Abstract
BACKGROUND
Salt-sensitive hypertension is often accompanied by insulin resistance in obese individuals, but the underlying mechanisms are obscure. Microvascular function is known to affect both salt sensitivity of blood pressure and metabolic insulin sensitivity. We hypothesized that excessive salt intake increases blood pressure and decreases insulin-mediated glucose disposal, at least in part by impairing insulin-mediated muscle microvascular recruitment (IMMR).
METHODS
In 20 lean and 20 abdominally obese individuals, we assessed mean arterial pressure (MAP; 24-hour ambulatory blood pressure measurements), insulin-mediated whole-body glucose disposal (M/I value; hyperinsulinemic-euglycemic clamp technique), IMMR (contrast-enhanced ultrasound), osmolyte and water balance, and excretion of mineralocorticoids, glucocorticoids, and amino and organic acids after a low- and high-salt diet during 7 days in a randomized, double-blind, crossover design.
RESULTS
On a low-, as compared with a high-salt, intake, MAP was lower, M/I value was lower, and IMMR was greater in both lean and abdominally obese individuals. In addition, natural logarithm IMMR was inversely associated with MAP in lean participants on a low-salt diet only. On a high-salt diet, free water clearance decreased, and excretion of glucocorticoids and of amino acids involved in the urea cycle increased.
CONCLUSION
Our findings imply that hemodynamic and metabolic changes resulting from alterations in salt intake are not necessarily associated. Moreover, they are consistent with the concept that a high-salt intake increases muscle glucose uptake as a response to high salt–induced, glucocorticoid-driven muscle catabolism to stimulate urea production and thereby renal water conservation.
TRIAL REGISTRATION
ClinicalTrials.gov, NCT02068781.
Keywords: Metabolism, Vascular Biology
Keywords: Hypertension, Insulin signaling, Obesity
Individuals on a low salt diet, compared to a high salt diet, had decreased blood pressure and improved microvascular insulin sensitivity, but exhibited deterioration in metabolic insulin sensitivity.
Introduction
In obesity, an increased susceptibility to the hypertensive effects of salt (salt sensitivity) is often seen in parallel with impaired insulin-mediated glucose disposal (insulin resistance) (1–6). The exact underlying mechanisms for the association of salt-sensitive hypertension with insulin resistance in obese individuals have not been clarified, although several explanations have been proposed, including inappropriate activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, sodium-induced elevation of circulating free fatty acids, and insulin-mediated sodium retention (2, 7, 8).
We and others (9–13) have proposed that impairment of microvascular function may contribute to the detrimental effects of salt on blood pressure and insulin sensitivity, particularly in obesity. First, if excess salt impairs microvascular dilatation, the resulting increase in peripheral resistance, other things being equal, will increase blood pressure. Indeed, (skin) capillary recruitment capacity during reactive hyperemia has been shown to be inversely associated with salt sensitivity of blood pressure in normotensive and hypertensive individuals (10). In addition, salt loading was found to impede skin postocclusive reactive hyperemia in healthy women (9). Conversely, a modest reduction in salt intake increased basal and maximal skin capillary density in mildly hypertensive individuals (11), while a larger decrease in sodium intake resulted in a higher bulbar conjunctival arteriolar density in essential hypertensive individuals, compared with controls (14). Second, microvascular dysfunction can impair insulin-stimulated glucose disposal. An important physiological function of insulin in muscle is to dilate arterioles and recruit capillaries, thus enhancing its own access and that of glucose to myocytes and increasing muscle glucose uptake (15). In addition, these microvascular actions of insulin may affect blood pressure by reducing peripheral vascular resistance (16, 17). As a consequence, impairment of insulin-mediated microvascular dilatation and capillary recruitment, as often observed in obese individuals, may hinder insulin-stimulated glucose disposal and increase peripheral vascular resistance, thereby contributing to the development of, and linking, insulin resistance and hypertension (12, 13). However, it is not known whether excess salt intake impairs insulin’s microvascular effects.
We hypothesized that excess salt intake can impair insulin-mediated microvascular recruitment by interfering with nitric oxide (NO) availability and thus contribute to salt-induced increases in blood pressure and decreases in insulin-mediated glucose disposal, especially in obesity.
Indeed, in lean rats, a high-salt diet impaired both insulin-stimulated microvascular recruitment and glucose uptake in muscle (18), whereas in obese rats, salt restriction prevented the development of hypertension and insulin resistance (19). Data in humans, however, are lacking.
In view of these considerations, we studied, in lean and abdominally obese individuals, insulin-mediated muscle microvascular recruitment and its associations with 24-hour ambulatory blood pressure and whole-body insulin-mediated glucose disposal after salt loading and salt restriction. We expected blood pressure to decrease and insulin’s metabolic and microvascular actions to improve on a low- compared with a high-salt diet. To investigate underlying mechanisms of potential changes in blood pressure and metabolic and microvascular insulin sensitivity, we also assessed urinary excretion of osmolytes, water excretion, and mineralo- and glucocorticoids. Because changes in water excretion might result from alterations in urea synthesis, which in turn could affect insulin-mediated glucose disposal via increased AMPK levels because of an energy deficit in skeletal muscle (20–22), we measured amino acid and organic acid excretion as well.
Results
General characteristics.
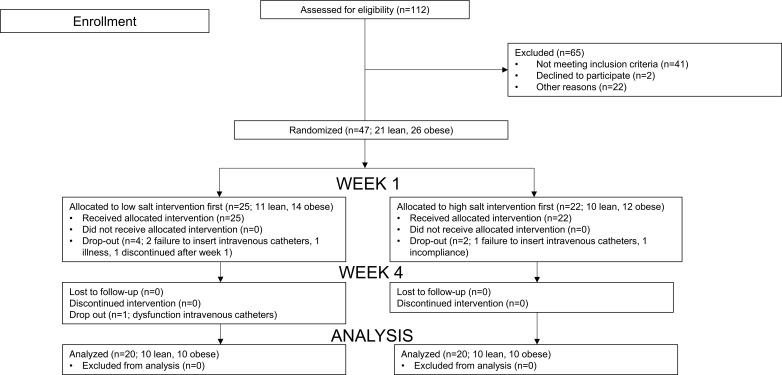
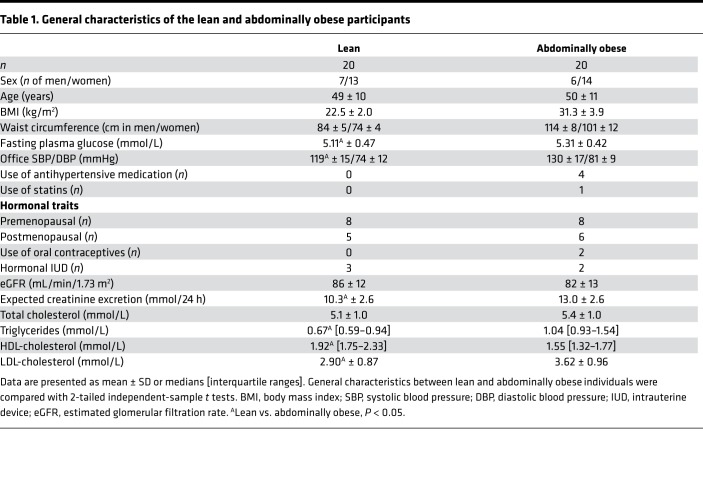
As shown in Figure 1 and Table 1, 21 lean and 26 abdominally obese individuals were randomized to start with either the low- or the high-salt intervention, and ultimately 20 lean and 20 abdominally obese individuals completed the study. Eleven lean and 10 abdominally obese participants followed a low-salt diet prior to the first set of measurements; the remaining participants started with a high-salt diet. Insulin levels during the hyperinsulinemic clamp after a low-salt diet were lacking in 1 lean participant due to hemolysis of blood samples; insulin-mediated muscle microvascular recruitment (IMMR) data after a high-salt diet were unavailable in 1 abdominally obese individual for technical reasons. Lean, compared with abdominally obese, participants had significantly lower systolic and mean arterial pressure (MAP), expected creatinine excretion and LDL-cholesterol and triglyceride levels, and higher HDL-cholesterol concentration. The numbers of pre- and postmenopausal women were comparable in both groups. Urinary sodium excretion showed adequate compliance to both the low- and the high-salt diets.
