Abstract
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic bladder disorder with epithelial thinning or ulceration, pain, urinary frequency and urgency, for which there is no reliably effective therapy. We previously reported that IC/PBS bladder epithelial cells make a glycopeptide antiproliferative factor or “APF” (Neu5Acα2-3Galβ1-3GalNAcα-O-TVPAAVVVA) that induces abnormalities in normal cells similar to those in IC/PBS cells in vitro, including decreased proliferation, decreased tight junction formation, and increased paracellular permeability. We screened inactive APF derivatives for their ability to block antiproliferative activity of asialylated-APF (“as-APF”) in normal bladder cells, and determined the ability of as-APF-blocking derivatives to normalize tight junction protein expression, paracellular permeability, and/or proliferation of IC/PBS cells. Only two of these derivatives [Galβ1-3GalNAcα-O-TV-(d-pipecolic acid)-AAVVVA and Galβ1-3GalNAcα-O-TV-(d-proline)-AAVVVA] blocked as-APF antiproliferative activity in normal cells (p<.001 for both). Both of these antagonists also 1) significantly increased mRNA expression of ZO-1, occludin, and claudins 1, 4, 8, and 12 in IC/PBS cells by qRT-PCR; 2) normalized IC/PBS epithelial cell tight junction protein expression and tight junction formation by confocal immunofluorescence microscopy; and 3) decreased paracellular permeability of 14C-mannitol and 3H-inulin between confluent IC/PBS epithelial cells on Transwell plates, suggesting that these potent APF antagonists may be useful for development as IC/PBS therapies.
Keywords: interstitial cystitis, antiproliferative factor, bladder, epithelium, tight junctions
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic disorder that is characterized by bladder epithelial thinning or ulceration, pain, urinary frequency and urgency (1-3). The etiology of IC/PBS remains unknown, and there is currently no reliably effective therapy for this disorder.
Epithelial cells that line the interior urinary bladder wall form an important barrier against noxious substances in urine. This barrier effectively results from the formation of intercellular junctions (including tight junctions as well as adherens junctions), which consist of both transmembrane components and cytosolic proteins that provide a direct or indirect link between the epithelial cell cytoskeleton and the surrounding mammalian bladder tissue (4-6). Tight junctions are the most apical junctional complexes and have been shown to include zonula occludens-1 (ZO-1), occludin, and claudin proteins in the mammalian urinary bladder (5,7).
Several reports have indicated that the bladder epithelial barrier may be abnormal in IC/PBS. IC/PBS patients have thinning or denudation of the bladder epithelium (3,8,9), decreased expression of ZO-1 (10), increased absorption of urea given intravesically (9,11), and increased pain following intravesical potassium chloride infusion (12), indicating that bladder epithelial permeability is abnormally increased in IC/PBS patients as compared to controls. Explanted bladder epithelial cells from IC/PBS patients have also been shown to have increased paracellular permeability and decreased expression of several tight junction proteins including ZO-1, occludin, and certain claudins in vitro (7,13,14).
We previously determined that IC/PBS bladder epithelial cells produce a small frizzled 8 protein-related factor (antiproliferative factor, or “APF”) that inhibits proliferation and regulates bladder epithelial cell gene expression resulting in a less proliferative cell phenotype (15-18). APF is a small sialoglycopeptide (Neu5Acα2-3Galβ1-3GalNAcα-O-TVPAAVVVA) whose peptide backbone bears 100% homology to a segment from the 6th transmembrane portion of Frizzled 8 (17,19). APF causes abnormalities in normal bladder epithelial cells that mimic changes seen in IC/PBS cells after passage in vitro, including profoundly inhibited cell proliferation (15), increased p53 and p21 expression (20), altered epithelial growth factor production [including significantly decreased heparin-binding epidermal growth factor-like growth factor (HB-EGF) and increased epidermal growth factor (EGF)] (15), a specifically altered gene expression pattern (21), and decreased tight junction protein expression with increased bladder epithelial paracellular permeability (13). Because decreased urine levels of HB-EGF (16,22), bladder epithelial thinning, ulceration, or leakiness (1-3,9,11,12), and abnormal expression of some of the same proteins (including ZO-1) (10) have all been described previously in IC/PBS patients in vivo, APF may play an important role in the pathogenesis of this disorder.
Our laboratories are collaborating on detailed structure-activity studies for APF, for which over 40 structural derivatives have been synthesized and their biological antiproliferative activity determined (17,23). The previous finding that removal of the sialic acid unit from APF did not affect its biological activity (17) prompted us to prepare all synthetic APF analogues with the more synthetically accessible Galβ1-3GalNAcα-O- disaccharide (asialylated-APF or “as-APF”). As part of these studies we screened 32 synthetic as-APF derivatives that did not have any antiproliferative activity for their ability to attenuate the antiproliferative activity of synthetic as-APF in normal bladder cells, and found only two such antagonists: Galβ1-3GalNAcα-O-TV-(d-Pro)-AAVVVA or “d-proline as-APF” and Galβ1-3GalNAcα-O-TV-(d-Pip)-AAVVVA or “d-pipecolic acid as-APF”. This report describes the ability of these two APF antagonists to normalize specific tight junction protein expression, paracellular permeability, and proliferation of IC/PBS cells in vitro, suggesting they may be useful for development as IC/PBS therapies.
METHODS AND MATERIALS
Patients
IC/PBS patients had previously undergone cystoscopy and fulfilled modified NIDDK diagnostic criteria for IC/PBS (without measurement of bladder capacity] (24); age- and gender-matched controls were asymptomatic for urinary tract disease. All participants were at least 18 years old and enrolled in accordance with guidelines of the Institutional Review Board of the University of Maryland School of Medicine.
