Summary
Fecal IgA production depends on colonization by a gut microbiota. However, the bacterial strains that drive gut IgA production remain largely unknown. Here, we assessed the IgA-inducing capacity of a diverse set of human gut microbial strains by monocolonizing mice with each strain. We identified Bacteroides ovatus as the species that best induced gut IgA production. However, this induction varied bimodally across different B. ovatus strains. The high IgA-inducing B. ovatus strains preferentially elicited more IgA production in the large intestine through the T-cell-dependent B cell-activation pathway. Remarkably, a low-IgA phenotype in mice could be robustly and consistently converted into a high-IgA phenotype by transplanting a multiplex cocktail of high IgA-inducing B. ovatus strains but not individual ones. Our results highlight the critical importance of microbial strains in driving phenotype variation in the mucosal immune system and provide a strategy to robustly modify a gut immune phenotype, including IgA production.
Graphical Abstract
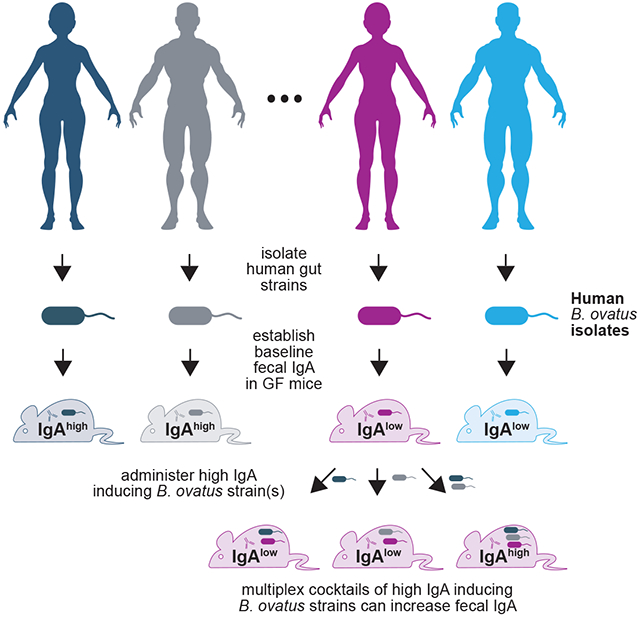
eTOC Blurb:
Yang et al. identify that different Bacteroides ovatus strains induce either high or low baseline fecal IgA in mice. Cocktails of IgAhigh B. ovatus strains convert mice from low IgA to high IgA producers. This demonstrates a role of microbial strains in immune variation and a microbiota-based immune modulation strategy.
Introduction
Immunoglobulin A (IgA) is the most abundant mucosal antibody and plays an essential role in maintaining gut homeostasis (Macpherson et al., 2012; Sutherland et al., 2016). Secretory IgA can limit the access of bacteria and bacteria-derived toxins to intestinal epithelial cells (Tokuhara et al., 2010), facilitate bacterial clearance (Fagarasan, 2008; Strugnell and Wijburg, 2010) regulate bacterial colonization (Donaldson et al., 2018; McLoughlin et al., 2016), and bind disease-associated bacteria (Kau et al., 2015; Palm et al., 2014; Viladomiu et al., 2017). The gut microbiota drives the production of IgA as germ-free (GF) mice have an almost undetectable level of fecal IgA (Macpherson et al., 2000), while upon bacteria colonization, even with a single bacterial strain (Fritz et al., 2011; Hapfelmeier et al., 2010), B cells undergo class-switch to IgA+ cells in gut-associated lymphoid tissues (Macpherson et al., 2008; Pabst, 2012). Much of the intestinal IgA is bacteria-specific (Bunker et al., 2015; Peterson et al., 2007) and the B-cell repertoire is highly influenced by the microbiota composition (Lindner et al., 2015). A few murine derived bacterial species have been identified to enhance or reduce intestinal IgA (Chudnovskiy et al., 2016; Lecuyer et al., 2014; Moon et al., 2015; Obata et al., 2010).
Apart from IgA-secreting cells, the gut microbiota has the capacity to influence numerous other immune cell populations (Atarashi et al., 2011; Ivanov et al., 2009; Mortha et al., 2014; Round and Mazmanian, 2010). Many of these responses seem to be bacterial strain-specific as communities with comparable species composition can drive gut immune responses characterized by largely different cell compositions (Britton et al., 2019) suggesting that manipulation of the gut microbiota, with appropriate bacterial strains, represents a potential therapeutic pathway for the treatment of immune mediated disease.
Here we demonstrate that, human isolates restricted to specific strains of the Bacteroides ovatus species are capable of inducing high mucosal IgA production in the large intestine. Cocktails of high IgA-inducing (IgAhigh) strains, but not individual strains, elicited higher fecal IgA levels upon administration to animals harboring a pre-existing microbiota with low IgA-inducing potential (IgAlow). Our work demonstrates the importance of strain-level variation in gut microbiota composition on mucosal immune responses and the potential utility of cultured multi-bacterial effector strain cocktails as a strategy to overcome phenotype transfer resistance in microbiota-based immunomodulation (Petrof and Khoruts, 2014).
Results
B. ovatus Elicits Robust Gut IgA Production
To determine if individual gut bacterial species have a distinct IgA-inducing potential, we monocolonized GF C57BL/6 mice with different human gut commensal bacteria (Table S1) with representatives from the most prominent phyla of the human gut including Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria (Human Microbiome Project, 2012; Turnbaugh et al., 2009). After three-week colonization to allow optimal steady-state gut IgA secretion (Figure S1A), we measured serum and fecal IgA levels in each group of mice and found B. ovatus monocolonized mice secreted significantly more fecal IgA compared with mice colonized with any of the other seven bacteria (Figure 1A; p < 0.001). Most species also increased serum IgA (Figure S1B), but fecal and serum IgA levels did not correlate significantly (Figure S1C; R2 = 0.226; p = 0.196) (Macpherson et al., 2008). GF mice with a cocktail of all eight bacterial species yielded as much fecal and serum IgA as mice monocolonized with B. ovatus.
Figure 1. Select B. ovatus Strains Dominate Fecal IgA Induction in Gnotobiotic Mice.
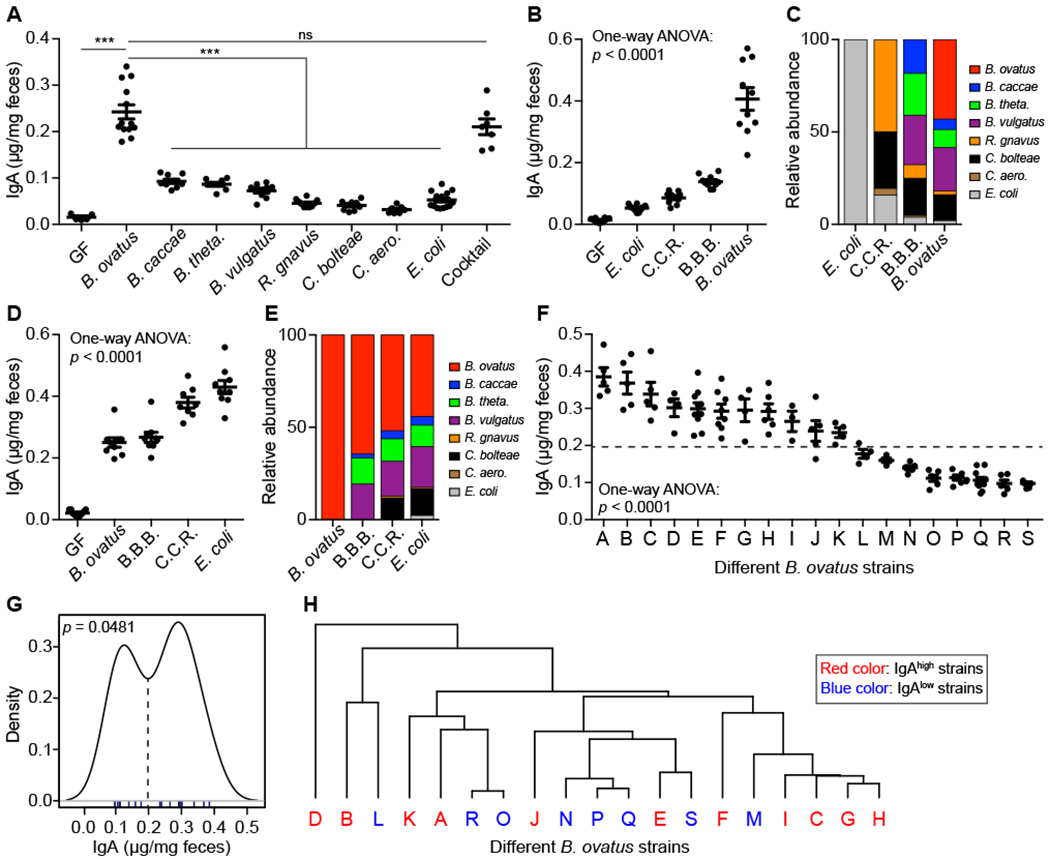
(A) Fecal IgA level in C57BL/6 gnotobiotic mice colonized with individual or a cocktail of human gut commensal bacteria. (B-E) Concentration of fecal IgA (B and D) and relative abundance of each bacterial strain (C and E) in the stool of gnotobiotic mice colonized sequentially with bacteria starting from E. coli (B and C) or B. ovatus (D and E). C.C.R.: mix of C. bolteae, C. aero. and R. gnavus; B.B.B.: mix of B. caccae, B. theta. and B. vulgatus. (F) Quantification of fecal IgA in monocolonized mice with an individual strain of B. ovatus. Dotted line separates high- and low-IgA phenotypes. (G) Statistical test of binomial distribution of B. ovatus strains based on fecal IgA induction in monocolonzied mice. (H) Dendrogram clustering of different B. ovatus strains based on genomic sequence. Data shown are mean ± SEM of 2-3 independent experiments and each dot represents the value for one mouse. Mann-Whitney test and one-way ANOVA were used; ***p < 0.001; ns, not significant. See also Figure S1 and Table S1, S2, S3.
To address if bacterial colonization order influence fecal IgA secretion, GF mice were sequentially colonized with low to high IgA inducers (i.e. E. coli to B. ovatus) (Figure S1Di). Fecal IgA increased gradually with the addition of bacterial species. However, the more striking (>2-fold) increase occurred after B. ovatus (Figure 1B). Metagenomic sequencing revealed colonization by each species, albeit with different proportions (Figure 1C). When the order of colonization was reversed (i.e. B. ovatus to E. coli) (Figure S1Dii), B. ovatus still elicited the largest response, with the other species leading to a smaller increase of fecal IgA production (Figure 1D). Intriguingly, the final relative abundance of each organism was similar regardless of colonization order (Figures 1C and 1E). These results demonstrate that B. ovatus is a robust gut IgA inducer and that species composition of the gut microbiota impacts IgA production more than the order of bacterial colonization in the cases studied.
To test the role of bacterial viability in gut IgA induction by B. ovatus (Kim et al., 2016), GF mice were administered heat-killed B. ovatus or B. ovatus metabolites for three weeks. Neither approach was capable of enhancing fecal IgA above the level detected in GF mice (Figure S1E). Similarly, heat-killed B. ovatus was also not able to enhance IgA production in E. coli pre-colonized mice (Figure S1E). Therefore, viable B. ovatus elicited the most robust gut IgA production among tested gut bacterial species in GF mice.
B. ovatus-driven Gut IgA Production Is Strain-specific
Given the remarkable microbial strain variation across individuals (De Filippis et al., 2019; Faith et al., 2013; Vatanen et al., 2018; Zhao et al., 2019), we wondered whether all B. ovatus strains within this species induced comparable levels of fecal IgA. GF mice monocolonized with one of 19 different B. ovatus strains (Table S2) showed a strain-specific gut IgA response (Figure 1F; p < 0.0001). Similar results were observed in Swiss Webster mice with identical bacterial strains (Figures S1F–S1H; R2 = 0.601, p = 0.0011). In contrast to the variability of fecal IgA levels, serum IgA levels and the density of gut bacteria were comparable across mice monocolonized with different B. ovatus strains (Figures S1I and SIJ). Of note, the distribution of IgA induction across multiple B. ovatus strains was bimodal (Figure 1G; p = 0.0481), allowing them to be categorized as IgAhigh or IgAlow strains. The genomic similarity of B. ovatus strains was not a significant predictor of their IgAhigh and IgAlow properties (Figure 1H), though some genes are enriched in IgAhigh or IgAlow strains (Table S3). These results suggest distinct IgA-inducing function is shared amongst the species rather than representing an evolutionarily distinct group within the species.
To explore if other species have common IgAhigh strains like B. ovatus, we tested distinct strains of B. caccae, B. theta., B. vulgatus and E. coli and found no differences in fecal IgA induction (Figure S1K). Additional common species such as P. johnsonii, B. intestinalis and B. fragilis also induced much less gut IgA than B. ovatus (Figure S1K). When comparing different SPF mice, Taconic mice, colonized by known IgA inducer SFB (Ivanov et al., 2009), produced more fecal IgA, which was comparable to mice colonized with a single IgAhigh B. ovatus strain (Figure 1F and S1K) demonstrating the efficient fecal IgA induction by IgAhigh B. ovatus stains.
We next examined the influence of B. ovatus strain variation on host fecal IgA production in the context of more complex gut microbiotas, GF mice were colonized with either a human microbiota arrayed culture collection containing a B. ovatus strain and 10 more other species or individual B. ovatus strain (Figure S1L). We found a significant positive correlation in IgA levels between culture collection and individual B. ovatus strain colonized mice (Figure S1M; R2 = 0.859, p = 0.0027). We also observed IgAhigh B. ovatus strain human donors trend towards more fecal IgA production (Figures S1L and S1N; R2 = 0.2071, p = 0.0765). These results suggest that the B. ovatus strain composition is a major contributor of gut IgA response even in complex microbial communities.
IgAhigh B. ovatus Strains Induce More IgA Production in the Large Intestine
To interrogate the mechanisms underpinning gut IgA induction by different B. ovatus strains, GF mice were monocolonized with a representative IgAhigh or IgAlow strain (B. ovatus strain E and Q, respectively). We first quantified different types of immunoglobulin isotypes (i.e. IgA, IgG1, IgG2a, IgG2b, IgG3, IgM and IgE) in serum, and no significant difference was observed in these monocolonized mice (Figures S1F and S2A).
Most fecal IgA is translocated into gut lumen across epithelial cells via polymeric immunoglobulin receptor (pIgR) (Johansen and Kaetzel, 2011). Expression of pIgR in gut epithelial cells is influenced by bacteria stimulation (Hooper et al., 2001). We found no noticeable difference in protein and mRNA level of pIgR in different B. ovatus strain monocolonized mice (Figures S2B and S2C). The two strains of B. ovatus also induced similar level of mRNA transcription of Muc2 and colonized the colon similarly without penetrating into epithelial cells (Figures S2C and S2D). However, we did find more IgA-secreting B cells in the colonic LP of mice harboring B. ovatus strain E compared with mice harboring strain Q, while no significant strain-specific difference was observed in small intestine, PPs and MLNs (Figures 2A–C, S2E and S2F).
Figure 2. IgAhigh B. ovatus Strain Elicits Stronger IgA Responses in the Large Intestine.
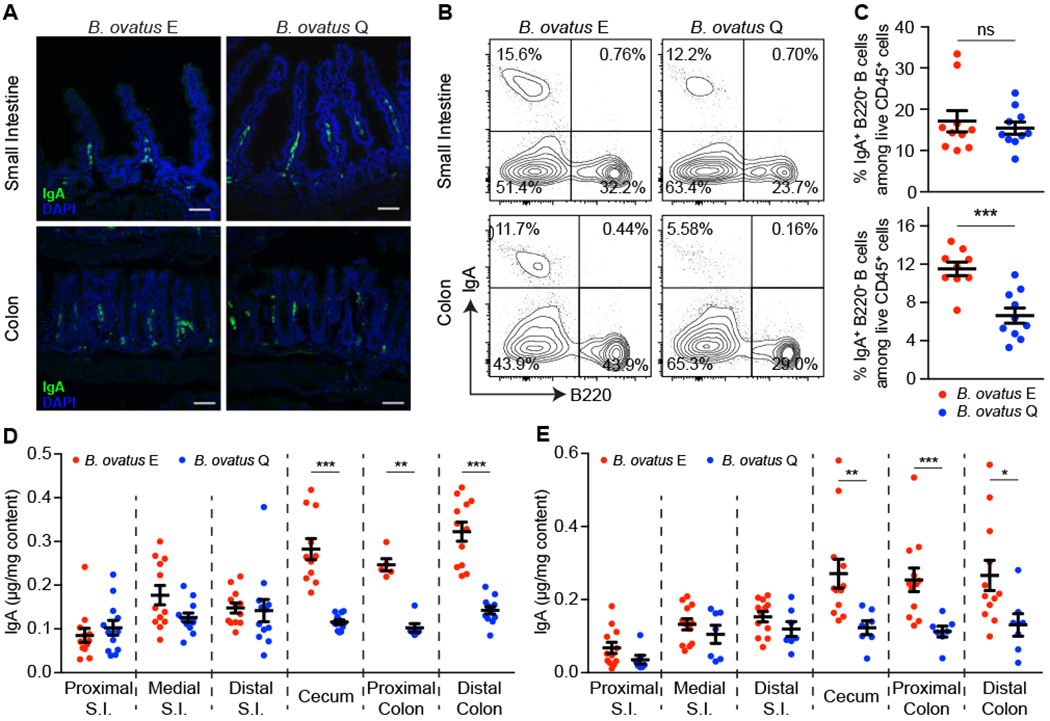
(A) Representative images of IgA+ cells in small intestine and the colon. IgA+ cells were stained with anti-IgA (green); Nuclei were counter-stained with DAPI (4’,6-diamidino-2-phenylindole) (blue). n = 5~. Scale bar = 50 μm. (B and C) Representative flow cytometry plot (B) and quantification of IgA-secreting cells (C) in small intestine and colon are shown. (D and E) Concentration of luminal IgA along the length of the intestinal tract in monocolonized C57BL/6 mice (D) and Swiss Webster mice (E). S.I.: small intestine. Data shown are mean ± SEM of 2-3 independent experiments and each dot represents the value for one mouse. Mann-Whitney test was used; **p < 0.01, ***p < 0.001; ns, not significant. See also Figure S2 and Table S2.
Given the preferential expansion of IgA-secreting B cells in the colon of monocolonized mice with strain E, we then explored whether luminal IgA levels would vary between small and large intestinal regions. We found comparable luminal IgA levels in the small intestine (Figure 2D) but significantly more luminal IgA from cecum to distal colon in those colonized with strain E (Figure 2D). Similar results were observed across all tested IgAhigh and IgAlow B. ovatus strains (Figure S2G) and also in Swiss Webster mice (Figure 2E). Thus, the IgAhigh B. ovatus strains induce more colonic IgA-secreting B cells than IgAlow B. ovatus strains, which results in enhanced IgA secretion to the large intestinal lumen.
B. ovatus Elicits Gut IgA Production Primarily via TD B Cell-activation Pathway
Gut IgA responses occur through T cell dependent (TD) and/or T cell independent (TI) B cell-activation pathways (Fagarasan et al., 2010). To determine the influence of CD4+ T cells on the gut IgA production induced by B. ovatus, we depleted CD4+ T cells in mice by injecting anti-CD4 antibody five days prior to and for three weeks after monocolonization with B. ovatus (Figures 3A and S3A–S3C). On day 7 post-colonization, fecal IgA increased in both T cell-depleted and T cell-sufficient gnotobiotic mice, suggesting CD4+ T cells are not a dominant factor in early IgA induction. By day 14 post-colonization, mice receiving an isotype-matched irrelevant control antibody generated significantly more fecal IgA than mice receiving anti-CD4 antibody suggesting the majority of the steady state B. ovatus induced IgA is T cell dependent, although the depletion of CD4 T cells had more influence on the production of IgA in the B. ovatus strain E colonized mice (Figure 3B). In addition, B. ovatus-bound IgA also decreased in the stool of CD4+ T cell-depleted mice (Figure 3C). Similarly, the frequency of IgA-secreting B cells was also reduced significantly in these mice (Figures 3D and S3D–S3G). The control mice showed more luminal IgA production than CD4+ T cell-depleted mice across the whole intestinal tract (Figure 3E). When cellular egress from secondary lymphoid tissues was blocked by FTY720 (Kunisawa et al., 2007), we found treated mice, compared with control mice, had similar fecal IgA level (Figure S3H) but significantly less luminal IgA in the distal small intestine (Figure S12B). These results suggest a mechanism that B. ovatus (especially the IgAhigh) strains might influence the local development of IgA-secreting B cells primarily via T cell dependent pathway and/or promote the survival of IgA-secreting cells better in the colon (Fagarasan et al., 2010; Masahata et al., 2014).
Figure 3. T-cell-dependent B Cell Activation Pathway Plays An Essential Role in B. ovatus Induced Fecal IgA Production.
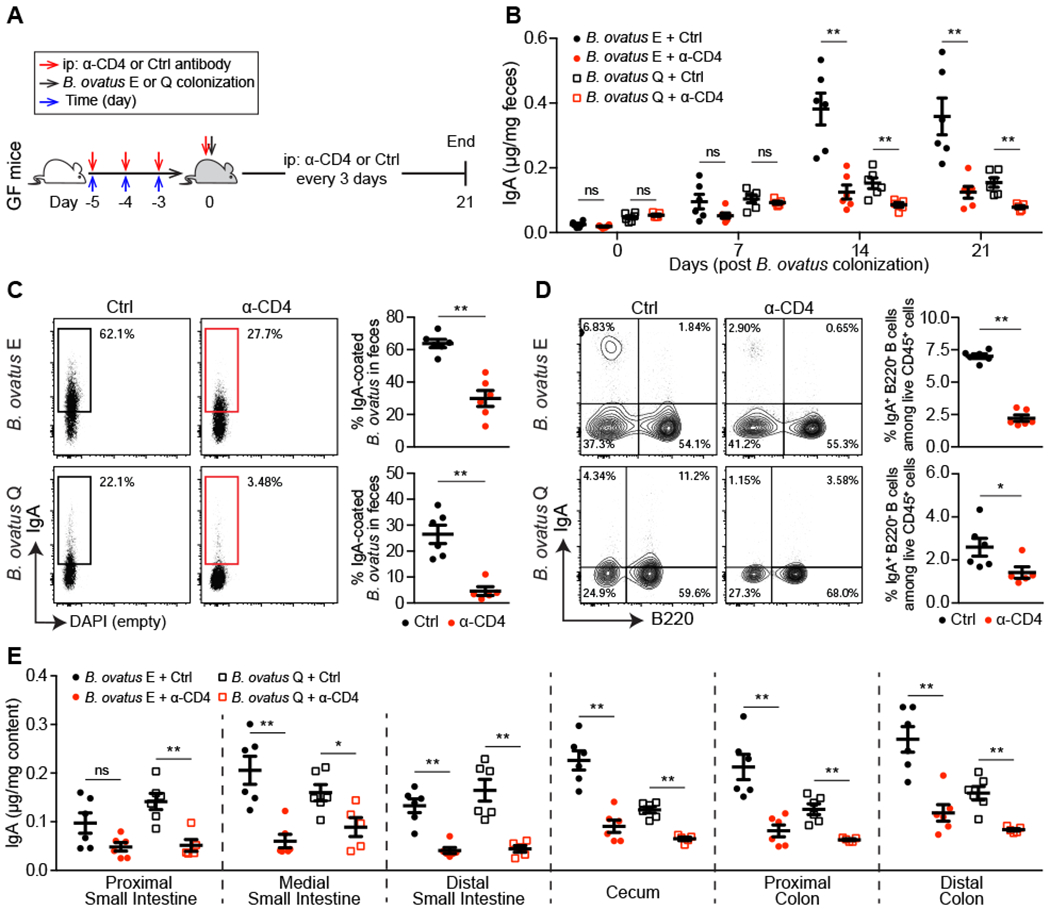
(A) Schematic representation of CD4+ T cells depletion in germ-free mice. (B) Dynamics of fecal IgA concentration in B. ovatus monocolonized mice w/o anti-CD4 antibody treatment. (C) Representative flow cytometry plot and quantification of IgA-coated bacteria in the feces of monocolonized mice w/o anti-CD4 antibody treatment. (D) Representative flow cytometry plot and percentage of IgA-secreting B cells in the colon of monocolonized mice w/o anti-CD4 antibody treatment. (E) Concentration of luminal IgA along the length of the intestinal tract of monocolonized mice w/o anti-CD4 antibody treatment. Data shown are mean ± SEM and each dot represents the value for one mouse. Mann-Whitney test was used; *p < 0.05, **p < 0.01; ns, not significant. See also Figure S3 and Table S2.
Multiplex Cocktail of B. ovatus Strains Robustly Modify Gut IgA Production
To determine if the high-IgA phenotype could be transferred, we cohoused C57BL/6 mice monocolonized with either B. ovatus strain E or Q (Figure 4A). After cohousing, mice monocolonized with B. ovatus strain Q showed no significant change in fecal IgA. In contrast, mice colonized initially with strain E had reduced fecal IgA, which raised the possibility that the low-IgA phenotype dominates in the context of this simple bacterial community (Figure 4B). Interestingly, the IgAlow B. ovatus strain Q also dominated the relative abundance of the microbiota over strain E (Figure 4C) in both WT and Rag1−/− mice (Lee et al., 2013). In an attempt to overcome this resistance to transfer of the high-IgA phenotype to mice with low-IgA phenotype, we performed a similar experiment but added three more IgAhigh B. ovatus strains. Under these conditions, the high-IgA phenotype was transferred successfully to the cohoused mice initially producing low fecal IgA (Figure S4A), while strain Q still represented a substantial relative abundance (32.5 - 53.8%) (Figure S4B). Thus, a multiplex cocktail of bacterial effector strains that each individually can induce a specific phenotype provides a more robust strategy for transferring a high-IgA phenotype.
Figure 4. Multiplex Microbial Strains Robustly Transfer High-IgA Phenotype to Low-IgA Producing Mice.
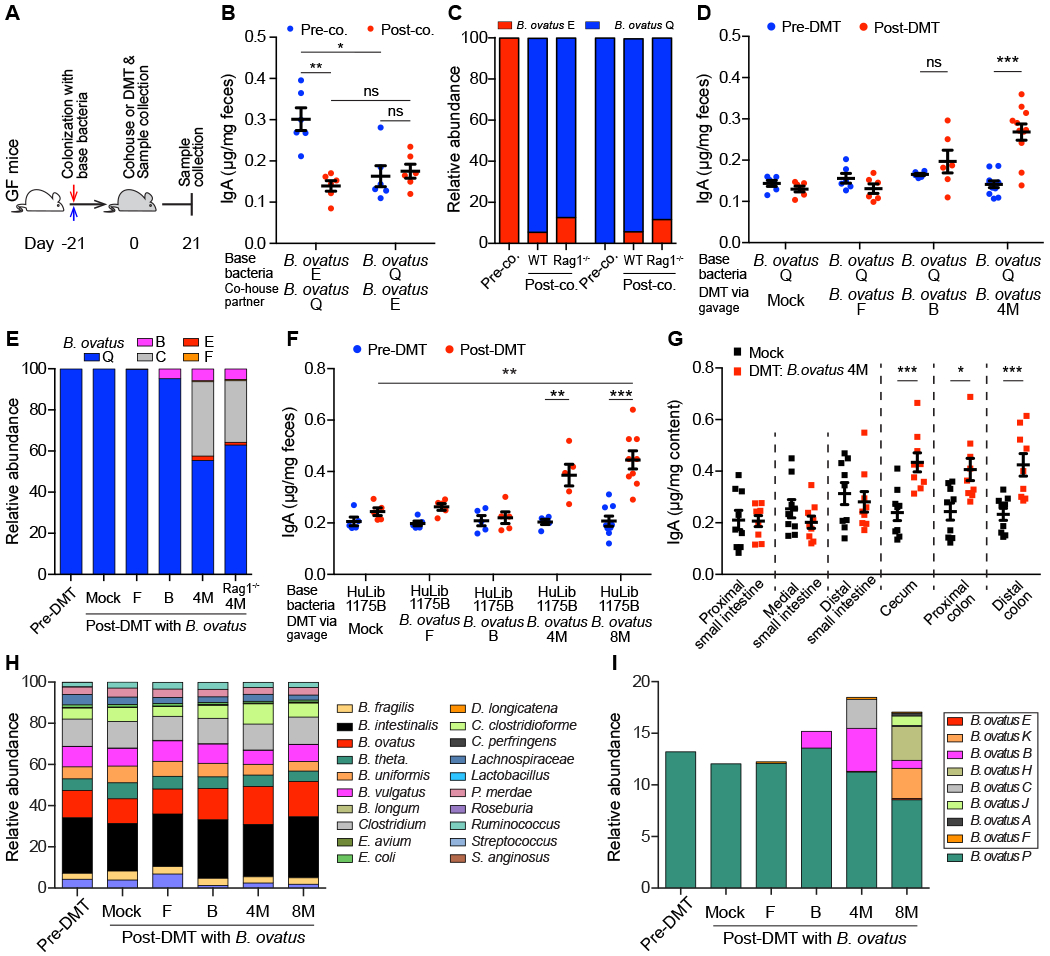
(A) Schematic representation of cohousing and defined microbial transplant (DMT) strategies. (B and C) Fecal IgA concentration (B) and relative abundance of each B. ovatus strain (C) in pre- and post-cohoused gnotobiotic mice, which were pre-colonized with either B. ovatus strain E or Q. (D and E) Fecal IgA concentration (D) and relative abundance of each B. ovatus strain (E) in mice pre- and post-DMT. Mice were pre-colonized with B. ovatus strain Q before DMT. (F) Fecal IgA concentration in mice pre- and post-DMT, which were pre-colonized with human microbiota culture collection (i.e. HuLib1175B). Mock: PBS; B. ovatus 4M/8M: a cocktail of 4/8 different IgAhigh B. ovatus strains (G) Concentration of luminal IgA along the length of the intestinal tract of mice after DMT with Mock or B. ovatus 4M. (H and I) Relative abundance of bacterial species (H) and different B. ovatus strains (I) in mice pre- and post-DMT. Data shown are mean ± SEM of 2-3 independent experiments and each dot represents the value for one mouse. Mann-Whitney test was used; *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. See also Figure S4 and Table S4.
Beyond cohousing, we further validated the above findings by transferring IgAhigh strains by oral gavage. Consistent with cohousing results, mice, pre-colonized with an IgAlow B. ovatus strain, did not alter gut IgA secretion after a defined microbial transplant (DMT) with an additional IgAhigh strain, unless a cocktail of four IgAhigh B. ovatus strains (B. ovatus 4M) was used (Figure 4D). Interestingly, IgAlow B. ovatus strain Q still dominated the relative abundance of the gut microbiota in individual strain transfers (Figure 4E). Total IgAhigh strains accounted for 44% of the gut microbiota in the B. ovatus 4M DMT group with each individual IgAhigh strain having a distinct relative abundance (Figure 4E). We also replicated these results in mice pre-colonized with another IgAlow B. ovatus strain R (Figures S4C and S4D).
To validate these results in the setting of more complex gut microbiotas, we performed experiments using either gnotobiotic mice pre-colonized by a synthetic cocktail of diverse bacterial species (8 member community) (Table S4) or arrayed culture collections established from donors (Figure S1J and Table S4). As with simpler communities, transfer of the high-IgA phenotype was robustly achieved with B. ovatus 4M or a multiplex cocktail of eight IgAhigh B. ovatus strains (B. ovatus 8M) but not by individual IgAhigh B. ovatus strains (Figures 4F and S4E). Consistently, IgA was elevated only in the large intestine (Figures 4G). The relative proportions of each IgAhigh strain and total relative abundance of all IgAhigh strains in the stool of multiplex bacterial cocktail recipient mice varied across recipient microbiota communities (Figures 4H, 4I, S4F and S4G). Regardless of its high relative abundance after DMT with B. ovatus 4M, transplantation of B. ovatus strain C did not significantly increase fecal IgA on its own (Figures 4E, S4H and S4I).
We further validate the IgA-inducing properties of our multiplex IgAhigh B. ovatus cocktails in two additional gnotobiotic mouse models pre-colonized by human microbiota arrayed culture collections with low-IgA potential (Table S4). Again, multiplex IgAhigh B. ovatus cocktails robustly increased fecal IgA (Figures S4J–S4O). In summary, our results demonstrate that transfer of multiplex IgAhigh B. ovatus strain cocktails, but not that of individual IgAhigh strains, consistently and robustly modulates the immune system (e.g. IgA phenotype) across several complex pre-existing gut microbiota.
Discussion
Strain-level functional differences of pathogenic bacteria are a fundamental component of infectious disease clinical practice and are becoming apparent in the context of the protective or disease-enhancing properties of the commensal microbiota (Arthur et al., 2012; Palm et al., 2014; Viladomiu et al., 2017). Here, we identified that about half of the tested B. ovatus strains robustly drive fecal IgA production. IgAhigh B. ovatus strains increased IgA production in distal but not proximal intestinal segments by increasing the proportion of IgA-secreting B cells. Interestingly, we did not find the fecal IgA variation induced by different B. ovatus strains was related to unique genetic lineages amongst strains. Through manipulation of gut microbiota composition, cocktails of IgAhigh B. ovatus strains were more efficient than individual strains in converting mice from low to high gut IgA producers.
IgAhigh B. ovatus strains increased IgA production by enhancing the proportion of IgA-secreting B cells in the large intestine. Remarkably, this did not result from the migration of IgA+ B cells from canonical IgA inductive sites like PPs and MLNs, suggesting local elicitation of IgA production in the large intestine including cecal patches, ILFs and LP (Cerutti and Rescigno, 2008; Masahata et al., 2014). Interestingly, protein antigens expressed on the surface of commensal bacteria were recently shown to elicit potent IgA responses in mice (Bunker et al., 2019; Wang et al., 2017). Thus, further studies will be needed to delineate the precise mechanisms whereby IgAhigh B. ovatus strain colonized mice produce more gut IgA. Moreover, our study also highlights the important contribution of CD4+ T cells in bacteria-mediated IgA production, especially in the large intestine (Kawamoto et al., 2014).
Microbiota manipulation of specific immune phenotypes could have a large range of applications (Honda and Littman, 2016). A key factor in their success will be largely dependent of identifying strategies that can robustly and consistently manipulate the desired immune populations. Using IgA induction as an example of immunomodulatory phenotype transfer, we demonstrated that multiplex bacterial cocktails of IgAhigh B. ovatus strains elicited a more robust phenotype transfer than any individual strain, even in mice with different complex gut ecosystems, providing one potential route to a consistent immune response that is robust to the variation in microbiome composition across individuals.
In summary, our results highlight the importance of bacterial strain variation on the IgA-inducing potential of the gut microbiota. In addition, we also identify a strategy (i.e. multiplex bacterial strain cocktail) to exploit strain variation to induce robust microbiota-based immune modulation.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeremiah J. Faith (jeremiah.faith@mssm.edu). All unique/stable reagents generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Housing and Husbandry of Experimental Animals
All germ-free mice were bred and maintained in flexible film gnotobiotic isolators (Class Biologically Clean, Ltd.) in the Gnotobiotic Mouse Facility in Icahn School of Medicine at Mount Sinai. Stool samples from germ-free mice were collected weekly and examined by PCR and culture-based methods to verify germ-free status. All mice were group housed with a 12-hour light/dark cycle and allowed ad libitum access to diet and water. All animal studies were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) in Icahn School of Medicine at Mount Sinai.
Growth and Isolation of Bacterial Strains
All bacterial strains were obtained from previously banked stool, public culture repositories or human gut microbiota arrayed culture collections (Faith et al., 2014). All bacterial strains isolated for this study were isolated from deidentified stool samples from individuals under a Mount Sinai IRB approved protocol (IRB-16-00008). All bacteria apart from E. coli were grown under anaerobic condition at 37° in Brain Heart Infusion (BHI) medium supplemented with 0.5% yeast extract (Difco Laboratories), 0.4% monosaccharide mixture, 0.3% disaccharide mixture, L-cysteine (0.5 mg/ml; Sigma-Aldrich), malic acid (1 mg/ml; Sigma-Aldrich) and 5 μg/ml hemin. E. coli was cultured in LB Broth Miller (EMD Chemicals, Inc.) under aerobic condition at 37°C. Cultured bacteria were stored at −80° freezer for future usage.
Colonization of Germ-free Mice with Cultured Bacteria
Germ-free mice (~8 weeks old) with same gender were pooled together and randomly assigned to different cages (2 mice per cage unless otherwise specified). Then mice were colonized with ~200-μl aliquot of bacteria suspension via oral gavage. For colonized mice, they were housed in flexible film vinyl isolators or in filter top cages using previously described techniques (Faith et al., 2014). Specifically, 30”x24”x24” vinyl flexible film isolators from Class Biologically Clean were maintained with filtered air and a sterilization port combined with sterilization cylinders to import sterilized supplies for all experiments. Filter top cages consisted of a filtered Allentown cage sterilized as a unit with bedding, a gavage needle, food, and a sealed cage. Prior to every opening, each cage was sterilized with Clidox with a five minute contact time.
METHOD DETAILS
Quantification of Immunoglobulins by ELISA
Total fecal and serum IgA were measured by sandwich ELISA. For total IgA detection, ELISA plates (Corning 3690) were coated with 1 μg/ml goat anti-mouse IgA (Southern Biotech, AL) capture antibody overnight at 4°C. Plates were washed and blocked with 1% BSA in PBS for 2 h at room temperature. Diluted samples and standards were added and incubated overnight at 4°C. Captured IgA was detected by horseradish peroxidase (HRP)-conjugated goat anti-mouse IgA antibody (Sigma-Aldrich). ELISA plates were developed by TMB microwell peroxidase substrate (KPL, Inc.) and quenched by 1 M H2SO4. Colorimetric reaction was measured at OD = 450 nm by a Synergy™ HTX Multi-Mode Microplate Reader (BioTek Instruments, Inc.). Other serum immunoglobulins (IgG1, IgG2a, IgG2b, IgG3, IgM and IgE) were also detected using sandwich ELISA with the following capture and detection antibody pairs (all the following antibodies were purchased from Southern Biotech, AL): goat anti-mouse IgG1, goat anti-mouse IgG2a, goat anti-mouse IgG2b, goat anti-mouse IgG3, rat anti-mouse IgE, rat anti-mouse IgM, goat anti-mouse IgG-HRP, goat anti-mouse IgE-HRP and goat anti-mouse IgM-HRP. For quantification of human stool IgA, the same ELISA procedure as described above was performed except using anti-human IgA and anti-human IgA-HRP antibodies (Southern Biotech, AL). Corresponding immunoglobulin isotypes were used as standards after serial dilutions.
Depletion of CD4+ T Cells in Germ-free Mice
In vivo depletion of CD4+ T cells was performed as described (Kruisbeek, 2001). Briefly, gnotobiotic mice (8 weeks old) were first injected intraperitoneally (i.p.) with anti-mouse CD4 monoclonal antibody (Bio X Cell, clone GK1.5) or matched isotype control (Bio X Cell, clone LTF-2) at 0.5 mg/day/mouse for 3 consecutive days. Then the injection was performed every 3 days for a period of 3 weeks. Five days after the first antibody injection, mice were inoculated via oral gavage with B. ovatus strain E or Q. Efficacy of T cell depletion was evaluated by flow cytometry.
Lymphocyte Isolation from Tissues
To isolate mononuclear cells from Peyer’s patches (PPs), PPs were excised from mouse small intestines and incubated in dissociation buffer, containing Hank’s Balanced Salt Solution (HBSS) without Ca2+ and Mg2+ (GIBCO), 10% fetal bovine serum (FBS), 5 mM EDTA and 15 mM HEPES, at 37°C for 30 min. Later, tissues were mechanically separated by pushing them through a 70 μm strainer into Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 2% FBS. Filtered cells were spun down, washed and resuspended in IMDM/2%FBS. Lamina propria lymphocytes were isolated as described (Faith et al., 2014). Briefly, small intestines and colons were excised, followed by removing visceral fat and intestinal contents. Tissues were opened longitudinally, washed twice in HBSS and incubated in dissociation buffer for 30 min at 37°C with mild agitation to remove ep ithelium and intraepithelial lymphocytes. Tissues were then washed three times in ice cold HBSS, cut into ~2 cm pieces and digested with collagenase (Sigma-Aldrich), DNase I (Sigma-Aldrich) and dispase I (Corning). Cell suspensions were filtered through 70 μm cell strainers, washed three times, and resuspended in IMDM/2%FBS. Mesenteric lymph nodes were separated from mesenteric fat and dissociated in IMDM/2%FBS by physically pressing the tissues between the frosted portions of two glass microscope slides. The cell suspension was filtered through a 70 μm cell strainer, washed three times and resuspended in IMDM/2%FBS.
Detection of IgA-coated Bacteria in Feces
IgA-coated fecal bacteria were measured by flow cytometry as previously described (Kau et al., 2015; Palm et al., 2014). Briefly, mouse fecal pellets were dissolved in PBS, homogenized in vortex mixer and centrifuged at 4°C to remove large particles. The s upernatant was passed through a 40 μm sterile nylon filter and an aliquot of the bacteria suspension was collected for staining. Bacteria were washed in PBS/1%BSA/2mM EDTA for 3 times. Non-specific binding sites were first blocked with PBS/1%BSA/20% rat serum for 20 min at 4°C. Bacteria were then stained with monoclonal rat anti-mouse IgA antibody (eBioscience, clone mA-6E1) for 30 min at 4°C. Afte r washing, bacteria were stained with SYBR Green I (Invitrogen). Samples were run through a BD LSR Fortessa cell analyzer and further analyzed by FlowJo software (Tree Star, Inc.).
Flow Cytometry Analysis and Antibodies
Isolated mononuclear cells were washed in PBS and incubated with Zombie Aqua™ dye (BioLegend) to distinguish live and dead cells. Non-specific binding of immunoglobulin to Fc receptors was blocked by anti-mouse CD16/32 antibody (BD Biosciences). Cells were stained in FACS buffer (PBS with 2% FBS and 2 mM EDTA) containing a mix of antibodies for 30 min at 4°C. The following antibodies were purchased from BioLegend if not indicated otherwise: anti-mouse CD45 (clone 30-F11), anti-mouse/human CD45R/B220 (clone RA3-6B2), anti-mouse GL7 (clone GL7), anti-mouse CD4 (clone GK1.5), anti-mouse IgA (eBioscience, clone mA-6E1). For the staining of IgA+ cells, both surface and intracellular staining were performed. Multi-parameter analysis was conducted with BD™ LSR II flow cytometry and analyzed with FlowJo software (Tree Star, Inc.).
Extraction of Bacterial DNA from Feces
Each murine fecal pellet was collected into a 2 ml screw cap tube (Axygen Scientific, SCT200SSC) and stored at −80°C freezer until processing. Each sample was mixed with 1.3 ml of buffer, composed of 282 μl of DNA buffer A (20 mM Tris pH 8.0, 2 mM EDTA and 200 mM NaCl), 200 μl of 20% SDS (v/w), 550 μl of Phenol:Chloroform:IAA (25:24:1) (Ambion, AM9732) and 268 μl of Buffer PM (Qiagen, 19083), and 400 μl of 0.1 mm diameter zirconia/silica beads (BioSpec, 11079101z). Next, the sample was mechanically lysed with a Mini-Beadbeater-96 (BioSpec, 1001) for 5 min. After centrifuging, all aqueous phase was collected, mixed with 650 μl of Buffer PM thoroughly before running through a Qiagen spin column. The column was washed twice with Buffer PE (Qiagen, 19065). Attached DNA was eluted with 100 μl of Buffer EB (Qiagen, 19086) and quantified with Qubit dsDNA Assay Kit (Thermo Fisher Scientific, Q32853/Q32854). Bacteria density was calculated by the following equation: Bacteria Density = DNA yield per sample (ug) / weight of sample (mg) (Contijoch et al., 2019).
Shotgun Metagenomic Sequencing
Purified bacterial template DNA (~250 ng) was sonicated and prepared using the NEBNext® Ultra™ II DNA Library Prep kit. Samples were pooled and sequenced with an Illumina HiSeq 4000 with pair-end 150nt reads. Metagenomic sequencing reads were mapped back to the reference genomes for each experiment to determine the relative abundance of each strain. To uniquely distinguish each strain, 100K sequencing reads for each sample were mapped to the unique regions of each genome and final abundances were scaled by the unique genome size of each strain (i.e. genome equivalents), as previously described (McNulty et al., 2013).
Bacterial Gene Enrichment Analysis in B. ovatus Strains
To identify candidate genes associated with the IgA high/low phenotype, we annotated each of the 19 B. ovatus genomes using prokka (Seemann, 2014) A gene presence/absence matrix was generated using the rapid large-scale prokaryotic pan genome analysis software (roary) (Page et al., 2015). Finally, genes enriched in the IgA high and IgA low groups were identified with the scoary analysis software (Brynildsrud et al., 2016).
Quantification of mRNA by Quantitative RT-PCR
Excised small intestine and colon from mice were kept in RNAlater RNA Stabilization Reagent (Qiagen, 76104) and stored at −20°C freezer until future processing. Total RNA was extracted with the RNeasy Mini Kit (Qiagen, 74104) according to manufacture’s protocol. cDNA was synthesized with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, 4368813). The StepOne Real-Time PCR System (Applied Biosystems, USA) was used for PCR amplification of the cDNAs with Applied Biosystems™ Power SYBR™ Green Master Mix and oligonucleotide primer pairs specific for pIgR, Muc2 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNAs. The primer sequences were as follows: GAPDH forward primer, 5’-TGAACGGGAAGCTCACTGG-3’; GAPDH reverse primer, 5’-TCCACCACCCTGTTGCTGTA-3’; pIgR forward primer, 5’-AGGCAATGACAACATGGGG-3’; pIgR reverse primer, 5’-ATGTCAGCTTCCTCCTTGG-3’ (Nakamura et al., 2012); Muc2 forward primer, 5’-GCTGACGAGTGGTTGGTGAATG-3’; Muc2 reverse primer, 5’-GATGAGGTGGCAGACAGGAGAC-3’ (Wlodarska et al., 2011). The following parameters were set for cDNA amplification and quantification: 30 seconds at 95°C, and then 40 cycles of denaturation at 95°C for 15 seconds and annealing at 60°C for 1 minutes. The mRNA level of test gene was normalized to GAPDH according to the formula: .
Scanning Electron Microscopy
The morphology of B. ovatus in mouse colonic tissue was observed under scanning electron microscopy (SEM). Colon tissues were excised from gnotobiotic mice and fixed in 3% glutaraldehyde buffer overnight at 4°C. Samples were then washed gently in 0.2 M so dium cacodylate buffer to remove residual fixative and re-fixed with 1% osmium tetraoxide/0.2 M cacodylate buffer for one hour. After complete drying, samples were first coated with gold particles and observed with a HITACHI S-4300 SEM (HITACHI, Japan).
Treatment of Mice with FTY720
Germ-free mice were administered 2-Amino-2-[2-(4-octyl-phenyl)-ethyl]-propane-1,3-diol hydrochloride (FTY720) (Sigma-Aldrich, SML0700) by i.p. at 1 μg/g body weight (Kunisawa et al., 2007). Three days after the initial treatment, mice were colonized with B. ovatus strain E and followed by treatment with FTY720 every three days for a period of three weeks. PBS was used in control mice. Content from different regions of the intestinal tract was harvested for IgA quantification.
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously (Kunisawa et al., 2013; Moon et al., 2015). Briefly, intestinal tissues were fixed in 10% neutral formalin overnight at 4°C, dehydrated in 15% and 30% sucrose buffer sequentially and mounted in O.C.T Embedding Compound (Electron Microscopy Sciences). Cryostat sections (~8 μm) were prepared, blocked with anti-CD16/32 antibody in 10% (v/v) rat serum/0.1% Triton-X100 in PBS for 30 min at room temperature and incubated with the indicated primary antibodies at 4°C overnight. The following primary antibodies were used: rat anti-mouse IgA-FITC (1/300 dilution; eBioscience, clone mA-6E1), goat anti-mouse pIgR (1/500 dilution; R&D Systems, cat #: AF2800). Slides were washed in PBS for three times, incubated with Alexa Fluor®-conjugated species-specific secondary antibody (1/400 dilution; Invitrogen) for 1 h at room temperature if needed and finally mounted with ProLong Gold Anti-fade Reagent with DAPI (Invitrogen). Fluorescence images of sections were acquired with a LSM780 confocal laser-scanning microscope (Carl Zeiss).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
Statistical significance between two groups was assessed by an unpaired, nonparametric, Mann-Whitney test, unless stated otherwise. In cases where multiple pairwise Mann-Whitney tests were applied, only those still significant after Bonferroni correction are displayed. Comparisons among three or more groups were performed using nonparametric Kruskal-Wallis test followed by Dunn’s test. Bimodality distribution of IgA level induced by different B. ovatus strains was performed in R (R package ‘diptest’) using the average fecal IgA level as input. For correlation test, Pearson correlation coefficient was employed. Data plotting, interpolation and statistical analysis were performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA) or R statistical software (version 3.2.2). Statistical details of experiments are described in the figure legends. A p-value less than 0.05 is considered statistically significant.
DATA AND SOFTWARE AVAILABILITY
Whole bacterial genome sequencing data for this study are available via NCBI BioProject accession number PRJNA518912.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse IgA | Southern Biotech | Cat#1040-01 |
| Anti-mouse IgG1 | Southern Biotech | Cat#1073-01 |
| Anti-mouse IgG2a | Southern Biotech | Cat#1083-01 |
| Anti-mouse IgG2b | Southern Biotech | Cat#1093-01 |
| Anti-mouse IgG3 | Southern Biotech | Cat#1103-01 |
| Anti-mouse IgM | Southern Biotech | Cat#1139-01 |
| Anti-mouse IgE | Southern Biotech | Cat#1130-01 |
| Anti-human IgA | Southern Biotech | Cat#2050-01 |
| Anti-mouse IgA-HRP | Sigma-Aldrich | Cat#A4789 |
| Anti-mouse IgG-HRP | Southern Biotech | Cat#1030-05 |
| Anti-mouse IgM-HRP | Southern Biotech | Cat#1021-05 |
| Anti-mouse IgE-HRP | Southern Biotech | Cat#1110-05 |
| Anti-human IgA-HRP | Southern Biotech | Cat#2053-05 |
| Mouse IgA | Southern Biotech | Cat#0106-01 |
| Mouse IgG1 | Southern Biotech | Cat#0102-01 |
| Mouse IgG2a | Southern Biotech | Cat#0103-01 |
| Mouse IgG2b | Southern Biotech | Cat#0104-01 |
| Mouse IgG3 | Southern Biotech | Cat#0105-03 |
| Mouse IgM | Southern Biotech | Cat#0101-01 |
| Mouse IgE | Southern Biotech | Cat#0114-01 |
| Human IgA | Southern Biotech | Cat#0155K-01 |
| Anti-mouse CD16/32 | BioLegend | Cat#101320 |
| APC/Cy7 Anti-mouse CD45 (clone: 30-F11) | BioLegend | Cat#103116 |
| FITC Anti-mouse CD4 (clone: GK1.5) | BioLegend | Cat#100406 |
| PE/Cy7 Anti-mouse CD4 (clone: RM4-4) | BioLegend | Cat#116016 |
| APC Anti-mouse B220 (clone: RA3-6B2) | BioLegend | Cat#103212 |
| FITC Anti-mouse GL7 (clone: GL-7) | BioLegend | Cat#144603 |
| PE Anti-mouse IgA (clone: mA-6E1) | eBioscience | Cat#124204-82 |
| FITC Anti-mouse IgA (clone: mA-6E1) | eBioscience | Cat#114204-82 |
| InVivoMAb Anti-mouse CD4 (clone: GK1.5) | Bio X Cell | Cat#BE0090 |
| InVivoMAb Rat IgG2b Isotype Control (clone: LTF-2) | Bio X Cell | Cat#BE0003-1 |
| Anti-mouse pIgR | R&D System | Cat#AF2800 |
| Anti-goat IgG (H+L), Alexa Fluor 594 | Invitrogen | Cat#A11058 |
| Bacterial and Virus Strains | ||
| B. ovatus | ATCC | Cat#8483 |
| B. caccae | ATCC | Cat#43185 |
| B. theta. | ATCC | Cat#VPI5482 |
| B. vulgatus | ATCC | Cat#8482 |
| R. gnavus | ATCC | Cat#29149 |
| C. bolteae | ATCC | Cat#BAA-613 |
| C. aero. | ATCC | Cat#25986 |
| E. coli | ATCC | Cat#K-12MG1655 |
| B. caccae A | This study | see Table S1 |
| B. caccae B | This study | see Table S1 |
| B. theta. A | This study | see Table S1 |
| B. theta. B | This study | see Table S1 |
| B. vulgatus A | This study | see Table S1 |
| B. vulgatus B | This study | see Table S1 |
| E. coli A | This study | see Table S1 |
| E. coli B | This study | see Table S1 |
| P. johnsonii | DSMZ | Cat#DSM18315 |
| B. intestinalis | DSMZ | Cat#DSM17393 |
| B. fragilis | This study | see Table S1 |
| B. ovatus A | This study | see Table S2 |
| B. ovatus B | This study | see Table S2 |
| B. ovatus C | This study | see Table S2 |
| B. ovatus D | This study | see Table S2 |
| B. ovatus E | ATCC | Cat#8483 |
| B. ovatus F | This study | see Table S2 |
| B. ovatus G | This study | see Table S2 |
| B. ovatus H | This study | see Table S2 |
| B. ovatus I | This study | see Table S2 |
| B. ovatus J | This study | see Table S2 |
| B. ovatus K | This study | see Table S2 |
| B. ovatus L | This study | see Table S2 |
| B. ovatus M | This study | see Table S2 |
| B. ovatus N | This study | see Table S2 |
| B. ovatus O | This study | see Table S2 |
| B. ovatus P | This study | see Table S2 |
| B. ovatus Q | This study | see Table S2 |
| B. ovatus R | This study | see Table S2 |
| B. ovatus S | This study | see Table S2 |
| 8member Community | This study | see Table S4 |
| HuLib1175B | This study | see Table S4 |
| HuLib1271b | This study | see Table S4 |
| HuLib2780_88b | This study | see Table S4 |
| Biological Samples | ||
| Human Stool Samples | This study | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Sulfuric Acid Solution | Sigma-Aldrich | Cat#72266 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#A9418 |
| Phenol:Chloroform:IAA | Invitrogen | Cat#AM9732 |
| Collagenase D | Sigma-Aldrich | Cat#C5138 |
| Dispase | Corning | Cat#354235 |
| DNase I | Sigma-Aldrich | Cat#DN25 |
| SYBR Green I Nucleic Acid Gel Stain | Invitrogen | Cat#S7563 |
| Zombie Aqua Fixable Viability Kit | BioLegend | Cat#423101 |
| Intracellular Fixation Buffer | eBioscience | Cat#00-8222 |
| Permeabilization Buffer | eBioscience | Cat#00-8333 |
| ProLong Gold Antifade Mountant with DAPI | Invitrogen | Cat#P36931 |
| LB Broth (MILLER) | EMD Millpore | Cat#110285 |
| Bacto Brain Heart Infusion | BD Biosciences | Cat#237500 |
| Yeast Extract | Difco Laboratories | Cat#212750 |
| L-cysteine Hydrochloride | Sigma-Aldrich | Cat#C1276 |
| Malic Acid | Sigma-Aldrich | Cat#M6413 |
| Histidine-HCL | Sigma-Aldrich | Cat#H7875 |
| Hematin Porcine | Sigma-Aldrich | Cat#H3281 |
| 2-Amino-2-[2-(4-octyl-phenyl)-ethyl]-propane-1,3-diol hydrochloride (FTY720) | Sigma-Aldrich | Cat#SML0700 |
| RNAlater RNA Stabilization Reagent | Qiagen | Cat#76104 |
| Power SYBR Green Mastermix | Applied Biosystems | Cat#4367659 |
| Critical Commercial Assays | ||
| NEB Next Ultra II DNA Library Prep Kit for Illumina | New England BioLabs | Cat#E7645S |
| Agencourt AMPure XP | Beckman Coulter | Cat#A63880 |
| Qubit dsDNA Assay Kit | Thermo Fisher Scientific | Cat#Q32853/Q32854 |
| QIAquick 96 PCR Purification Kit | Qiagen | Cat#28183 |
| RNeasy Mini Kit | Qiagen | Cat#74104 |
| High-Capacity cDNA Reverse Transcription Kits | Applied Biosystems | Cat#4368813 |
| TMB 2-Component Microwell Peroxidase Substrate Kit | KPL, Inc. | Cat#50-76-03 |
| Deposited Data | ||
| Shotgun Bacterial Whole-genome Sequencing Data | This study | BioProject # PRJNA518912 |
| Experimental Models: Organisms/Strains | ||
| Germ-free C57BL/6 WT Mice | This study | N/A |
| Germ-free Swiss Webster Mice | This study | N/A |
| Germ-free Rag1−/− Mice | This study | N/A |
| Oligonucleotides | ||
| GAPDH primer, fwd: 5’-TGAACGGGAAGCTCACTGG-3’ | (Nakamura et al., 2012) | N/A |
| GAPDH primer, rev: 5’-TCCACCACCCTGTTGCTGTA-3’ | (Nakamura et al., 2012) | N/A |
| pIgR primer, fwd: 5’-AGGCAATGACAACATGGGG-3’ | (Nakamura et al., 2012) | N/A |
| pIgR primer, rev: 5’-ATGTCAGCTTCCTCCTTGG-3’ | (Nakamura et al., 2012) | N/A |
| Muc2 primer, fwd: 5’-GCTGACGAGTGGTTGGTGAATG-3’ | (Wlodarska et al., 2011) | N/A |
| Muc2 primer, rev: 5’-GATGAGGTGGCAGACAGGAGAC-3’ | (Wlodarska et al., 2011) | N/A |
| Software and Algorithms | ||
| R | N/A | https://www.r-project.org |
| FlowJo | N/A | https://www.flowjo.com |
| GraphPad Prism | N/A | https://www.graphpad.com |
Highlights:
Bacteroides ovatus strain variation drives either high or low fecal IgA
CD4+ T cells are critical in B. ovatus-mediated gut IgA production
Cocktails of IgAhigh B. ovatus strains convert mice from low- to high-IgA producers
Acknowledgments
We are grateful to C. Fermin, E. Vazquez, and G. Escano for the husbandry of gnotobiotic mice; Drs. Anuk A. Das, Dirk D. Gevers, Charlotte Cunningham-Rundles, Brian Brown and Thomas Moran for helpful discussions and comments. This work was supported in part by the staff and resources of the Gnotobiotic Mouse Core Facility, the Microbiome Translational Center, the Flow Cytometry Core Facility, the Microscopy CoRE and the Scientific Computing Division in Icahn School of Medicine at Mount Sinai. This work was supported by National Institutes of Health Grants (NIGMS GM108505, NCCIH AT008661, NIDDK DK112978) and Janssen Human Microbiome Institute (to J.J.F.) and NIH DK112679 (to E.J.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
J.J.F. serves as a consultant for Janssen Research & Development LLC. All remaining authors declare no conflict of interests.
References
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. (2011). Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, et al. (2019). Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORgammat(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity 50, 212–224 e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynildsrud O, Bohlin J, Scheffer L, and Eldholm V (2016). Rapid scoring of genes in microbial pangenome-wide association studies with Scoary. Genome Biol 17, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker JJ, Drees C, Watson AR, Plunkett CH, Nagler CR, Schneewind O, Eren AM, and Bendelac A (2019). B cell superantigens in the human intestinal microbiota. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, et al. (2015). Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 43, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, and Rescigno M (2008). The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, et al. (2016). Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell 167, 444–456 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contijoch EJ, Britton GJ, Yang C, Mogno I, Li Z, Ng R, Llewellyn SR, Hira S, Johnson C, Rabinowitz KM, et al. (2019). Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pasolli E, Tett A, Tarallo S, Naccarati A, De Angelis M, Neviani E, Cocolin L, Gobbetti M, Segata N, et al. (2019). Distinct Genetic and Functional Traits of Human Intestinal Prevotella copri Strains Are Associated with Different Habitual Diets. Cell Host Microbe 25, 444–453 e443. [DOI] [PubMed] [Google Scholar]
- Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, Conner ME, Earl AM, Knight R, Bjorkman PJ, et al. (2018). Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S (2008). Evolution, development, mechanism and function of IgA in the gut. Curr Opin Immunol 20, 170–177. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, and Suzuki K (2010). Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol 28, 243–273. [DOI] [PubMed] [Google Scholar]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J, and Gordon JI (2014). Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6, 220ra211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. (2013). The long-term stability of the human gut microbiota. Science 341, 1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, et al. (2011). Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. (2010). Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, and Littman DR (2016). The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, and Gordon JI (2001). Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project C (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen FE, and Kaetzel CS (2011). Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol 4, 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu TC, Stappenbeck TS, Maleta KM, et al. (2015). Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med 7, 276ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, et al. (2014). Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41, 152–165. [DOI] [PubMed] [Google Scholar]
- Kim M, Qie Y, Park J, and Kim CH (2016). Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 20, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisbeek AM (2001). In vivo depletion of CD4- and CD8-specific T cells. Curr Protoc Immunol Chapter 4, Unit 4 1. [DOI] [PubMed] [Google Scholar]
- Kunisawa J, Gohda M, Hashimoto E, Ishikawa I, Higuchi M, Suzuki Y, Goto Y, Panea C, Ivanov II, Sumiya R, et al. (2013). Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun 4, 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisawa J, Kurashima Y, Gohda M, Higuchi M, Ishikawa I, Miura F, Ogahara I, and Kiyono H (2007). Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 109, 3749–3756. [DOI] [PubMed] [Google Scholar]
- Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, et al. (2014). Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40, 608–620. [DOI] [PubMed] [Google Scholar]
- Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, and Mazmanian SK (2013). Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501, 426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner C, Thomsen I, Wahl B, Ugur M, Sethi MK, Friedrichsen M, Smoczek A, Ott S, Baumann U, Suerbaum S, et al. (2015). Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol 16, 880–888. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, and Zinkernagel RM (2000). A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222-+. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, and McCoy KD (2012). Homeland Security: IgA immunity at the frontiers of the body. Trends Immunol 33, 160–167. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD, Johansen FE, and Brandtzaeg P (2008). The immune geography of IgA induction and function. Mucosal Immunol 1, 11–22. [DOI] [PubMed] [Google Scholar]
- Masahata K, Umemoto E, Kayama H, Kotani M, Nakamura S, Kurakawa T, Kikuta J, Gotoh K, Motooka D, Sato S, et al. (2014). Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun 5, 3704. [DOI] [PubMed] [Google Scholar]
- McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AL, and Foster KR (2016). Host Selection of Microbiota via Differential Adhesion. Cell Host Microbe 19, 550–559. [DOI] [PubMed] [Google Scholar]
- McNulty NP, Wu M, Erickson AR, Pan C, and Erickson BK (2013). Effects of Diet on Resource Utilization by a Model Human Gut Microbiota Containing Bacteroides cellulosilyticus WH2, a Symbiont with an Extensive Glycobiome. PLoS biology 11, e1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Baldridge MT, Wallace MA, Burnham D,CA, Virgin HW, and Stappenbeck TS (2015). Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521, 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, and Merad M (2014). Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Terahara M, Iwamoto T, Yamada K, Asano M, Kakuta S, Iwakura Y, and Totsuka M (2012). Upregulation of Polymeric Immunoglobulin Receptor Expression by the Heat-Inactivated Potential Probiotic Bifidobacterium bifidum OLB6378 in a Mouse Intestinal Explant Model. Scand J Immunol 75, 176–183. [DOI] [PubMed] [Google Scholar]
- Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, et al. (2010). Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A 107, 7419–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O (2012). New concepts in the generation and functions of IgA. Nature Reviews Immunology 12, 821–832. [DOI] [PubMed] [Google Scholar]
- Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, and Parkhill J (2015). Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. (2014). Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, and Gordon JI (2007). IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339. [DOI] [PubMed] [Google Scholar]
- Petrof EO, and Khoruts A (2014). From stool transplants to next-generation microbiota therapeutics. Gastroenterology 146, 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, and Mazmanian SK (2010). Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107, 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. [DOI] [PubMed] [Google Scholar]
- Strugnell RA, and Wijburg OLC (2010). The role of secretory antibodies in infection immunity. Nat Rev Microbiol 8, 656–667. [DOI] [PubMed] [Google Scholar]
- Sutherland DB, Suzuki K, and Fagarasan S (2016). Fostering of advanced mutualism with gut microbiota by Immunoglobulin A. Immunological Reviews 270, 20–31. [DOI] [PubMed] [Google Scholar]
- Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, et al. (2010). Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci U S A 107, 8794–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T, Plichta DR, Somani J, Munch PC, Arthur TD, Hall AB, Rudolf S, Oakeley EJ, Ke X, Young RA, et al. (2018). Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A, et al. (2017). IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu L, Moore DJ, Shen X, Peek RM, Acra SA, Li H, Ren X, Polk DB, and Yan F (2017). An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol 10, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, and Finlay BB (2011). Antibiotic Treatment Alters the Colonic Mucus Layer and Predisposes the Host to Exacerbated Citrobacter rodentium-Induced Colitis. Infection and Immunity 79, 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lieberman TD, Poyet M, Kauffman KM, Gibbons SM, Groussin M, Xavier RJ, and Alm EJ (2019). Adaptive Evolution within Gut Microbiomes of Healthy People. Cell Host Microbe 25, 656–667 e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole bacterial genome sequencing data for this study are available via NCBI BioProject accession number PRJNA518912.