Figure 1. Enrollment, randomization, and dropout of participants.
Table 1. General characteristics of the lean and abdominally obese participants.
MAP, M/I value, and IMMR on a low- as compared with a high-salt diet in the total study population.
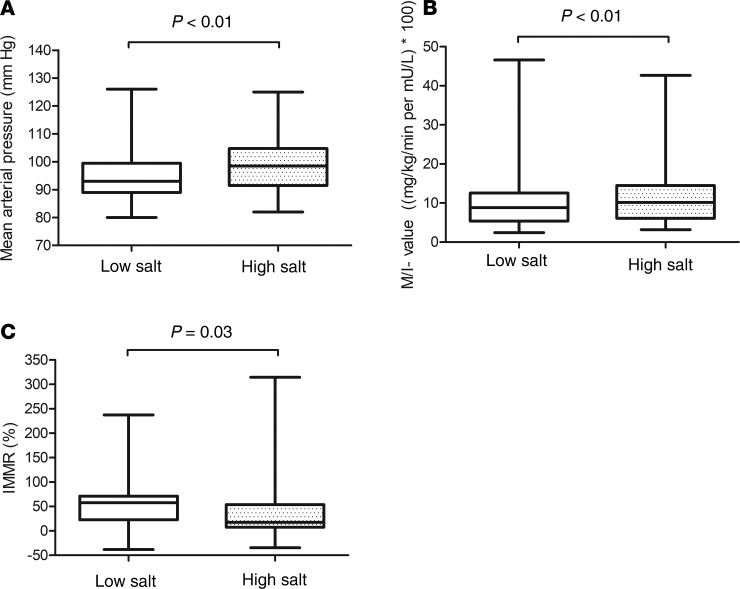
On a low- as compared with a high-salt diet, MAP was lower, whole-body glucose disposal per unit of plasma insulin concentration (M/I value) was lower, and IMMR was greater (low vs. high salt: MAP: 96 ± 11 vs. 100 ± 11 mmHg, P < 0.01; M/I value: 8.8 [interquartile range 5.4–12.6] vs. 10.2 [6.1–14.5] ([mg/kg/min per mU/L] × 100), P < 0.01; IMMR: 58 [23–71]% vs. 17 [7–54]%, P = 0.03; Figure 2). Similar conclusions were reached after adjustment for group, age, and sex (low vs. high salt: MAP: 96 ± 10 vs. 100 ± 11 mmHg, P < 0.01; M/I value: 8.8 [5.6–12.9] vs. 10.1 [7.0–15.2] ([mg/kg/min per mU/L] × 100), P < 0.01; and IMMR: 38 [32–60]% vs. 19 [14–41]%, P = 0.03).
Figure 2. MAP, M/I value, and IMMR on a low-salt, as compared with a high-salt, diet in the total study population.
(A) MAP: low salt: n = 40 (20 lean and 20 abdominally obese individuals) vs. high salt: n = 40 (20 lean and 20 abdominally obese individuals. (B) M/I value: low salt: n = 39 (19 lean; no insulin levels available in 1 participant and 20 abdominally obese individuals) vs. high salt: n = 40 (20 lean and 20 abdominally obese individuals). (C) IMMR: low salt: n = 40 (20 lean and 20 abdominally obese individuals) vs. high salt: n = 39 (20 lean and 19 abdominally obese individuals; IMMR data unavailable in 1 participant). MAP was assessed with 24-hour ambulatory blood pressure measurement (ABPM), M/I value with a hyperinsulinemic-euglycemic clamp, and IMMR with contrast-enhanced ultrasound before and during hyperinsulinemia, on both a low-salt (50 mmol/24 h) and high-salt (250 mmol/24 h) diet during 7 days in randomized order. Data are presented as median (black line), first and third quartiles (box edges), and minimum and maximum (whiskers). Data were analyzed with repeated-measures ANCOVA, adjusted for group, age, and sex.
Changes in MAP, M/I value, and IMMR during low- and high-salt intake were not statistically significantly different between lean and abdominally obese individuals (P for interaction all > 0.26) and were not affected by pre- versus postmenopausal status.
MAP, M/I value, and IMMR in lean versus abdominally obese individuals on a low- and high-salt diet.
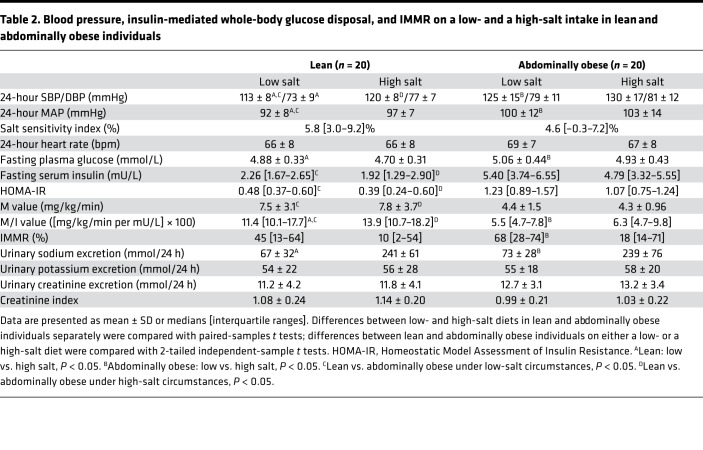
In lean, as compared with abdominally obese individuals, and on both a low- and a high-salt diet, MAP was lower, M/I value was higher, and IMMR was not statistically significantly different (Table 2).
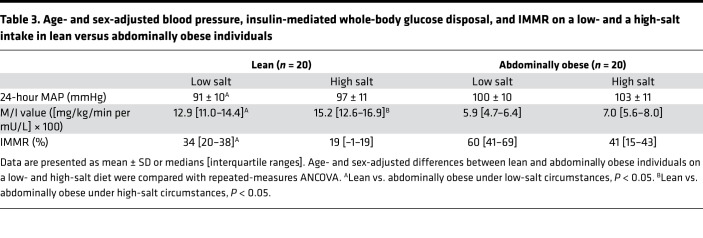
Table 2. Blood pressure, insulin-mediated whole-body glucose disposal, and IMMR on a low- and a high-salt intake in lean and abdominally obese individuals.
Adjustment for age and sex gave comparable findings with regard to MAP and M/I value, but IMMR was significantly greater in abdominally obese, compared with lean, individuals, on a low-salt diet (lean vs. abdominally obese: MAP: low salt 91 ± 10 vs. 100 ± 10 mmHg, P = 0.01; high salt 97 ± 11 vs. 103 ± 11 mmHg, P = 0.08; M/I value: low salt 12.9 [11.0–14.4] vs. 5.9 [4.7–6.4] ([mg/kg/min per mU/L] × 100), P < 0.01; high salt 15.2 [12.6–16.9] vs. 7.0 [5.6–8.0] ([mg/kg/min per mU/L] × 100), P < 0.01; and IMMR: low salt: 34 [20–38]% vs. 60 [41–69]%, P = 0.03; high salt: 19 [–1–19]% vs. 41 [15–43]%, P = 0.22; Table 3).
Table 3. Age- and sex-adjusted blood pressure, insulin-mediated whole-body glucose disposal, and IMMR on a low- and a high-salt intake in lean versus abdominally obese individuals.
The salt sensitivity index (SSI) was comparable between lean and abdominally obese participants (lean: 5.8 [3.0–9.2]%, abdominally obese: 4.6 [–0.3–7.2]%, P = 0.124; difference lean vs. abdominally obese adjusted for age and sex: 2.8 [–0.9–6.5]%).
Carryover effects were not detected in any of the above analyses (P values all > 0.11).
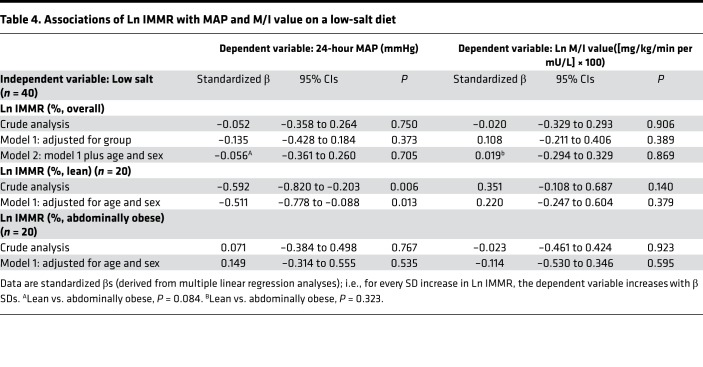
Associations of natural logarithm IMMR with MAP and M/I value on a low- and a high-salt diet.
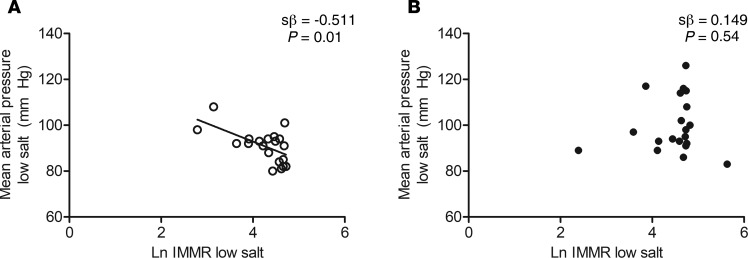
On a low-salt diet, natural logarithm (Ln) IMMR was not associated with MAP or Ln M/I value in the total study population, either without or with adjustment for potential confounders (Table 4). Interaction analyses, however, showed a significant and independent inverse association of Ln IMMR with MAP in lean participants (crude: standardized β = –0.592 [–0.820 to –0.203], and P = 0.006; age and sex adjusted: standardized β = –0.511 [–0.778 to –0.088], and P = 0.013]), while there was no association in abdominally obese participants (crude: standardized β = 0.071 [–0.384 to 0.498], and P = 0.767; age and sex adjusted: standardized β = 0.149 [–0.314 to 0.555], and P = 0.535; P for interaction 0.084) (Table 4 and Figure 3).
Table 4. Associations of Ln IMMR with MAP and M/I value on a low-salt diet.
Figure 3. Association of Ln IMMR with MAP on a low-salt diet in lean and abdominally obese individuals.
MAP was assessed with 24-hour ABPM and IMMR with contrast-enhanced ultrasound before and during hyperinsulinemia, on a low-salt diet (50 mmol/24 h) during 7 days. (A) Association of Ln IMMR with MAP on a low-salt diet in lean individuals (n = 20). (B) Association of Ln IMMR with MAP on a low-salt diet in abdominally obese individuals (n = 20). Standardized regression coefficients (sβ; derived from multiple linear regression analyses) are adjusted for age and sex; P lean vs. abdominally obese = 0.084. Lean (○): n = 20; abdominally obese (●): n = 20.
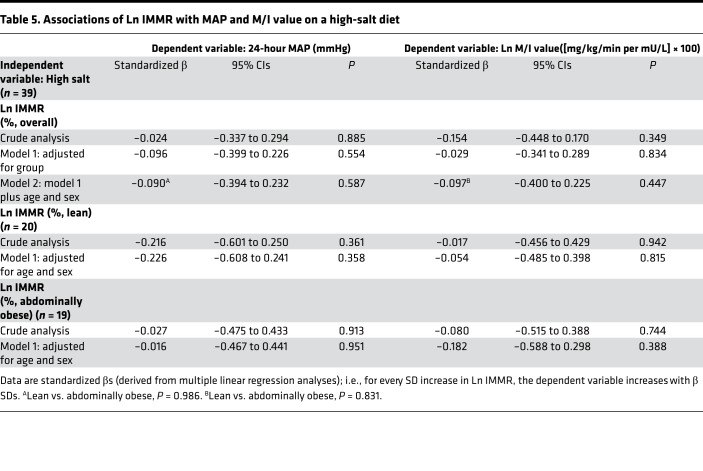
On a high-salt diet, Ln IMMR was not associated with MAP or Ln M/I value in the study population as a whole (Table 5) or in lean and abdominally obese individuals separately (MAP: P for interaction = 0.986; Ln M/I value: P for interaction = 0.831).
Table 5. Associations of Ln IMMR with MAP and M/I value on a high-salt diet.
Urinary excretion of osmolytes, mineralo- and glucocorticoids, and amino and organic acids on a high- and a low-salt diet.
Copeptin levels on a high-salt diet were lacking in 1 lean participant, due to a laboratory error.
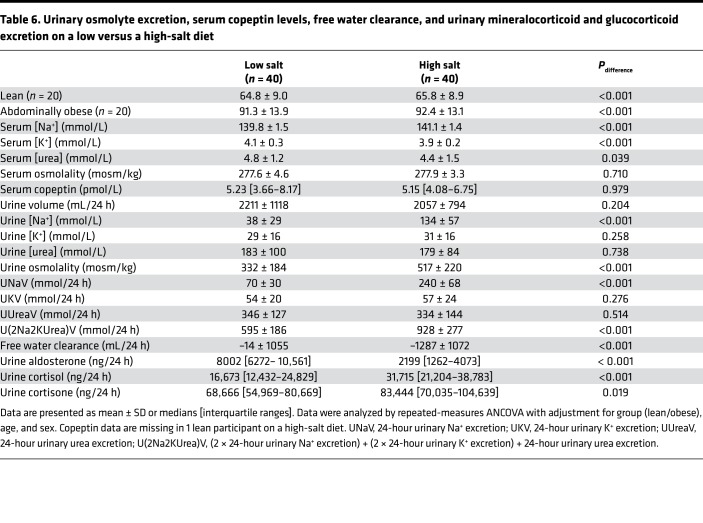
On a high-salt, as compared with a low-salt, diet, 24-hour urinary sodium and total osmolyte excretion increased (UNaV, 70 ± 30 vs. 240 ± 68 mmol/24 h; P < 0.001; U[2Na2KUrea]V, 595 ± 186 vs. 928 ± 277 mmol/24 h; P < 0.001), but urine volume was unchanged. Thus, free water clearance decreased (–14 ± 1055 vs. –1287 ± 1072 mL/24 h; P < 0.001). Urea excretion and serum copeptin levels were comparable on a low- and high-salt diet (Table 6 and Table 7).
Table 6. Urinary osmolyte excretion, serum copeptin levels, free water clearance, and urinary mineralocorticoid and glucocorticoid excretion on a low versus a high-salt diet.
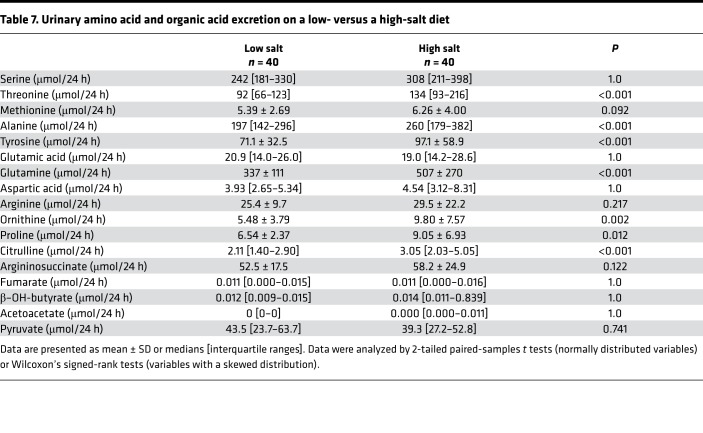
Table 7. Urinary amino acid and organic acid excretion on a low- versus a high-salt diet.
On a high-salt, as compared with a low-salt, diet, urinary aldosterone excretion decreased (8002 [6272– 10,561] vs. 2199 [1262–4073] ng/24 h; P < 0.001), whereas urinary cortisol and cortisone excretion increased (cortisol, 16,673 [12,432–24,829] vs. 31,715 [21,204–38,783] ng/24 h; P < 0.001; cortisone, 68,666 [54,969–80,669] vs. 83,444 [70,035–104,639] ng/24 h; P = 0.019) (Table 6 and Table 7).
Results of the analyses above were not different between lean and abdominally obese individuals (P all > 0.107). Carryover effects were not detected (P values all > 0.08) (Table 6 and Table 7).
