
| Mean (sd) | 58.5 (9.8) | 61.6 (8.3) |
| Median (range) | 57.5 (47–76) | 59.2 (54–78) |
| Mean Education (years) | 13.5 (4.8) | 12.9 (5.6) |
| Mean Est.VIQ | 107 (12.1) | 106 (13.4) |
Patients who completed the 24-month follow-up
Est.VIQ = Estimated Verbal IQ (North American Adult Reading Test-NAART or Barona Index)
Table 2 presents disease and treatment history of the 12 patients. At the time of diagnosis, 11 patients were on corticosteroids and eight were taking anticonvulsants; four patients had seizures within one month of the baseline cognitive evaluation. There were no significant differences on cognitive test performance between patients who were on anticonvulsants or had seizures, and the ones who were medication or seizure free. After completion of post-induction chemotherapy and at the 6- and 12-month follow-ups, corticosteroids had been discontinued in all patients, and three remained on anticonvulsants; none of the patients had seizures within a month of the follow-up cognitive evaluations.
Table 2.
Disease and treatment history (n = 12)
| Tumor location | |
| Frontal | 4 (33%) |
| Frontal-parietal | 2 (17%) |
| Temporal-parietal-occipital | 3 (25%) |
| Cortical/subcortical | 3 (25%) |
| Tumor side | |
| Left | 3 (25%) |
| Right | 6 (50%) |
| Bilateral | 3 (25%) |
| Number of lesions | |
| 1 | 9 (75%) |
| 2 or more | 3 (25%) |
| Biopsy/resection | 10/2 |
| Seizures (Y/N)a | 4/8 |
| Antiepileptics (Y/N)a | 8/4 |
| Corticosteroids (Y/N)a | 11/1 |
At baseline
Spearman correlation coefficients for the subtests that comprised each of the three cognitive domains were as follows: Executive (Trails A and B = 0.67; Trails A and BTA = 0.52; Trails B and BTA = 0.70), Verbal Memory (HVLT-R: Total Learning and Delayed Recall = 0.72), Motor (Grooved Pegboard Test, Dominant and Non-Dominant hand = 0.90). Cognitive tests mean raw and z-scores and mood and quality of life scores for all patients at each assessment time are presented in Table 3. At baseline, patients had z-scores in the impaired range in the Executive (mean z-score = −1.61 ± 0.29), Verbal Memory (mean z-score = −1. 92 ± 0.30), and Motor Speed (mean z-score = −1.68 ± 0.40) domains. A comparison between baseline and post-induction chemotherapy evaluations showed a significant improvement in the Executive (t (8) = −4.78, P < 0.01) and Verbal Memory domains (t (10) = −2.47, P < 0.05), but not in the Motor domain (Fig. 1). In the Executive domain, significant improvements were evident in all three tests (Trail Making Test A & B and BTA; P < 0.01); in the Verbal Memory Domain, there was a significant improvement in the HVLT-R Total Learning (P < 0.05) but not in Delayed Recall. Although there was a trend toward improvement in the Verbal Memory and Motor Speed domains between the post-induction chemotherapy visit and the 6- and 12-month follow-ups (Fig. 1), the comparisons did not reach statistical significance and scores remained about 1 to 1.5 standard deviations below normative values.
Table 3.
