Abstract
Purpose
Antidepressant consumption has risen in recent years, driven by longer treatment duration. The objective of this study was to measure the prevalence of antidepressant long-term and chronic use in the Bologna area, Italy, and to identify their main determinants.
Materials and Methods
We conducted a retrospective claims-based cohort study by using the Bologna Local Health Authority data. A cohort of 18,307 incident users of antidepressant drugs in 2013 was selected, and subjects were followed for three years. A long-term utilization was defined as having at least one prescription claimed during each year of follow-up, while chronic utilization was defined as claiming at least 180 defined daily doses per year. Factors associated with chronic and long-term use were identified by univariate and multivariate logistic regressions.
Results
In our cohort, 5448 (29.8%) and 1817 (9.9%) subjects were dispensed antidepressants for a long-term course and in a chronically way, respectively. Older age, antidepressant polytherapy, polypharmacy, and being prescribed the first antidepressant by a hospital physician were all factors independently associated with chronic and long-term prescriptions of antidepressant drugs. Results were reported separately for men and women.
Conclusion
Antidepressant long-term and chronic prescriptions are common in the Bologna area. Because longer treatment should be clinically motivated, these results strongly prompt the need to evaluate the actual relevance, as they may indicate potentially inappropriate prescription patterns.
Keywords: antidepressants, depression, anxiety, anxiety disorders, primary care, pharmacotherapy, adherence, epidemiology, pharmacoepidemiology, treatment
Introduction
Antidepressant drugs are widely used, especially in developed countries, and their use has increased over the last few decades.1–8 The higher number of prescriptions is associated with the increasing number of subjects being treated with these medications in primary care, along with the increased duration of treatment.7,9 Despite the fact that antidepressant drugs are being prescribed for different conditions, including neurological and rheumatoid diseases, as well as some off-label uses,10,11 the major indications are depressive and anxiety disorders,3 which often co-occur in the same subject,12 accounting for about 75% of all prescriptions.3,5,13 Recent studies show, however, that the incidence of new prescriptions has not increased in the last few years, with the rise in prevalence of antidepressant use mainly due to an increased duration of treatment.7,9,14,15
Pharmacological treatment of depression3,5 is aimed at reducing depressive symptoms and preventing relapse.16 According to the National Institute for Health and Care Excellence (NICE) guidelines, antidepressant drugs should be continued for 6 months after remission of symptoms and should be maintained for a maximum of 2 years.16,17 Maintenance treatments longer than 2 years should be clinically motivated, such as for individuals with recurrent depression, or those with residual mood symptoms, or having a treatment-resistant depression.16
Similar treatment patterns should be used for anxiety disorders,18,19 with a delay of 2 to 8 weeks for symptom relief, and up to 12 weeks for a complete response. Similar to depression, long-term therapy should be applied for continuous improvement of symptoms and a reduced likelihood of relapse, with an optimal treatment duration of 12 to 24 months, even without studies on the efficacy and safety of antidepressant treatment with a follow-up of more than 12 months.18,19
Antidepressant drugs are associated with many adverse effects, and their discontinuation may engender withdrawal symptoms.20,21 According to a recent systematic review, withdrawal symptoms largely vary in incidence rates (from 27% to 86%, with a weighted mean of 56%), duration and severity of symptoms.21 Notwithstanding this variation, which may depend on population studied, type of antidepressant used and duration of treatment, withdrawal symptoms seem very common and may increase the duration of antidepressant use without a clinical exigency. There is a lack of evidence on their efficacy and safety for long-term use22 since the majority of clinical trials conducted had follows-up up to 8 months.23–25 Nevertheless, there is evidence that they are continued for long periods.15,26,27 Moreover, the use of antidepressants is associated with clinically important adverse events in susceptible populations, including weight gain, falls, and relevant cardiovascular events.28,29
To the best of our knowledge, very few studies specifically analyze individual antidepressant drug use with follow-ups longer than two years, as well as factors associated with long-term or chronic use.15,30 This study was conducted due to the paucity of studies on long-term antidepressant prescription patterns. Also, it is important to identify long-term user characteristics to develop greater knowledge on this topic and highlight whether further study is warranted to verify a possible misuse of these drugs.
The objectives of this study were: 1. to estimate the prevalence of long-term antidepressant use, namely longer than two years; 2. to estimate the prevalence of chronic use, namely 180 daily defined doses (DDDs) of antidepressant medications per year for three years; 3. to examine the factors associated with long-term and chronic use in a cohort of subjects newly prescribed an antidepressant treatment in 2013, in Bologna, Italy.
Materials and Methods
Source of Data and Study Population
We conducted a retrospective population-based cohort study using prescription claims from the Bologna Local Health Authority (BLHA) during the period 2012–2016. In Italy, medical services are administered by the publicly funded National Health System (NHS), covering all the citizens and residents in the national territory. The Italian universal public healthcare system offers, thus, its population a universal public drug plan that allows all citizens to have equitable access to prescribed medications. Throughout the Italian territory, the NHS is organized at a regional level into Regional Health Authorities (RHA), and a local level into multiple Local Health Authorities (LHAs), responsible for providing health services to their local residents. RHA databases gather information collected by their LHAs for administrative purposes (reimbursing) on main health services (drug prescription, hospital admission, ambulatory visits, etc.). As for BLHA, all these databases cover a population of about 850,000 inhabitants living in the urban-rural area of Bologna, the main city of the Emilia-Romagna region in northern Italy. The BLHA administrative database contains information on insured subjects (unique identification number, gender, and age), on prescriptions claimed (drug names, trade names, claim date, number of packages dispensed and Anatomical Therapeutic Chemical Classification System [ATC] code), and on prescribers (identifier numbers and specialty), all linked through patient identification number. All essential drugs prescribed by general practitioners (GPs) and family pediatricians (FPs), such as antidepressants, are entirely (or partially, depending on income) reimbursed according to the Italian National Drug Formulary criteria, and almost all over-the-counter drugs, herbal medicines, and drugs used in hospitals or nursing homes are not included in these databases. Similarly, there is no information on the duration of the prescribed therapy, but the most common prescription contains two packages of 30 tablets and covers a maximum of 60 days of therapy.