Because reduced free water clearance and higher glucocorticoid levels on a high-salt diet might reflect increased urea generation for renal medullary water reabsorption (20), we also measured amino acid and organic acid excretion (Table 7). On a high-salt, as compared with a low-salt, diet, there were increases in the excretion of threonine, methionine, alanine, and tyrosine, which serve as nitrogen donors for ureagenesis; of ornithine, which is an early reactant in the urea cycle and a by-product of urea synthesis; and of citrulline, which is an early product in the urea cycle. Urinary excretion of other by-products of urea generation, i.e., glutamine and proline, also increased on a high-salt diet. Excretion of serine (another nitrogen donor), glutamic acid (as a measure of glutamate, the amino source for urea synthesis), and late reactants and products (aspartic acid, as a measure of aspartate; argininosuccinate; arginine; and fumarate) was not statistically significantly different between the low- and high-salt diets. Pyruvate excretion, which is used to generate alanine (20, 23), was also unchanged.
It has been suggested that a high-salt diet induces ketogenesis to reprioritize energy expenditure in favor of urea production (20). However, both on a low- and a high-salt diet, ketone bodies were demonstrable in only a few participants, and on a high-salt diet, ketone body excretion was not significantly increased.
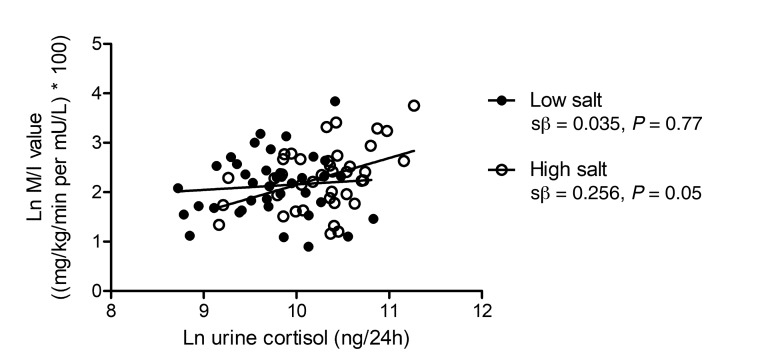
We and others hypothesized that salt-induced glucocorticoid synthesis might induce a catabolic state, and, consequently, an energy deficit in skeletal muscle (20), resulting in increased insulin-mediated glucose disposal (21, 22). Indeed, cortisol excretion was directly associated with M/I value on a high-salt diet (crude: standardized β = 0.416 [0.120 to 0.644], P = 0.008; group, age, and sex adjusted: standardized β = 0.256 [–0.060 to 0.523], P = 0.051; Figure 4). This association was similar in lean and abdominally obese individuals (P for interaction = 0.384). Cortisol excretion was not associated with M/I value on a low-salt diet in the study population as a whole (crude: standardized β = 0.088 [–0.234 to 0.393], P = 0.592; group, age, and sex adjusted: standardized β = 0.035 [–0.284 to 0.347], P = 0.770) (Figure 4), or in lean and abdominally obese individuals separately (P for interaction = 0.270), and was not associated with MAP or IMMR on either diet (data not shown).
Figure 4. Association of Ln urine cortisol with Ln M/I value on a low- and a high-salt diet.
Standardized regression coefficients (derived from multiple linear regression analyses) are adjusted for group (lean/obese), age, and sex. Low salt (●): n = 39; high salt (○): n = 40. Urinary cortisol excretion was measured by supported liquid extraction (SLE+) followed by liquid chromatography tandem mass spectrometry detection (LC-MS/MS), and M/I value was assessed with a hyperinsulinemic-euglycemic clamp, on both a low-salt (50 mmol/24 h) and high-salt (250 mmol/24 h) diet during 7 days in randomized order.
Discussion
The present study demonstrates that on a low-salt, compared with a high-salt, diet, blood pressure decreases, whole-body insulin-mediated glucose disposal decreases, and microvascular insulin sensitivity increases in both lean and abdominally obese individuals. In addition, greater IMMR is associated with lower MAP on a low salt diet in lean, but not in abdominally obese, participants and is not associated with whole-body insulin-mediated glucose disposal on either a low- or a high-salt diet.
A major finding is the improvement of IMMR in humans following salt restriction, similar to earlier observations in rats (18) and in line with previous observations of improved microvascular function and structure in normotensive and hypertensive humans in other vascular beds (skin, conjunctiva) and in response to other stimuli (postocclusive reactive hyperemia, venous congestion) (9, 11, 24). Increased salt intake may interfere with insulin-mediated endothelial NO production and thus insulin-stimulated microvascular dilatation at several levels, notably by reducing endothelial NOS (eNOS) protein expression and activation, by accelerating NO degradation through superoxide generation by NADH oxidase and eNOS when tetrahydrobiopterin availability is reduced, and by inducing superoxide dismutase deficiency, which further increases oxidative stress (25). It logically follows that reducing salt intake improves microvascular insulin signaling via opposite mechanisms. Whether basal (as opposed to insulin-stimulated) muscle microvascular perfusion is also diminished after a high-salt diet cannot be derived from our data because the ultrasound method we used has a large interindividual variation under basal circumstances (26, 27). However, it is likely that functional muscle microvascular density under basal circumstances is also diminished after a high-salt diet, given the fact that an elevation in blood pressure occurring during high-salt intake is eventually the result of increased peripheral resistance (28, 29), which is determined largely at the microvascular level (12). The rise in (muscle) microvascular resistance can be ascribed to failure of the microvasculature to dilate in response to an initial expansion of the cardiac output induced by increasing salt intake and/or to direct microvascular actions of salt (25, 28).
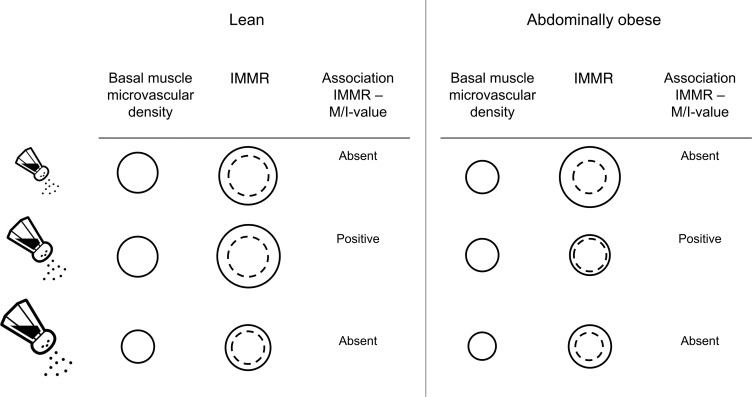
After a low-salt diet, IMMR was greater in abdominally obese than in lean participants, whereas after a high-salt diet IMMR was more or less equally diminished, and of similar magnitude, in lean and abdominally obese individuals.
In a previous study, we demonstrated impaired IMMR in obese, compared with lean, men under ad libitum salt intake (26). Because the net effect of variation in salt intake on IMMR will be determined by changes in both basal functional muscle microvascular density and microvascular insulin signaling, the discrepancy in responses of IMMR to low-, ad libitum–, and high-salt diets between lean and abdominally obese individuals may be related to differences in the relative contributions of both factors. Earlier observations by us and others indicate that under unspecified or ad libitum salt intake, both functional muscle capillary density under basal circumstances and IMMR are diminished in (abdominally) obese, compared with lean, individuals (26, 30, 31), due to increased levels of free fatty acids and inflammatory cytokines and changes in adipokine signaling in obese individuals (12, 32). An explanation for our current findings may be that also under low-salt circumstances and through similar mechanisms, basal functional muscle microvascular density is diminished in abdominally obese versus lean individuals, while salt restriction improves microvascular insulin signaling in both lean and abdominally obese participants, thus resulting in greater IMMR in the abdominally obese participants. Vice versa, the intrinsic capacity of the muscle microvasculature to dilate in response to salt loading might be greater in lean than abdominally obese individuals. Therefore, deterioration of microvascular insulin sensitivity, which is already impaired under ad libitum salt intake in the abdominally obese individuals, will ultimately lead to a comparable IMMR after a high-salt diet in both groups (Figure 5).
Figure 5. Schematic proposal of basal functional muscle microvascular density, IMMR (i.e., microvascular insulin sensitivity), and the association of IMMR with M/I value (i.e., metabolic insulin sensitivity) during low-, ad libitum– and high-salt intake in lean compared with abdominally obese individuals.
The continuous line represents the insulin-mediated increase of muscle microvascular density, relative to basal density (represented with the dashed line).