Neuropsychological test results, mean raw and z-scores (SD)
| Baseline | Post-R-MPV | 6-Month | 12-Month | 18-Month | 24-Month | ||
|---|---|---|---|---|---|---|---|
| Executive | |||||||
| TMTA | Raw | 56.92 (28.07) | 36.73 (14.66) | 35.50 (12.23) | 38.33 (14.36) | 34.56 (8.96) | 35.22 (10.89) |
| z-score | −1.34 (1.03) | −0.35 (0.95) | −0.22 (0.88) | −0.34 (1.08) | −0.04 (0.80) | 0.01 (1.03) | |
| TMTB | Raw | 182.70 (83.58) | 106.90 (73.98) | 94.91(58.39) | 90.45 (26.14) | 96.25 (32.80) | 104.88 (46.33) |
| z-score | −1.80 (1.07) | −0.69 (1.04) | −0.05 (1.01) | −0.36 (0.56) | −0.15 (0.51) | −0.15 (0.89) | |
| BTA | Raw | 9.50 (5.05) | 14.09 (4.35) | 13.92 (4.32) | 15.00 (3.59) | 14.33 (4.12) | 15.56 (3.84) |
| z-score | −1.92 (1.31) | −0.82 (1.28) | −0.77 (1.23) | −0.42 (1.00) | −0.62 (1.17) | −0.17 (1.16) | |
| Memory | |||||||
| HVLTRTL | Raw | 18.25 (6.41) | 22.55 (6.56) | 21.58 (6.10) | 22.83 (6.94) | 20.22 (6.26) | 21.67 (6.38) |
| z-score | −1.90 (0.98) | −1.24 (1.51) | −1.23 (1.10) | −0.58 (1.73) | −1.49 (1.32) | −1.22 (1.38) | |
| HVLTRD | Raw | 5.08 (3.53) | 6.82 (3.76) | 7.25 (3.49) | 7.50 (3.03) | 6.67 (3.39) | 6.22 (2.82) |
| z-score | −1.94(1.25) | −1.50(1.33) | −1.19 (1.52) | −1.09 (1.40) | −1.39 (1.54) | −1.60(1.23) | |
| Motor | |||||||
| GPD | Raw | 153.00 (91.52) | 113.78 (64.89) | 96.91(34.60) | 92.00 (32.57) | 99.67 (47.09) | 85.11 (15.07) |
| z-score | −1.74 (1.36) | −1.67 (1.20) | −1.35 (1.00) | −1.17 (1.26) | −1.17 (1.05) | −0.78 (0.81) | |
| GPND | Raw | 146.00 (86.37) | 122.78 (68.33) | 112.18(65.12) | 106.82 (65.21) | 118.89(70.29) | 114.78 (70.36) |
| z-score | −1.63 (1.22) | −1.59(1.04) | −1.18 (1.26) | −0.86 (1.21) | −1.20 (0.99) | −1.08 (1.05) | |
| Mood and QOL | |||||||
| BDI | Raw | 8.67 (5.31) | 7.18 (4.81) | 7.67 (7.64) | 6.00(7.71) | 6.78 (6.76) | 6.00 (4.85) |
| FACT-Br | Raw | 122.75 (26.06) | 140.55 (17.17) | 153.50 (25.05) | 154.92 (24.12) | 146.89 (28.31) | 153.89 (14.19) |
TMTA–Trail Making Test A; TMTB–Trail Making Test B; BTA–Brief Test of Attention; HVLTRTL–Hopkins Verbal Learning Test-Revised– Total Learning; HVLTRD–Hopkins Verbal Learning Test-Revised—Delayed Recall; GPTD–Grooved Pegboard Test Dominant; GPTND– Grooved Pegboard Test Non-Dominant; QOL–Quality of Life; BDI–Beck Depression Inventory; FACT-Br–Functional Assessment of Cancer Therapy-Brain
