Abstract
Introduction
Athletes have attempted to glean the ergogenic benefits of recombinant human erythropoietin (rHuEPO) since it became available in the 1980s. However, there is limited consensus in the literature regarding its true performance-enhancing effects. In fact, some studies suggest there is no conclusive evidence; therefore, it is necessary to evaluate and quantify the strength of the evidence.
Objective
To determine the effects of erythropoietin on enhancing athletic performance.
Design
At least two independent reviewers conducted citation identification through abstract and full-text screening, and study selection, and extracted raw data on demographics, descriptions of interventions and all outcomes to predesigned abstraction forms. Outcomes were stratified by treatment periods and dosages. Study quality was assessed using the Cochrane Risk of Bias Tool and Cochrane Grading of Recommendations Assessment Development and Education (GRADE) scale. Where appropriate, quantitative analysis was performed.
Data sources
EMBASE, MEDLINE and SPORTDiscus were searched from their inception to January 2020.
Eligibility criteria
Trials that examined any enhancement in sport in healthy participants aged 18–65 using rHuEPO compared with placebo were included.
Results
Overall, there is low-to-moderate quality evidence suggesting rHuEPO may be more beneficial than placebo in enhancing haematological parameters, pulmonary measures, maximal power output and time to exhaustion independent of dosage. However, these improvements are almost exclusively seen during maximal exercise intensities, which may be less relevant to athletic competition conditions.
Conclusion
Due to heterogeneity among trials, more high-quality randomised controlled trials with larger sample sizes in conditions that mirror actual competition are needed to further elucidate these effects.
Keywords: EPO, erythropoietin, doping, athletic performance, systematic review
Summary box.
What is already known?
Recombinant human erythropoietin (rHuEPO) is used clinically to improve circulating erythrocyte levels.
The literature is inconsistent regarding the performance-enhancing effects of erythropoietin; some studies suggest it is ergogenic, while other studies suggest there is no evidence to support the claim.
What are the new findings?
There is low-to-moderate quality evidence suggesting that, independent of dosage, rHuEPO may be more beneficial than placebo in enhancing haematological, pulmonary measures, maximal power output and time to exhaustion.
These improvements are almost exclusively seen during maximal exercise intensities.
Introduction
In 2012, the US Anti-Doping Agency (USADA) published an investigative report on Lance Armstrong, winner of seven consecutive Tour de France, with evidence that Armstrong had doped throughout his professional cycling career.1 Based on these findings, USADA imposed a lifetime ban on Armstrong and revoked his championship titles from 1998 onwards.1 Despite initially denying these allegations, in January 2013, Armstrong admitted to using erythropoietin (EPO) among other performance-enhancing drugs and blood transfusions.1 2 This is just one of many examples that draw attention towards the controversy surrounding the ergogenic effects of EPO and its prohibition in sport.
EPO is a naturally produced glycoprotein hormone that induces erythropoiesis, and maturation and proliferation of oxygen-delivering erythrocytes.3 The body uses EPO to control the number of circulating erythrocytes, thus maintaining tissue oxygen delivery levels within a narrow range.3 Recombinant human erythropoietin (rHuEPO) has also been developed in the laboratory and successfully administered to patients with late-stage renal failure to improve circulating erythrocyte levels.4
While the effects of rHuEPO have proven to be significant in medicine, endurance athletes have attempted to glean the benefits of this substance as an ergogenic drug, particularly due to rHuEPO’s potential to increase maximal oxygen uptake capacity and thus endurance. rHuEPO became readily available in Europe in the late 1980s, and the use of rHuEPO in sports was banned in the early 1990s.3
However, the evidence for the ergogenic effects of rHuEPO might not be as straightforward; some studies suggest there is no conclusive evidence that supports the claim that rHuEPO can enhance the performance of endurance athletes, particularly elite cyclists.5 6 Despite the vast amounts of money poured into the doping industry and reports of widespread use of rHuEPO in endurance sports, the use of this drug may not be based on evidence.
Previous reviews of the ergogenic effects of rHuEPO have been published with various shortcomings.7–10 The review by Lodewijkx et al7 did not include a search strategy, which increases the risk of selection bias and does not rule out the likelihood of the search being performed long before the study was published. Next, the review only explored maximal oxygen consumption (VO2 max) and maximal power output (POmax) as surrogates for athletic performance.7 Many relevant outcomes including submaximal oxygen consumption (submaximal VO2) and time to exhaustion (TTE) were not included.7 Additionally, the dosing threshold and duration of the ergogenic effects of rHuEPO were not explored.7 Many of the pooled studies did not have a placebo group; hence, estimates should be interpreted with caution. Similarly, reviews by Bird et al,9 Sgrò et al8 and Heuberger10 were limited by their lack of a systematic approach, search strategies, up-to-date methodological assessment tools and/or meta-analyses. Furthermore, these authors did not appear to follow the guidelines set out by the Cochrane Handbook for Systematic Reviews of Interventions.11 It is therefore necessary to conduct an updated review that includes a Cochrane risk of bias and Grading of Recommendations Assessment Development and Education (GRADE) analysis to quantify the strength of the evidence, in addition to performing a quantitative analysis through meta-analysis.11 12 This article aims to clarify the relationship of varying treatment periods and dosages of rHuEPO with multiple parameters of performance enhancement to determine the validity of its ban from competition.
Methods
Data sources and searches
In consultation with two research librarians, we developed search strategies to identify potentially relevant studies from the EMBASE, MEDLINE and SPORTDiscus databases from their inception to January 2020 (online supplementary appendix 1). Reference lists of included studies were hand-searched using titles and abstracts for potential studies.
bmjsem-2019-000716supp001.pdf (106.6KB, pdf)
Design and study selection
At least two independent reviewers conducted citation identification through abstract and full-text screening, as well as study selection. Disagreements were resolved through a third assessor. We sought reports of any qualitative or quantitative trials in relation to rHuEPO (eg, epoetin alfa, epoetin beta, darbepoetin alfa) use for its ergogenic effect. Clinical judgement was used to review the search and retrieve potentially relevant studies. Inclusion criteria for studies are as follows:
Types of studies
Any published quantitative or qualitative study design in the English language was included. The study must have specifically investigated the ergogenic effects of rHuEPO.
Types of participants
Participants were healthy men and women between the age of 18 and 65 with no other comorbid conditions.
Types of interventions
rHuEPO was the sole substance administered to participants at a given time. Studies that looked at other substances were included if participants were not administered both substances simultaneously or if we were able to separate the effects of these substances to analyse the effects of the intervention. The presence of a placebo group and reported between-group differences were also necessary for inclusion to allow for comparisons.
Types of outcome measures
Outcome measures determined a priori included any enhancement in sport above baseline, with respect to aerobic, anaerobic and laboratory parameters.
Studies were excluded if the intervention effects of rHuEPO could not be isolated, there was no placebo group, inappropriate outcomes were measured, between-group analyses were not performed, participants had comorbidities, there was an inappropriate study design (eg, review article), animal models were used, or if the source was not an original scientific journal article (online supplementary appendix 2).
bmjsem-2019-000716supp002.pdf (142.3KB, pdf)
Treatment and dosage ranges
Modified from the Cochrane Handbook, the treatment period was defined as starting from the first administered dose and categorised into the following11:
Immediate-term treatment: up to 2 weeks.
Short-term treatment: longer than 2 weeks and up to 3 months.
Intermediate-term treatment: longer than 3 months and up to 1 year.
Long-term treatment: longer than 1 year.
Dosage ranges were calculated using the prespecified dose categories of epoetin alfa used by Annaheim et al13 adjusted per week. These ranges were categorised into the following:
Low dosage: <6875 IU/week.