Contrary to expectation, insulin-mediated whole-body glucose disposal decreased after 7 days of low, compared with 7 days of high, salt intake. Controlled experiments in healthy and Dahl salt-sensitive animals have demonstrated that salt loading impairs insulin-mediated glucose disposal (3, 33–35). Similar findings were obtained using other measures of insulin sensitivity in hypertensive and obese rats (36, 37), whereas salt restriction has been shown to improve the HOMA index (which reflects both hepatic and muscle insulin sensitivity) in obese rats (19). However, controlled experiments in humans are limited. Nevertheless, there are several reports of decreased insulin-mediated glucose disposal, as assessed by the hyperinsulinemic-euglycemic clamp technique, in healthy (38, 39), hypertensive (40), and hypertension-prone (41) individuals after both moderate and more extreme salt restriction. Comparable results have been acquired with the HOMA index (42, 43).
We speculate that increased insulin-mediated glucose disposal on a high-salt diet may be explained by salt-induced, glucocorticoid-driven muscle catabolism to increase urea production and thereby renal water conservation (20, 44). In skeletal muscle of mice fed a high-salt diet, AMPK levels increased (20), which in turn may promote whole-body glucose disposal (21, 22, 45, 46). Indeed, we observed that on a high-salt, compared with a low-salt, diet, free water clearance was reduced, which occurred independent of changes in antidiuretic hormone secretion (as reflected by similar copeptin levels on the high- and low-salt diets), and glucocorticoid excretion increased. In addition, urinary excretion of amino acids serving as nitrogen donors for urea synthesis and of early reactants and (by-)products in the urea cycle were higher on a high- versus a low-salt diet, suggesting increased urea production. Excretion of later products and reactants in the urea cycle, i.e., of products of reactions involving argininosuccinate synthetase, which is the rate-limiting enzyme in urea synthesis (47), and of reactants and products after this part of the cycle (23) was not statistically significantly increased on a high-salt diet. It is likely that measurement of urinary excretion is a relatively insensitive method of detecting changes in amino and organic acid fluxes, particularly in the later part of the urea cycle, which have been demonstrated previously directly in murine muscle and liver (20). Although a higher salt intake during at least 4 weeks increased plasma urea concentration in mice, and decreased urinary urea excretion in both mice and healthy men (20, 44), we did not observe differences in serum urea levels or urinary urea excretion between the low- and high-salt diets. However, noticeable changes in circulating or urine urea levels might only occur after several weeks because urea has been shown to be actively transported to and accumulate in the renal medulla to increase water reabsorption on a high-salt diet (20), and it may take some time before a steady state has been reached. Cortisol excretion was directly associated with insulin-mediated glucose disposal on a high-salt diet, but not on a low-salt diet, which we interpret as consistent with the concept that, under circumstances of high salt intake, greater insulin-mediated glucose disposal is a compensating mechanism for the glucocorticoid-induced energy deficit in skeletal muscle.
To the best of our knowledge, this is the first study investigating the association of IMMR with whole-body insulin-mediated glucose disposal on a low- versus a high-salt diet. At usual or nonspecified salt intake, IMMR has been demonstrated to be a direct determinant of whole-body insulin-mediated glucose disposal (26, 30, 48), but this seems not to be the case at a very low or a very high salt intake (Figure 5). Dissociated effects of low- and high-salt diets on vascular and metabolic insulin signaling have been reported previously in rats and healthy humans, although in these studies, insulin-induced vasodilatation was assessed in larger vessels (39, 49, 50).
Thus, impairment of microvascular function on a high-salt diet is not sufficient to impair insulin-mediated glucose disposal, possibly because the energy deficit in skeletal muscle, caused by salt-induced glucocorticoid synthesis, is a greater stimulus for (insulin-mediated) muscle glucose uptake. Although higher AMPK levels resulting from glucocorticoid-induced muscle catabolism have also been demonstrated to promote microvascular insulin signaling in animals and humans (51–53), this effect is presumably offset by direct interference of salt with (insulin-mediated) vasodilatation.
As expected, and in agreement with previous studies performed in normotensive, hypertensive, and obese individuals, salt reduction lowered blood pressure in lean and abdominally obese participants (54, 55), although to a similar extent in both groups, indicating a comparable degree of salt sensitivity. Previous investigations have shown greater salt sensitivity in obese, compared with lean, Zucker rats (56) and human adolescents (6), and in Chinese nondiabetic individuals with versus without metabolic syndrome (5). These seemingly contradictory findings might be explained by differences in degree of obesity and ethnicity between the current and other study populations because the mean BMI in the obese adolescent population was 33.6 kg/m2, versus 31.3 kg/m2 in our abdominally obese population, and Asian individuals tend to be more salt sensitive (57) and generally consume diets with a higher salt content than White individuals (58–60).
Although a low-salt diet reduced blood pressure and improved insulin-mediated muscle microvascular recruitment in both lean and abdominally obese individuals, a higher IMMR was associated with lower blood pressure under low-salt circumstances in the lean individuals only, which may be ascribed to a contribution of insulin’s microvascular actions to decreased peripheral vascular resistance (17). In the abdominally obese participants, MAP was higher than in the lean participants after a low-salt diet, probably due to interaction of several factors, including overactivity of the renin-angiotensin-aldosterone system and sympathetic nervous system, and physical compression of the kidneys (61), and these might overrule the contribution of an improvement in IMMR following salt restriction to blood pressure regulation.
A limitation of the present study is the fact that IMMR and whole-body insulin-induced glucose disposal were not measured under ad libitum–salt ingestion, which is about 140 mmol per day in the Netherlands (59).
In addition, the underlying mechanisms of the improvement in microvascular insulin signaling, which was presumed to involve enhanced NO availability, were not identified in the current investigation. Last, water intake was not registered, which would have allowed estimation of water balance. An important strength of this study is its randomized, placebo-controlled, blinded design, with a washout period between the low- and high-salt diets. In addition, we assessed insulin-mediated microvascular function directly in skeletal muscle, which is the main site of peripheral glucose uptake, and we used the gold standard for the determination of metabolic insulin sensitivity.
In conclusion, a low-salt, as compared with a high-salt, diet during 7 days reduces blood pressure and impairs insulin-mediated glucose disposal, but improves insulin-mediated muscle microvascular recruitment in both lean and abdominally obese participants. In addition, the enhancement of IMMR was associated with decreased MAP but only in lean individuals. The higher insulin-mediated glucose disposal on a high-salt diet may reflect an energy deficit in skeletal muscle caused by salt-induced glucocorticoid synthesis with the goal of increasing urea production and thereby renal water conservation.
An important question is whether the observed increase in insulin-mediated glucose disposal on a high-salt diet should be regarded as a beneficial effect of salt or merely a compensating mechanism. This could be investigated by exposing individuals to a high-salt diet with and without increasing water intake and comparing insulin-mediated glucose disposal between these conditions. In addition, the mechanisms underlying the improvement of IMMR on a low-salt diet require further elucidation. Nevertheless, our findings indicate that determinants of insulin-mediated glucose disposal are dynamic, i.e., are affected by salt status. Moreover, hemodynamic benefits of reductions in salt intake are not necessarily paralleled by metabolic advantages.
Methods
Study population.
Lean and abdominally obese individuals were recruited at the Maastricht University Medical Center, Maastricht, the Netherlands, between September 2014 and August 2016 via advertisements in local newspapers and among participants in previous investigations. A sample size of 20 individuals per group was calculated to be sufficient for detecting a mean difference of 5 mmHg in MAP, of 1 ([mg/kg/min per mU/L] × 100) in M/I value between the low- and high-salt diets with a power (1–β) of 0.80 and α = 0.95, and of 7 mmHg in MAP and 4 ([mg/kg/min per mU/L] × 100) in M/I value between lean and abdominally obese individuals with the same power and α. Although data on relevant differences in and variation of IMMR were limited, we also expected this sample size to be large enough to demonstrate a difference in IMMR because previous investigators have observed an average difference in IMMR of 40% between 10 lean and 11 abdominally obese participants (30). Thus, we aimed at 20 lean and 20 abdominally obese White individuals to complete this randomized double-blind crossover trial with masked analyses. Participants were 18–65 years of age, nonsmoking, nondiabetic, and free of cardiovascular disease and had a waist circumference below 80 cm (lean women) or 94 cm (lean men) or above 88 cm (abdominally obese women) or 102 cm (abdominally obese men). Exclusion criteria were fasting plasma glucose more than 6.1 mmol/L, office blood pressure more than 180/110 mmHg, unstable or severe pulmonary or thyroid disease, a recent history of malignancy, inflammatory diseases, impairment of renal or hepatic function, pregnancy or lactation, and use of glucose-lowering medication, nonsteroidal antiinflammatory drugs, or corticosteroids. Four abdominally obese participants were taking antihypertensive medication at the time of inclusion (calcium channel blocker: n = 1; angiotensin receptor blocker in combination with a thiazide diuretic: n = 1; angiotensin-converting enzyme [ACE] inhibitor combined with a beta blocker: n = 1; ACE inhibitor combined with a thiazide-like diuretic; n = 1). Antihypertensives were discontinued 3 weeks before measurements; statin use was not interrupted (n = 1 abdominally obese man).