Fig. 1.
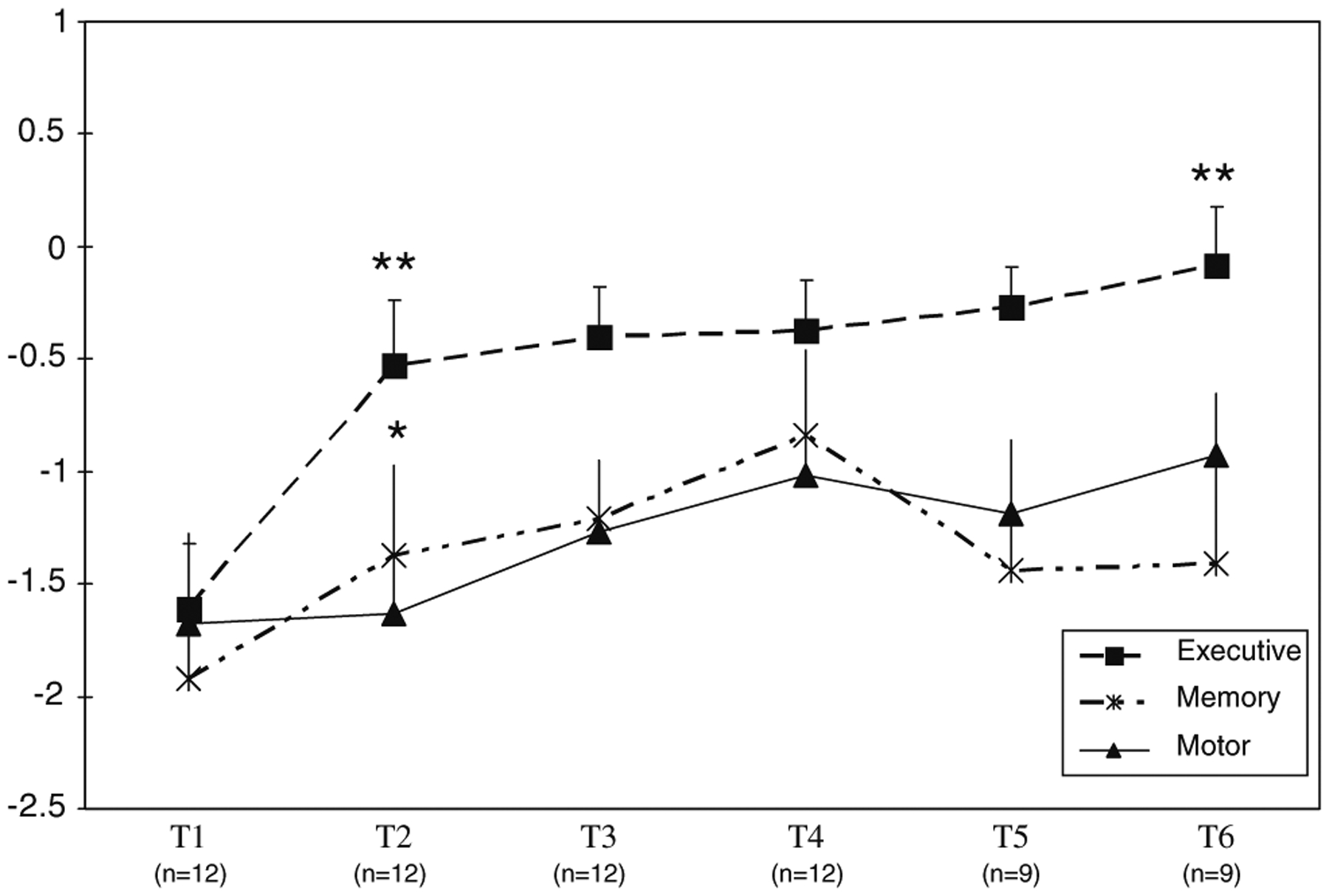
Executive, Verbal Memory, and Motor Domain Scores at Baseline and Follow-up. T1 = Pre-Treatment (n = 12); T2 = post-induction chemotherapy (n = 12); T3 = 6 months Post-RT ± ARA-C (n = 12), T4 = 12 months Post-RT ± ARA-C (n = 12), T5 = 18 months Post-RT ± ARA-C (n = 9), T6 = 24 months Post-RT ± ARA-C (n = 9). Mean z score ± SEM. ** Time 1-Time 2 and Time 3-Time 6 (Executive), P < 0.01.* Time 1-Time 2 (Memory), P < 0.05
FACT-BR mean scores improved significantly between baseline and induction chemotherapy (t (10) = −3.16, P < 0.05), and between the 6- and 12-month post-WBRT follow-ups (t (10) = −2.99, P < 0.05). There were no significant correlations between the cognitive domain scores and the FACT-BR or the BDI. BDI mean scores suggested no evidence of depressed mood and there were no significant changes over time.
18- and 24-month follow-ups (n = 9)
Nine patients completed neuropsychological evaluations approximately 18 and 24 months after completion of reduced-dose WBRT and ARA-C. There was no evidence of disease recurrence or change in work status for any of these patients during this period. Only one patient remained on anticonvulsants, and none had seizures.
There was a significant improvement in the Executive domain between the 12- and 24-month follow-ups (t (8) = −3.54, P < 0.01) (Fig. 1); this was evident in the BTA but not on the Trail Making Test. At the 18-month follow-up, there was a mild decline in the Verbal Memory domain (Delayed Recall), but comparisons did not reach significance. At the 24-month follow-up, scores remained stable but in the impaired range for Verbal Memory and one standard deviation below the mean on the Motor domain. There were no significant changes in the FACT-BR and BDI scores over this follow-up period.
Neuroimaging
The extent of white matter disease on MRI scans was rated at each assessment interval, and correlated with the cognitive domain scores. Inter-rater reliability yielded a significant Spearman correlation coefficient (r = 0.69, P < 0.01). The ratings of white matter disease used for subsequent analyses were based on a consensus between the two raters.