Medium dosage: 6875–13 750 IU/week.
High dosage: >13 750 IU/week.
If included studies used other rHuEPO formulations, the respective dose equivalency to epoetin alfa per week was calculated based on clinical guidelines.14
Data extraction and quality assessment
At least two independent reviewers extracted raw data on demographics, descriptions of interventions and all outcomes to predesigned abstraction forms. Disagreements were resolved by repeated review and consensus. If specific values were not reported, values were estimated from any given graphs. When data were not extractable, the primary authors were contacted.
Outcomes from each study were classified into their respective dosage ranges by calculating the dosage per week. For studies where subjects followed multiple dosage regimens, the weight-adjusted dosage per week was calculated and used to appropriately categorise the outcome.
At least two independent reviewers assessed each study for bias and methodological quality, based on the Cochrane Collaboration Tool for assessing risk of bias and the Cochrane GRADE scale.11 12 Disagreements were resolved through a third assessor. Bias and methodological quality were graded using two sets of criteria:
Risk of bias: based on selection, performance, detection, attrition, reporting and other biases.12
Cochrane GRADE table: began with the highest quality rating for randomised trial evidence or depending on clinical judgements, non-randomised trial evidence, with downgrades to moderate, low or very low depending on the presence of limitations in design, indirectness of evidence, inconsistency of results, imprecision of results, and high probability of publication bias.12
Data synthesis and analysis
Outcomes were stratified by treatment periods and dosages. Only differences between intervention and placebo groups were included for analysis to control for the placebo effect. Where appropriate, quantitative analysis was performed for outcomes with two or more studies and with low heterogeneity using a random-effects analysis model. Review Manager V.5.3 was used to calculate the I² statistics for heterogeneity, overall effect and standard mean difference (SMD), as well as to construct forest plots. Effect sizes were measured using SMDs with 95% CIs to allow for standardisation of different unit measurements per outcome. A priori sensitivity analyses for baseline participant training status (untrained vs prior training) would be carried out for analyses with moderate heterogeneity as per the Cochrane interpretation guide (40%<I²<60%).11 When quantitative analysis was not performed (eg, substantial heterogeneity with I² >60%),11 a qualitative analysis for combining evidence was performed. At least two independent reviewers analysed the data. Disagreements were resolved by repeated review and consensus.
Results
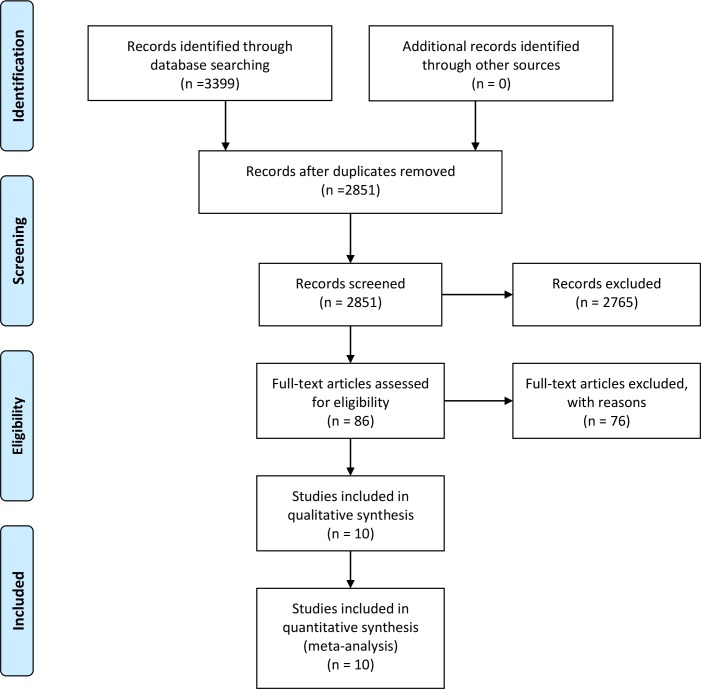
Out of the 3399 articles retrieved from EMBASE, MEDLINE and SPORTDiscus databases, 548 duplicates were removed. The 2851 remaining articles proceeded through title and abstract screening, after which 86 were selected for full-text screening. Of these 86, only 10 met all inclusion and exclusion criteria and were used in this systematic review (table 1). Hand searches of reference lists of all included studies did not yield any additional articles. All included studies were used in the quantitative synthesis. Figure 1 illustrates the selection of studies depicted in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
Table 1.
Summary of included studies
| Study | Design | Sample size | Participants | Main interventions | Outcomes measured |
| Annaheim et al13 | Double-blind, RCT with 4 arms: receiving high-dose rHuEPO, medium-dose rHuEPO, low-dose rHuEPO and placebo. | 40 total; high-dose rHuEPO (n=10), medium-dose rHuEPO (n=10), low-dose rHuEPO (n=10), placebo (n=10). | Healthy, endurance-trained men; none of the participants suffered from cardiovascular and ventilatory diseases or allergies; ferritin (30–400 µg/L), hct% <50%. | rHuEPO or placebo (0.9% NaCl) injection every 2–3 days for 4 weeks; dosages were 10 000 IU, 5000 IU and 2500 IU for high-dose, medium-dose and low-dose groups, respectively; all subjects were given intravenously 100 mg of Fe-III-saccharate. | Red cell parameters (hct%, Hb), plasma volume, VO2 max, time limit of constant-load test, maximum power, rate of perceived exertion, time to exhaustion. |
| Birkeland et al18 | Double-blind, RCT with 2 arms: rHuEPO and placebo; matched for type of sport and training level. | 20 total; rHuEPO (n=10), placebo (n=10). | Healthy, well-trained male athletes (cycling, orienteering, running, triathlon, swimming, cross-country skiing) with normal haematological parameters, no history of thromboembolic disease and no risk factors for cardiovascular disease. | rHuEPO (5000 U) or placebo (1 mL NaCl, 9 g/L) subcutaneous injection three times weekly for 30 days (4 weeks) or until hct% greater than or equal to 50%; all subjects were given iron supplementation with 270 mg/day Fe2+ in liquid formula. | Hct%, Hb, VO2 max, serum EPO concentration, serum ferritin concentration, time course to soluble transferrin receptor levels. |
| Caillaud et al24 | RCT with 2 arms: rHuEPO and placebo. | 12 total; rHuEPO (n=6), placebo (n=6). | Healthy aerobically trained men with no comorbidities. | rHuEPO (50 U/kg) or placebo (0.9% NaCl) subcutaneous injection three times weekly for 4 weeks; all subjects given appropriate oral dose of iron sulfate, vitamin B9 and vitamin B12. | Hct%, Hb, respiratory exchange ratio, substrate utilisation, body weight, % fat, max power, VO2 max, submaximal VO2, training load. |
| Connes et al20 | Double-blind, RCT with 2 arms: rHuEPO and placebo. | 16 total; rHuEPO (n=9), placebo (n=7). | Endurance-trained men (cyclists, runners and triathletes). | rHuEPO (50 IU/kg) or placebo (0.9% NaCl) subcutaneous injection three times weekly for 4 weeks; oral dose of 200 mg iron sulfate during 4 weeks. | Hct%, Hb, VO2 max and POmax via incremental maximal exercise test vs submaximal exercise. |