Women on oral contraceptives were instructed to continue using them throughout the study period (n = 2 abdominally obese women).
Measurements were performed in either the follicular or the luteal phase of the menstrual cycle, if applicable, with the exception of 2 lean women (in one, the first study day took place in the follicular phase and the second in the luteal phase; in the other, vice versa). Data on the menstrual cycle phase were unavailable in 3 lean and 2 abdominally obese women, due to the presence of a hormonal intrauterine device without menstrual bleeding (n = 4) or a very irregular cycle (n = 1).
Study design and general procedures.
Prior to the first and second sets of measurements, participants adhered to a diet aimed at either a high-salt (250 mmol NaCl/24 h) or a low-salt (50 mmol NaCl/24 h) intake for 7 days in randomized order in a 1:1 ratio, with a washout period of 14 days. Randomization was performed by an independent investigator using block randomization with variable block sizes. Every individual participant was provided a personalized diet by a dietician, which was used during both the low- and high-salt phases. This diet contained 50 mmol NaCl and 70–80 mmol K+ per day and the same daily amount of calories that he or she ingested before the intervention started (and in the wash-out period). It was supplemented with sodium capsules in the high-salt week (9 per day, containing 1.3 g [22.2 mmol] NaCl per capsule; BasicPharma) and with matched placebo capsules (BasicPharma) in the same amount in the low-salt week, to reach a salt intake of 14.6 and 2.9 g salt/day, respectively. Thus, the energy content and energy sources, although different for every individual, were kept constant throughout the intervention periods. To prevent side effects, capsules with delayed-release properties were used (DRcaps, Capsugel). The containers with capsules were labeled in accordance with the randomization numbers and handed over to the participants by a member of the research team; both were unaware of the treatment allocation. Study data were unblinded only upon completion of all analyses by an independent investigator.
On the seventh day of both the low-salt and high-salt weeks, 24-hour urine was collected for assessment of sodium, potassium, and creatinine excretion, and 24-hour ABPMs were performed (Mobilograph, New Generation, I.E.M.) at the nondominant arm with appropriately sized cuffs at 15-minute intervals from 8 a.m. to 11 p.m. and at 30-minute intervals from 11 p.m. to 8 a.m.
MAP values collected with ambulatory blood pressure monitoring during the low- and high-salt diets were used to compute the SSI. The SSI is the difference in MAP between the low- and high-salt diet divided by MAP during the low-salt diet (62).
Assessments of whole-body insulin-stimulated glucose disposal and IMMR were conducted in a temperature-controlled room (24°C ± 0.5°C) after a 12-hour overnight fast with participants in the supine position. Individuals were instructed to refrain from alcohol and meals rich in lipids for a period of 24 hours before each study day and from strenuous physical exercise for a period of 48 hours before each study day. After insertion of 2 i.v. catheters and a 30-minute acclimatization period with the participants in the supine position, we took blood samples for determination of glucose and creatinine levels.
Assessment of whole-body insulin-mediated glucose disposal.
We determined metabolic insulin sensitivity by means of a modified version of the hyperinsulinemic-euglycemic clamp technique as described by DeFronzo et al. (63). Briefly, insulin (Insuman Rapid, Sanofi) was administered in a primed continuous manner at a rate of 1 mU/kg/min during 180 minutes. Isoglycemia was maintained by adjusting the rate of a 20% d-glucose infusion based on plasma glucose measurements performed at 5-minute intervals. Whole-body glucose disposal (M value) was estimated from the steady-state glucose infusion rate between 90 and 150 minutes after initiation of insulin administration. M was expressed per kilogram body weight per unit of plasma insulin concentration (M/I value), thus correcting for variation in steady-state insulin concentrations. For convenience, the M/I ratio was multiplied by 100.
Assessment of IMMR.
IMMR was assessed with contrast-enhanced ultrasound as described previously (26). Briefly, microvascular blood volume (MBV) of forearm skeletal muscle was measured before and during hyperinsulinemia with a Toshiba Aplio XG ultrasound system during continuous i.v. administration of sulfur hexafluoride gas-filled microbubbles (SonoVue, Bracco Diagnostics).
After steady-state microbubble concentration was achieved (3 minutes), 5 real-time replenishment curves of 30 seconds were acquired. These replenishment curves were stored and analyzed offline in a blinded fashion after completion of the trial using CHI-Q software (Toshiba).
The replenishment curves were fitted to the exponential function y = A(1–e–βt), where t is time since high mechanical index pulse, y is the video intensity at any given t, A is the plateau video intensity (representing MBV), and β is the microvascular flow velocity. IMMR was calculated as the relative increase in muscle MBV during hyperinsulinemia.
Blood and urine measurements.
Plasma glucose was determined with a YSI2300 glucose analyzer (YSI, Yellow Springs). Blood samples were analyzed for total cholesterol, HDL-cholesterol, and triglycerides (enzymatic colorimetric method, Roche Diagnostics). LDL-cholesterol was calculated with the Friedewald formula (64). Sodium and potassium in serum and urine were determined with the ion-selective electrode method (Roche Diagnostics); creatinine and urea in serum and urine were measured with enzymatic assays (Roche Diagnostics). eGFR was calculated using the CKD Epidemiology Collaboration equation (65). Expected creatinine excretion was computed as 879.89 + (12.51 × weight in kg) – (6.19 × age) – 379.42 if female, as proposed by Ix et al. (66), and the creatinine index, i.e., the ratio of observed versus expected 24-hour urinary creatinine excretion, was used to assess the completeness of the 24-hour urine collection (67). Serum insulin levels before and during the hyperinsulinemic clamp were measured with a sandwich immunoassay (MSD; intra-assay CV = 4.2%, and interassay CV = 5.4%).
Copeptin concentration was determined with an automated immunofluorescent assay (B●R●A●H●M●S; intra-assay CV < 15%, and interassay CV < 18%). Osmolality in serum and urine was determined by assessment of freezing point depression. Free water clearance was calculated as: urine volume × (1 – urine osmolality/plasma osmolality).
Urinary aldosterone, cortisol, and cortisone excretion were measured by SLE+ followed by LC-MS/MS detection (intra-assay CV: aldosterone, 6%; cortisol, 5%; cortisone, 7%; interassay CV: aldosterone, 7%; cortisol, 6%; cortisone, 8%).
To assess amino acid excretion, urine samples were diluted to a creatinine concentration of about 1 mmol/L.