Cell Culture
Cystoscopy was performed under general anesthesia, and 4-mm2 pieces of transitional epithelium with submucosal bladder tissue were obtained from IC/PBS patients and controls for the growth of primary bladder epithelial cells, as previously described (15,16). Epithelial cells were propagated in DMEM-F12 (Media-Tech, Herndon VA) with 10% heat-inactivated FBS, 1% antibiotic/antimycotic solution, 1% l-glutamine, 0.25 units/ml insulin (all from Sigma, St. Louis, MO), and 5 ng/ml hEGF (R & D Systems, Minneapolis, MN) at 37°C in a 5% CO2 atmosphere and characterized by binding of AE-1/AE-3 pancytokeratin antibodies (Signet, Dedham, MA), as previously described.
Synthesis of APF Derivatives
All glycopeptides were synthesized according to the procedure described earlier (23) with minor modifications. Briefly, the synthesis of Galβ1-3GalNAcα-O-TV-(d-Pip)-AAVVVA was performed manually using standard Fmoc solid-phase peptide chemistry on 2ClTrt resin. All Fmoc-protected amino acids (5 eq) were coupled using HATU (5 eq) and HOAt (5 eq) reagents in the presence of DIPEA (10 eq), except for Fmoc-Thr-(Ac4Galβ1-3Ac2GalNAcα-O-)-OH (0.5 eq), which was coupled using BEP/HOAt/DIPEA (0.5 eq:0.5 eq:1.5 eq) in NMP. The Fmoc group was removed with 20% piperidine in NMP, and each glycopeptide was cleaved from the resin with TFA:TIS:H2O (95:2.5:2.5). Acetyl groups were removed with NaOMe/MeOH. Preparative HPLC was performed on a Waters Prep LC 4000 System equipped with PDA detector [Waters 2996] on C18 column (mobile phase: Solvent A, 0.1% TFA in H2O; Solvent B, 0.1% TFA in CH3CN). All intermediates and final products were verified by HPLC-MS (Agilent 1200, Agilent Technologies, Inc., Santa Clara, CA). Purity of each final product was confirmed by HPLC trace analysis with UV detection at 227 nm.
3H-Thymidine Incorporation
Cell proliferation was measured by 3H-thymidine incorporation into explanted normal human bladder epithelial cells, as previously described (15,17). Significant inhibition of 3H-thymidine incorporation was defined as a mean decrease in counts per minute of greater than 2 standard deviations from the mean of control cells for each plate.
qRT-PCR
Total RNA was extracted from IC/PBS and normal control epithelial cell explants using the RNEasy Plus Mini Kit (Qiagen) according to the manufacturer’s protocol. qRT-PCR for tight junction gene expression was performed using Quantitect Primers (Qiagen), SYBR Green RT-PCR kit reagents (Qiagen), and a Roche 480 Light-Cycler. Samples were tested in triplicate runs, and specific mRNA levels quantified and compared to mRNA levels for β-actin using LightCycler 480 real-time PCR analysis software (version 1.5).
Paracellular Permeability Assay
Cells were plated in Corning T75 tissue culture flasks (VWR Scientific Products, Bridgeport, NJ) at a density of 104 cells/ml and cultured in DMEM-F12 (Media-Tech, Herndon, Virginia) with 10% heat inactivated FBS, 1% antibiotic/antimycotic solution, 1% l-glutamine, 0.25 U/ml insulin (Sigma Chemical Co., St. Louis, MO) and 5 ng/ml human EGF (R & D Systems, Minneapolis, MN) at 37°C in a 5% CO2 atmosphere. When the cells were confluent, medium was changed to serum-free MEM (GIBCO-BRL; Life Technologies, Grand Island, NY) containing 1% antibiotic/antimycotic solution, and 1% l-glutamine, and cells were incubated at 37°C in a 5% CO2 atmosphere overnight prior to the first treatment with as-APF, its inactive unglycosylated peptide control, or APF derivatives. Thereafter, serum-free medium containing each treatment was replaced twice weekly until transfer of the cells to Transwell plates for performance of the flux assay.
Flux assays were performed using 12-mm Transwell culture plates (Corning Incorporated, Corning, NY), as previously described (14). Briefly, cells that had been treated with APF derivatives, inactive control peptide, or diluent alone for varying periods of time were plated at 4 × 105 cells/cm2 on the insert and grown in DMEM-F12 medium containing 10% heat-inactivated FBS, 1% antibiotic solution, 1% l-glutamine, 0.25 units/ml insulin (all from Sigma, St. Louis, MO), and 5 ng/ml hEGF (R & D Systems, Minneapolis, MN) to establish tight monolayers. The next day the medium was changed to MEM (GIBCO/Invitrogen) containing 1% antibiotic/antimycotic solution and 1% l-glutamine (Sigma). On the following day, synthetic as-APF, its inactive unglycosylated peptide control, or an APF derivative was added to the medium for a final treatment, and all cells were then cultured for an additional 48 hours prior to performing the flux assay.
Two different membrane impermeable molecules, [14C]-mannitol (molecular weight: 184 Daltons) and [3H]-inulin (molecular weight: 5,200 Daltons) served as paracellular tracers. At the beginning of the flux assay, both sides of the bathing wells of Transwell filters were replaced with fresh medium containing either 5 mM unlabeled mannitol or 0.5 mM unlabeled inulin. Each tracer was added at a final concentration of 3.6 nM for [14C]-mannitol and 0.36 nM for [3H]-inulin to the apical bathing wells. The basal bathing well contained the same medium as the apical compartment but without tracers. Flux assays were performed at 37°C; basal medium was collected at 0.5 - 6 hrs after addition of [14C]-mannitol or [3H]-inulin, and the amount of radioactivity determined using a Beckman LS 5000 scintillation counter. Results were expressed as percentage of total counts for each tracer.