| Heuberger et al6 | Double-blind, RCT with 2 arms: rHuEPO and placebo. | 49 total, 2 dropouts. 48 participants included in the analyses: rHuEPO (n=24), placebo (n=24). | Healthy male cyclists, age 18–50. | rHuEPO or placebo (0.9% NaCl) subcutaneous injection once weekly for 8 weeks; rHuEPO group received 5000 IU per injection for the first 4 rHuEPO injections; if Hb was below the target range, then dose increased to 6000 IU, 8000 IU or 10 000 IU; if it was in target range, dose decreased to 2000 IU, and if it was above target range placebo was administered; all subjects were given open-label, daily oral doses of 200 mg ferrous fumarate and 50 mg ascorbic acid. | Hct%, Hb, VO2 max, POmax, lactate threshold VO2, lactate threshold power, ventilatory threshold, gross efficiency, heart rate, tidal volume, respiratory frequency, respiratory minute ventilation, respiratory quotient, average power output, average VO2, average heart rate, submaximal lactate, cycling economy, treatment-emergent adverse events. |
| Ninot et al21 | Double-blind, RCT with 3 arms: rHuEPO, placebo and no treatment. | 17 total; rHuEPO (n=6), placebo (n=5), no treatment (n=6). | Endurance-trained men, same middle-class socioeconomic background, no psychiatric disorder or acute medical illness, no negative life events occurring over a 3-month period, no comorbidities. | rHuEPO or placebo (0.9% NaCl) subcutaneous injection three times weekly for 6 weeks; rHuEPO group was given 50 U/kg three times weekly for the first 4 weeks followed by 20 U/kg three times weekly for the remaining 2 weeks; treatment was stopped if hct% ≥50%; all subjects were given a daily oral dose of 200 mg of iron sulfate during the 6 weeks of injections. | Hct%; Hb; psychological measures of global self-esteem, physical self-worth and 4 physical subdomains (physical condition, sport competence, physical strength and attractive body) using the Physical Self Inventory-6 Scale; VO2 max, training load. |
| Rasmussen et al22 | Double-blind, crossover RCT with 2 arms: rHuEPO and placebo. | 15 total; 3 days group (n=7), 3 months group (n=8); however, participants from the 3 months group were excluded due to the lack of placebo. |
Healthy men, age 18–34. | rHuEPO (30 000 IU) or placebo (saline) subcutaneous injection once daily for three consecutive days; washout period of 3 months. | Hct%, VO2 max, respiratory frequency, tidal volume, EPO assay, blood glucose and lactate content, voluntary activation, RPE, visual perception, visual memory, selective attention, mental concentration, visual scanning abilities, perceptual speed. |
| Sieljacks et al15 | Single-blind, RCT with 4 arms: sedentary placebo, sedentary rHuEPO, training placebo and training rHuEPO. | 38 total, 2 dropouts; sedentary placebo (n=9), training placebo (n=10), sedentary rHuEPO (n=9), training rHuEPO (n=8). | Healthy, non-smoking, untrained men, age 18–35; BMI: 18–29 kg/m2, BP: <135/85 mm Hg, hct%: <45%, VO2 max:<50 mL/kg/min. | rHuEPO or placebo (isotonic saline) subcutaneous injection once weekly for 10 weeks; dosing variable; all subjects were given 100 mg of oral iron daily. | Hct%, Hb, reticulocytes, mean cell volume, VO2 max, wattmax, total training workload and estimated energy consumption. |
| Thomsen et al19 | Single-blind, controlled clinical trial with 2 arms: rHuEPO and placebo. | 16 total; rHuEPO (n=8), placebo (n=8). | Healthy men of reasonable age, weight and height. | rHuEPO (5000 IU) or placebo (saline) subcutaneous injection every other day for the first 2 weeks, 3 injections on three consecutive days for the third week, 1 injection weekly for weeks 4–13; all subjects were given 100 mg iron daily for 2 weeks prior and throughout treatment. | Hct%, Hb, VO2 max, time to exhaustion, VO2, VCO2, SaO2 (arterial oxygen saturation). |
| Wilkerson et al23 | Double-blind, RCT with 2 arms: experimental and placebo. | 15 total; rHuEPO (n=8), placebo (n=7). | Healthy men, age 25±4 years, recreationally active, no hypertension or hct% baseline. | rHuEPO (150 IU/kg) or placebo (saline) subcutaneous injection once weekly for 4 weeks; all subjects given appropriate iron tablets and vitamin C tablets. | Hct%, Hb, blood pressure, VO2 consumption rate, pulmonary gas exchange, ventilation, lactate levels, pulmonary VO2, heart rate, peak VO2, time to exhaustion, peak power output. |
BMI, body mass index; BP, blood pressure; EPO, erythropoietin; Hb, haemoglobin concentration; hct%, haematocrit percentage; IU/kg, IU per kilogram body mass; POmax, maximal power output; RCT, randomised controlled trial; rHuEPO, recombinant human erythropoietin; RPE, Borg Scale Rating of Perceived Exhaustion; VO2 max, maximal oxygen consumption.
Figure 1.
PRISMA flow diagram depicting 2851 studies screened following the removal of search duplicates. Eighty-six studies underwent full-text screening, of which 10 eligible studies were included in both qualitative and quantitative syntheses. Adapted from Moher et al.32 PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Notably, full-text screening found three studies (Sieljacks et al,15 Larsen et al16 and Christensen et al17) that used the same cohort. Only the study by Sieljacks et al15 was included in our analyses as it was the latest study of this cohort and contained all the relevant outcomes from the previous studies.15 Meanwhile, two other studies did not explicitly report numerical data, so their data were extracted through graph estimations.18 19
From the 10 included studies, the following were determined to be relevant outcomes: Borg Scale Rating of Perceived Exhaustion (RPE), TTE, training load, total work, POmax and submaximal PO, Mont Ventoux race times, haematological parameters (ie, haematocrit percentage (hct%), haemoglobin concentration (Hb)), and pulmonary measures (ie, pulmonary ventilation, VO2 max, submaximal VO2).6 13 15 18–24 Outcomes were further stratified by treatment periods and dosages. Where appropriate, quantitative analysis was performed (figure 2.1–2.4).
Figure 2.
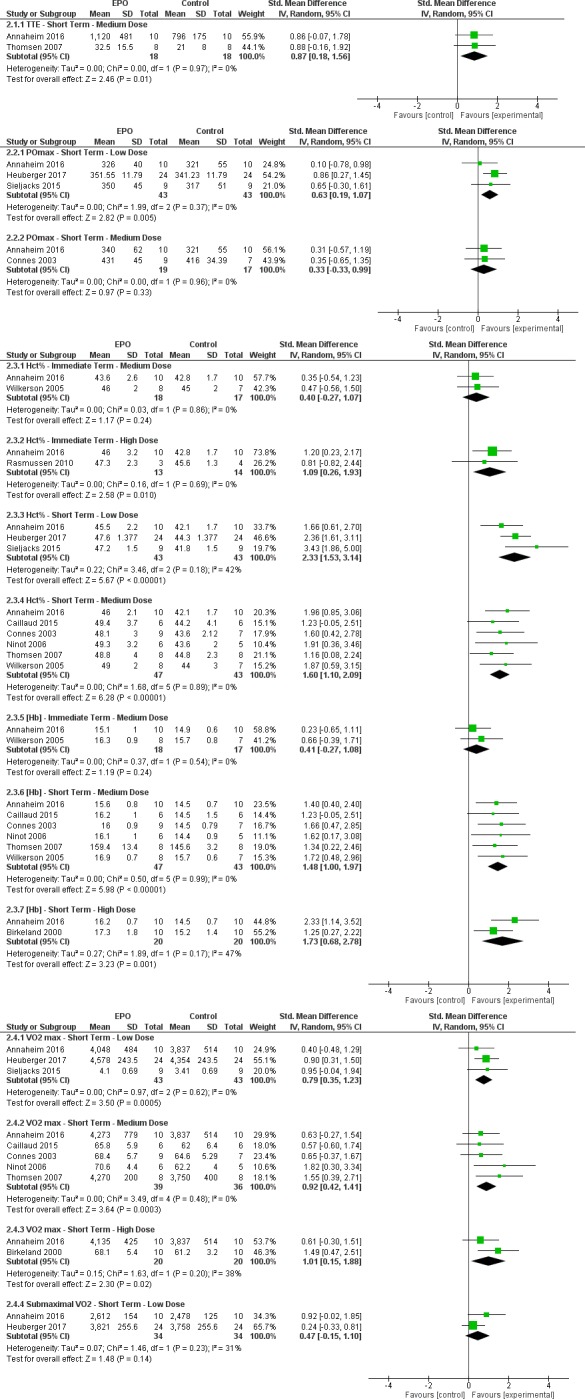
Summary of all meta-analyses completed for relevant outcomes. The black diamond represents the overall effect size of each meta-analysis. The right side of the forest plot favours experimental (rHuEPO), while the left side favours control (placebo). EPO, erythropoietin; Hb, haemoglobin concentration; hct%, haematocrit percentage; POmax, maximal power output; rHuEPO, recombinant human erythropoietin; TTE, time to exhaustion; VO2 max, maximal oxygen consumption; IV, inverse variance.