For analysis, 10 μL urine sample was diluted in 1500 μL of 0.5 mM tridecafluoroheptanoic acid in ultrapure water (buffer A). Five microliters of the diluted sample was analyzed. Quantitation was performed using ultra-performance high-pressure liquid chromatography–tandem mass spectrometry on a configuration of an Acquity UHPLC and a Micromass Quattro Premier XE Tandem Mass Spectrometer (Waters) equipped with a Acquity UHPLC BEH C18, 1.7-μm, 2.1 × 100 mm column. The mobile phase consisted of buffer A and 0.5 mM tridecafluoroheptanoic acid in acetonitrile. Mass spectrometry was performed in multiple reaction monitoring mode using electrospray ionization in positive mode. Concentrations were determined using stable isotope–labeled internal standards and external calibration curves. For full details see the previous publication (68). For the measurement of organic acid excretion, urine samples were diluted to a creatinine concentration of about 1 mmol/L. Twenty-five microliters of diluted urine sample was mixed with 25 μL of an internal standard mixture and 350 μL 0.1% v/v formic acid in ultrapure water analyzed using liquid chromatography–quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) on an LC-QTOF/MS configuration (Agilent Technologies) consisting of an Infinity II1290 UHPLC coupled to a 6550 iFunnel QTOF equipped with an electrospray ionization source. LC separation was done on an Acquity C18 column UPLC HSS T3 1.8-μm, 2.1 × 100 mm, with Acquity VanGuard PreColumn UPLC HSS T3, 2.1 × 5 mm (Waters). The mobile phase buffers were 0.1% v/v formic acid in water and 0.1% formic acid in 95% acetonitrile/5% water v/v. The mass spectrometer was tuned for low mass range (≤1700 m/z) at high-resolution slicer mode and 2G Hz extended dynamic range and run in the negative mode for a full scan. Concentrations were determined using stable isotope–labeled internal standards and external calibration curves. For full details see the previous publication (69).
Statistics.
Normally distributed variables were expressed as mean ± SD, variables with a skewed distribution were displayed as median and interquartile range, and natural logarithmic transformation was performed before further analyses where appropriate (triglycerides, HDL-cholesterol, insulin, HOMA, M/I value, IMMR, copeptin, aldosterone, cortisol, cortisone). Because IMMR results were partially negative, natural logarithmic transformation could be performed only after adding 40 to each value (lowest value, –38).
We used 2-tailed independent-sample t tests to compare general characteristics between lean and abdominally obese individuals and 2-tailed paired-samples t tests to compare 24-hour ambulatory blood pressure, M/I value, and IMMR between the low- and high-salt diets in the study population as a whole. Next, we used repeated-measures ANCOVA to adjust these comparisons for group (lean or abdominally obese), age, and sex. We then compared 24-hour ambulatory blood pressure, M/I value, and IMMR on the low- and high-salt diets between lean and abdominally obese individuals with 2-tailed independent-sample t tests, followed by repeated-measures ANCOVA with adjustment for age and sex. We performed interaction analyses (group × low- or high-salt condition) to investigate whether effects of low- and high-salt conditions were different between lean and abdominally obese individuals. Where appropriate, stratified analyses are presented. To establish whether IMMR was a potential determinant of MAP and M/I value under low- and/or high-salt conditions, we used multiple linear regression analysis with IMMR as an independent variable and MAP or M/I value as dependent variables, adjusted for group, age, and sex; we performed interaction analyses to study whether these associations differed between lean and abdominally obese individuals. We compared osmolyte excretion, copeptin levels, free water clearance, and mineralocorticoid and glucocorticoid excretion between the low- and high-salt diets with repeated-measures ANCOVA with adjustment for group, age, and sex, and we performed interaction analyses to establish whether results differed between lean and abdominally obese individuals. We analyzed differences in amino acid and organic acid excretion between the low- and high-salt conditions with 2-tailed paired-samples t tests or Wilcoxon’s signed-rank tests, where appropriate. Last, to investigate whether cortisol was a potential determinant of M/I value, IMMR, and MAP on a low- and a high-salt diet, we used multiple linear regression analysis with cortisol as an independent variable and M/I value, IMMR, or MAP as dependent variables, adjusted for group, age, and sex.
We also carried out interaction analyses to determine whether associations were different between lean and abdominally obese individuals. Analyses were performed using the SPSS statistical software package (IBM SPSS Statistics version 20). Two-tailed P values of less than 0.05 and less than 0.10 were considered statistically significant in the main and interaction analyses, respectively.
Study approval.
All participants gave written informed consent. The study was approved by the committee of the Maastricht University Medical Center, performed in accordance with the Declaration of Helsinki, and registered at ClinicalTrials.gov (NCT02068781).
Author contributions
MTJS, YHAMK, AJHMH, PWDL, and CDAS designed the study. MTJS, HEN, JOTR, JLJMS, and MPVDW performed the measurements included in this article, and MTJS and YHAMK performed the analyses. MTJS and CDAS wrote the manuscript. MTJS, YHAMK, AJHMH, HEN, JOTR, JLJMS, MPVDW, CGS, PWDL, and CDAS critically read and commented on the manuscript.
Acknowledgments
We thank the dieticians of the Maastricht University Medical Center for the dietary counseling of the participants in this trial and V. Vermeulen (Department of Internal Medicine, Maastricht University Medical Center) for assistance during the execution of this study.
Version 1. 02/27/2020
In-Press Preview
Version 2. 03/26/2020
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: JCI Insight. 2020;5(6):e127530.https://doi.org/10.1172/jci.insight.127530.
Contributor Information
Monica T.J. Schütten, Email: m.schutten@maastrichtuniversity.nl.
Yvo H.A.M. Kusters, Email: y.kusters@maastrichtuniversity.nl.
Alfons J.H.M. Houben, Email: b.houben@maastrichtuniversity.nl.
Casper G. Schalkwijk, Email: c.schalkwijk@maastrichtuniversity.nl.
Peter W. de Leeuw, Email: p.deleeuw@maastrichtuniversity.nl.
Coen D.A. Stehouwer, Email: cda.stehouwer@mumc.nl.
References
- 1.Baudrand R, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf) 2014;80(5):677–684. doi: 10.1111/cen.12225. [DOI] [PubMed] [Google Scholar]
- 2.Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010;7(10):577–584. doi: 10.1038/nrcardio.2010.123. [DOI] [PubMed] [Google Scholar]
- 3.Ogihara T, et al. Insulin resistance with enhanced insulin signaling in high-salt diet-fed rats. Diabetes. 2001;50(3):573–583. doi: 10.2337/diabetes.50.3.573. [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, et al. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373(9666):829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocchini AP, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321(9):580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 7.Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 8.Goodfriend TL, Ball DL, Weinberger MH, Moore TJ, Weder AB, Egan BM. Salt loads raise plasma fatty acids and lower insulin. Hypertension. 1991;17(6 pt 2):958–964. doi: 10.1161/01.hyp.17.6.958. [DOI] [PubMed] [Google Scholar]
- 9.Cavka A, et al. The role of cyclo-oxygenase-1 in high-salt diet-induced microvascular dysfunction in humans. J Physiol (Lond) 2015;593(24):5313–5324. doi: 10.1113/JP271631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jongh RT, Serné EH, IJzerman RG, Stehouwer CD. Microvascular function: a potential link between salt sensitivity, insulin resistance and hypertension. J Hypertens. 2007;25(9):1887–1893. doi: 10.1097/HJH.0b013e32825e1db7. [DOI] [PubMed] [Google Scholar]
- 11.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56(2):253–259. doi: 10.1161/HYPERTENSIONAHA.110.155747. [DOI] [PubMed] [Google Scholar]
- 12.Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 13.Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda) 2017;32(3):197–209. doi: 10.1152/physiol.00037.2016. [DOI] [PubMed] [Google Scholar]
- 14.Houben AJ, Willemsen RT, van de Ven H, de Leeuw PW. Microvascular adaptation to changes in dietary sodium is disturbed in patients with essential hypertension. J Hypertens. 2005;23(1):127–132. doi: 10.1097/00004872-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Kusters YH, Barrett EJ. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab. 2016;310(6):E379–E387. doi: 10.1152/ajpendo.00443.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer MP, Serné EH, Smulders YM, Eringa EC, Meijer RI. Phenotyping the microcirculation with contrast-enhanced ultrasound. Hypertension. 2012;60(5):e38; author reply e39. doi: 10.1161/HYPERTENSIONAHA.112.202465. [DOI] [PubMed] [Google Scholar]
- 17.Hornstra JM, et al. Insulin’s microvascular vasodilatory effects are inversely related to peripheral vascular resistance in overweight, but insulin-sensitive subjects. Obesity (Silver Spring) 2013;21(12):2557–2561. doi: 10.1002/oby.20406. [DOI] [PubMed] [Google Scholar]