Immunofluorescence Confocal Microscopy
For immunofluorescence, cells were fixed using ethanol/acetone (1:1) for 15 minutes at room temperature, washed 3 times with PBS, and incubated with FITC-labeled mouse monoclonal anti-ZO-1 (5 μg/ml); or unlabeled mouse monoclonal anti-occludin (5 μg/ml) or anti-claudin 4 (3 μg/ml); or unlabeled rabbit polyclonal anti-claudin 1 (3 μg/ml), anti-claudin 8 (8 μg/ml), or anti-claudin 12 (5 μg/ml) antibodies (all from Zymed, South San Francisco, California) diluted in PBS, for 2 hours at 37°C. Cells incubated with unlabeled mouse monoclonal primary antibodies were then washed 3 times with PBS and further incubated with FITC-labeled secondary goat anti-mouse IgG antibody (Zymed) diluted in PBS, while cells incubated with unlabeled rabbit polyclonal primary antibodies were washed and further incubated for 2 hours at 37°C with goat anti-rabbit IgG (Zymed) diluted in PBS. Following an additional 5 washes with PBS, the cells were examined using an LSM510 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany). Negative controls for the method included cells incubated without primary and secondary antibodies as well as cells incubated with secondary antibody alone.
Statistical Analysis
For the permeability assay, the percentage of total counts applied to the apical medium that were recovered in the basal medium was determined and expressed as mean ± standard deviation. Crossover point analysis was performed for qRT-PCR data, and expression of each gene was quantified relative to β-actin; this value was expressed as mean ± standard error of the mean for duplicate runs performed on two separate occasions. 3H-thymidine incorporation was determined in triplicate on two separate occasions, and the CPM expressed as mean ± standard deviation. The significance of the difference between mean values was determined by an analysis of variance for data expressed as noted above for each assay.
RESULTS
Inhibition of APF Antiproliferative Activity in Normal Bladder Epithelial Cells by APF Derivatives
To date, over 40 synthetic APF derivatives have been tested for their ability to inhibit normal bladder epithelial cell proliferation; 32 of these were found to be completely inactive in our cell proliferation assay (17, 23, Figure 2, and unpublished data], including “d-proline as-APF” and “d-pipecolic acid as-APF” (Figure 1). We therefore preincubated primary normal bladder cells with each of the 32 inactive synthetic APF derivatives prior to incubation with active synthetic as-APF, to determine their ability to block as-APF activity. Only d-proline as-APF and d-pipecolic acid as-APF were able to significantly attenuate as-APF antiproliferative activity in as-APF-treated normal bladder epithelial cells, and they did so in a dose-dependent manner starting at nanomolar concentrations (Figure 3; p<.001 for either APF derivative at concentrations ≥ 1.25 nM; data not shown for remaining 30 inactive derivatives).
Figure 2. Antiproliferative activity of as-APF, d-proline as-APF, and d-pipecolic acid as-APF derivatives in normal bladder epithelial cell explant cultures.
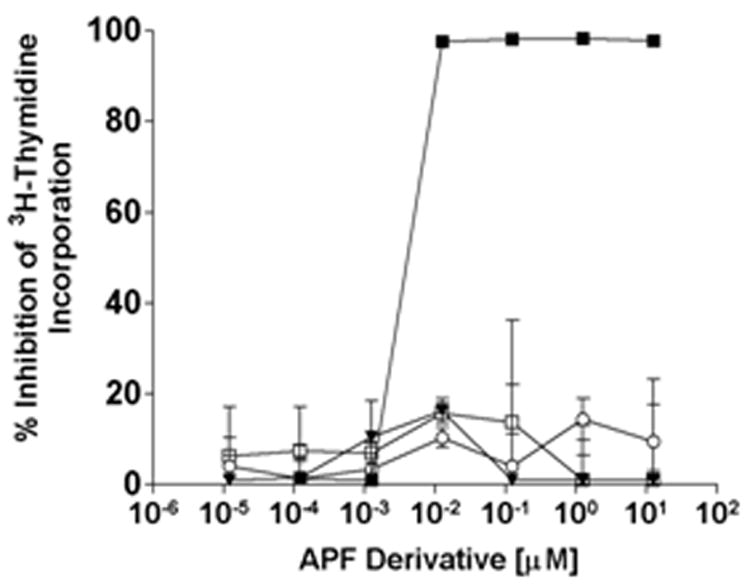
Explanted normal bladder epithelial cells were treated with varying concentrations of as-APF (■), inactive control peptide (□), d-proline as-APF (▼), or d-pipecolic acid as-APF (○) for 48 hours prior to determination of 3H-thymidine incorporation. Assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/- standard deviation.
Figure 1. Structures of as-APF, d-proline as-APF, and d-pipecolic acid as-APF derivatives.
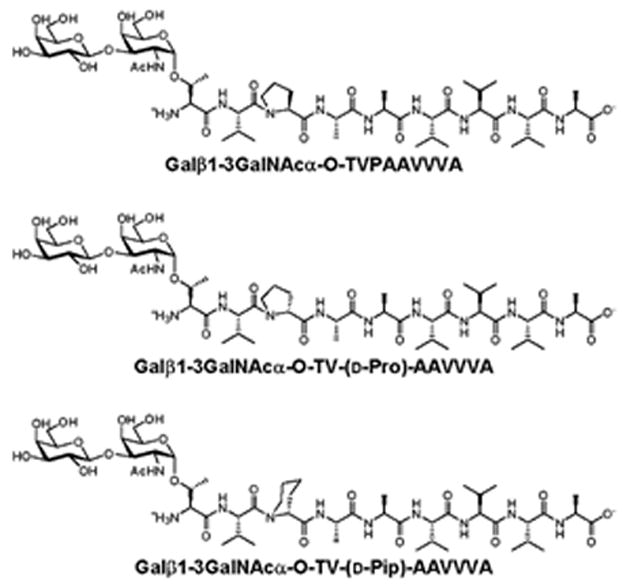
Figure 3. Blocking of as-APF activity by d-proline as-APF and d-pipecolic acid as-APF in normal bladder epithelial cells.