Borg Scale Rating of Perceived Exhaustion
There are two studies in this category.13 22 The first study measured RPE at the highest power output during an incremental cycling test as well as at the time of failure during a constant-load test (85% of maximum power).13 The latter study measured RPE at 5 min intervals of 20 min submaximal and maximal tests; however, it was unclear which of the two tests conditions was significant.22
Immediate-term outcome
One study reported no significant difference in RPE between the placebo group and the groups that received low, medium or high doses of rHuEPO under both testing conditions.13 In contrast, another study reported that a high dose of rHuEPO increased RPE compared with the placebo group (p<0.05).22
Short-term outcome
One study reported no significant difference in RPE between the placebo group and the groups that received low, medium or high doses of rHuEPO under both testing conditions.13
Time to exhaustion
There are three studies in this category.13 18 19 Outcomes were attained through submaximal testing for two studies13 19 and maximal testing for the remaining study.18
Immediate-term outcome
One study reported no significant difference between the placebo group and the groups that received low, medium or high doses of rHuEPO.13
Short-term outcome
Low dose
One study reported no significant difference in TTE between the placebo group and the rHuEPO group.13
Medium dose
One study reported no significant difference between the placebo group and the rHuEPO group.13 In contrast, a different study reported that TTE was longer in the rHuEPO group in comparison with the placebo group (p<0.05).19
Heterogeneity between the two studies is low (I2=0%). Test for overall effect: Z=2.46 (p=0.01) (SMD=0.87 (0.18 to 1.56)). The forest plot suggests that medium-dose rHuEPO improves TTE more than placebo in the short term (figure 2.1.1).
High dose
Two studies reported that TTE was longer for the rHuEPO group compared with the placebo group (p<0.05, p=0.04). Heterogeneity between the two studies is substantial (I2=71%); hence, quantitative analysis was not performed.13 18
Training load
There are three studies in this category that measured training load.20 21 24 This outcome was assessed by using the questionnaire of Millet et al25 which asked athletes to report the volume and intensity of their training (in swimming, cycling, running and miscellaneous).
Short-term outcome
All studies reported no significant difference between the placebo group and the medium-dose rHuEPO group.20 21 24
Maximal power output
There are four studies in this category.6 13 15 20 Outcomes were attained through maximal testing for all studies.6 13 15 20
Immediate-term outcome
One study reported no significant difference between the placebo group and the groups receiving either low, medium or high dose of rHuEPO.13
Short-term outcome
Low dose
Two studies reported no significant difference between the placebo group and the rHuEPO group.13 15 In contrast, another study reported that POmax was significantly higher in the rHuEPO group compared with the placebo group (p=0.0040).6
Heterogeneity between the three studies is low (I2=0%). Test for overall effect: Z=2.82 (p=0.005) (SMD=0.63 (0.19–1.07)). The forest plot suggests that low-dose rHuEPO improves POmax more than placebo in the short term (figure 2.2.1).
Medium dose
One study reported no significant difference between the placebo group and the rHuEPO group.13 The other study reported that POmax was significantly higher in the rHuEPO group compared with the placebo group (p<0.05).20
Heterogeneity between the two studies is low (I2=0%). Test for overall effect: Z=0.97 (p=0.33) (SMD=0.33 (−0.33 to 0.99)). The forest plot suggests that medium-dose rHuEPO does not improve POmax more than placebo in the short term (figure 2.2.2).
High dose
One study reported that POmax was significantly higher in the rHuEPO group compared with the placebo group (p<0.01).13
Submaximal power output
There is only one study in this category.6 This outcome was attained through submaximal exercise testing.6
Short-term outcome
This study reported no significant difference between the placebo group and the low-dose rHuEPO group (p=0.086).6
Total work
There is one study in this category.22 This outcome was attained through incremental exercise testing.22
Immediate-term outcome
This study reported no significant difference between the placebo group and the high-dose rHuEPO group.22
Mont Ventoux race time
There is one study in this category.6 This outcome was attained through a timed trial.6
Short-term outcome
This study reported no significant difference between the placebo group and the low-dose rHuEPO group.6
Haematological parameters
Haematocrit percentage
There are 10 studies in this category.6 13 15 18–24
Immediate-term outcome
Three studies examined the immediate-term effect.13 22 23
Low dose
One study reported no significant difference between the placebo group and the rHuEPO group.13
Medium dose
Two studies reported no significant difference between the placebo group and the rHuEPO group.13 23
Heterogeneity between the two studies was low (I²=0%). Test for overall effect: Z=1.17 (p=0.24) (SMD=0.40 (−0.27 to 1.07)). The forest plot suggests that medium-dose rHuEPO does not improve hct% more than placebo in the immediate term (figure 2.3.1).
High dose
One study reported that hct% was significantly higher in the rHuEPO group compared with the placebo group (p<0.01).13 In contrast, another study reported that hct% was similar between the rHuEPO and placebo groups.22
Heterogeneity between the two studies is low (I2=0%). Test for overall effect: Z=2.58 (p=0.01) (SMD=1.09 (0.26–1.93)). The forest plot suggests a high dose of rHuEPO improves hct% more than placebo in the immediate term (figure 2.3.2).
Short-term outcome
Eight of these studies examined the short-term effects.6 13 15 19–21 23 24
Low dose
Three studies reported that hct% was significantly higher in the rHuEPO group compared with the placebo group (p<0.001, p<0.00001, p<0.001).6 13 15
Heterogeneity was moderate (I2=42%). Quantitative analysis was performed, but results should be considered with caution. Test for overall effect: Z=5.67 (p<0.00001) (SMD=2.33 (1.53–3.14)). The forest plot suggests low-dose rHuEPO improves hct% more than placebo in the short term (figure 2.3.3).
Medium dose
Six studies reported that hct% was significantly higher in rHuEPO group compared with the placebo group (p<0.001, p<0.01, p<0.01, p<0.05, p<0.05, p<0.01).13 19–21 23 24
Heterogeneity between the studies was low (I2=0%). Test for overall effect: Z=6.28 (p<0.00001) (SMD=1.60 (1.10–2.09)). The forest plot suggests medium-dose rHuEPO improves hct% more than placebo in the short term (figure 2.3.4).
High dose
One study reported that hct% was significantly higher in rHuEPO group compared with the placebo group (p<0.001).13
Haemoglobin concentration
There are eight studies in this category.6 13 15 19–21 23 24
Immediate-term outcome
There are two studies in this category.13 23
Low dose
One study reported that Hb was significantly higher in the rHuEPO group compared with the placebo group (p<0.01).13
Medium dose
Two studies reported no significant difference between the placebo group and the rHuEPO group.13 23
Heterogeneity between the two groups is low (I²=0%). Test for overall effect: Z=1.19 (p=0.24) (SMD=0.41 (−0.27 to 1.08)). The forest plot suggests that medium-dose rHuEPO does not improve Hb more than placebo in the immediate term (figure 2.3.5).
High dose
One study reported that Hb was significantly higher in the rHuEPO group compared with the placebo group (p<0.05).13
Short-term outcome
Low dose
Three studies reported that Hb was significantly higher in the rHuEPO group compared with the placebo group (p<0.01, p<0.0001, p<0.001).6 13 15
Heterogeneity is substantial (I²=62%); hence, quantitative analysis was not performed.
Medium dose
Six studies reported that Hb was significantly higher in the rHuEPO group compared with the placebo group (p<0.01, p<0.01, p<0.01, p<0.05, p<0.05, p<0.01).13 19–21 23 24
Heterogeneity was low (I2=0%). Test for overall effect: Z=5.98 (p<0.00001) (SMD=1.48 (1.00–1.97)). The forest plot suggests medium-dose rHuEPO improves Hb more than placebo in the short term (figure 2.3.6).
High dose
Two studies reported that Hb was significantly higher in the rHuEPO group compared with the placebo group (p<0.001, p<0.05).13 18
Heterogeneity is moderate (I²=47%). Quantitative analysis was performed, but results must be considered with caution. Test for overall effect: Z=3.23 (p=0.001) (SMD=1.73 (0.68–2.78)). The forest plot suggests high-dose rHuEPO improves Hb more than the placebo in the short term (figure 2.3.7).
Pulmonary measures
Pulmonary ventilation
There is only one study in this category.22 Outcomes were attained through submaximal and maximal testing.22
Immediate-term outcome
This study reported that pulmonary ventilation was significantly higher in the high-dose rHuEPO group compared with the placebo group.22 This difference was observed in settings of submaximal low-intensity exercise in normoxia (p<0.01) and submaximal low-intensity exercise in hypoxia (p<0.001).22
However, no significant between-group difference was observed when pulmonary ventilation was measured during maximal high-intensity exercise in normoxia.13
Maximal oxygen consumption
There are eight studies in this category (VO2 max).6 13 15 18–21 24 Outcomes were attained through maximal testing for all studies.6 13 15 18–21 24
Immediate-term outcome
One study reported that VO2 max was significantly higher in the groups receiving low, medium or high doses of rHuEPO compared with the placebo group (p<0.05, p<0.01, p<0.05).13
Short-term outcome
Low dose
There are three studies in this category.6 13 15 One study reported no significant difference in VO2 max between the placebo group and the rHuEPO group.13 However, the other two studies reported that VO2 max was significantly higher in the rHuEPO group compared with the placebo group (p=0.0026, p<0.01).6 15
Heterogeneity is low (I2=0). Test for overall effect: Z=3.50 (p=0.0005) (SMD=0.79 (0.35–1.23)). The forest plot suggests low-dose rHuEPO improves VO2 max more than placebo in the short term (figure 2.4.1).
One of these studies also reported that if training level was kept constant (ie, both rHuEPO and placebo group received training over the duration of the observation period), there was no significant difference in VO2 max between the two groups.15
Medium dose
Five studies reported that VO2 max was significantly higher in the rHuEPO group compared with the placebo group (p<0.01, p<0.05, p<0.05, p<0.05, p<0.05).13 19–21 24
Heterogeneity is low (I2=0%). Test for overall effect: Z=3.64 (p=0.0003) (SMD=0.92 (0.42–1.41)). The forest plot suggests medium-dose rHuEPO improves VO2 max more than placebo in the short term (figure 2.4.2).
High dose
Two studies reported that VO2 max was higher in the rHuEPO group compared with the placebo group (p<0.01, p<0.001).13 18
Heterogeneity is low (I2=38%). Test for overall effect: Z=2.30 (p=0.02) (SMD=1.01 (0.15–1.88)). The forest plot suggests high-dose rHuEPO improves VO2 max more than placebo in the short term (figure 2.4.3).
Submaximal oxygen consumption
There are two studies in this category.6 13 Outcomes were attained through submaximal testing for both studies.6 13
Immediate-term outcome
One study reported no significant difference between the placebo group and the groups that received low, medium or high doses of rHuEPO.13
Short-term outcome
Low dose
Two studies reported no significant difference between the placebo group and the rHuEPO group.6 13
Heterogeneity between the two studies is low (I2=31%). Test for overall effect: Z=1.48 (p=0.14) (SMD=0.47 (−0.15 to 1.10)). The forest plot suggests low-dose rHuEPO does not improve submaximal VO2 more than placebo in the short term (figure 2.4.4).
Medium dose
One study reported no significant difference between the placebo group and the rHuEPO group.13
High dose
One study reported no significant difference between the placebo group and the rHuEPO group.13
Discussion
Quantitative analyses revealed moderate-quality evidence that in the immediate term, a high dose of rHuEPO (>13 750 IU/week) significantly increases hct% more than placebo. Additionally, moderate-quality evidence revealed that in the short term, low and high doses (<6875 and >13 750 IU/week) significantly improve VO2 max; low doses (<6875 IU/week) increase hct%; and low doses (<6875 IU/week) do not significantly increase submaximal VO2 (table 2). Furthermore, there was low-quality evidence that in the immediate term low doses significantly increase Hb. Additionally, low-quality evidence revealed that in the short term low doses significantly improve POmax; medium doses (6875–13 750 IU/week) do not significantly increase POmax, but significantly improve TTE, hct%, Hb and VO2 max; and high doses (>13 750 IU/week) increase Hb (table 2). Moreover, quantitative and qualitative analyses revealed that most outcomes measured under submaximal conditions appeared similar between groups (table 2).
Table 2.
Summary of findings
| Recombinant human erythropoietin vs placebo | ||||||
| Patient or population: male and female patients with no comorbidities between the age of 18 and 65. Setting: track or gym. Intervention: rHuEPO administered at low, medium or high doses. Comparison: placebo. | ||||||
| Outcomes* | Duration | Intervention† | Participants (n) (studies)‡ |
Quality of the evidence§¶ (GRADE) |
||
| rHuEPO, low-dose (<6875 IU/week) | rHuEPO, medium-dose (6876–13 750 IU/week) | rHuEPO, high-dose (>13 750 IU/week) | ||||
| Borg Scale Rating of Perceived Exhaustion | Immediate | No statistically significant improvement (NS) in 1 study, n=20 (13) | NS, n=20 (13) | Significant improvement in 1 study (p<0.05; n=7) (22); NS, n=20 (13) | ||
| Short | NS, n=20 (13) | NS, n=20 (13) | NS, n=20 (13) | |||
| Time to exhaustion | Immediate | NS, n=20 (13) | NS, n=20 (13) | NS, n=20 (13) | ||
| Short | NS, n=20 (13) | Heterogeneity: I2=0% (13 19) Test for overall effect: Z=2.46 (p=0.01) SMD (95% CI): 0.87 (0.18 to 1.56) |
Significant improvement using a submaximal exercise test in 2 studies (p<0.05, p=0.04; n=40) (13 18) |
Medium-dose 36 (2) (13 19) |
Medium-dose ⊕⊕○○ Low Limitations 0 Imprecision −1** Inconsistency −1†† Indirectness 0 Other 0 |
|
| Training load | Short | N/A | NS, n=39 (20 21 24) | N/A | ||
| Maximum power output | Immediate | NS, n=20 (13) | NS, n=20 (13) | NS, n=20 (13) | ||
| Short | Heterogeneity: I2=0% (6 13 15) Test for overall effect: Z=2.82 (p=0.005) SMD (95% CI): 0.63 (0.19 to 1.07) |
Heterogeneity: I2=0% (13 20) Test for overall effect: Z=0.97 (p=0.33) SMD (95% CI): 0.33 (−0.33 to 0.99) |
Significant improvement in 1 study (p<0.01; n=20) (13) |
Low-dose 86 (3) (6 13 15) |
Low-dose ⊕⊕○○ Low Limitations 0 Imprecision −1** Inconsistency −1†† Indirectness 0 Other 0 |
|
|
Medium-dose 36 (2) (13 20) |
Medium-dose ⊕⊕○○ Low Limitations 0 Imprecision −1** Inconsistency −1†† Indirectness 0 Other 0 |
|||||
| Submaximal power output | Short | NS, n=48 (6) | N/A | N/A | ||
| Total work | Immediate | N/A | N/A | NS, n=7 (22) | ||
| Mont Ventoux race time | Short | NS, n=48 (6) | N/A | N/A | ||
| Haematocrit percentage | Immediate | NS, n=20 (13) | Heterogeneity: I2=0% (13 23) Test for overall effect: Z=1.17 (p=0.24) SMD (95% CI): 0.40 (−0.27 to 1.07) |
Heterogeneity: I2=0% (13 22) Test for overall effect: Z=2.58 (p=0.010) SMD (95% CI): 1.09 (0.26 to 1.93) |
Medium-dose 35 (2) (13 23) |
Medium-dose ⊕⊕○○ Low Limitations 0 Imprecision −2** Inconsistency 0 Indirectness 0 Other 0 |
|
High-dose 27 (2) (13 22) |
High-dose ⊕⊕⊕○ Moderate Limitations 0 Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|||||
| Short | Heterogeneity: I2=42% (6 13 15) Test for overall effect: Z=5.67 (p<0.00001) SMD (95% CI): 2.33 (1.53 to 3.14) |
Heterogeneity: I2=0% (13 19–21 23 24) Test for overall effect: Z=6.28 (p<0.00001) SMD (95% CI): 1.60 (1.10 to 2.09) |
Significant improvement in 1 study (p<0.001; n=20) (13) |
Low-dose 86 (3) (6 13 15) |
Low-dose ⊕⊕⊕○ Moderate Limitations 0 Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|
|
Medium-dose 90 (6) (13 19–21 23 24) |
Medium-dose ⊕⊕○○ Low Limitations −1‡‡ Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|||||
| Haemoglobin concentration | Immediate | Significant improvement in 1 study (p<0.01; n=20) (13) | Heterogeneity: I2=0% (13 23) Test for overall effect: Z=1.19 (p=0.24) SMD (95% CI): 0.41 (−0.27 to 1.08) |
Significant improvement in 1 study (p<0.05; n=20) (13) |
Medium-dose 35 (2) (13 23) |
Medium-dose ⊕⊕○○ Low Limitations 0 Imprecision-2** Inconsistency 0 Indirectness 0 Other 0 |
| Short | Significant improvements in 3 studies (p<0.01, p<0.0001, p<0.001; n=86) (6 13 15) | Heterogeneity: I2=0% (13 19–21 23 24) Test for overall effect: Z=5.98 (p<0.00001) SMD (95% CI): 1.48 (1.00 to 1.97) |
Heterogeneity: I2=47% (13 18) Test for overall effect: Z=3.23 (p=0.001) SMD (95% CI): 1.73 (0.68 to 2.78) |
Medium-dose 90 (6) (13 19–21 23 24) |
Medium-dose ⊕⊕○○ Low Limitations −1‡‡ Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|
|
High-dose 40 (2) (13 18) |
High-dose ⊕⊕○○ Low Limitations 0 Imprecision −1** Inconsistency −1†† Indirectness 0 Other 0 |
|||||
| Pulmonary ventilation | Immediate | N/A | N/A | Significant improvement using a submaximal exercise test in 1 study (p<0.01; n=7) (22) NS using a maximal exercise test (n=7) (22) |
||
| Absolute maximal oxygen consumption | Immediate | Significant improvement in 1 study (p<0.05; n=20) (13) | Significant improvement in 1 study (p<0.01; n=20) (13) | Significant improvement in 1 study (p<0.05; n=20) (13) | ||
| Short | Heterogeneity: I²=0% (6 13 15) Test for overall effect: Z=3.50 (p=0.0005) SMD (95% CI): 0.79 (0.35 to 1.23) |
Heterogeneity: I2=0%(13 19–21 24) Test for overall effect: Z=3.64 (p=0.0003) SMD (95% CI): 0.92 (0.42 to 1.41) |
Heterogeneity: I2=38% (13 18) Test for overall effect: Z=2.30 (p=0.02) SMD (95% CI): 1.01 (0.15 to 1.88) |
Low-dose 86 (3) (6 13 15) |
Low-dose ⊕⊕⊕○ Moderate Limitations 0 Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|
|
Medium-dose 75 (5) (13 19–21 24) |
Medium-dose ⊕⊕○○ Low Limitations −1‡‡ Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|||||
|
High-dose 40 (2) (13 18) |
High-dose ⊕⊕⊕○ Moderate Limitations 0 Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|||||
| Absolute submaximal oxygen consumption | Immediate | NS, n=20 (13) | NS, n=20 (13) | NS, n=20 (13) | ||
| Short | Heterogeneity: I2=31% (6 13) Test for overall effect: Z=1.48 (p=0.14) SMD (95% CI): 0.47 (−0.15 to 1.10) |
NS, n=20 (13) | NS, n=20 (13) |
Low-dose 68 (2) (6 13) |
Low-dose ⊕⊕⊕○ Moderate Limitations 0 Imprecision −1** Inconsistency 0 Indirectness 0 Other 0 |
|
*Outcomes were stratified by treatment duration (rows) and dosages (columns).
†Qualitative and quantitative findings for outcomes are summarised in the grey boxes.
‡Number of participants and studies for outcomes that underwent quantitative analysis were listed below. For outcomes that underwent qualitative analysis, number of participants and studies were listed in the grey boxes.
§A quality assessment (GRADE) was only given for outcomes that underwent quantitative analysis.
¶GRADE Working Group grades of evidence: high quality (⊕⊕⊕⊕): further research is very unlikely to change our confidence in the estimate of effect; moderate quality (⊕⊕⊕○): further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality (⊕⊕○○): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality (⊕○○○): we are very uncertain about the estimate.
**Small study group or wide CI.
††Heterogeneity of participants, interventions or outcomes.
‡‡Lack of allocation concealment or blinding.
GRADE, Grading of Recommendations Assessment Development and Education; N/A, not available; rHuEPO, recombinant human erythropoietin; SMD, standard mean difference; TTE, time to exhaustion.
It has been argued that submaximal performance testing may be more clinically relevant to athletic competition conditions.6 26 For instance, endurance competitions are performed at a wide range of submaximal intensities rather than strictly at maximal intensity.13 Unfortunately, only 4 of the 10 studies conducted submaximal exercise tests.6 13 19 22 While outcomes measured using submaximal exercise tests were similar between rHuEPO and placebo groups for RPE, TTE, power output, Mont Ventoux race times and VO2, only one meta-analysis for submaximal VO2 could be performed. The results revealed moderate-quality level of evidence that in the short term a low dose of rHuEPO did not improve absolute submaximal VO2.6 13 In contrast, there were only 3 out of 23 outcomes measured under submaximal testing conditions which yielded significant between-group differences.13 19 22 The first study that reported significant differences under submaximal conditions reported that high doses of rHuEPO improved TTE in the short term, and a second study found similar results but for medium doses.13 19 A third study reported that pulmonary ventilation was significantly higher in the high-dose rHuEPO group compared with the placebo group.22 However, the first and third studies possessed a limited sample size and used a relatively higher dose compared with any comparisons that generated non-significant submaximal testing results.13 22 The second study possessed a limited sample size and suffered from a high risk of bias.19 The first and second studies also used tests that required participants to pedal until failure, which is similar to a maximal exercise test; therefore, the submaximal test used may be a poor representative of competition-like settings.6 19 26 Despite this limitation, outcomes measured during maximal exercise intensities are still important and should not be disregarded when assessing performance. Endurance sports still involve instances of maximal effort, for instance sprinting to the finish line of a long race. Due to the limited number of studies investigating the ergogenic effects of rHuEPO during submaximal versus maximal exercise intensities, there remains uncertainty and more original research needs to be conducted.