- 18.Premilovac D, Richards SM, Rattigan S, Keske MA. A vascular mechanism for high-sodium-induced insulin resistance in rats. Diabetologia. 2014;57(12):2586–2595. doi: 10.1007/s00125-014-3373-y. [DOI] [PubMed] [Google Scholar]
- 19.Hattori T, et al. Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. J Am Heart Assoc. 2014;3(6):e001312. doi: 10.1161/JAHA.114.001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitada K, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017;127(5):1944–1959. doi: 10.1172/JCI88532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuthbertson DJ, et al. 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56(8):2078–2084. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 22.Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94(4):1373–1379. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- 23.Morris SM. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 24.Houben AJ, Willemsen RT, van de Ven H, de Leeuw PW. Microvascular adaptation to changes in dietary sodium is disturbed in patients with essential hypertension. J Hypertens. 2005;23(1):127–132. doi: 10.1097/00004872-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29(3):415–424. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 26.Kusters YH, et al. Independent tissue contributors to obesity-associated insulin resistance. JCI Insight. 2017;2(13):89695. doi: 10.1172/jci.insight.89695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjøberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am J Physiol Heart Circ Physiol. 2011;301(2):H450–H458. doi: 10.1152/ajpheart.01174.2010. [DOI] [PubMed] [Google Scholar]
- 28.Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28(5):1362–1369. doi: 10.1681/ASN.2016080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyton AC, Coleman TG, Young DB, Lohmeier TE, DeClue JW. Salt balance and long-term blood pressure control. Annu Rev Med. 1980;31:15–27. doi: 10.1146/annurev.me.31.020180.000311. [DOI] [PubMed] [Google Scholar]
- 30.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 31.Gavin TP, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol. 2005;98(1):315–321. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 32.Sorop O, et al. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res. 2017;113(9):1035–1045. doi: 10.1093/cvr/cvx093. [DOI] [PubMed] [Google Scholar]
- 33.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64(6):2163–2171. doi: 10.1046/j.1523-1755.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 34.Ogihara T, et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension. 2002;40(1):83–89. doi: 10.1161/01.HYP.0000022880.45113.C9. [DOI] [PubMed] [Google Scholar]
- 35.Sechi LA, et al. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertension. 1996;27(4):955–961. doi: 10.1161/01.HYP.27.4.955. [DOI] [PubMed] [Google Scholar]
- 36.Cho TM, et al. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology. 2007;148(11):5396–5402. doi: 10.1210/en.2007-0245. [DOI] [PubMed] [Google Scholar]
- 37.Hayashida T, et al. Salt-loading elevates blood pressure and aggravates insulin resistance in Wistar fatty rats: a possible role for enhanced Na+ -H+ exchanger activity. J Hypertens. 2001;19(9):1643–1650. doi: 10.1097/00004872-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Perry CG, et al. Decreased insulin sensitivity during dietary sodium restriction is not mediated by effects of angiotensin II on insulin action. Clin Sci. 2003;105(2):187–194. doi: 10.1042/CS20020320. [DOI] [PubMed] [Google Scholar]
- 39.Townsend RR, Kapoor S, McFadden CB. Salt intake and insulin sensitivity in healthy human volunteers. Clin Sci. 2007;113(3):141–148. doi: 10.1042/CS20060361. [DOI] [PubMed] [Google Scholar]
- 40.Gomi T, Shibuya Y, Sakurai J, Hirawa N, Hasegawa K, Ikeda T. Strict dietary sodium reduction worsens insulin sensitivity by increasing sympathetic nervous activity in patients with primary hypertension. Am J Hypertens. 1998;11(9):1048–1055. doi: 10.1016/S0895-7061(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 41.Melander O, Groop L, Hulthén UL. Effect of salt on insulin sensitivity differs according to gender and degree of salt sensitivity. Hypertension. 2000;35(3):827–831. doi: 10.1161/01.HYP.35.3.827. [DOI] [PubMed] [Google Scholar]
- 42.Garg R, Sun B, Williams J. Effect of low-salt diet on insulin resistance in salt-sensitive versus salt-resistant hypertension. Hypertension. 2014;64(6):1384–1387. doi: 10.1161/HYPERTENSIONAHA.114.03880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metab Clin Exp. 2011;60(7):965–968. doi: 10.1016/j.metabol.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rakova N, et al. Increased-salt consumption induces body water conservation and decreases fluid intake. J Clin Invest. 2017;127(5):1932–1943. doi: 10.1172/JCI88530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buhl ES, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51(7):2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 46.Iglesias MA, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51(10):2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 47.Grøfte T, et al. Effects of growth hormone and insulin-like growth factor-I singly and in combination on in vivo capacity of urea synthesis, gene expression of urea cycle enzymes, and organ nitrogen contents in rats. Hepatology. 1997;25(4):964–969. doi: 10.1002/hep.510250429. [DOI] [PubMed] [Google Scholar]
- 48.Meijer RI, et al. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation. 2012;19(6):494–500. doi: 10.1111/j.1549-8719.2012.00174.x. [DOI] [PubMed] [Google Scholar]
- 49.Foo M, Denver AE, Coppack SW, Yudkin JS. Effect of salt-loading on blood pressure, insulin sensitivity and limb blood flow in normal subjects. Clin Sci. 1998;95(2):157–164. [PubMed] [Google Scholar]
- 50.Vecchione C, Morisco C, Fratta L, Argenziano L, Trimarco B, Lembo G. Dietary sodium restriction impairs endothelial effect of insulin. Hypertension. 1998;31(6):1261–1265. doi: 10.1161/01.HYP.31.6.1261. [DOI] [PubMed] [Google Scholar]
- 51.Bradley EA, et al. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol. 2010;30(6):1137–1142. doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]
- 52.Bradley EA, Zhang L, Genders AJ, Richards SM, Rattigan S, Keske MA. Enhancement of insulin-mediated rat muscle glucose uptake and microvascular perfusion by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside. Cardiovasc Diabetol. 2015;14:91. doi: 10.1186/s12933-015-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Boer MP, et al. Globular adiponectin controls insulin-mediated vasoreactivity in muscle through AMPKα2. Vascul Pharmacol. 2016;78:24–35. doi: 10.1016/j.vph.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Andersson OK, Persson B, Hedner T, Aurell M, Berglund G, Fagerberg B. Central haemodynamics, baroreceptor sensitivity and alpha 1-adrenoceptor-mediated vascular reactivity during weight-stable sodium restriction in obese men with hypertension. J Hypertens. 1986;4(1):101–107. doi: 10.1097/00004872-198602000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pamidimukkala J, Jandhyala BS. Effects of salt rich diet in the obese Zucker rats: studies on renal function during isotonic volume expansion. Clin Exp Hypertens. 2004;26(1):55–67. doi: 10.1081/CEH-120027331. [DOI] [PubMed] [Google Scholar]
- 57.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26(7):521–525. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 58.Ando K, et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension(1) Role of salt in hypertension and cardiovascular diseases. Hypertens Res. 2013;36(12):1009–1019. doi: 10.1038/hr.2013.102. [DOI] [PubMed] [Google Scholar]
- 59.Hendriksen MA, van Raaij JM, Geleijnse JM, Wilson-van den Hooven C, Ocké MC, van der A DL. Monitoring salt and iodine intakes in Dutch adults between 2006 and 2010 using 24 h urinary sodium and iodine excretions. Public Health Nutr. 2014;17(7):1431–1438. doi: 10.1017/S1368980013001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Y, et al. Salt intake, knowledge of salt intake, and blood pressure control in Chinese hypertensive patients. J Am Soc Hypertens. 2014;8(12):909–914. doi: 10.1016/j.jash.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yatabe MS, et al. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92(1):77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 63.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 64.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 65.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ix JH, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6(1):184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.John KA, et al. Accuracy and usefulness of select methods for assessing complete collection of 24-hour urine: a systematic review. J Clin Hypertens (Greenwich) 2016;18(5):456–467. doi: 10.1111/jch.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterval WA, Scheijen JL, Ortmans-Ploemen MM, Habets-van der Poel CD, Bierau J. Quantitative UPLC-MS/MS analysis of underivatised amino acids in body fluids is a reliable tool for the diagnosis and follow-up of patients with inborn errors of metabolism. Clin Chim Acta. 2009;407(1-2):36–42. doi: 10.1016/j.cca.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 69.Körver-Keularts IMLW, et al. Fast and accurate quantitative organic acid analysis with LC-QTOF/MS facilitates screening of patients for inborn errors of metabolism. J Inherit Metab Dis. 2018;41(3):415–424. doi: 10.1007/s10545-017-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]