At baseline and prior to treatment, 8 of 12 patients had either no or minimal white matter changes on MRI (grades 0 or 1; 67%), whereas 4 patients showed white matter confluence (grades 2 or 3; 34%) (Table 4); 2 of these 4 patients were 60 years and older (i.e., ages = 60 and 64 years) and had at least two vascular risk factors (i.e., hypertension, hypercholesterolemia, smoking history).
Table 4.
White matter (WM) disease
| MRI grade | Baseline (n = 12) | Post-chemo (n = 12) | 6 months (n = 12) | 12 months (n = 12) | 18 months (n = 9) | 24 months (n = 9) |
|---|---|---|---|---|---|---|
| No WM disease (Grade 0) | 6 | 5 | 3 | 2 | 0 | 0 |
| Minimal WM disease (Grade 1) | 2 | 2 | 3 | 2 | 2 | 2 |
| Start of confluence (Grade 2) | 3 | 3 | 4 | 6 | 5 | 5 |
| Large confluent areas (Grade 3) | 1 | 2 | 2 | 1 | 2 | 2 |
| Confluence, Cortical/subcortical (Grade 4) | 0 | 0 | 0 | 1 | 0 | 0 |
After the completion of induction chemotherapy, 7 of 12 patients had either no or minimal white matter changes on MRI (grades 0 or 1; 58%), whereas 5 patients showed white matter confluence (grades 2 or 3; 42%). There was an increase in white matter changes in 2 patients after induction chemotherapy (i.e., grade 0–1, age = 54; grade 1–3, age = 71); the 71-year-old patient had vascular risk factors.
At the 6-month follow-up, 6 patients had white matter confluence (50%) and at the 12-month follow-up 7 patients showed this pattern (58%). There was an increase in white matter changes in 3 patients 6 months post-WBRT and ARA-C (i.e., grade 0–1, ages = 57; 1–2, age = 76), and in 4 patients 12 months post-treatment (i.e., grade 0–1, age = 60; 1–2, ages = 54 and 57; 3–4 age = 64); the 60 and 64-year-old patients had vascular risk factors. Among the 9 patients who completed 18- and 24-month post-treatment follow-ups, 7 (58%) had white matter confluence (Table 4) and none showed an increase in white matter changes within this time period.
Overall, there were no significant correlations between white matter changes and cognitive test performance in this group of patients. However, an examination of cognitive domain scores according to the extent of white matter disease in the 9 patients who completed all follow-ups, suggested that patients with white matter confluence on MRI obtained lower Executive and Verbal Memory domain scores than patients with no or minimal white matter changes at most assessments (Fig. 2).
Fig. 2.
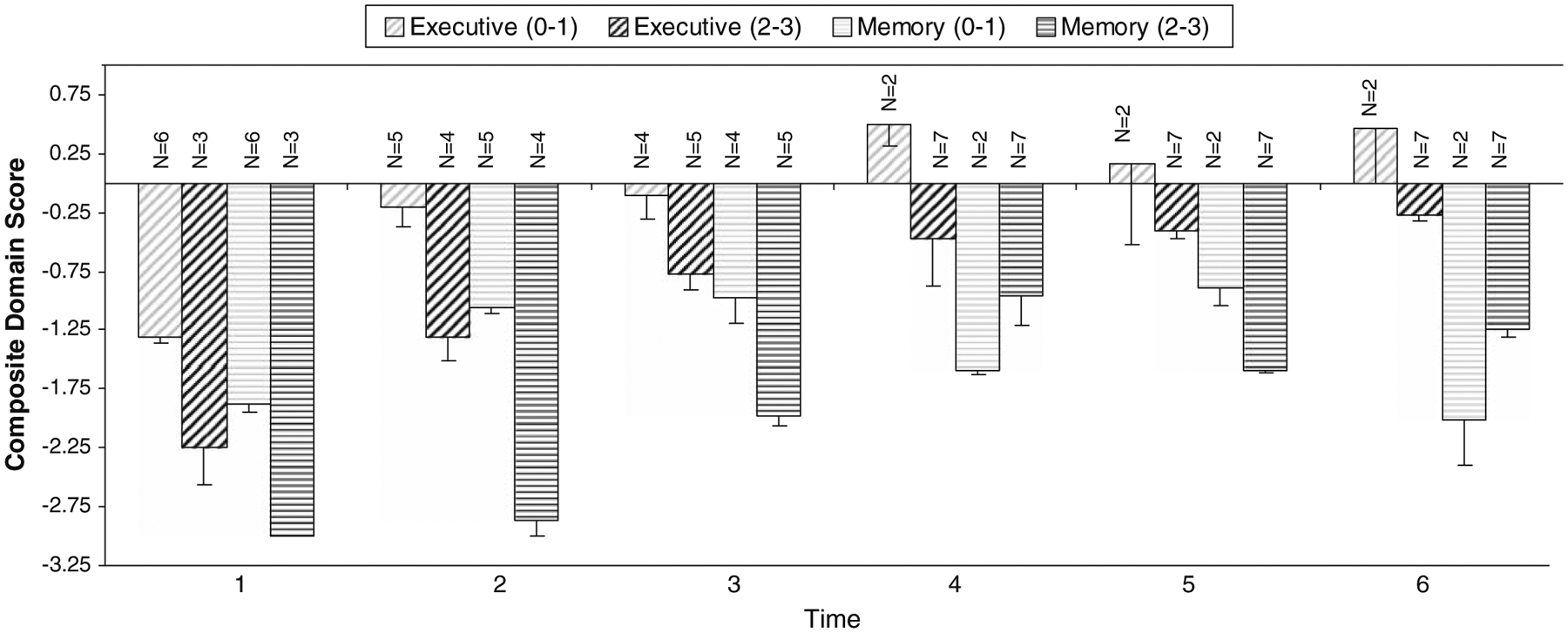
Cognitive Test Performance by White Matter Grade for patients who completed all follow-ups (n = 9). (0–1) = no/minima white matter change; (2–3) = white matter confluence. T1 = baseline; T2 = post-induction chemotherapy; T3 = 6 months Post-RT ± ARA-C; T4 = 12 months Post-RT ± ARA-C; T5 = 18 l months Post-RT ± ARA-C, T6 = 24 months Post-RT ± ARA-C.Values plotted represent mean z score ± SEM
Discussion