For the purpose of this study, we identified a cohort of subjects having claimed at least one antidepressant (ATC code N06A) in 2013, without any prescription the previous year, and who were continuously enrolled in the public drug plan of the BLHA from 2012 to 2016. Subjects who died during follow-up were excluded from the cohort because the assessment of treatment duration could not have been evaluated, otherwise. The date of the first prescription in 2013 was considered the index date. All subjects were followed for three years from their index date.
Definitions
Long-Term and Chronic Use of Antidepressant Drugs
Long-term use of antidepressants was defined as the presence of at least one claim recorded during each year of the three-year follow-up. Long-term use was further indicated as “chronic use”, if at least 180 DDDs31 of antidepressants were claimed during each year of the three-year follow-up.
Independent Variables
We collected the demographic characteristics of subjects present in the BLHA database (gender; age group; area of residence) and the information on prescriptions claimed: first antidepressant drug claimed [fifth level of the ATC system]; the presence of polytherapy (yes/no), defined as the presence of more than one antidepressant during follow-up; polypharmacy (yes/no), defined as the presence of claims for at least five different drugs32,33 (third level of the ATC system), other than antidepressants, during each year of follow-up; specialty of the first prescriber.
Statistical Analysis
Descriptive statistics were carried out to describe baseline characteristics of the study population. Differences between chronic and non-chronic use were evaluated with chi-square tests for categorical variables and Student’s t-tests for continuous variables. Logistic regression analyses were used to identify factors associated with long-term and chronic use of antidepressants, testing for each variable, and calculating the unadjusted and adjusted odds ratios (OR and aOR, respectively) with a 95% confidence interval (CI) for all covariates. A variable was considered as statistically-significant for chronic or long-term use if it was associated with the outcome with a p-value ≤ 0.05. Interactions among independent variables were also analyzed and considered present if the p-value was ≤ 0.05. All analyses were performed using RStudio and PostgreSQL statistical software.
Results
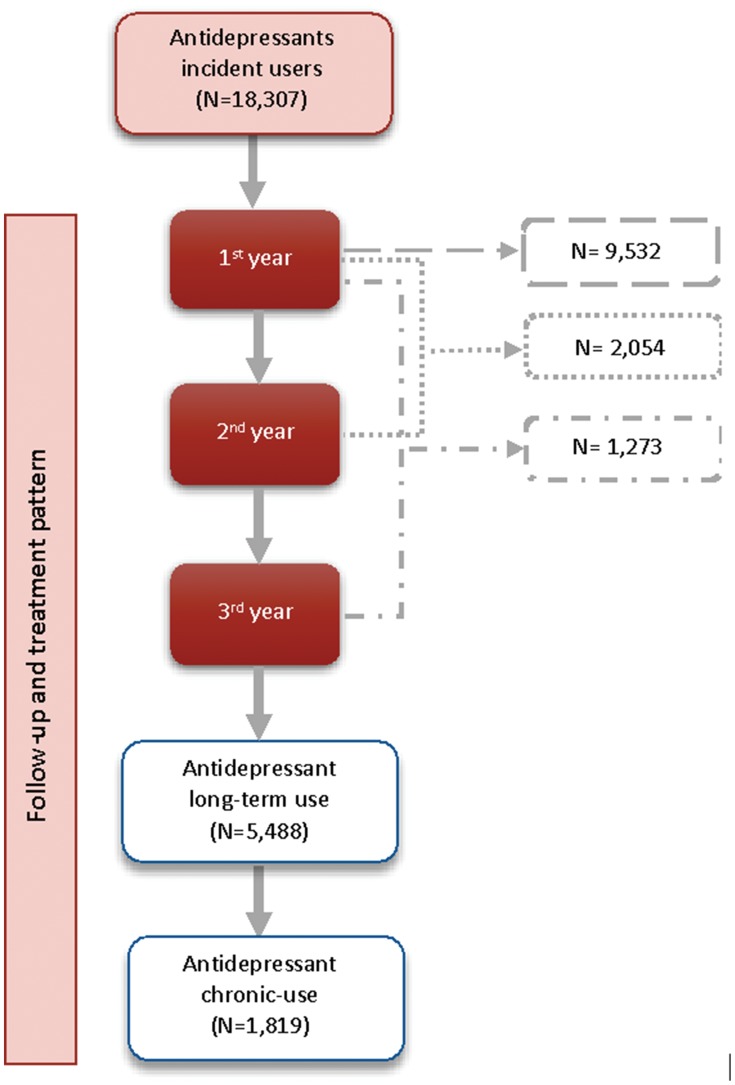
As shown in Figure 1, out of 870,507 inhabitants in the Bologna area in 2013 insured by the public drug plan of the BLHA, 18,307 were prescribed at least one antidepressant, without any prescriptions the previous year, with an incidence of 2.10%. Among them, 5488 subjects (29.9%) claimed at least one antidepressant during each year of the 3-year period and were therefore considered as having a long-term use for the purpose of this study. 1819 subjects (9.9% of the whole cohort) were dispensed at least 180 DDDs during each year of follow-up and were considered to have a chronic use of antidepressants.