Haematological parameters, hct% and Hb generally increased with rHuEPO treatment as expected, likely attributed to increased haemoconcentration.27 Meanwhile, improvements in VO2 max at similar treatment durations and dosages were also observed. These findings confirm previous notions that improvements in VO2 max are attributed to increases in Hb.28 Meanwhile, some studies suggest that total red cell mass may be a more relevant parameter given its closer correlation with VO2 max.28 29 However, no conclusions can be drawn as there were no published data available.
Moreover, there does not appear to be a dose-dependent relationship between rHuEPO and athletic performance, and the specific dosage threshold for the ergogenic effects of rHuEPO remains unclear. Additionally, only 6 of the 10 studies in this review recruited participants who were trained athletes.6 13 18 20 21 24 Two studies used untrained participants.15 23 Meanwhile, two studies did not report training status of the participants.19 22 The effects of rHuEPO may differ in endurance trained athletes and untrained individuals.19 22 A priori sensitivity analyses for training status were not conducted given the limited number of studies per analysis. Hence, more studies with larger sample sizes are needed in order to better elucidate these relationships.
With regard to policy implications, it is important to acknowledge that a substance’s potential to enhance performance is only one of the three criteria that WADA considers when determining its inclusion in the prohibited list.30 Doping policymakers must also examine any health risks to athletes and potential violations to the ‘spirit of sport’. Therefore, even if one of these dimensions is inconclusive, prohibition may still be justifiable.
Limitations of this review include participant demographics, heterogeneity, risk of bias, dissimilar baseline values and limitations in our search. First, the effects of rHuEPO on athletic performance were solely investigated in male participants, thus limiting generalisability. Next, heterogeneity between the included trials appeared at several levels, including study methodology, sample size, participant training status, duration of the study, intervention and outcomes measured. As a result of heterogeneity, stratification based on treatment duration and dosages yielded very few studies in each category. With so few studies in each category and small sample sizes in each study, the real estimate of the effect may be less confident. Moreover, all included studies except two were assessed to have a low risk of bias (table 3).19 24 The first exception did not declare random sequence generation, and the rHuEPO group was aware that they were receiving rHuEPO.19 The second exception was completely unblinded.24 Hence, both studies were downgraded to having a high risk of bias. Finally, the meta-analyses were completed using a comparison of final measurements between rHuEPO and placebo groups. Despite how all our analyses included randomised controlled trials, baseline values between intervention and control groups may not have been truly balanced given limited sample sizes. It was not possible to make any adjustments because correlation coefficients between pretreatment and post-treatment values were unknown, and independence could not be assumed given the effects of time, training and test–retest learning.31 Furthermore, despite the aid of two librarians, some relevant studies may still have been missed in this review as only literature published in the English language was included. Finally, experts, including several authors of included and excluded studies, were contacted by email for guidance and missing data. These authors either did not respond or no longer had access to their original data and were unable to provide additional help. This might have had an effect on the grouping of our studies and the results in each of the categories. Overall, results should be interpreted with caution due to heterogeneity among trials and inclusion of only male participants.
Table 3.
Risk of bias
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding (performance bias and detection bias) for all outcomes— patients? | Blinding (performance bias and detection bias) for all outcomes— providers? | Blinding (performance bias and detection bias) for all outcomes—outcome assessors? | Incomplete outcome data (attrition bias) for all outcomes— dropouts? | Intention-to-treat analysis (attrition bias) for all outcomes |
Selective reporting (reporting bias) | Overall impression | |
| Annaheim et al13 | Low risk. ‘Subjects were randomised’. |
Unclear risk. Not addressed. |
Low risk. ‘double blind’. |
Low risk. ‘researchers involved in the assessment of blood volume compartments and exercise testing were blinded’. |
Low risk. | Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Birkeland et al18 | Low risk. ‘randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘double blind’. |
Low risk. ‘double blind’. |
Low risk. ‘blinded to technicians and investigators engaged in blood sampling, sample analyses, and exercise testing’. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Caillaud et al24 | Low risk. ‘Randomly’. |
Unclear risk. Not addressed. |
Unclear risk. Not addressed. |
Unclear risk. Not addressed. |
Unclear risk. Not addressed. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
High risk. |
| Connes et al20 | Low risk. ‘randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘double blind’. |
Low risk. ‘double blind’. |
Unclear risk. Not addressed. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Heuberger et al6 | Low risk. ‘randomly assigned’. |
Low risk. ‘randomization code’ was generated by a statistician who was not involved in the execution of the study. |
Low risk. ‘double blind’. |
Low risk. ‘double blind’. |
Unclear risk. Not addressed. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Ninot et al21 | Low risk. ‘randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘double blind’. |
Low risk. ‘double blind’. |
Low risk. ‘blinded to the technicians and investigators engaged in blood sampling, sample analysis and exercise testing’. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Rasmussen et al22 | Low risk. ‘randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘double blind’. |
Low risk. ‘double blind’. |
Unclear risk. Not addressed. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Sieljacks et al15 | Low risk. ‘randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘single blind’. |
Low risk. Not blinded but not likely to affect outcomes. |
Unclear risk. Not addressed. |
Low risk. 2 excluded, addressed and justified. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
| Thomsen et al19 | Unclear risk. Not addressed. |
Unclear risk. Not addressed. |
High risk. ‘single blind’ for placebo group only, rHuEPO group was ‘aware’. |
High risk. Providers were not blinded. |
Unclear risk. Not addressed. |
Low risk. No dropouts. |
Low risk. | Low risk. All prespecified outcomes reported. |
High risk. |
| Wilkerson et al23 | Low risk. ‘Randomly assigned’. |
Unclear risk. Not addressed. |
Low risk. ‘Subjects … were blind to the group assignment’. |
Unclear risk. Not addressed. |
Low risk. ‘Investigators involved in the administration of the exercise tests and the subsequent data analysis were blind to the group assignment’. |
Low risk. ‘One subject in the control group sustained an injury unrelated to the study and was unable to complete the post-intervention tests’. |
Low risk. | Low risk. All prespecified outcomes reported. |
Low risk. |
rHuEPO, recombinant human erythropoietin.
Conclusion
There is low-to-moderate evidence suggesting that low, medium and high doses of rHuEPO may be more beneficial than placebo in enhancing athletic performance. These ergogenic effects, however, are almost exclusively seen during maximal exercise intensities. Athletic performance mostly appears similar between placebo and intervention groups during submaximal exercise conditions, which may be more relevant to performance in sporting competitions, especially in endurance sports.
As a result of its prohibited and contentious nature, there is a lack of current research on the effects of rHuEPO in professionally trained athletes in conditions that mirror actual competition. Therefore, there is a need for large, high-quality randomised controlled trials to determine the exact role of rHuEPO in athletic performance.
Acknowledgments
We thank Rachel Coubon and her team at the National Pain Centre at McMaster University for her work on the search strategy. We also thank Timothy Kwan and Abhiti Kuhad for their contributions to study selection and data extraction.
Footnotes
Contributors: KVT and DD contributed to design, data abstraction, study appraisal, data analysis, manuscript drafting and approval of the final manuscript. KJQC contributed to data abstraction, study appraisal, data analysis, manuscript drafting and approval of the final manuscript. LH contributed to study appraisal, data analysis, manuscript drafting and approval of the final manuscript. OG contributed to data abstraction, manuscript drafting and approval of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Research ethics approval was not obtained because this study did not directly involve human participants. There was no personal and/or medical information about an identifiable living individual in our study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.de Bruijn H, Groenleer M, van Ruijven T. The dynamics of doping: Lance Armstrong, the United States anti-doping agency and the regulatory governance of professional cycling. Regulation & Governance 2016;10:284–97. 10.1111/rego.12085 [DOI] [Google Scholar]
- 2.Albergotti R, O’Connell V. Wheelmen: Lance Armstrong, the tour de France, and the greatest sports conspiracy ever. New York: Gotham Books, 2014. [Google Scholar]
- 3.Robinson N, Giraud S, Saudan C, et al. Erythropoietin and blood doping. Br J Sports Med 2006;40 Suppl 1:i30–4. 10.1136/bjsm.2006.027532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souma T, Suzuki N, Yamamoto M. Renal erythropoietin-producing cells in health and disease. Front Physiol 2015;6:167 10.3389/fphys.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuberger JAAC, Cohen Tervaert JM, Schepers FML, et al. Erythropoietin doping in cycling: lack of evidence for efficacy and a negative risk-benefit. Br J Clin Pharmacol 2013;75:1406–21. 10.1111/bcp.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuberger JAAC, Rotmans JI, Gal P, et al. Effects of erythropoietin on cycling performance of well trained cyclists: a double-blind, randomised, placebo-controlled trial. Lancet Haematol 2017;4:e374–86. 10.1016/S2352-3026(17)30105-9 [DOI] [PubMed] [Google Scholar]
- 7.Lodewijkx HF, Brouwer B, Kuipers H, et al. Overestimated effect of EPO administration on aerobic exercise capacity: a meta–analysis. Am J Sports Sci Med 2013;1:17–27. [Google Scholar]
- 8.Sgrò P, Sansone M, Sansone A, et al. Effects of erythropoietin abuse on exercise performance. Phys Sportsmed 2018;46:105–15. 10.1080/00913847.2018.1402663 [DOI] [PubMed] [Google Scholar]
- 9.Bird SR, Goebel C, Burke LM, et al. Doping in sport and exercise: anabolic, ergogenic, health and clinical issues. Ann Clin Biochem 2016;53:196–221. 10.1177/0004563215609952 [DOI] [PubMed] [Google Scholar]
- 10.Heuberger J. The clinical pharmacology of performance enhancement and doping detection in sports [Doctoral dissertation. Leiden, Netherlands: Leiden University, 2019. [Google Scholar]
- 11.Higgins JP, Green S. Cochrane Handbook for systematic reviews of interventions. John Wiley & Sons, 2011. [Google Scholar]
- 12.Brozek J, Guyatt G, Oxman A. Grade Handbook for grading quality of evidence and strength of recommendations, 2013. [Google Scholar]
- 13.Annaheim S, Jacob M, Krafft A, et al. RhEPO improves time to exhaustion by non-hematopoietic factors in humans. Eur J Appl Physiol 2016;116:623–33. 10.1007/s00421-015-3322-6 [DOI] [PubMed] [Google Scholar]
- 14.Deicher R, Hörl WH. Differentiating factors between erythropoiesis-stimulating agents. Drugs 2004;64:499–509. 10.2165/00003495-200464050-00004 [DOI] [PubMed] [Google Scholar]
- 15.Sieljacks P, Thams L, Nellemann B, et al. Comparative effects of aerobic training and erythropoietin on oxygen uptake in untrained humans. J Strength Cond Res 2016;30:2307–17. 10.1519/JSC.0000000000001314 [DOI] [PubMed] [Google Scholar]
- 16.Larsen MS, Vissing K, Thams L, et al. Erythropoietin administration alone or in combination with endurance training affects neither skeletal muscle morphology nor angiogenesis in healthy young men. Exp Physiol 2014;99:1409–20. 10.1113/expphysiol.2014.080606 [DOI] [PubMed] [Google Scholar]
- 17.Christensen B, Nellemann B, Larsen MS, et al. Whole body metabolic effects of prolonged endurance training in combination with erythropoietin treatment in humans: a randomized placebo controlled trial. Am J Physiol Endocrinol Metab 2013;305:E879–89. 10.1152/ajpendo.00269.2013 [DOI] [PubMed] [Google Scholar]
- 18.Birkeland KI, Stray-Gundersen J, Hemmersbach P, et al. Effect of rhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc 2000;32:1238–43. 10.1097/00005768-200007000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Thomsen JJ, Rentsch RL, Robach P, et al. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol 2007;101:481–6. 10.1007/s00421-007-0522-8 [DOI] [PubMed] [Google Scholar]
- 20.Connes P, Perrey S, Varray A, et al. Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch 2003;447:231–8. 10.1007/s00424-003-1174-0 [DOI] [PubMed] [Google Scholar]
- 21.Ninot G, Connes P, Caillaud C. Effects of recombinant human erythropoietin injections on physical self in endurance athletes. J Sports Sci 2006;24:383–91. 10.1080/02640410500131340 [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen P, Foged EM, Krogh-Madsen R, et al. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol 2010;109:476–83. 10.1152/japplphysiol.00234.2010 [DOI] [PubMed] [Google Scholar]
- 23.Wilkerson DP, Rittweger J, Berger NJA, et al. Influence of recombinant human erythropoietin treatment on pulmonary O 2 uptake kinetics during exercise in humans. J Physiol 2005;568:639–52. 10.1113/jphysiol.2005.089920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caillaud C, Connes P, Ben Saad H, et al. Erythropoietin enhances whole body lipid oxidation during prolonged exercise in humans. J Physiol Biochem 2015;71:9–16. 10.1007/s13105-014-0374-8 [DOI] [PubMed] [Google Scholar]
- 25.Millet GP, Candau RB, Barbier B, et al. Modelling the transfers of training effects on performance in elite triathletes. Int J Sports Med 2002;23:55–63. 10.1055/s-2002-19276 [DOI] [PubMed] [Google Scholar]
- 26.Noakes TD. Testing for maximum oxygen consumption has produced a brainless model of human exercise performance. BMJ 2008;42:551–5. [DOI] [PubMed] [Google Scholar]
- 27.Lundby C, Thomsen JJ, Boushel R, et al. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol 2007;578:309–14. 10.1113/jphysiol.2006.122689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt W, Prommer N. Impact of alterations in total hemoglobin mass on VO 2max. Exerc Sport Sci Rev 2010;38:68–75. 10.1097/JES.0b013e3181d4957a [DOI] [PubMed] [Google Scholar]
- 29.Kanstrup IL, Ekblom B. Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med Sci Sports Exerc 1984;16:256–62. 10.1249/00005768-198406000-00010 [DOI] [PubMed] [Google Scholar]
- 30.World Anti-Doping Agency Prohibited List Q&A. Available: https://www.wada-ama.org/en/questions-answers/prohibited-list-qa [Accessed 20 Jul 2019].
- 31.Cuijpers P, Weitz E, Cristea IA, et al. Pre-Post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci 2017;26:364–8. 10.1017/S2045796016000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2019-000716supp001.pdf (106.6KB, pdf)
bmjsem-2019-000716supp002.pdf (142.3KB, pdf)