The findings of this study suggested that R-MPV followed by dose-reduced WBRT and ARA-C was not associated with a statistically significant cognitive decline up to 2 years post-treatment in this group of PCNSL patients. Although it is possible that cognitive decline may occur in the ensuing years post-treatment, these initial results represent an improvement relative to the cognitive deterioration described in many PCNSL patients, often within 6 months after treatment with conventional MTX-based chemotherapy and standard dose WBRT [1].
At diagnosis and prior to treatment, patients had impairments in executive, verbal memory and motor functions. The relatively diffuse pattern of cognitive difficulties is most likely associated with the tumor, and possibly the adverse effects of corticosteroids and anticonvulsants. Cognitive impairment at diagnosis has been reported in some of the studies that performed pre-treatment neuropsychological evaluations in this population [15–17]. There was a significant improvement after induction chemotherapy with R-MPV in executive and verbal memory functions, probably related to complete tumor response to treatment. Discontinuation of corticosteroids in all patients and anticonvulsants in most may have also contributed to the change, as these medications can disrupt cognitive functioning [18, 19]. However, considering the marked impairment at the initial evaluation, regression to the mean may have also played a role in the improved scores seen after induction chemotherapy. In the verbal memory domain, total learning but not delayed recall improved; this could be in part related to the improvement in executive functions, as learning often requires the ability to generate organizational strategies for acquisition of new information. The absence of change in the Motor domain suggested that the improved performance on the Trail Making Test involved primarily the executive components of the task and not motor speed. There was also a significant improvement in self-reported quality of life following the induction chemotherapy and 6 months after completion of all therapy; six of eight patients resumed their work activities one-year after treatment but two were working at a lower capacity due mostly to persistent cognitive difficulties.
Among the nine patients who completed the 2-year post-treatment follow-up, cognitive performance and self-reported quality of life remained relatively stable and suggested that treatment with R-MVP and reduced-dose WBRT is not associated with significant cognitive decline, at least within this time frame. Retrospective neuropsychological studies of PCNSL patients treated with conventional combined modality therapy reported significant cognitive impairment [11, 20]; although this was likely related to the adverse effects of standard dose WBRT and chemotherapy, the current results raise the possibility that some portion of the cognitive deficits were also due to the tumor. Patients continued to have persistent difficulties in verbal memory and motor speed likely to interfere with their daily functioning; however, there was no decline in self-reported quality of life.
There was a mild increase in treatment-related white matter disease following treatment, and this was first evident after induction chemotherapy in two patients and 6 and 12 months following completion of all treatment in three and four patients, respectively; four of these patients were 60 years and older. These findings are consistent with the reported delayed onset of chemotherapy and radiation injury [3] and the increased risk of neurotoxicity in the elderly [21]. However, no further increase in white matter disease was seen at the 18 and 24 month follow-ups. Four patients had white matter confluence at baseline and among these two had vascular risk factors; there was no evidence that treatment contributed to a further increase in these changes over the 2-year follow-up among these patients. There were no significant correlations between treatment-related white matter changes and cognitive test performance in this small sample. However, the observations of a mild decline in delayed recall of verbal information and the lower verbal memory and executive scores obtained by patients with white matter confluence are intriguing and require further investigation with a larger sample. In addition, several of the patients with white matter confluence were also older, which supports prior studies suggesting that the risk of delayed neurotoxicity increases with age [22], albeit these changes were less pronounced than seen after standard dose WBRT [1, 23]. These observations are particularly relevant given evidence that more extensive white matter disease is associated with executive dysfunction [24], and recent animal and human studies documenting a disruption in hippocampal neurogenesis after treatment with radiotherapy and chemotherapy [5, 6, 25]. It has been suggested that the mechanisms of chronic RT and chemotherapy injury may involve the combined effects of damage to the vasculature, neural precursor cells, and myelin, and disruption of neurogenesis [3, 7, 25–27]. Recent studies have shown impaired performance in rats treated with low dose radiation on non-matching to sample tasks when intervals between sample and test trials were long [8] and on long-term spatial memory [28]. Future animal studies that investigate the biological basis of neurotoxicity, and clinical studies including imaging techniques that assess both gray and white matter integrity (e.g., diffusion tensor imaging, voxel-based morphometry) would assist in further understanding the substrate of treatment-related changes.
The relatively small sample size and attrition are significant limitations of this study, and may have diminished the power to detect small changes in cognitive test performance and correlations with white matter changes. Nevertheless, the current findings highlight the importance of conducting prospective longitudinal studies to determine the contribution of both disease and treatment to cognitive outcome in this clinical population. Collaborative studies using recently published guidelines for cognitive assessment would be important to advance our understanding of cognitive outcome in PCNSL patients. Continued long-term follow-up of our patient cohort is planned to monitor delayed neurotoxicity. In addition, we expect to increase the sample size as this phase II clinical trial is ongoing and new patients are being recruited and followed over time.
Acknowledgment
This study was supported in part by Genentech, Inc.
Footnotes
Preliminary results of this study were presented at: (1) International Primary CNS Lymphoma Collaborative Group (IPCG) Meeting in Orlando, Florida—December, 2006.
(2) International Neuropsychological Society (INS) 36th Annual Meeting in Waikoloa, Hawaii—February, 2008.
Contributor Information
Rima Dolgoff-Kaspar, Department of Psychiatry, University of Arizona College of Medicine, Tucson, AZ, USA.
Fabio Iwamoto, Department of Neurology, Memorial-Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10021, USA.
Joachim Yahalom, Department of Radiation Oncology, Memorial-Sloan Kettering Cancer Center, New York, NY, USA.
Lauren E. Abrey, Department of Neurology, Memorial-Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10021, USA
References
- 1.Abrey LE, DeAngelis LM, Yahalom J (1998) Long-term survival in primary CNS lymphoma. J Clin Oncol 16:859–863 [DOI] [PubMed] [Google Scholar]
- 2.Deangelis LM, Seiferheld W, Schold SC et al. (2002) Combination chemotherapy and radiotherapy for primary central nervous lymphoma: Radiation Therapy Oncology Group 93–100. J Clin Oncol 21:4643–4648. doi: 10.1200/JCO.2002.11.013 [DOI] [PubMed] [Google Scholar]
- 3.Behin A,Delattre J-Y (2003) Neurologic sequelae ofradiotherapyon the nervous system In: Schiff D, Wen PY (eds) Cancer Neurology in clinical practice. Humana Press Inc, Totowa, NJ, pp 173–191 [Google Scholar]
- 4.Tuxen MK, Hansen SW (1994) Neurotoxicity secondary to antineoplastic drugs. Cancer Treat Rev 20:191–214. doi: 10.1016/0305-7372(94)90027-2 [DOI] [PubMed] [Google Scholar]
- 5.Santarelli L, Saxe M, Gross C et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. doi: 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- 6.Dietrich J, Han R, Yang Y et al. (2006) CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and vivo. J Biol 5:1–23. doi: 10.1186/jbiol50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monje ML, Vogel H, Masek M et al. (2007) Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 62:515–520. doi: 10.1002/ana.21214 [DOI] [PubMed] [Google Scholar]
- 8.Winocur G, Wojtowicz JM, Sekeres M et al. (2006) Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16:296–304. doi: 10.1002/hipo.20163 [DOI] [PubMed] [Google Scholar]
- 9.Correa DD, Maron L, Harder H et al. (2007) Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 18:1145–1151. doi: 10.1093/annonc/mdl464 [DOI] [PubMed] [Google Scholar]
- 10.Shah GD, Yahalom J, Correa DD et al. (2007) Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 25:4730–4735. doi: 10.1200/JCO.2007.12.5062 [DOI] [PubMed] [Google Scholar]
- 11.Correa DD, DeAngelis LM, Shi W et al. (2004) Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 62:548–555 [DOI] [PubMed] [Google Scholar]
- 12.Fazekas F, Chawluk JB, Alavi A et al. (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol 8:421–426 [DOI] [PubMed] [Google Scholar]
- 13.Blair JR, Spreen O (1989) Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol 3:129–136. doi: 10.1080/13854048908403285 [DOI] [Google Scholar]
- 14.Barona A, Reynolds CR, Chastain R (1984) A demographically based index of premorbid intelligence for the WAIS-R. J Consult Clin Psychol 52:885–887. doi: 10.1037/0022-006X.52.5.885 [DOI] [Google Scholar]
- 15.Fliessbach K, Helmstaedter C, Urbach H et al. (2005) Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology 64:1184–1188 [DOI] [PubMed] [Google Scholar]
- 16.Schlegel U, Pels H, Glasmacher A et al. (2001) Combined systemic and intraventricular chemotherapy in primary CNS lymphoma: a pilot study. J Neurol Neurosurg Psychiatry 71:118–122. doi: 10.1136/jnnp.71.1.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister LD, Doolittle ND, Guastadisegni PE et al. (2000) Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 46:51–61. doi: 10.1097/00006123-200001000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Aldenkamp AP (2001) Effects of antiepileptic drugs on cognition. Epilepsia 41:46–49. doi: 10.1046/j.1528-1157.2001.00516.x [DOI] [PubMed] [Google Scholar]
- 19.Young AH, Sahakian BJ, Robbins TW et al. (1999) The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology 145:260–266. doi: 10.1007/s002130051057 [DOI] [PubMed] [Google Scholar]
- 20.Harder H, Holtel H, Bromberg JEC et al. (2004) Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology 62:544–555 [DOI] [PubMed] [Google Scholar]
- 21.Omuro AMP, Ben-Porat LS, Panageas KS et al. (2005) Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 62:1595–1600. doi: 10.1001/archneur.62.10.1595 [DOI] [PubMed] [Google Scholar]
- 22.Corry J, Smith JG, Wirth A et al. (1998) Primary central nervous system lymphoma: age and performance status are more important than treatment modality. Int J Radiat Oncol Biol Phys 41:615–620. doi: 10.1016/S0360-3016(97)00571-3 [DOI] [PubMed] [Google Scholar]
- 23.Poortmans PMP, Kluin-Nelemans HC, Haaxma-Reiche H et al. (2003) High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European organization for research and treatment of cancer lymphoma group phase II trial 20962. J Clin Oncol 21:4483–4488. doi: 10.1200/JCO.2003.03.108 [DOI] [PubMed] [Google Scholar]
- 24.Tullberg M, Fletcher E, DeCarli C et al. (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63:246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134. doi: 10.1097/00019052-200304000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Andres-Mach M, Rola R, Fike JR (2008) Radiation effects on neural precursor cells in the denate gyrus. Cell Tissue Res 331:251–262. doi: 10.1007/s00441-007-0480-9 [DOI] [PubMed] [Google Scholar]
- 27.Rzeski W, Pruskil S, Macke A et al. (2004) Anticancer agents are potent neurotoxins in vivo and in vitro. Ann Neurol 56:351–360. doi: 10.1002/ana.20185 [DOI] [PubMed] [Google Scholar]
- 28.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. doi: 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]