Figure 1.
Synopsis of cohort selection and pattern distribution.
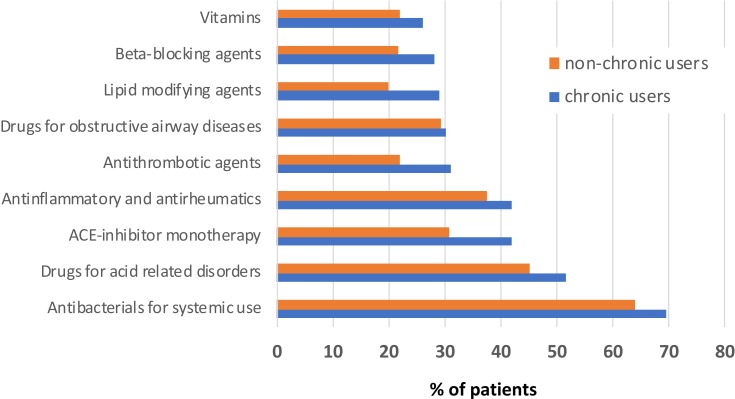
Demographic and clinical characteristics of the entire cohort and of sub-cohorts having received chronic and non-chronic prescriptions are shown in Table 1. The mean age of the study population was 58.46 years (18.24 SD) and 66.98% were women. Those being classified as having received long-term prescriptions claimed a mean of 257.1 DDDs in the first year of follow-up, 235.5 in the second year, and 232.2 in the third year. Among them, the most frequent antidepressants being dispensed were selective serotonin reuptake inhibitors (SSRIs), with sertraline as the most prescribed drug (26.2% of total drugs claimed), followed by paroxetine (16.1%), and citalopram (13.9%). Trazodone was received by 11.3% of patients. Among those having received chronic treatment, the most prescribed antidepressants were sertraline (36.9%), paroxetine (15.0%) and citalopram (13.4%). Description of the first ten prescribed concomitant drug classes (at ATC II level) among chronic-prescribed and non-chronic-prescribed antidepressants during the follow-up are reported in Figure 2. There was little difference between those having chronic and non-chronic use: systemic antibacterials (ATC: J01) and drugs against acid-related disorders (ATC: A02, mainly represented by proton pump inhibitors) were the first and second class in both groups of patients, followed by agents acting on the renin–angiotensin system (ATC: C09) and anti-inflammatory and antirheumatic agents (ATC: M01).
Table 1.
Demographic Characteristics of Selected Cohort
| Characteristics | Full Cohort N=18,307 | Chronic Use N=1819 | Non-Chronic Use N=16,488 | P value |
|---|---|---|---|---|
| Gender | 0.65 | |||
| Male | 6065 (33.13%) | 594 (32.66%) | 5471 (33.26%) | |
| Female | 12,242 (66.87%) | 1225 (67.34%) | 11,017 (66.98%) | |
| Age [mean (SD)] | 58.46 (18.24) | 60.40 (17.52) | 58.24 (18.30) | <0.001 |
| Age class | <0.001 | |||
| 0–17 | 93 (0.51%) | 10 (0.55%) | 83 (0.50%) | |
| 18–39 | 2979 (16.27%) | 230 (12.64%) | 2749 (16.71%) | |
| 40–59 | 6432 (35.13%) | 597 (32.82%) | 5835 (35.48%) | |
| 60–79 | 6096 (33.30%) | 704 (38.70%) | 5392 (32.78%) | |
| 80+ | 2707 (14.79%) | 278 (15.28%) | 2429 (14.77%) | |
| Living area | <0.001 | |||
| Urban | 8159 (44.57%) | 820 (45.08%) | 7339 (44.62%) | |
| Rural | 6669 (36.43%) | 729 (40.08%) | 5940 (36.11%) | |
| Missing | 3479 (19.00%) | 270 (14.84%) | 13,279 (19.46%) | |
| First prescriber | 0.85 | |||
| GP | 15,226 (83.17%) | 1533 (84.28%) | 13,693 (83.05%) | |
| Specialist | 2743 (14.98%) | 280 (15.39%) | 2463 (14.94%) | |
| Missing | 338 (1.85%) | 6 (0.33%) | 332 (02.01%) | |
| Initial antidepressant drug | <0.001 | |||
| Sertraline | 4798 (26.21%) | 671 (36.89%) | 4127 (25.09%) | |
| Paroxetine | 2951 (16.21%) | 273 (15.01%) | 2678 (16.28%) | |
| Citalopram | 2535 (13.85%) | 244 (13.41%) | 2291 (13.93%) | |
| Trazodone | 2061 (11.26%) | 60 (3.30%) | 2001 (12.17%) | |
| Escitalopram | 1512 (8.26%) | 186 (10.23%) | 1326 (8.06%) | |
| Amitriptyline | 1390 (7.59%) | 25 (1.37%) | 1365 (8.30%) | |
| Venlafaxine | 894 (4.88%) | 105 (5.77%) | 789 (4.80%) | |
| Duloxetine | 675 (3.69%) | 79 (4.39%) | 596 (3.62%) | |
| Fluoxetine | 496 (2.71%) | 46 (2.53%) | 450 (2.74%) | |
| Mirtazapine | 336 (1.84%) | 38 (2.09%) | 298 (1.81%) | |
| Other | 317 (1.73%) | 18 (0.99%) | 299 (1.82%) | |
| Polytherapy | 342 (1.87%) | 74 (4.07%) | 268 (1.63%) | |
| Polypharmacy | <0.001 | |||
| Yes | 5411 (29.56%) | 394 (21.66%) | 5017 (30.50%) | |
| No | 12,896 (70.44%) | 1425 (78.34%) | 11,471 (69.74%) |
Notes: Chronic use: at least 180 Defined Daily Dose (DDD) of antidepressant drug claimed in every year of follow-up. P values: all p-values but for age [mean (SD)], which was performed with independent Student t tests, were obtained by using chi square tests. Specialist: hospital or mental health center physician. Polytherapy: a combination of more than one antidepressant drugs as the initial treatment. Polypharmacy: 5 or more drugs claimed concomitantly.
Abbreviations: SD, standard deviation; GP, general practitioner.
Figure 2.
Description of the first 10 prescribed concomitant drugs classes (at ATC II level) among chronic and non-chronic users during the 3-year follow-up.
In the multivariate logistic regression analysis of chronic use (Table 2), gender and age, and age and living area resulted in having a significant interaction (p value< 0.05). We indeed performed the regressions separately for men and women. As reported in Table 2, risk factors determining a higher likelihood of chronic use were older age (especially in women and in rural settings), being prescribed the first antidepressant by a hospital or mental health center physician (especially in women, with an aOR of 3.45 and a 95% CI of 2.33–5.03), meeting criteria for polypharmacy. Compared to sertraline, the majority of antidepressant drugs were associated with a lower likelihood of chronic use (especially trazodone and amitriptyline), whereas receiving antidepressant polytherapy increased the likelihood of chronic use only among women.
Table 2.
Logistic Regression Analysis of Factor Associated with Chronic Use of Antidepressant Drugs Among Men and Women Separately
| Men | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Univariate Regression | Multivariate Regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age – Urban Area | – | – | ||||
| 0–17 | 2.16 | 0.48–7.08 | 0.25 | 2.02 | 0.44–6.84 | 0.30 |
| 18–39 | 1 | - | - | 1 | - | - |
| 40–59 | 0.84 | 0.58–1.22 | 0.34 | 0.76 | 0.52–1.11 | 0.15 |
| 60–79 | 1.13 | 0.79–1.63 | 0.51 | 0.88 | 0.60–1.30 | 0.52 |
| 80+ | 0.82 | 0.49–1.34 | 0.44 | 0.69 | 0.40–1.16 | 0.17 |
| Age – Rural Area | ||||||
| 0–17 | 3.74 | 0.80–13.07 | 0.05 | 4.60 | 0.95–17.26 | 0.03 |
| 18–39 | 1 | - | - | 1 | - | |
| 40–59 | 1.21 | 0.78–1.93 | 0.41 | 1.11 | 0.71–1.79 | 0.65 |
| 60–79 | 1.77 | 1.15–2.80 | 0.01 | 1.43 | 0.92–2.30 | 0.12 |
| 80+ | 1.95 | 1.14–3.36 | 0.02 | 1.91 | 1.08–3.38 | 0.03 |
| First Antidepressant Prescriber | ||||||
| GP | 1 | - | - | 1 | - | - |
| Specialist | 1.04 | 0.83–1.29 | 0.74 | 2.30 | 1.34–3.80 | <0.01 |
| Antidepressant Drug | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 0.62 | 0.48–0.79 | <0.01 | 0.58 | 0.44–0.77 | <0.01 |
| Citalopram | 0.55 | 0.42–0.72 | <0.01 | 0.62 | 0.45–0.83 | <0.01 |
| Trazodone | 0.15 | 0.09–0.23 | <0.01 | 0.14 | 0.08–0.23 | <0.01 |
| Escitalopram | 0.67 | 0.48–0.92 | 0.02 | 0.72 | 0.51–1.01 | 0.06 |
| Amitriptyline | 0.08 | 0.03–0.18 | <0.01 | 0.09 | 0.03–0.19 | <0.01 |
| Venlafaxine | 0.63 | 0.41–0.92 | 0.02 | 0.48 | 0.27–0.80 | 0.01 |
| Duloxetine | 0.49 | 0.29–0.78 | <0.01 | 0.39 | 0.22–0.65 | <0.01 |
| Fluoxetine | 0.58 | 0.30–1.03 | 0.08 | 0.66 | 0.32–1.20 | 0.20 |
| Mirtazapine | 0.84 | 0.48–1.39 | 0.53 | 0.84 | 0.41–1.56 | 0.61 |
| Other | 0.44 | 0.21–0.81 | 0.02 | 0.42 | 0.18–0.83 | 0.02 |
| Polytherapy | 1.47 | 0.89–2.34 | 0.11 | 1.34 | 0.66–2.55 | 0.39 |
| Polypharmacy | ||||||
| Non | 1 | - | - | 1 | - | - |
| Yes | 1.63 | 1.34–1.98 | <0.01 | 1.64 | 1.28–2.10 | <0.01 |
| Women | ||||||
| Characteristics | Univariate Regression | Multivariate Regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age – Urban Area | ||||||
| 0–17 | 0.68 | 0.04–3.41 | 0.71 | 0.70 | 0.04–3.53 | 0.73 |
| 18–39 | 1 | - | - | 1 | - | - |
| 40–59 | 1.10 | 0.82–1.49 | 0.54 | 1.07 | 0.80–1.47 | 0.64 |
| 60–79 | 1.51 | 1.14–2.03 | 0.01 | 1.39 | 1.03–1.89 | 0.03 |
| 80+ | 1.55 | 1.13–2.16 | 0.01 | 1.56 | 1.11–2.20 | 0.01 |
| Age – Rural Area | ||||||
| 0–17 | 1.45 | 0.23–5.13 | 0.63 | 1.74 | 0.27–6.36 | 0.47 |
| 18–39 | 1 | - | - | 1 | - | |
| 40–59 | 1.71 | 1.22–2.45 | <0.01 | 1.63 | 1.16–2.35 | 0.01 |
| 60-79 | 2.51 | 1.80–3.58 | <0.01 | 2.26 | 1.60–3.24 | <0.01 |
| 80+ | 3.00 | 2.07–4.41 | <0.01 | 3.22 | 2.18–4.28 | <0.01 |
| First Antidepressant Prescriber | ||||||
| GP | 1 | - | - | 1 | - | |
| Specialist | 1.00 | 0.85–1.18 | 0.97 | 3.45 | 2.33–5.03 | <0.01 |
| Antidepressant Drug | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 0.63 | 0.52–0.76 | <0.01 | 0.73 | 0.60–0.89 | <0.01 |
| Citalopram | 0.71 | 0.59–0.86 | <0.01 | 0.77 | 0.62–0.94 | 0.01 |
| Trazodone | 0.21 | 0.15–0.28 | <0.01 | 0.19 | 0.13–0.27 | <0.01 |
| Escitalopram | 0.96 | 0.78–1.18 | 0.73 | 1.07 | 0.86–1.33 | 0.54 |
| Amitriptyline | 0.13 | 0.08–0.19 | <0.01 | 0.14 | 0.08–0.22 | <0.01 |
| Venlafaxine | 0.93 | 0.71–1.21 | 0.61 | 0.80 | 0.56–0.12 | 0.21 |
| Duloxetine | 1.02 | 0.76–1.35 | 0.89 | 1.14 | 0.84–1.54 | 0.39 |
| Fluoxetine | 0.65 | 0.44–0.93 | 0.02 | 0.69 | 0.45–1.03 | 0.08 |
| Mirtazapine | 0.73 | 0.45–1.14 | 0.19 | 0.84 | 0.46–1.41 | 0.53 |
| Other | 0.30 | 0.13–0.58 | <0.01 | 0.19 | 0.06–0.46 | <0.01 |
| Polytherapy | 1.82 | 1.30–2.51 | <0.01 | 2.21 | 1.39–3.44 | <0.01 |
| Polypharmacy | ||||||
| Non | 1 | - | - | 1 | - | - |
| Yes | 1.56 | 1.35–1.81 | <0.01 | 1.21 | 1.01–1.45 | 0.04 |
Notes: Specialist: hospital or mental health center physician; Polytherapy: a combination of more than one antidepressant drugs as the initial treatment. Polypharmacy: 5 or more drugs claimed concomitantly.
Abbreviations: OR, odds ratio; 95% CI, 95% Confidence Interval; GP, general practitioner.
Similarly, we performed separate regression analyses for men and women for long-term use since a statistically significant interaction was present between gender and polypharmacy and between initial drug and polypharmacy (p values< 0.005). Factors associated with overall long-term use are reported in Table 3. Older age was associated with a higher likelihood of long-term use among both genders, but among women, also those under 18 years old were at higher risk for long-term use, with an OR of 2.45 (compared to the class 18–39 years). Receiving the first prescription by a specialist showed similar ORs than those for chronic use. Amitriptyline was the only antidepressant associated with a lower risk of long-term use among men without polypharmacy. Among both men and women with polypharmacy, also paroxetine, citalopram and trazodone showed lower risks. Among women without polypharmacy, citalopram and duloxetine resulted in a higher likelihood of long-term use, if compared to sertraline.
Table 3.
Logistic Regression Analysis of Factor Associated with Long Term Use of Antidepressants Among Men and Women Separately
| Men | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Univariate Regression | Multivariate Regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | ||||||
| 0–17 | 1.95 | 0.98–3.78 | 0.47 | 2.22 | 0.98–4.82 | 0.05 |
| 18–39 | 1 | - | - | 1 | - | - |
| 40-–59 | 1.04 | 0.88–1.22 | 0.66 | 1.00 | 0.83–1.22 | 0.97 |
| 60–79 | 1.33 | 1.13–1.57 | <0.01 | 1.31 | 1.07–1.61 | 0.01 |
| 80+ | 1.26 | 1.02–1.55 | 0.03 | 1.60 | 1.23–2.07 | <0.01 |
| Living Area | ||||||
| Urban | 1 | - | - | 1 | - | |
| Rural | 0.92 | 0.82–1.05 | 0.21 | 0.92 | 0.81–1.05 | 0.22 |
| First Antidepressant Prescriber | ||||||
| GP | 1 | - | - | 1 | - | - |
| Specialist | 1.06 | 0.91–1.22 | 0.47 | 2.69 | 1.77–4.11 | <0.01 |
| Antidepressant Drug – No Polypharmacy | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 1.05 | 0.77–1.44 | 0.74 | 1.33 | 0.93–1.92 | 0.12 |
| Citalopram | 0.99 | 0.71–1.36 | 0.94 | 1.03 | 0.69–1.53 | 0.88 |
| Trazodone | 0.60 | 0.37–0.95 | 0.03 | 0.59 | 0.31–1.08 | 0.10 |
| Escitalopram | 1.19 | 0.81–1.73 | 0.36 | 1.34 | 0.88–2.04 | 0.17 |
| Amitriptyline | 0.36 | 0.18–0.65 | <0.01 | 0.38 | 0.18–0.74 | 0.01 |
| Venlafaxine | 0.89 | 0.57–1.38 | 0.62 | 1.13 | 0.63–1.96 | 0.67 |
| Duloxetine | 0.63 | 0.29–1.23 | 0.20 | 0.66 | 0.29–1.37 | 0.29 |
| Fluoxetine | 0.95 | 0.47–1.79 | 0.87 | 1.17 | 0.54–2.38 | 0.68 |
| Mirtazapine | 0.91 | 0.43–1.79 | 0.80 | 0.87 | 0.31–2.13 | 0.78 |
| Other | 1.49 | 0.80–2.69 | 0.19 | 1.25 | 0.57–2.56 | 0.56 |
| Polytherapy | 0.59 | 0.20–1.45 | 0.29 | 0.61 | 0.09–2.42 | 0.53 |
| Antidepressant Drug - Polypharmacy | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 0.77 | 0.62–0.95 | 0.01 | 0.77 | 0.61–0.96 | 0.02 |
| Citalopram | 0.75 | 0.59–0.94 | 0.01 | 0.73 | 0.56–0.93 | 0.01 |
| Trazodone | 0.70 | 0.57–0.87 | <0.01 | 0.77 | 0.60–0.97 | 0.03 |
| Escitalopram | 0.82 | 0.62–1.08 | 0.16 | 0.85 | 0.63–1.14 | 0.28 |
| Amitriptyline | 0.39 | 0.27–0.55 | <0.01 | 0.43 | 0.30–0.61 | <0.01 |
| Venlafaxine | 0.71 | 0.50–1.01 | 0.06 | 0.77 | 0.50–1.16 | 0.21 |
| Duloxetine | 0.46 | 0.31–0.66 | <0.01 | 0.42 | 0.27–0.62 | <0.01 |
| Fluoxetine | 0.68 | 0.40–1.13 | 0.15 | 0.75 | 0.42–1.28 | 0.30 |
| Mirtazapine | 1.23 | 0.78–1.92 | 0.37 | 1.38 | 0.78–2.41 | 0.26 |
| Other | 0.54 | 0.31–0.88 | 0.02 | 0.54 | 0.30–0.94 | 0.04 |
| Polytherapy | 1.85 | 1.18–2.92 | 0.01 | 1.56 | 0.83–2.94 | 0.16 |
| Women | ||||||
| Characteristics | Univariate Regression | Multivariate Regression | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | ||||||
| 0–17 | 1.30 | 0.69–2.32 | 0.39 | 2.45 | 1.23–4.74 | 0.01 |
| 18–39 | 1 | - | - | 1 | - | - |
| 40–59 | 1.45 | 1.28–1.65 | <0.01 | 1.30 | 1.12–1.49 | <0.01 |
| 60–79 | 1.76 | 1.55–2.00 | <0.01 | 1.64 | 1.42–1.90 | <0.01 |
| 80+ | 1.89 | 1.64–2.19 | <0.01 | 2.25 | 1.90–2.67 | <0.01 |
| Living Area | ||||||
| Urban | 1 | - | - | 1 | - | - |
| Rural | 0.94 | 0.87–1.03 | 0.19 | 0.96 | 0.88–1.05 | 0.36 |
| First Antidepressant Prescriber | ||||||
| GP | 1 | - | - | 1 | - | - |
| Specialist | 0.79 | 0.71–0.89 | <0.01 | 2.38 | 1.70–3.33 | <0.01 |
| Antidepressant Drug – No Polypharmacy | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 1.16 | 0.90–1.50 | 0.25 | 1.31 | 0.98–1.75 | 0.07 |
| Citalopram | 1.34 | 1.03–1.73 | 0.03 | 1.42 | 1.05–1.92 | 0.02 |
| Trazodone | 0.79 | 0.54–1.12 | 0.20 | 0.82 | 0.52–1.27 | 0.38 |
| Escitalopram | 1.60 | 1.19–2.15 | <0.01 | 1.66 | 1.20–2.30 | <0.01 |
| Amitriptyline | 0.40 | 0.25–0.62 | <0.01 | 0.40 | 0.23–0.66 | <0.01 |
| Venlafaxine | 1.40 | 0.92–1.99 | 0.06 | 0.96 | 0.57–1.58 | 0.89 |
| Duloxetine | 1.80 | 1.10–2.88 | 0.02 | 2.14 | 1.26–3.59 | <0.01 |
| Fluoxetine | 0.59 | 0.33–0.98 | 0.05 | 0.50 | 0.26–0.90 | 0.03 |
| Mirtazapine | 1.01 | 0.55–1.76 | 0.98 | 1.17 | 0.50–2.48 | 0.70 |
| Other | 0.95 | 0.49–1.75 | 0.88 | 0.65 | 0.26–1.43 | 0.32 |
| Polytherapy | 1.53 | 0.86–2.62 | 0.13 | 2.27 | 0.75–6.45 | 0.13 |
| Antidepressant Drug - Polypharmacy | ||||||
| Sertraline | 1 | - | - | 1 | - | - |
| Paroxetine | 0.78 | 0.68–0.90 | <0.01 | 0.84 | 0.72–0.98 | 0.02 |
| Citalopram | 0.80 | 0.69–0.92 | <0.01 | 0.81 | 0.69–0.95 | 0.01 |
| Trazodone | 0.80 | 0.69–0.94 | 0.01 | 0.81 | 0.68–0.96 | 0.01 |
| Escitalopram | 1.09 | 0.92–1.29 | 0.34 | 1.15 | 0.96–1.37 | 0.13 |
| Amitriptyline | 0.45 | 0.37–0.55 | <0.01 | 0.48 | 0.39–0.58 | <0.01 |
| Venlafaxine | 0.86 | 0.68–1.08 | 0.20 | 0.77 | 0.58–1.01 | 0.06 |
| Duloxetine | 0.90 | 0.71–1.14 | 0.38 | 0.98 | 0.76–1.26 | 0.88 |
| Fluoxetine | 0.81 | 0.61–1.07 | 0.14 | 0.83 | 0.61–1.13 | 0.24 |
| Mirtazapine | 0.76 | 0.52–1.11 | 0.17 | 0.70 | 0.44–1.07 | 0.11 |
| Other | 0.88 | 0.59–1.30 | 0.53 | 0.80 | 0.51–1.23 | 0.32 |
| Polytherapy | 1.45 | 1.05–1.99 | 0.02 | 1.64 | 1.07–2.54 | 0.02 |
Notes: Specialist: hospital or mental health center physician; Polytherapy: a combination of more than one antidepressant drugs as the initial treatment. Polypharmacy: 5 or more drugs claimed concomitantly.
Abbreviations: OR, odds ratio; 95% CI, 95% Confidence Interval; GP, general practitioner.
Discussion
Prevalence of Antidepressant Drug Utilization
This study provided new insights on chronic and long-term use of antidepressants and their determinants. The main finding of this study was that among patients having started an antidepressant treatment in 2013, about 30% were still being treated after more than two years, and about 10% of them claimed at least 180 DDDs each year for the three-year follow-up. Despite the extensive literature on antidepressant drug utilization,1–8,34,35 only one study, conducted in the United States, analyzed trends of antidepressant use with a follow-up period longer than two years at the patient level, ie, from 1999–2010.15 In that study, the authors found that the proportion of long-term use was extremely higher compared to our study, ranging from 46% in 1999–2000 to 67% in 2009–2010. Mojtabai et al used a self-reported measure of antidepressant duration, rather than an objective measure through pharmacy records; this may have overestimated the duration of treatment in that population.15 Moreover, the study was cross-sectional, with participants having longer treatment durations more likely to be selected for the study. These high proportions could be explained by the method for assessment of antidepressant use and the study design.
A more recent study in the Netherlands analyzed long-term antidepressant use (at least 15 months) from 1995 to 2015.30 They found a trend similar to that reported by Mojtabai et al, but with lower proportions.15,30 In that study, more than 40% of their population claimed antidepressants for longer than 15 months (calculated from the first to the last prescription).30 Despite the lower proportion than that reported by Mojtabai et al, the prevalence of long-term use was considerably higher than found in our study.30 This can be partly explained by their definition of long-term use of 15 months, compared to our definition of treatment longer than two years. Moreover, they allowed interruptions between two consecutive prescriptions, while we imposed for our definition that the patient claimed at least one prescription of an antidepressant during each year of the three-year follow-up (and 180 DDDs per year for chronic use).30 Therefore, the proportion of long-term use was lower than reported in other studies in Europe and the United States,15,30 even if comparisons are not evident due to differences in the definitions of drug exposure and study designs.
It must be acknowledged that, despite the majority of subjects with depression who recover rapidly (within a few months), a minority of these patients experience a longer course of depressive symptoms.36 In 2004, a systematic review analyzed the prevalence of recovery, recurrence, and chronicity of depression in general practice and the community.36 The authors, examining the results of published prospective longitudinal studies, reported that the prevalence of chronic courses of depression ranged from 10 to 17%.36 The prevalence of long-term users in our population could be explained by the proportion of subjects experiencing chronic courses of depression or those at higher risk of recurrence. Recently, in a study on the duration of depressive episodes in the general Dutch population, the authors reported that about 74% of subjects in their cohort recovered from depression within one year, 84% within two years, and 88% within three years.37 The authors estimated about 20% for the prevalence of chronic depression, which is in accordance with the proportion of long-term use in our study.
Moreover, antidepressants such as SSRIs and selective serotonin-norepinephrine reuptake inhibitors (SNRIs) are considered as first-line pharmacological treatment choices, not only for depression, but also for anxiety disorders.38 Due to the frequent chronic course of anxiety disorders,39 pharmacological treatment may be continued for long periods. We found frequent prescriptions of antidepressants that are second-line treatments for depression (ie, trazodone and paroxetine). A higher sedative effect of these drugs may suggest a possible use for treating anxiety symptoms. Trazodone is also used off-label for insomnia in elderly, and paroxetine can be the first-choice SSRI in patients receiving polypharmacy or with liver failure since it is the only SSRI excreted without CYP metabolism. Another possible explanation is that trazodone and paroxetine can also be taken at low doses since trazodone is available in drops and paroxetine in scored tablets. A recent meta-analysis on the risk of relapse of anxiety disorders after antidepressant discontinuation found that patients on treatment for at least one year had a lower relapse rate compared to subjects who were discontinued earlier.40 Unfortunately, there is a lack of evidence for longer duration of treatment for anxiety disorders in terms of efficacy, safety (withdrawal effect), and patient preferences.22–25 Yet, given the chronicity of anxiety disorders, a certain proportion of long-term antidepressant treatments may be expected. Besides, patients suffering from anxiety symptoms could be more afraid of stopping antidepressants and having negative experiences with drawbacks.41 Qualitative studies have investigated the reasons behind the difficulties of withdrawing antidepressants.42,43 They found that patients are generally more afraid of discontinuing medications than persist taking them. They also consider that their wellbeing is linked to the continued use of antidepressants because of the chronicity of their conditions.42,43
Although long-term treatment with antidepressants should be suitable for individuals with chronic depression, those with a high risk of relapse, or patients with anxiety disorders as defined by clinical guidelines, there are no sufficient evidence-based studies for the appropriate duration of antidepressant treatment for these subgroups of patients.16,44
As for the percentage of patients with long-term but not chronic use (about 20%), it should be recognized that they probably received recurrent very short courses of antidepressants, which, per se, suggest stronger inappropriateness in comparison with chronic use. Six-month cycles seem not reached at all indeed, and recurrence of disease episodes could be the reason for subsequent cycles.
Determinants of Long-Term and Chronic Use
This study also identified some demographic and clinical factors associated with chronic and long-term use. We found that older age and polypharmacy were associated with a higher risk for a longer duration of antidepressant treatment, both in the univariate and multivariate analyses, with higher odd ratios for the age group of 80-years-old and above. This finding is in line with what has been reported in other studies on long-term treatment,15,30 which could be due to the higher level of chronicity of depression in the elderly,45 or the fact that older patients may take more time to show a response and are less likely to discontinue their treatment.18 Older subjects are also more likely to be affected by multiple chronic conditions (multimorbidity) and to take many medications (polypharmacy).46 Regardless of age, a higher number of drugs has been associated with a higher probability of depression,47 while a recent systematic review found that depression was more frequent in subjects with multimorbidity.48 Some medications may cause depression as an adverse effect, so a recent study found that patients taking antidepressants along with potential depression-inducing medications, such as hormonal contraceptives, β-blockers, corticosteroids, or anti-obesity drugs were more likely to report depressive symptoms than patients taking only antidepressant drugs.49–51 Women are more likely to suffer from anxiety and depressive disorders. In our analyses, gender interacted with age (for chronic use) and initial drug (for long-term use), so the analyses were reported separately for men and women. Other studies found no association between gender and chronic or long-term use.15,30
Being prescribed the first antidepressant by hospital physicians or those specializing in mental health was associated with a higher risk of chronic and long-term use, with significant aORs of 2.30–3.45, depending on gender. The higher risk for more prolonged antidepressant use among those prescribed by a specialist could be justified by the fact that more severe cases of depression, which are possibly those with a high risk of relapse or with treatment-resistant depression, are likely referred directly to mental health specialists by general practitioners.52 Other studies on antidepressants53 or other drugs54,55 have used the specialty of the prescriber as a proxy for disease severity or complexity. Another possible explanation of these results is that patients receiving a prescription by a specialist rather than a GP may be more likely to feel that its treatment was helpful,56 and they might be more persistent.13,57 A study by Pomerantz et al,13 based on administrative data of a health maintenance organization in the United States linked to a physicians’ survey, showed that specialist practitioners (ie, psychiatrists) prescribed more prolonged courses of antidepressant treatment, with their patients being more prone to follow their recommendations. Although some studies reported a higher prevalence of diagnosed depression in urban vs rural living areas,47,58 we did not find any association between the area and duration of antidepressant treatment. This finding may indicate that in our population, patients have equitable access to mental health resources, irrespective of residence, access that is assured by the diffused presence of family physicians and LHAs of the Italian healthcare system integrated with a specific collaboration program started in 1999.59
Among antidepressants as initial treatment, we found that only sertraline was associated with a higher risk for chronic use. Men and women with polypharmacy (and, therefore, probably multimorbid) showed similar associations between first drug and long-term use. SSRIs, which are generally used for their favorable pharmacological profile in the general population, can cause significant side effects due to drug-drug interactions.60,61 Among SSRIs, sertraline is usually preferred, especially in the elderly, due to its favorable risk-benefit profile, because of its lower likelihood of interaction with other drugs in multimorbid patients.17 Sertraline could, therefore, be preferred in more complicated clinical cases (eg older or multimorbid patients). Among patients not exposed to polypharmacy, only amitriptyline showed a lower risk for long-term use, and citalopram and duloxetine showed a higher risk only among women. Amitriptyline is also used for short periods, at low dosages, for insomnia or headache, and duloxetine can be used for long periods for chronic pain related to fibromyalgia, diabetic neuropathy or other painful syndromes.62,63 Patients who use duloxetine may be afraid to come back to pain if they stop treatment. These results are nevertheless more complicated to interpret, and further studies are needed to investigate the reasons for prescribers’ clinical choices regarding different antidepressants.
Strengths and Limitations
The main strength of this study is its large population that represents virtually all residents of the Bologna area having started a treatment course with an antidepressant drug in 2013. The Italian health system is fully public and covers all the resident population. Another strength is that we identified subjects newly treated with antidepressants since they did not have any prescription in the previous year. Moreover, we followed all the patients for a period of three years, starting from the first prescription claimed, recording all the prescriptions claimed in this period. Furthermore, the BLHA databases are of high quality, as they are meant for reimbursement purposes, and they are largely used for research and surveillance purposes.1,64–66
This study, however, has some limitations. First, the BLHA database does not have information on diagnosis, clinical evaluations, or indications for prescriptions. We were thus unable to characterize subjects according to a diagnosis of depressive or anxiety disorder and did not know the reason for the prescriptions received. The indications could have been other than depression or anxiety, such as other psychiatric or non-psychiatric diseases, which could need longer durations of treatment. However, no other possible indication of use could justify an evidence-based long-term or chronic use, so far. