Abstract
Ezrin is a member of the ERM (ezrin, radixin, moesin) protein family and links F-actin to the cell membrane following phosphorylation. Ezrin has been associated with tumor progression and metastasis in several cancers including the pediatric solid tumors, osteosarcoma and rhabdomyosarcoma. In this study, we were surprised to find that ezrin was not constitutively phosphorylated but rather was dynamically regulated during metastatic progression in osteosarcoma. Metastatic osteosarcoma cells expressed phosphorylated ERM early after their arrival in the lung, and then late in progression, only at the invasive front of larger metastatic lesions. To pursue mechanisms for this regulation, we found that inhibitors of PKC (protein kinase C) blocked phosphorylation of ezrin, and that ezrin coimmunoprecipitated in cells with PKCα, PKCι and PKCγ. Furthermore, phosphorylated forms of ezrin and P KC had identical expression patterns at the invasive front of pulmonary metastatic lesions in murine and human patient samples. Finally, we showed that the promigratory effects of P KC were linked to ezrin phosphorylation. These data are the first to suggest a dynamic regulation of ezrin phosphorylation during metastasis and to connect the PKC family members with this regulation.
Keywords: ezrin, ERM, PKC, tumor metastasis
Introduction
Metastatic disease continues to be the most common cause of death for patients with cancer. We have shown that ezrin is required for metastasis in two pediatric solid tumors of mesenchymal origin, osteosarcoma and rhabdomyosarcoma (Khanna et al., 2004; Yu et al., 2004). The expression of ezrin has been linked to poor survival in several cancers including carcinomas of the breast, colon, endometrium and ovary, cutaneous and uveal melanomas, brain tumors and soft tissue sarcomas (Ohtani et al., 1999; Khanna et al., 2004; Yu et al., 2004; Elliott et al., 2005; Ilmonen et al., 2005; Weng et al., 2005; Kobel et al., 2006; Bruce et al., 2007).
Ezrin is a member of the ERM (ezrin,radixin, moesin) protein family (Berryman et al., 1993; Bretscher et al., 2000). ERM proteins provide a physical link from F-actin to membrane-associated proteins on the surface of cells (Tsukita et al., 1994; Reczek et al., 1997). This linker function makes ezrin essential for many fundamental cellular processes, including the determination of cell shape, polarity and surface structure, cell adhesion, motility, cytokinesis, phagocytosis and integration of membrane transport with signaling pathways (Serrador et al., 1999; Ng et al., 2001; Bretscher et al., 2002; Wu et al., 2004). Ezrin is expressed in many normal tissues and has been demonstrated to be important during embryogenesis (Dard et al., 2004; Polesello and Payre, 2004; Saotome et al., 2004). Nonetheless, the redundancy that exists between ezrin and the other ERM proteins may be sufficient to compensate for the loss of ezrin in some physiological processes. Indeed, ezrin knockout mice survive for 21 days after birth, suggesting some ERM functional redundancy during embryogenesis. Interestingly, the lethal phenotypes in these mice are restricted to the intestinal villi, a site in which other ERM proteins are not expressed (Kivela et al., 2000; Saotome et al., 2004). Death in these mice is believed to result from the intestinal villous malformations. In our studies of ezrin’s role in cancer, a functional redundancy of ERM proteins may be suggested. Following suppression of ezrin in murine osteosarcoma cells, there were no changes in in vitro viability, proliferation or primary tumor growth in vivo (Khanna et al., 2004). Despite expression of other ERM proteins, the suppression of ezrin in several murine and human cancer models resulted in the inhibition of metastasis (Makitie et al., 2001; Khanna et al., 2004; Pang et al., 2004; Yu et al., 2004; Ilmonen et al., 2005; Weng et al., 2005). This suggested that ezrin, rather than other ERM proteins, contributed a unique and necessary function to cells undergoing metastasis.
Phosphorylation of the C-terminal threonine of ERM proteins is important for their activation. ERM proteins exist in inactive forms in which the C-terminal tail binds to, and masks, the N-terminal FERM domain (band 4.1, ezrin, radixin, moesin homology domains) (Pearson et al., 2000). The activation of ERM proteins is mediated by both C-terminal threonine phosphorylation (T567 in ezrin, T564 in radixin, T558 in moesin) (Matsui et al., 1999; Gautreau et al., 2000; Pearson et al., 2000) and exposure to polyphosphoinositides (Fievet et al., 2004). It is most likely that phosphorylation of other residues in ERM proteins are needed to maintain an open activated conformation and to direct specific effects in cells (Krieg and Hunter, 1992; Srivastava et al., 2005). Several protein kinases have been found to phosphorylate the C-terminal threonine residue of ERM proteins. Examples include protein kinase Cα (PKCα) (Ng et al., 2001; Chuan et al., 2006), PKCθ (Pietromonaco et al., 1998), PKCι (Wald et al., 2008), Rho kinases/ROCK (Matsui et al., 1998; Oshiro et al., 1998), G protein-coupled receptor kinase 2 (GRK2) (Cant and Pitcher, 2005) and myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) (Nakamura et al., 2000). Most studies that have investigated the kinase responsible for ezrin T567 phosphorylation have been conducted in cell-free systems or non-cancer cell lines.
In this study, we were surprised to find that ezrin phosphorylation was dynamically regulated during the metastatic process in osteosarcoma. On the basis of our previous data, we had assumed that the phosphorylated, ‘activated’, form of ezrin would be constitutively expressed during metastasis. By following the progression of metastasis in highly metastatic murine and human osteosarcoma cells, high expression of phosphorylated ezrin was observed early after cells arrived in the lung. Surprisingly, at later points in the metastatic process there was a loss of phosphorylated ezrin, most notable several days after metastatic cells arrived in the lung, and most evident as metastatic lesions progressed in size, particularly within the central portions of large metastases. Re-expression of phosphorylated ezrin was then found at the invasive front of larger metastatic lesions. Using pharmacological inhibitors, to uncover the kinase responsible for this regulation, we found the phosphorylation of ezrin at T567 to be dependent on PKC family members (BIM, Ro31–8220, Gö 6976) but not by inhibition of Rho-kinase (Y27632) or PI3 kinase (LY294002). Furthermore, ezrin and several PKC isoforms were found to coimmunoprecipitate in osteosarcoma cells. In support of this in vitro data, staining of lung metastases in mice showed that phospho-PKCα and phosphorylated ERM were both expressed in cells found at the leading front of metastatic lesions. The connection between PKC and ezrin expression was further supported by similar decreases in osteosarcoma cell migration following either PKC or ezrin inhibition. Lastly, cells overexpressing an activated phospho-mimetic ezrin mutant (T567D) were less responsive to the PKC inhibitors suggesting that PKC-induced motility was in part ezrin dependent. These data are the first to describe a dynamic regulation of ezrin phosphorylation during metastatic progression. This regulation is linked with PKC activation of ezrin and reciprocally suggests a role for ezrin in mediating some PKC prometastatic effects. Targeting PKC activation of ezrin during specific times in metastatic progression may be considered as a means to improve outcome for patients with metastasis.
Results
Phosphorylated ERM proteins are regulated during metastatic progression
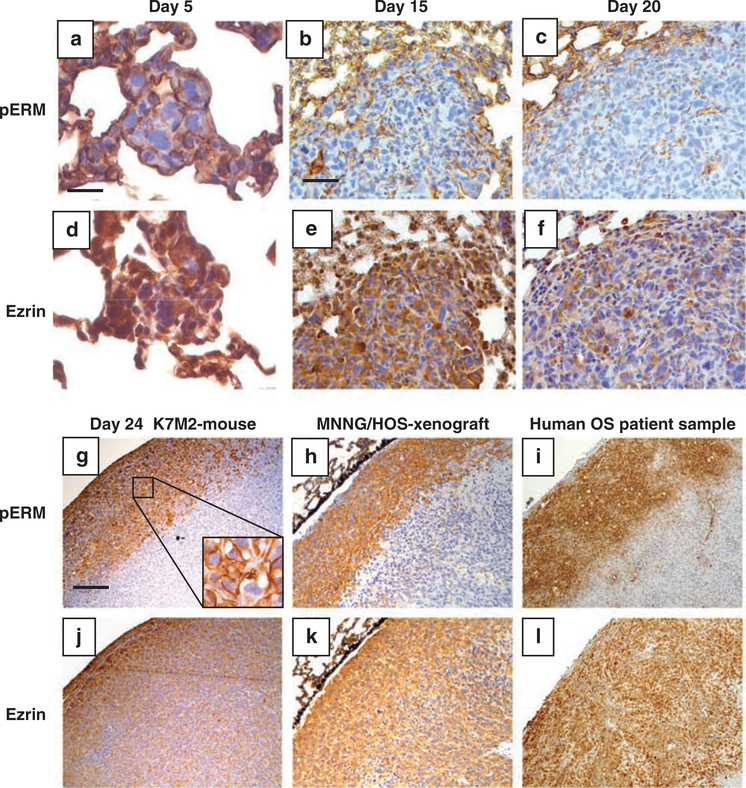
Human and murine osteosarcoma cells were injected intravenously into mice. Lung samples were harvested at various time points to assess the expression of ezrin and phosphorylated ERM (Figure 1 and Supplementary Figure S1). At present, an antibody that is specific for phosphorylated ezrin does not exist. Early (5 days) after K7M2 osteosarcoma cells arrived in the lung, both ezrin and phosphorylated ERM expression were uniformly high (Figure 1). It is important to note that most ezrin in K7M2 cells cultured in vitro is not phosphorylated (Supplementary Figure S2). At this time point, the metastatic cells were identified as single cells or in small clusters of 5–20 cells, with both total ezrin and phosphorylated ERM expression distributed throughout the cytosol and cell membrane. Fifteen days after arrival of the cells in the lung, small metastatic lesions (0.1–0.5mm) were observed throughout the lung. These metastatic foci continued to express total ezrin uniformly in the cytoplasm; however, phosphorylated ERM was dramatically decreased. With further progression of the metastatic lesions, the majority of the cells, particularly those in the center of the mass expressed very low phosphorylated ERM. Interestingly, as these larger metastatic lesions progressed, phosphorylated ERM began to be re-expressed but only at the periphery of the expanding metastatic lesions. This result was seen consistently following both experimental (Figure 1g) and spontaneous metastasis assays (data not shown) using K7M2 murine osteosarcoma cells in mice, using human MNNG/HOS osteosarcoma cells in mice (Figure 1h) and in six of six human metastatic osteosarcoma patient samples (Figure 1i). Observations of single metastatic cells and the expression of ezrin and phosphorylated ERM in these cells were limited to the use of the experimental metastasis assay (tail vein injection of tumor cells). Subsequent events during metastatic progression were assessed and found concordant following both experimental metastasis and spontaneous metastasis that developed from orthotopic primary appendicular osteosarcoma tumors. These data suggested that phosphorylation of ERM proteins was dynamically regulated during metastatic progression, and furthermore that the expression of phosphorylated ERM was not necessary throughout the metastatic process as we had expected. Phosphorylated ERM expression appeared to be important early after arrival of metastatic cells in the lung and during further progression at the invasive front of larger metastatic lesions.
Figure 1.
Phosphorylation (activation) of ERM proteins is dynamically regulated during metastatic progression. Metastatic lung nodules were evaluated by immunohistochemistry for expression of phosphorylated ERM and total ezrin. Samples were derived after tail vein injection of K7M2 murine osteosarcoma cells (a–g and j) or MNNG/HOS human osteosarcoma cells (h and k), and from human patient tissue samples (i and l). K7M2 lung metastases were examined at days 5, 15, 20 and 24 after injection (a–g and j). A similar pattern of immunoreactivity for ezrin and phosphorylated ERM were seen in K7M2 pulmonary metastases derived from spontaneous lung metastasis from an appendicular primary tumor in mice (data not shown). Total ezrin and phosphorylated ERM were stained on adjacent lung sections. a and d, Bar = 40 μm; b, c, e and f, bar = 100 μm; g–l, bar = 200 μm.
PKC is responsible for phosphorylation (activation) of ezrin on Thr 567 in osteosarcoma
To determine the kinase involved in the regulation of ezrin phosphorylation, the mouse ezrin protein sequence was included in a MotifScan (http://scansite.mit.edu) search for kinase candidate(s). As shown in Table 1, six kinases were identified in this scan as potential kinases for ezrin phosphorylation at threonine 567, four of which were PKC isoforms (classical PKCα, β, γ and ζ). For unknown reasons, although structurally similar to PKCζ the motif scan did not identify PKCi. The scan scores start at 0.000 if the sequence optimally matches a given motif, and the scores increase for sequences as they diverge from the optimal match. These data suggested the hypothesis that PKC family kinases are responsible for the phosphorylation of ezrin on Thr 567 (activation) in osteosarcoma. Outside the setting of cancer, several kinases (PKC, ROCK, GRK2, p38, MRCK and so on) have been previously shown to contribute to the phosphorylation of ezrin (Matsui et al., 1998; Oshiro et al., 1998; Pietromonaco et al., 1998; Ng et al., 2001; Weng et al., 2005; Wald et al., 2008). Very recently, PKC phosphorylation of ezrin in androgen-primed prostate cancer cells was reported, lending further support to our hypothesis in metastasis (Chuan et al., 2006).
Table 1.
MotifScan results for ezrin T567 site
| Kinasesa | Site | Scoreb | Percentilec |
|---|---|---|---|
| PKCα, β, γ | T567 | 0.5410 | 3.449% |
| PKCζ | T567 | 0.5566 | 1.407% |
| 14–3-3 Mode 1 | T567 | 0.5680 | 2.576% |
| PKA | T567 | 0.6074 | 4.058% |
Predicted Ser/Thr kinases for ezrin T567 phosphorylation site.
The scan scores start at 0.000 if the sequence optimally matches a given motif and the scores increase for sequences as they diverge from the optimal match. Lower scores in the output are thus better matches.
The percentile ranking of ezrin T567 phosphorylation site in respect to all potential motifs in vertebrate proteins in Swiss-Prot.
To test the hypothesis that PKC family kinases are active in phosphorylation of ezrin at threonine 567 in osteosarcoma cells, pharmacological inhibitors of selected kinases were exposed to K7M2 murine osteosarcoma cells. Following exposure, cell lysates were collected and analysed for phosphorylation of ERM proteins at the C-terminal threonine. As shown in Figure 2a, BIM (inhibits PKC and other kinases) suppressed threonine phosphorylation of ERM proteins in a dose-dependent manner. Exposure of cells to Y27632 (inhibits Rho kinase), LY294002 (inhibits PI3 kinase) and U0126 (inhibits MEK1/2) did not change phosphorylated ERM at the C-terminal threonine (Figure 2a). The specific modulation of either AKT (LY294002) or MAPK-44/42 (U0126) confirmed each agent’s biological activity and the presence of the pathway in K7M2 cells (Figure 2a). To further demonstrate that the inhibition of phosphorylated ERM was mediated by PKC, isoform selective PKC inhibitors were evaluated in both murine K7M2 and human MNNG/HOS cells as shown in Figure 2b. In both cell lines, treatment with Ro31–8220 (inhibits PKC) and Gö 6976 (inhibits PKC isoforms; PKCα, βI, βII and γ) resulted in decreased phosphorylated ERM levels in a time- and concentration-dependent manner. In situ evaluation demonstrated that phosphorylated ERM proteins were mainly localized on the cell membrane, largely concentrated in the membrane protrusions (microspikes) (Figure 2c). Following the treatment of cells with the PKC inhibitor Ro31–8220, this membranous and microspike expression of phosphorylated ERM was markedly diminished (Figure 2c). It should be noted that PKC inhibitors were active at concentrations as low as 1 μM within 10min of exposure. The rapid effects of PKC inhibitors on ERM phosphorylation may be explained by the rapid intrinsic turnover of T567 phosphorylation (Zhu et al., 2007) or through the activation of an ERM-specific phosphatase following PKC inhibition (Forte et al., 2008). The physiological relevance of these exposures is not known. However, it is important to note that significantly greater exposures to other kinase inhibitors had no effect on phosphorylated ERM expression in these cells. Nonetheless, the lack of complete specificity of pharmacological inhibitors of kinases, including the isoform-specific PKC inhibitors used in our studies, should be considered when interpreting these data.
Figure 2.
Ezrin (T567) phosphorylation is dependent on protein kinase C (PKC). (a) Murine osteosarcoma K7M2 cells were incubated with various concentrations of PKC inhibitor BIM, Rho kinase inhibitor Y27632, PI3 kinase inhibitor LY294002 and MEK inhibitor U0126. Phospho-ERM expression is shown by western blot analysis (phosphorylated ERM is comprised of a phospho-ezrin/radixin band * and a phospho-moesin band **). The blots were probed for β-actin as the loading control, and phospho-Akt (Y374) or phospho-MAPK 42/44. (b) K7M2 and MNNG/HOS cells were treated with PKC inhibitors BIM, Ro 31–8220 or Gö 6976 at various concentrations for 60min or for different time periods as indicated. The level of phospho-ERM was analysed by western blotting. (c) Imuunofluorescent detection of phospho-ERM in untreated K7M2 cells or in K7M2 cells treated with 5 μM of Ro 31–8220 for 60min. Bar = 20 μm in low magnification photographs and 5 μm in high magnification photographs.
PKC isoforms α, γ and ι complex with ezrin in vivo
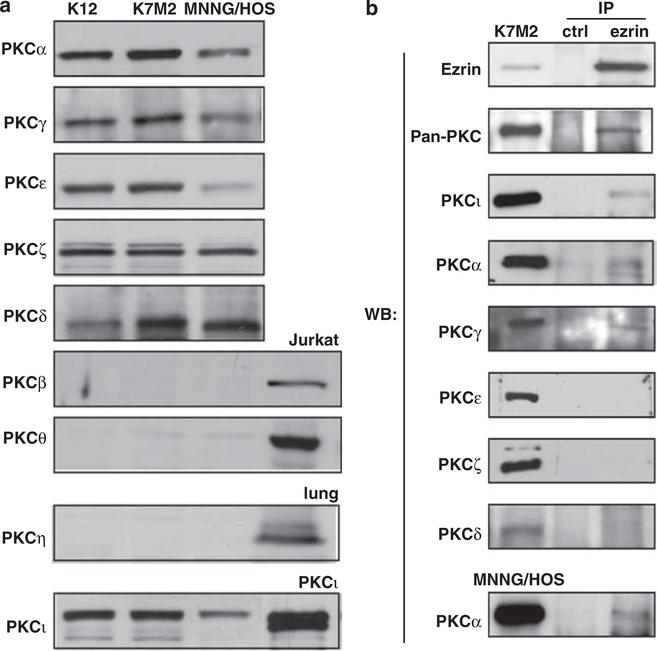
To further explore the connection of ezrin’s phosphorylation by PKC isoforms, we screened human and murine osteosarcoma cells for the expression of all PKC isoforms by immunoblot analysis. PKCα, γ, ε, ζ, ι and δ were expressed in murine K7M2, K12 and human MNNG/HOS osteosarcoma cell lines (Figure 3a). However, PKCβ, θ and η were not detectable. In addition, ezrin and the PKC isoforms α and γ and ι coimmunoprecipitated in the osteosarcoma cells (Figure 3b). We could not demonstrate PKCδ, PKCε and PKCζ coimmunoprecipitation with ezrin. On the basis of PKC coimmunoprecipitation results, we next used small interfering RNAs (siRNAs) to knockdown the expression of PKCα, γ and ι in the K7M2 osteosarcoma cells. The individual knockdown of PKCγ and ι was nearly 100%; however, no changes were seen in expression of phosphorylated ERM (data not shown). Consistent with reports in the literature, despite repeated attempts with several siRNAs and siRNA pools, we could not knock PKCα by more than 50% (data not shown). PKCα expression was not further modulated when pools of PKCα, γ and ι siRNAs were combined. Accordingly, the siRNA studies were unable to definitively answer if PKCα, PKCγ and PKCι are responsible for the regulation of ezrin phosphorylation in ostoesarcoma cells (Supplementary Figure S3).