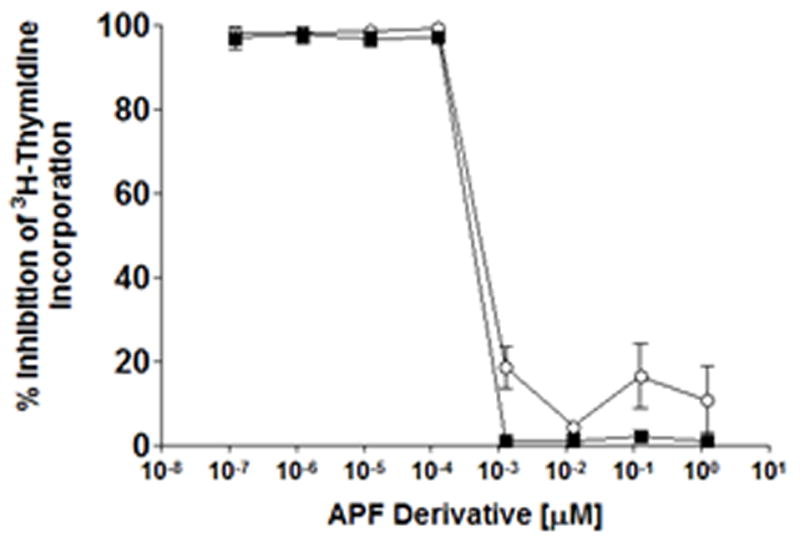
Explanted normal bladder epithelial cells were preincubated with varying concentrations of d-proline as-APF (■) or d-pipecolic acid as-APF (○) for 1.5 hours at 37°C, after which 125 nM as-APF was added to the medium; cell proliferation was assessed by 3H-thymidine incorporation 48 hours later. The assay was performed in triplicate twice; data are expressed as percent inhibition of thymidine incorporation compared to control cells incubated with medium alone +/- standard deviation.
Normalization of IC/PBS Cell Proliferation by APF Derivatives
IC/PBS bladder epithelial cells produce APF and as a result have a profound decrease in cell proliferation (18). We therefore next determined whether D-proline as-APF and D-pipecolic acid as-APF could also block APF activity in bladder epithelial cells from IC/PBS patients (i.e., whether they could stimulate, or normalize, the proliferation of IC/PBS cells that produce APF). Because the exact quantity of APF and half-life of secreted native APF produced by IC/PBS bladder epithelial cells is unknown, and the half-lives of the synthetic d-pipecolic acid and d-proline as-APF derivatives are also unknown, we performed these experiments using up to 1,000 fold greater concentrations of these antagonists, respectively, than the lowest concentration previously required to significantly inhibit a single dose of 125 nM as-APF over 48 hours in normal bladder cells. Cells from 3 different IC/PBS (1 male and 2 female) donors were treated with d-proline or d-pipecolic acid as-APF, and thymidine incorporation was determined at 48 hours. As shown in Figure 4, 125 nM of each of these APF derivatives significantly (p < .05) stimulated IC/PBS cell proliferation, as compared to control cells treated with diluent (PBS) alone.
Figure 4. Stimulation of IC/PBS cell proliferation by d-proline as-APF and d-pipecolic acid as-APF.
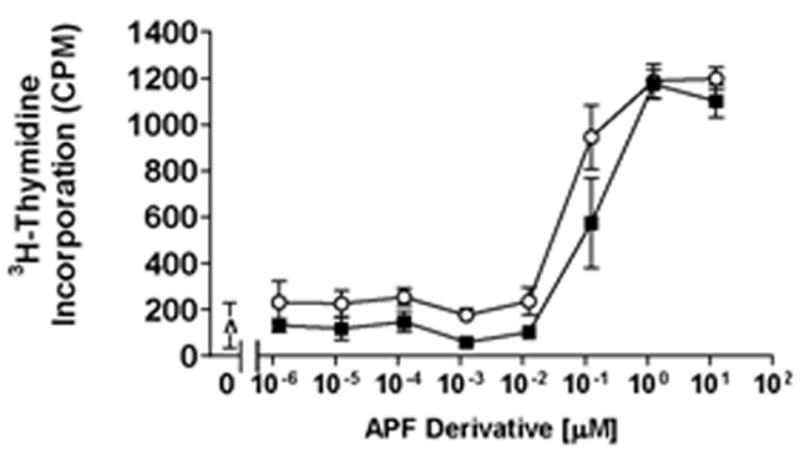
Explanted cells from 3 IC/PBS donors were treated with varying concentrations of d-proline as-APF (■) or d-pipecolic acid as-APF (○); controls were incubated with culture medium alone (Δ). Cell proliferation was assessed by 3H-thymidine incorporation 48 hours later. The assay was performed in triplicate twice for each donor, and data points combined for all donors; data are expressed as counts per minute (CPM) +/- standard error of the mean.
Increased IC/PBS Cell Tight Junction Protein Gene Expression by APF Derivatives
In addition to thinning and denudation, increased permeability of the IC/PBS bladder epithelium is thought possibly to contribute to the pain associated with this IC/PBS (9,11,12). Therefore, we determined whether d-proline and/or d-pipecolic acid as-APF could also abrogate the effects of APF on tight junction protein gene expression. As shown in Figure 5, by day 16 both APF derivatives were also able to significantly (p<.05) stimulate mRNA expression for ZO-1, occludin, and specific claudins (1, 4, 8, and 12) in IC/PBS cells in vitro as compared to the inactive peptide control or diluent alone, resulting in mRNA levels similar to those seen in normal bladder cells (data shown for the same female cell donor treated with each derivative; d-proline APF was tested on cells from a total of 4 IC/PBS donors, including 3 female and 1 male donor, with similar results).
Figure 5. RT-PCR analysis of tight junction protein mRNA expression in IC/PBS cells following treatment with d-proline as-APF or d-pipecolic acid as-APF.
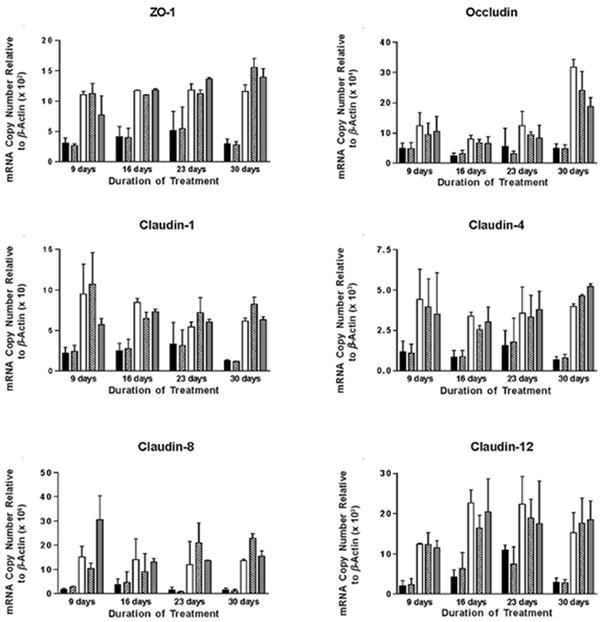
Explanted cells from IC/PBS donors were fed and treated with 2.5 μM d-proline as-APF (□), d-pipecolic acid as-APF (
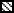 ), inactive peptide (
), inactive peptide (
 ), or diluent control (■) twice weekly, and RNA was extracted at 9, 16, 23 and 30 days for qRT-PCR. By day 16, both APF derivatives were able to significantly (p < .05) stimulate tight junction protein expression in IC/PBS cells in vitro resulting in levels similar to those seen in bladder cells from age- and gender-matched normal donors (
), or diluent control (■) twice weekly, and RNA was extracted at 9, 16, 23 and 30 days for qRT-PCR. By day 16, both APF derivatives were able to significantly (p < .05) stimulate tight junction protein expression in IC/PBS cells in vitro resulting in levels similar to those seen in bladder cells from age- and gender-matched normal donors (
 ). PCR was performed in duplicate on three separate occasions for each sample; data are expressed as mean +/- standard error of the mean. (Data shown from experiment with the same IC/PBS cell donor treated simultaneously with either d-pipecolic acid as-APF or d-proline as-APF; d-proline as-APF was tested on cells from a total of 4 IC/PBS donors, with similar results)
). PCR was performed in duplicate on three separate occasions for each sample; data are expressed as mean +/- standard error of the mean. (Data shown from experiment with the same IC/PBS cell donor treated simultaneously with either d-pipecolic acid as-APF or d-proline as-APF; d-proline as-APF was tested on cells from a total of 4 IC/PBS donors, with similar results)
In addition, immunofluorescence confocal microscopy confirmed that expression of the same tight junction proteins also increased in IC/PBS cells following treatment with d-proline or d-pipecolic acid as-APF (as compared to diluent control), and all of these upregulated proteins were localized in the tight junctions between cells (Figure 6) (both derivatives were tested on cells from 3 IC/PBS donors with similar results).
Figure 6. Immunofluorescence confocal microscopy of IC/PBS cell explants treated with d-pipecolic acid as-APF, d-proline as-APF, or vehicle (PBS) alone for 9 days.
Explanted cells from an IC/PBS donor were treated with 2.5 μM d-proline as-APF, d-pipecolic acid as-APF, or diluent (PBS) control twice weekly. On day 9, cells were fixed with acetone/ethanol and incubated with FITC-labeled anti-ZO-1 antibody, or primary antibodies against claudin or occludin proteins followed by FITC-labeled secondary antibodies. Images were obtained on a Zeiss LSM510 confocal laser scanning microscope. (Figure is representative of three separate experiments using cells from 3 IC/PBS donors)
Decreased IC/PBS Monolayer Paracellular Permeability by APF Derivatives
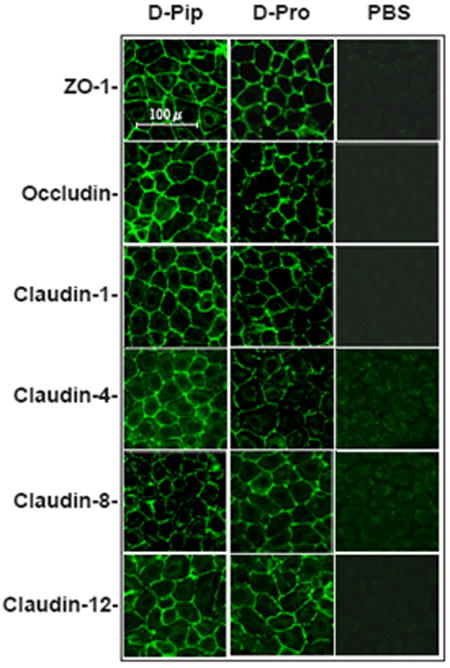
Although tight junction protein expression and tight junction formation had clearly normalized following treatment with the two APF derivatives, functional normalization of paracellular permeability remained to be demonstrated. Therefore, IC/PBS cells were fed and treated twice weekly with equimolar concentrations of d-proline APF, d-pipecolic acid as-APF, or inactive control peptide for 16 days (total of 5 treatments), after which paracellular permeability to two radiolabeled tracers (3H-inulin and 14C-mannitol) was determined over a 6 hour period. Experiments were performed with cells from 4 (d-proline APF) and 3 (d-pipecolic acid as-APF) IC/PBS donors, with male and female patients in each group. As shown in Figure 7 and Figure 8, treatment with either of these APF derivatives significantly (p<.05) decreased paracellular permeability of both tracer molecules in IC/PBS cell monolayers within one hour of exposure to tracer, restoring levels to those seen previously in normal bladder cells (13). In comparison, treatment with inactive control peptide did not decrease paracellular permeability of either radiolabeled tracer as compared to cells cultured in calcium free medium during the 6 hour assay period (run as a positive control for the permeability assay).
Figure 7. Decreased paracellular permeability of IC/PBS cells by d-proline as-APF.
Paracellular flux of [3H]-inulin (molecular weight: 5,200) or [14C]-mannitol (molecular weight: 184) was measured in IC/PBS cells cultured on Transwell membranes and treated with 2.5 μM d-proline as-APF (□), inactive peptide control (
 ), diluent control (■), or calcium free medium (
), diluent control (■), or calcium free medium (
 ) for 16 days prior to incubation with tracer. Data are expressed as mean percent of the radioactivity applied to the apical medium that was recovered in the basal medium at 0.5 hr, 1 hr, 2 hrs, 4 hrs, 6 hrs +/- standard deviation. (Data shown from 4 experiments using cells from 4 different IC/PBS donors).
) for 16 days prior to incubation with tracer. Data are expressed as mean percent of the radioactivity applied to the apical medium that was recovered in the basal medium at 0.5 hr, 1 hr, 2 hrs, 4 hrs, 6 hrs +/- standard deviation. (Data shown from 4 experiments using cells from 4 different IC/PBS donors).
Figure 8. Decreased paracellular permeability of IC/PBS cells by d-pipecolic acid as-APF.
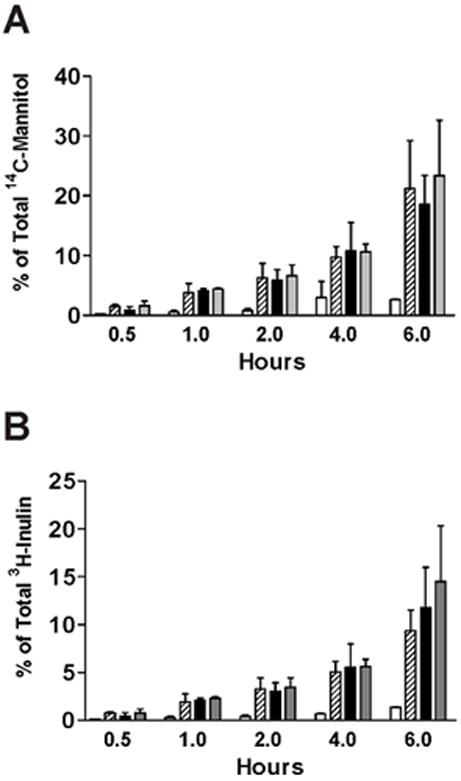
Paracellular flux of [3H]-inulin (molecular weight: 5,200) or [14C]-mannitol (molecular weight: 184) was measured in IC/PBS cells cultured on Transwell membranes and treated with 2.5 μM d-pipecolic acid as-APF (□), inactive peptide control (
 ), diluent control (■), or calcium free medium (
), diluent control (■), or calcium free medium (
 ) for 16 days prior to incubation with tracer. Data are expressed as mean percent of the radioactivity applied to the apical medium that was recovered in the basal medium at 0.5 hr, 1 hr, 2 hrs, 4 hrs, 6 hrs +/- standard deviation. (Data shown from 3 experiments using cells from 3 different IC/PBS donors).
) for 16 days prior to incubation with tracer. Data are expressed as mean percent of the radioactivity applied to the apical medium that was recovered in the basal medium at 0.5 hr, 1 hr, 2 hrs, 4 hrs, 6 hrs +/- standard deviation. (Data shown from 3 experiments using cells from 3 different IC/PBS donors).
DISCUSSION
In this manuscript we present evidence that two synthetic APF derivatives [Galβ1-3GalNAcα-O-TV-(d-pipecolic acid)-AAVVVA and Galβ1-3GalNAcα-O-TV-(d-proline)-AAVVVA] block APF’s inhibitory effects on cell proliferation in both as-APF-treated primary normal bladder epithelial cells and bladder epithelial cells explanted from IC/PBS patients. In addition, our data show that as-APF treatment of normal bladder epithelial cells in vitro can cause changes in mRNA and protein expression of claudin 1, 4, 8, and 12 similar to those seen in IC/PBS cells; expression of these tight junction proteins, plus ZO-1 and occludin, is also normalized by treatment with either APF antagonist glycopeptide in vitro. These findings suggest that these molecules may be useful for development as IC/PBS therapies.
APF is a nonapeptide comprised of primarily hydrophobic amino acids including one proline unit. The constrained cyclic backbone of proline confers special properties to this residue that affect a kink in peptide/protein structure and induce a reversal in the backbone conformation leading to its frequent presence in β-type turns. As such turns are sometimes important for biological activity (25), SAR studies of APF naturally included modifications to the proline residue to examine their effect on antiproliferative potency.
The first proline modification of as-APF therefore involved substituting d-proline for l-proline in the peptide backbone. d-amino acid isomers are often used in the synthesis of peptide drugs because they can impart resistance to proteolytic cleavage and/or increase drug activity (26,27), and this substitution was made in an attempt to stabilize as-APF and/or increase its activity. However, this substitution eliminated as-APF’s antiproliferative effects in vitro (23), indicating that the conformation achieved by l-proline in as-APF is important for mediating its biological activity. Substitution of amino acids normally found in peptides with peptidomimetic amino acids (including piperidine-2-carboxylic acid or “pipecolic acid”) that are rarely or never found in natural proteins is another means by which peptides can be stabilized against proteolysis (28). We previously discovered that ring expansion of l-proline as-APF to l-pipecolic acid as-APF did not alter its antiproliferative activity (23), prompting us to explore a similar substitution of l-proline in as-APF with d-pipecolic acid. Like the d-proline analog, d-pipecolic acid as-APF was also completely inactive, confirming that the conformation allowed by either l-proline or l-pipecolic acid is important for activity.
However, as we show in this report, the conformations of both the d-proline and d-pipecolic acid derivatives seem correctly folded for potent antagonistic activity. It is interesting to note that other d-proline-containing molecules have been designed as specific enzyme inhibitors (29). The unique ability of both d-proline and d-pipecolic acid APF (but none of 30 other inactive APF derivatives) to block as-APF activity suggests the possibility that these molecules may be able to interfere with as-APF binding to its receptor CKAP4 (30). However, the exact mechanism by which either d-proline APF or d-pipecolic acid APF blocks as-APF activity remains to be determined.
As noted above, this report also includes the first demonstration of as-APF’s effect on specific claudin gene expression, along with the ability of both d-proline and d-pipecolic acid derivatives to normalize this gene expression in explanted cells from IC patients. Along with the previously described decreased expression of ZO-1 (10,13) and occludin (13) by IC/PBS bladder epithelial cells, the decreased expression of claudins 1, 4, 8, and 12 in IC/PBS vs. normal bladder cells, or as-APF-treated vs. inactive peptide-treated normal bladder cells, as described in this report, could contribute to the increased epithelial paracellular permeability described in IC/PBS (9-12,14). Claudins belong to a large (multigene) family of tetraspan cell membrane tight junction proteins that appear to regulate paracellular permeability of small ions based on charge, cell proliferation and invasion (31-33). The function of each claudin protein (e.g., as cation pore, cation barrier, anion pore, anion barrier, or neutral charge pore) is apparently dependent on the cell type. Of the proteins examined in our study, claudins 1, 4, and 8 often function as cation barriers (the specific function of claudin 12 is not yet known) (32). Compromise of the bladder epithelial barrier could result in increased exposure of submucosal cells to urinary metabolites, conceivably resulting in irritative voiding symptoms, as demonstrated in rodent models of IC/PBS in which the epithelium was damaged (33,34). We are currently developing an animal model of IC/PBS based on APF that hopefully will be able to provide more direct evidence as to whether epithelial abnormalities caused by APF (including increased permeability) play a role in the pathophysiology of this poorly understood syndrome. However, whether increased permeability of the bladder epithelial barrier caused by APF is directly or indirectly related to the pathophysiology of IC/PBS remains unknown at this time.
Because an objective criterion for the diagnosis of IC/PBS does not yet exist, it is unclear whether patients who fulfill clinical criteria for IC, IC/PBS, and/or bladder pain syndrome (24,38) represent a homogeneous group with a single disease, or whether they represent a heterogeneous group with a similar clinical syndrome caused by more than one disease. However, the current study and all of our previous studies on APF production, explanted cell proliferation, tight junction and other gene expression, plus paracellular permeability, used only cell explants from patients who fulfilled the previous NIDDK criteria (24), a fairly homogeneous population with respect to APF production [approximately 95% of patients defined by these criteria have APF activity in their urine in past studies involving over 400 patients (16,39)]. The cells used in the current study, which were randomly selected from explants of male and female patients who had APF activity in their urine, exhibited similar abnormalities in tight junction protein expression, cell proliferation and sensitivity to both APF proline derivatives. However, whether cells derived from IC/PBS patients whose urine is negative for APF activity or who do not fulfill all of the previous NIDDK criteria for this syndrome would have the same abnormalities and/or response to these agents remains to be determined.
It is interesting to note that transepithelial resistance, as well as claudin 4 and 8 expression, are increased by p38/MAPK inhibitors in mammary epithelial cells (35), as HPLC-purified native APF has been shown to stimulate p38/MAPK activity in T24 bladder carcinoma cells (36), suggesting that it may inhibit claudin gene expression via stimulation of p38/MAPK. It is also interesting to note that claudin 4 expression, which is decreased by as-APF in normal bladder epithelial cells, has been shown to be increased in a variety of tumors including bladder cancer (31,33), as as-APF has been shown to have potent antiproliferative activity against various carcinoma cells (including T24 bladder carcinoma and HeLa cells) in vitro (16,37). Studies to determine the specific effect of as-APF on claudin gene expression in bladder cancer cells are in progress.
CONCLUSIONS AND FUTURE DIRECTIONS
IC/PBS is a poorly understood painful chronic disorder characterized by increased bladder epithelial thinning/ulceration and permeability, for which there is currently no reliably effective therapy. The novel strategy to inhibit a toxin (APF) produced uniquely by bladder cells in IC/PBS patients as therapy for this disorder has not yet been explored. We have identified two synthetic derivatives of APF that normalize cell proliferation, tight junction protein production, tight junction formation, and paracellular permeability in vitro. These compounds could be used as leads to develop effective therapies for IC/PBS.
Supplementary Material
Acknowledgments
The authors thank Toby Chai (Division of Urology, Department of Surgery, University of Maryland School of Medicine) for providing bladder biopsy specimens from which the explanted bladder epithelial cells were propagated, and Eunice Katz for assistance with the preparation of this manuscript. They also gratefully acknowledge the Biophysics Resource, Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute at Frederick.
Funding/support information: This work was supported by grants from the National Institutes of Health Grant NIDDK R01 DK52596 and the Merit Review Funding from Veterans Affairs (to SK) and by the Intramural Research Program of the NCI, National Institutes of Health (JB and CM).
Footnotes
None of the authors has a conflict of interest related to the work described in this manuscript.
References
- 1.Johansson SL, Fall M. Clinical features and spectrum of light microscopic changes in interstitial cystitis. J Urol. 1990;143:1118–1124. doi: 10.1016/s0022-5347(17)40201-1. [DOI] [PubMed] [Google Scholar]
- 2.Skoluda D, Wegner K, Lemmel EM. Critical Notes: Respective immune pathogenesis of interstitial cystitis. (article in German) Urologe A. 1974;13:15–23. [PubMed] [Google Scholar]
- 3.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the Interstitial Cystitis Database Study Group. Urology. 2001;57:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 4.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol. 2002;283:F242–F253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 5.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol. 2004;287:F305–F318. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 6.Kreft ME, Romih R, Sterle M. Antigenic and ultrastructural markers associated with urothelial cytodifferentiation in primary explant outgrowths of mouse bladder. Cell Biol Int. 2002;26:63–74. doi: 10.1006/cbir.2001.0829. [DOI] [PubMed] [Google Scholar]
- 7.Southgate J, Varley CL, Garthwaite MAE, Hinley J, Marsh F, Stahlschmidt J, Trejdosiewicz LK, Eardley I. Differentiation potential of urothelium from patients with benign bladder dysfunction. BJU International. 2007;99:1506–1516. doi: 10.1111/j.1464-410X.2007.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BH, Dehner LP. Chronic ulcerating interstitial cystitis (Hunner’s ulcer) Arch Path. 1972;93:76–81. [PubMed] [Google Scholar]
- 9.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis) J Urol. 1991;145:732–735. doi: 10.1016/s0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 10.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554–1559. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 11.Eldrup J, Thorup J, Nielsen SL, Hald T, Hainau B. Permeability and ultrastructure of human bladder epithelium. Br J Urol. 1983;55:488–492. doi: 10.1111/j.1464-410x.1983.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 12.Parsons CL. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–1866. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C-O, Wang JY, Koch K, Keay SK. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from interstitial cystitis patients. J Urol. 2005;174:2382–2387. doi: 10.1097/01.ju.0000180417.11976.99. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C-O, Guo L, Keay SK. Decreased expression of claudins 1, 4, and 8 in bladder epithelial cell explants from interstitial cystitis patients as compared to normal controls. J Urol. 2007;177:100. [Google Scholar]
- 15.Keay S, Kleinberg M, Zhang C-O, Hise MK, Warren JW. Bladder epithelial cells from interstitial cystitis patients produce an inhibitor of HB-EGF production. J Urol. 2000;64:2112–2118. [PubMed] [Google Scholar]
- 16.Keay S, Zhang C-O, Shoenfelt J, Erickson DR, Whitmore K, Warren JW, Marvel R, Chai T. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57(6 Suppl 1):9–14. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- 17.Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ, Jr, Zhang C-O, Koch KR, Michejda CJ. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004;101:11803–11808. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keay S, Zhang C-O, Shoenfelt JL, Chai TC. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology. 2003;61:1278–1284. doi: 10.1016/s0090-4295(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh T, Hirai M, Katoh M. Molecular cloning and characterization of human Frizzled-8 gene on chromosome 10p11.2. Int J Oncol. 2001;18:991–996. doi: 10.3892/ijo.18.5.991. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Keay SK, Dimitrakov JD, Freeman MR. p53 mediates interstitial cystitis antiproliferative factor (APF)-induced growth inhibition of human urothelial cells. FEBS Lett. 2007;581:3795–3799. doi: 10.1016/j.febslet.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keay S, Seillier-Moiseiwitsch F, Zhang C-O, Chai TC, Zhang J. Changes in human bladder cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiological Genomics. 2003;14:107–115. doi: 10.1152/physiolgenomics.00055.2003. [DOI] [PubMed] [Google Scholar]
- 22.Keay S, Zhang C-O, Kagen DI, Hise MK, Jacobs SC, Hebel JR, Gordon D, Whitmore K, Bodison S, Warren JW. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol. 1997;158:1983–1988. doi: 10.1016/s0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- 23.Kaczmarek P, Keay SK, Tocci GM, Koch KR, Zhang C-O, Barchi JJ, Jr, Grkovic D, Guo L, Michejda CJ. Structure-activity relationship studies for the peptide portion of the bladder epithelial cell antiproliferative factor from interstitial cystitis patients. J Med Chem. 2008;51:5974–5983. doi: 10.1021/jm8002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis - National Institutes of Health, Bethesda, Maryland, August 28–29, 1987. J Urol. 1988;140:203–206. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 25.Cline LL, Waters ML. The structure of well-folded beta-hairpin peptides promotes resistance to peptidase degradation. Biopolymers. 2009;92:502–507. doi: 10.1002/bip.21266. [DOI] [PubMed] [Google Scholar]
- 26.Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Develop Res. 1995;35:20–32. [Google Scholar]
- 27.Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Cur Med Chem. 2002;9:963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 28.Hanessian S, Auzzas L. The practice of ring constraint in peptidomimetics using bicyclic and polycyclic amino acids. Accounts Chem Res. 2008;41:1241–1251. doi: 10.1021/ar8000052. [DOI] [PubMed] [Google Scholar]
- 29.Hanessian S, MacKay DB, Moitessier N. Design and synthesis of matrix metalloproteinase inhibitors guided by molecular modeling. Picking the S1 pocket using conformationally constrained inhibitors. J Med Chem. 2001;44:3074–3082. doi: 10.1021/jm010096n. [DOI] [PubMed] [Google Scholar]
- 30.Conrads TP, Tocci GM, Hood BL, Zhang C-O, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem. 2006;281:37836–37843. doi: 10.1074/jbc.M604581200. [DOI] [PubMed] [Google Scholar]
- 31.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186–193. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelow S, Ahlstrom R, Yu ASL. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F875. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang YC, Chancellor MB, Seki S, Yoshimura N, Tyagi P, Huang L, Lavelle JP, DeGroat WC, Fraser MO. Intravesical protamine sulfate and potassium chloride as a model for bladder hyperactivity. Urology. 2003;61:665–670. doi: 10.1016/s0090-4295(02)02280-x. [DOI] [PubMed] [Google Scholar]
- 34.Anton E. Delayed toxicity of cyclophosphamide on the bladder of DBA/2 and C57BL/6 female mouse. Int J Exp Pathol. 2002;83:47–53. doi: 10.1046/j.1365-2613.2002.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol. 2009;297:C775–C787. doi: 10.1152/ajpcell.00084.2009. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Keay SK, Freeman MR. Heparin-binding epidermal growth factor-like growth factor functionally antagonizes interstitial cystitis antiproliferative factor via Mitogen-activated protein kinase pathway activation. BJU International. 2009;103:541–546. doi: 10.1111/j.1464-410X.2008.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planey SL, Keay SK, Zhang C-O, Zacharias DA. Palmitoylation of CKAP4/p63 by DHHC2 regulates APF-mediated signaling. Mol Biol Cell. 2009;20:1454–1463. doi: 10.1091/mbc.E08-08-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanno P, Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol Urodyn. 2009;28:274–286. doi: 10.1002/nau.20687. [DOI] [PubMed] [Google Scholar]
- 39.Keay S, Reeder JE, Koch K, Zhang C-O, Grkovic D, Peters K, Zhang Y, Kusek JW, Nyberg LM, Payne CK, Propert KJ. Prospective evaluation of candidate urine and cell markers in patients with interstitial cystitis enrolled in a randomized clinical trial of Bacillus Calmette Guerin (BCG) World J Urol. 2007;25:499–504. doi: 10.1007/s00345-007-0205-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