Figure 3.
Ezrin forms protein–protein complexes with PKCα, ι and γ in osteosarcoma cells. (a) Western blot analysis shows expression of the PKC isoforms in K12, K7M2 and MNNG/HOS osteosarcoma cell lines. PKCβ, η and θ isoforms are not expressed in osteosarcoma cells, although they are expressed in Jurkat cells or lung tissue. (b) Immunoprecipitated ezrin from K7M2 or MNNG/ HOS cell extracts were probed for pan-PKC, PKCα, γ, δ, ι, ε, ζ and ezrin. Ezrin interactions were detected with PKC isoforms α, γ and ι. Normal rabbit serum was used as the negative control.
Phospho-PKCα and phospho-ERM show identical expression patterns in osteosarcoma lung metastases
To study the association between ezrin and PKC in vivo, we performed immunohistochemistry using phospho-PKCα (Ser657) and phospho-ERM in lung nodules of K7M2 tumor-bearing mice. The phosphorylation of PKC on serine 657 controls accumulation of the active enzyme and contributes to the maintenance of the phosphatase-resistant conformation (Bornancin and Parker, 1997) of PKC. As shown in Figure 4, intense staining of phospho-PKCa (membranous, cytoplasmic and nuclear) was seen at the leading edges of metastastatic lesions (Figures 4a and b). At these same locations within the metastatic lesions, phospho-ERM was also highly expressed and localized predominantly on the tumor cell membrane (Figures 4d and e). Conversely, both active PKCα expression and phosphorylated ERM expression were low in the central portions of these lesions (Figures 4c and f). Interestingly, low phospho-PKCα expression in the central portions of the metastatic lesions was largely localized to the nucleus with no membranous or cytoplasmic staining.
Figure 4.
Matching expression patterns of phospho-ERM and phospho-PKCα in metastatic lesions. Immunohistochemistry detection of phosphorylated ERM and phospho-PKCα in K7M2 murine osteosarcoma lung nodules (harvested 24 days after cell injection to mice). Adjacent lung sections were labeled with antibodies against either phospho-PKCα (Ser 657) (a–c) or phospho-ERM (d–f). Phospho-PKCα (a and b) and phospho-ERM (d and e) immunoreactivity was evident at the periphery of metastatic lesions. Both active PKCα expression and phosphorylated ERM expression was low in the central portions of the metastatic lesions (Figures 4c and f). Bar = 200 μm in low magnification photographs and 50 μm in high magnification photographs.
PKC-mediated tumor cell migration is in part mediated by ezrin
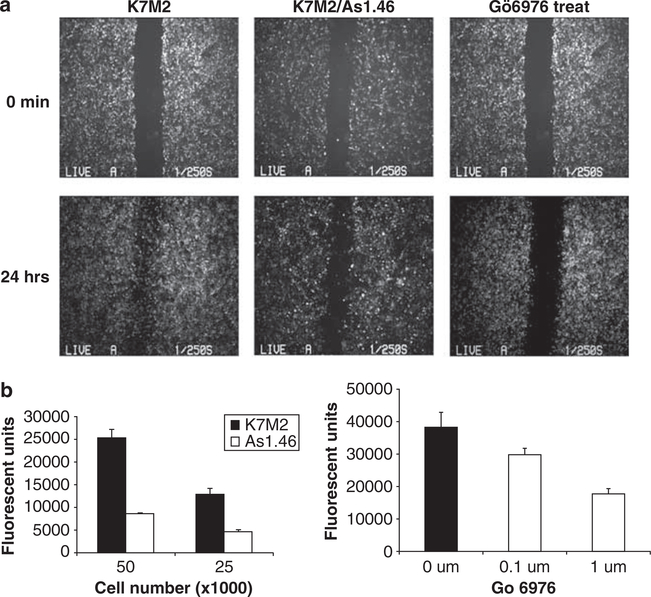
As previously reported, the suppression of total ezrin protein, using full-length antisense constructs (K7M2 As1.46) resulted in an inhibition of metastasis in mice without any measurable changes in primary tumor growth (Khanna et al., 2004). To extend these findings and explore the biological significance of PKC regulation on ERM phosphorylation, we independently examined the effects of ezrin and PKC on cell motility using wound healing and transwell migration assays. The cells with knockdown of ezrin (K7M2 As1.46 cells; low ezrin, poorly metastatic) had a significant delay in migration in the wound-healing migration assay compared with the parental K7M2 cells (high ezrin, highly metastatic; Figure 5a). Exposure of cells to the PKC inhibitor Gö 6976 at 1 μM also blocked cell migration through the wounded area (Figure 5a). Treatment of cells with Gö 6976 at 1 μM had no effect on proliferation in K7M2 cells, supporting the antimigratory role. Similar results were also seen with the PKC inhibitor Ro31–8220 (data not shown). To confirm the antimigratory phenotype associated with downregulation of ezrin and by PKC inhibition, we performed a modified Boyden chamber transwell assay. These results demonstrated significant inhibition of cell motility following ezrin suppression (antisense Ezrin-As1.46; 5 × 104 cells, P = 0.004; 2.5 × 104 cells, P = 0.01) or following either treatment with the PKC inhibitor Gö 6976 (0.1 μM, P = 0.05; 1 μM, P = 0.03) (Figure 5b).
Figure 5.
Suppression of ezrin or inhibition of PKC decreases osteosarcoma cell migration. (a) Nearly confluent wild-type ezrin (K7M2) or antisense mediated ezrin knockdown cells (As 1.46) were ‘wounded’ using a P-200 pipette, and images of the denuded area were taken at 0 and 24h. Migration of cells with suppression of ezrin (As1.46) was markedly decreased at 24h compared to K7M2 control cells (high ezrin). K7M2 cells that had been treated with 1 μM PKC inhibitor Gö 6976 also showed a significant reduction in migration. (b) Ezrin knock down (As1.46) and control K7M2 cells were seeded on transwell plates in complete medium (left panel). After an 18-h incubation, cells migrating to the lower chamber were stained with Calcein AM and the fluorescent intensity was quantitated. Ezrin knockdown cells (As1.46) had a marked decrease in migration compared to control cells (K7M2) using either 5 × 104 cells (P = 0.004) or 2.5 × 104 cells (P = 0.01) as starting material. Similarly, K7M2 cells were seeded on transwell plates with 0.1% dimethylsulfoxide or PKC inhibitor Gö 6976 (right panel). Cell migration was inhibited in the presence of Gö 6976 at 0.1 μM (P = 0.05) and at 1 μM (P = 0.03).
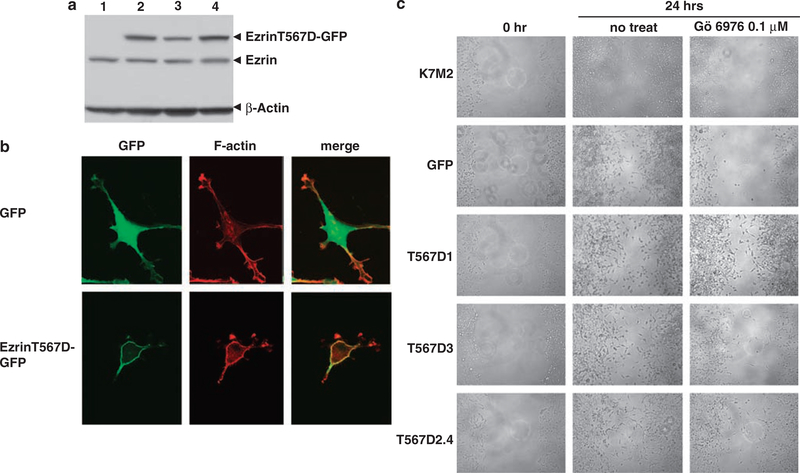
To link the similar anti-migratory results obtained following either ezrin knockdown or PKC inhibition, we performed similar experiments using an ezrin mutant that has been previously described to mimic the activated ‘open’ confirmation of ezrin (Pearson et al., 2000). The Thr 567 to Asp (EzrinT567D-GFP, activated-ezrin-GFP) mutation was detected by western blot analysis by the virtue of its larger size at 110kDa (Figure 6a). As predicted, the expression of the active ezrin (EzrinT567D-GFP) was localized to the cell membrane (Figure 6b). Treatment of both parental K7M2/GFP cells with the PKC inhibitor Gö 6976 resulted in decreased migration as expected. However, K7M2/EzrinT567D-GFP cells were more able to migrate in the presence of a PKC inhibitor (Figure 6c) compared with parental and GFP-expressing K7M2 cells. These data provide a link between PKC-mediated activation of ezrin and the metastatic phenotype of enhanced cell motility.
Figure 6.
PKC-mediated cell migration is in part dependent on ezrin. Expression of constitutively activated phospho-mimetic ezrin mutant (T567D-GFP) in K7M2 osteosarcoma cells allowed association between PKC, ezrin and cell migration to be assessed. (a) Expression of endogenous ezrin and GFP-tagged ezrin mutant proteins in K7M2 parental cells were resolved by western blot analysis and with an anti-ezrin antibody. Lane 1, GFP transfectants; lanes 2–4, three different clones of EzrinT567D-GFP transfectants. (b) Localization of GFP and EzrinT567D-GFP in K7M2 cells was assessed by GFP fluorescence. Whereas GFP was distributed throughout the cytoplasm and nucleus, EzrinT567D-GFP (active ezrin) was discretely localized to the cell membrane with F-actin. (c) Cell migration (wound healing) assay using K7M2 control, GFP control, and three different clones of EzrinT567D-GFP cells (T567D1, T567D3 and T567D2.4) before and after 24h of treatment with either 0.1% dimethylsulfoxide or 0.1 μM Gö 6976. The constitutive activation of ezrin in the EzrinT567D-GFP prevented PKC inhibitors from suppressing osteosarcoma cell migration.
Discussion
To study the mechanisms associated with ezrin’s role in metastasis, we followed the expression of ezrin and its phosphorylation serially during the metastatic cascade. Our previous studies have confirmed a unique dependence of metastatic cells on ezrin rather than the other ERM proteins (Khanna et al., 2004; Yu et al., 2004; Krishnan et al., 2006). As expected, we found high levels of total ezrin expressed in all lesions at all time points during metastasis. Our expectation was that the expression of phosphorylated ezrin would be equally and continuously high. We were surprised to find that phosphorylated ERM was not expressed throughout all stages of progression. ERM was phosphorylated early after metastatic cells arrived in the lung. As these multicellular lesions progressed, they lost the expression of phosphorylated ERM. However, as these lesions grew even larger, phosphorylated ERM was again expressed, but only at the leading edge, or invasive front, of the lesions. These data, confirmed in human osteosarcoma metastases, suggested that phosphorylated ezrin was not needed throughout metastatic progression and that the phosphorylation of ezrin was regulated in metastatic osteosarcoma.
The high expression of phosphorylated ERM in the early stages of metastasis is consistent with an existing hypothesis that ezrin contributes to the survival of metastatic cancer cells following their arrival in the lung. ERM proteins are regulated by an intramolecular association of the N-terminal and C-terminal domains that masks their protein–protein binding sites (Bretscher et al., 2002). Unfolding of the molecule into an active conformation occurs following binding to phosphoinositides and phosphorylation of the C-terminal threonine (T558 in moesin, T567 in ezrin, T564 in radixin) (Fievet et al., 2004). The open molecules bind with various membrane proteins (Tsukita et al., 1994; Bretscher et al., 1997; Reczek et al., 1997; Simons et al., 1998; Yonemura et al., 1998) at the N-terminal region and F-actin through the C-terminal domain (Bretscher et al., 1997; Hishiya et al., 1999; Pearson et al., 2000). As osteosarcoma cells in culture express high levels of ezrin and phosphorylated ERM, it was possible that the high levels of phosphorylated ERM seen in single cells that arrived in the lung following tail vein injection was merely a residual effect of their growth in vitro. However, it was not expected that the residual expression of phosphorylated ERM would persist for 5 days. The loss of ERM phosphorylation later in the course of metastatic progression was a consistent finding that suggested two possibilities. Namely, that phosphorylation of ERM proteins was not required during this stage of metastatic growth or that dephosphorylation of ERM proteins was necessary for progression. Dephosphorylation of C-terminal threonine of moesin is a crucial step for transendothelial migration of lymphocytes (Brown et al., 2003). Accordingly, it is interesting to speculate that ezrin dephosphorylation is an active and necessary step, required for the development of cell– cell contacts needed during progression of metastatic cells to multicellular clusters. Again, consistent with our hypothesis that ezrin is needed for the survival of metastatic cells, especially those encountering the foreign microenvironment of a secondary metastatic site, we observed the re-expression of phosphorylated ERM at the invasive front of metastatic lesions. At this time, we do not have data to causally associate the expression of phosphorylated ERM in single metastatic cells, either early after their arrival in the lung or at the periphery of established grossly detectable lesions, with metastatic success. Nonetheless, these data suggest a dynamic regulation of ERM during cancer progression rather than our initial expectation that constitutive activation of ezrin would be a requirement for metastases; furthermore, it appears that the phosphorylated and dephosphorylated states of ezrin are dynamically regulated during the process of metastatic progression.
Several kinases have been implicated in the regulation of ERM protein function. PKCα has been shown to interact with ezrin, both in vitro and in vivo, and can phosphorylate ezrin at T567 in vitro (Ng et al., 2001). Using a MotifScan program, we were able to identify six kinases that had the potential to phosphorylate ezrin at T567, three of which were classical (or conventional) PKCs and one was PKCζ. A screen using a panel of kinase inhibitors showed that only PKC inhibitors could decrease phosphorylation of ERM in osteosarcoma cells. Immunoprecipitation experiments then verified that PKCα, PKCγ and PKCι interact with ezrin. SiRNA-mediated knockdown PKCγ, and PKCι failed to decrease phosphorylation of ERM in osteosarcoma cells. SiRNA knockdown of PKCα was incomplete following the exposure of cells to siRNAs for PKCα alone, or siRNAs for PKCα, PKCγ and PKCι used in combination, as such were unable to ask if PKCα alone or if the combination of PKCα, PKCγ and PKCι regulated ezrin phosphorylation in osteosarcoma cells. The use of selective pharmacological inhibitors or more effective genetic knockdown strategies for specific PKC isoforms are required to better understand which PKC isoforms are individually or collectively involved in the dynamic regulation of ezrin phosphorylation during metastasis.
Protein kinase C isoforms have been connected with several aspects of cancer biology including carcinogenesis, progression and chemotherapy resistance (Blobe et al., 1994). Several of the steps associated with metastatic progression have been linked to PKC, including resistance to apoptosis, migration and invasion (Herbert, 1993; Musashi et al., 2000; Sullivan et al., 2000; Koivunen et al., 2004). To further connect our growing understanding of ezrin’s role in the metastatic phenotype, with our finding that PKC isoforms regulate ezrin phosphorylation, we hypothesized that some of the previously established effects of PKC on metastasisspecific functions, (that is, motility) were mediated through ezrin. To test this hypothesis, we mutated the C-terminal threonine of ezrin to aspartic acid. The result of this site-directed mutation was a constitutively phosphorylated conformation (phospho-mimetic, T567D). Using motility as an example of a PKC-related function, we found osteosarcoma cells with wild-type expression of ezrin to have significantly reduced migration following exposure to PKC inhibitors; whereas, cells expressing the phospho-mimetic ezrin (active) were not affected by PKC inhibitors. These data suggest that part of the PKC-mediated effects on cell migration are mediated through ezrin phosphorylation and may suggest that other recognized effects of PKC on metastasis are also mediated through ezrin.
In summary, our data suggest that the phosphorylation of ERM proteins, including ezrin, is regulated during the metastasis process in osteosarcoma. PKC is responsible for the phosphorylation of ezrin C-terminal threonine both early after cells arrive at secondary sites and during the invasion of the metastatic lesion into the surrounding parenchyma. Efforts are underway to develop isoform-specific PKC inhibitors as therapeutic drugs to treat cancer. The connection between ezrin and PKC suggest that cancers that are dependent on ezrin may be responsive to PKC inhibitors and that these inhibitors may be especially active in patients at high risk for metastasis.
Materials and methods
Cell culture
K7M2 and K12 murine osteosarcoma cell lines have been described previously (Khanna et al., 2001). MNNG/HOS human osteosarcoma cell lines were obtained from ATCC (Manassas, VA, USA) and grown in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum. Geneticin (0.8mg/ml) (Invitrogen) was added to the medium of K7M2 cells that had been transfected with antisense ezrin or ezrin mutant constructs.
In vivo studies and immunohistochemistry
The experimental and spontaneous metastasis assays were performed as previously described (Khanna et al., 2000). For the experimental metastasis assay, the entire lung was harvested from mice on days 5, 15, 20 and 24 after injection. For spontaneous metastasis assay, the lungs were collected from days 0 to 50 after the removing of primary tumor. Lungs were inflated by tracheobronchial injection of 1.0ml neutral buffered 10% formalin (Fisher, Newark, DE, USA). All tissues were fixed in formalin immediately after collection for 24h, and then transferred to 80% ethanol. All tissues were embedded in paraffin, sectioned at 5 μm thickness and mounted on glass slides. Slides were deparaffinized and rehydrated as previously described (Khanna et al., 2001). Slides were incubated in preheated target retrieval solution (Dako, Carpinteria, CA, USA), pH6, in a steam cooker for 20min. Anti-ezrin antibody (Sigma, St Louis, MO, USA) was used at 1:500 dilution, anti-phospho-ezrin(Thr567)/radixin(Thr564)/moesin(Thr558) (phosphorylated ERM) antibody (Cell Signaling Technology Inc., Danvers, MA, USA) and anti-phospho-PKCα (Ser657) antibody (Upstate, Swampscott, MA, USA) at 1:100 dilution. The samples were counterstained with hematoxylin (Dako) for 30s and mounted.
Immunoprecipitation and western blotting
Cells were lysed in RIPA buffer (150mM NaCl, 50mM Tris, pH 8.0, 0.1% SDS, 0.25% deoxycholate, 1% NP-40) with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) and 1 μM calyculin A (Alexis Biochemicals, Lausen, Switzerland). Lysates containing 1–2mg protein were precleared with A/G agarose beads (Pierce Biotechnology Inc., Rockford, IL, USA) and incubated overnight at 4°C with 7 μl of anti-ezrin serum (kindly provided by Dr Anthony Bretscher, Cornell University) or normal rabbit serum. Proteins were resolved on a 6% SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. To detect the expression of PKC isoforms, OS cells were lysed in SDS sample loading buffer. Proteins were resolved on a 4–12% SDS– polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Western blotting was performed with anti-ezrin (1:4000 dilution) (Sigma), anti-PKCα (1:1000 dilution) (Upstate), anti-PKCγ (1:1000 dilution) (BD Biosciences, Palo Alto, CA, USA), anti-PKCδ (1:500 dilution) (BD Biosciences), anti-PKCι (1:250 dilution) (BD Biosciences), anti-PKCζ (1:500 dilution) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-PKCη (1:500 dilution) (Santa Cruz Biotechnology Inc.), anti-PKCZ (1:500 dilution) (Santa Cruz Biotechnology Inc.), anti-PKCβ (1:250 dilution) (BD Biosciences), anti-PKCθ (1:200 dilution) (Santa Cruz Biotechnology Inc.) and anti-panPKC (1:500 dilution) (Santa Cruz Biotechnology Inc.) antibodies.
Immunofluorescence staining
Cells were fixed with 3% paraformaldehyde/phosphate-buffered saline, and permeablized with 0.2% Triton-X 100. After blocking with 0.2% bovine serum albumin for 30min, cells were incubated with anti-phosphorylated ERM antibody (Cell Signaling Technology Inc.) at 1:100 dilution for 30– 60min, followed by FITC-labeled secondary antibody (Molecular Probe, Eugene, OR, USA). Stained cells were mounted with VectaShield (Vector Laboratories, Burlingame, CA, USA) mounting medium and visualized using a Leica DMIRB fluorescent microscope at 10 × or 40 × magnification.
Kinase inhibitor treatments
Equal number of cells were plated in six-well tissue culture plates, grown to 70% confluence and then treated with the pharmacological inhibitors for PKC (BIM, Ro31–8220, Gö 6976) (Alexis Biochemicals), Rho-kinase (Y27632) (Santa Cruz), PI3 kinase (LY294002) (Cell Signaling Technology Inc.) or MEK1/2 (U0126) (Sigma). Dose titration (0, 0.1, 1, 5, 10, 20 μM) at 60min and time course (0, 10, 30, 60, 120 min) studies at optimal doses were performed. Dimethylsulfoxide was used as the control for the treatments. The treated cells were lysed in 200 μl of 1 × Laemmli’s buffer. Western blot analysis was performed using anti-phosphorylated ERM antibody (1:1000 dilution). The phosphorylation of Akt and ERK1/2 (p44/42) confirmed the inhibition of PI3 kinase and MEK1/2.
Wound-healing assay
K7M2 cells were plated in six-well tissue culture plates and grown to 70% confluence in complete medium. A ‘wound’ was made by scraping with a P200 pipette tip in the middle of the cell monolayer. Floating cells were removed by washing with phosphate-buffered saline and fresh complete medium containing dimethylsulfoxide or 1 μM Gö 6976 was added. Cells were incubated at 37°C for 24h. Phase contrast images were then taken using a Leica DMIRB inverted microscope.
Cell migration assay
In vitro tumor cell migration was assessed using a 24-Multiwell Insert System (HTS FluoroBlok, BD Biosciences) containing an 8-μm pore size polyethylene terephthalate membrane. Briefly, 0.5ml of tumor cells (5 × 104 or 2.5 × 104 cells per ml) resuspended in DMEM medium containing 5% fetal bovine serum was added to the upper chamber (in triplicate). DMEM medium containing 10% fetal bovine serum was added to the lower chamber. The PKC inhibitor (Ro31–8220 or Gö 6976) was added in both upper and lower chambers. The cells were incubated for 18h at 37°C and 5% CO2. Migrated cells were then stained with 5 μM Calcein AM (Molecular Probe) in HBSS buffer for 1h at 37°C. To quantitate tumor cell migration, fluorescently labeled cells were detected at an excitation wavelength of 485nm and emission wavelength of 530nm using a Wallac Victor 3 microplate reader (PerkinElmer, Wellesley, MA, USA). Migration experiments were repeated at least three times. Unpaired t-test with Welch’s correction was used for the migration studies. Statistical analyses were performed using GraphPad Prism version 4.0b for the Macintosh (GraphPad Software).
Transfection and expression of ezrin mutant
K7M2 cells in six-well tissue culture plates were grown in DMEM with 10% fetal bovine serum to 70% confluence. Transfection of the mutant ezrin construct (pEGFP-N1ezrinT567D-GFP) was performed with Trans IT-LT1 (Mirus Bio Co. Madison, WI, USA) transfection reagent following the manufacturer’s protocol. Two days after transfection, selection was started using media containing 0.8mg/ml G418. Stable transfected single cell clones (T567D1, T567D3 and T567D2.4) were utilized for all experiments.
Confocal microscopy
K7M2 cells expressing GFP or ezrin-T567D-GFP were fixed with 3% paraformaldehyde/phosphate-buffered saline, and permeabilized with 0.2% Triton-X 100. Cells were incubated with rhodamine phalloidin (Invitrogen) at 1:200 dilution for 20min and then washed with phosphate-buffered saline. Confocal images were obtained in Zeiss confocal microscope LSM510 using Zeiss LSM imagine browser software (Carl Zeiss, Oberkochen, Germany).
Supplementary Material
Acknowledgements
We would like to thank Drs Joseph Briggs and Luowei Li for experimental advice and their critical review of this manuscript.
References
- Berryman M, Franck Z, Bretscher A. (1993). Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105(Part 4): 1025–1043. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Obeid LM, Hannun YA. (1994). Regulation of protein kinase C and role in cancer biology. Cancer Metastasis Rev 13: 411–431. [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker PJ. (1997). Phosphorylation of protein kinase C-alpha on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J Biol Chem 272: 3544–3549. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. (2000). ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 16: 113–143. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. (2002). ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Reczek D, Berryman M. (1997). Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 110(Part 24): 3011–3018. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL et al. (2003). Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 102: 3890–3899. [DOI] [PubMed] [Google Scholar]
- Bruce B, Khanna G, Ren L, Landberg G, Jirstrom K, Powell C et al. (2007). Expression of the cytoskeleton linker protein ezrin in human cancers. Clin Exp Metastasis 24: 69–78. [DOI] [PubMed] [Google Scholar]
- Cant SH, Pitcher JA. (2005). G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell 16: 3088–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. (2006). Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem 281: 29938–29948. [DOI] [PubMed] [Google Scholar]
- Dard N, Louvet-Vallee S, Santa-Maria A, Maro B. (2004). Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol 271: 87–97. [DOI] [PubMed] [Google Scholar]
- Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. (2005). The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res 7: R365–R373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D et al. (2004). Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E, Orsatti L, Talamo F, Barbato G, De Francesco R, Tomei L. (2008). Ezrin is a specific and direct target of protein tyrosine phosphatase PRL-3. Biochim Biophys Acta 1783: 334–344. [DOI] [PubMed] [Google Scholar]
- Gautreau A, Louvard D, Arpin M. (2000). Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol 150: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM. (1993). Protein kinase C: a key factor in the regulation of tumor cell adhesion to the endothelium. Biochem Pharmacol 45: 527–537. [DOI] [PubMed] [Google Scholar]
- Hishiya A, Ohnishi M, Tamura S, Nakamura F. (1999). Protein phosphatase 2C inactivates F-actin binding of human platelet moesin. J Biol Chem 274: 26705–26712. [DOI] [PubMed] [Google Scholar]
- Ilmonen S, Vaheri A, Asko-Seljavaara S, Carpen O. (2005). Ezrin in primary cutaneous melanoma. Mod Pathol 18: 503–510. [DOI] [PubMed] [Google Scholar]
- Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C et al. (2001). Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res 61: 3750–3759. [PubMed] [Google Scholar]
- Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. (2000). An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis 18: 261–271. [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A et al. (2004). The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10: 182–186. [DOI] [PubMed] [Google Scholar]
- Kivela T, Jaaskelainen J, Vaheri A, Carpen O. (2000). Ezrin, a membrane-organizing protein, as a polarization marker of the retinal pigment epithelium in vertebrates. Cell Tissue Res 301: 217–223. [DOI] [PubMed] [Google Scholar]
- Kobel M, Gradhand E, Zeng K, Schmitt WD, Kriese K, Lantzsch T et al. (2006). Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol 25: 121–130. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. (2004). Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res 64: 5693–5701. [DOI] [PubMed] [Google Scholar]
- Krieg J, Hunter T. (1992). Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem 267: 19258–19265. [PubMed] [Google Scholar]
- Krishnan K, Bruce B, Hewitt S, Thomas D, Khanna C, Helman LJ. (2006). Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin Exp Metastasis 23: 227–236. [DOI] [PubMed] [Google Scholar]
- Makitie T, Carpen O, Vaheri A, Kivela T. (2001). Ezrin as a prognostic indicator and its relationship to tumor characteristics in uveal malignant melanoma. Invest Ophthalmol Vis Sci 42: 2442–2449. [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K et al. (1998). Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-totail association. J Cell Biol 140: 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Yonemura S, Tsukita S. (1999). Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol 9: 1259–1262. [DOI] [PubMed] [Google Scholar]
- Musashi M, Ota S, Shiroshita N. (2000). The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol 72: 12–19. [PubMed] [Google Scholar]
- Nakamura N, Oshiro N, Fukata Y, Amano M, Fukata M, Kuroda S et al. (2000). Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells 5: 571–581. [DOI] [PubMed] [Google Scholar]
- Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A et al. (2001). Ezrin is a downstream effector of trafficking PKCintegrin complexes involved in the control of cell motility. EMBO J 20: 2723–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. (1999). Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett 147: 31–38. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Fukata Y, Kaibuchi K. (1998). Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem 273: 34663–34666. [DOI] [PubMed] [Google Scholar]
- Pang ST, Fang X, Valdman A, Norstedt G, Pousette A, Egevad L et al. (2004). Expression of ezrin in prostatic intraepithelial neoplasia. Urology 63: 609–612. [DOI] [PubMed] [Google Scholar]
- Pearson MA, Reczek D, Bretscher A, Karplus PA. (2000). Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101: 259–270. [DOI] [PubMed] [Google Scholar]
- Pietromonaco SF, Simons PC, Altman A, Elias L. (1998). Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J Biol Chem 273: 7594–7603. [DOI] [PubMed] [Google Scholar]
- Polesello C, Payre F. (2004). Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol 14: 294–302. [DOI] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. (1997). Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 139: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. (2004). Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6: 855–864. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Nieto M, Sanchez-Madrid F. (1999). Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol 9: 228–233. [DOI] [PubMed] [Google Scholar]
- Simons PC, Pietromonaco SF, Reczek D, Bretscher A, Elias L. (1998). C-terminal threonine phosphorylation activates ERM proteins to link the cell’s cortical lipid bilayer to the cytoskeleton. Biochem Biophys Res Commun 253: 561–565. [DOI] [PubMed] [Google Scholar]
- Srivastava J, Elliott BE, Louvard D, Arpin M. (2005). Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Mol Biol Cell 16: 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stone M, Marshall JF, Uberall F, Rotenberg SA. (2000). Photo-induced inactivation of protein kinase calpha by dequalinium inhibits motility of murine melanoma cells. Mol Pharmacol 58: 729–737. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. (1994). ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJ. (2008). Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci 121(Part 5): 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng WH, Ahlen J, Astrom K, Lui WO, Larsson C. (2005). Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res 11: 6198–6204. [DOI] [PubMed] [Google Scholar]
- Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA et al. (2004). The NHE1 Na+/H+ exchanger recruits ezrin/ radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem 279: 26280–26286. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. (1998). Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 140: 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. (2004). Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 10: 175–181. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J et al. (2007). High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 293: C874–C884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.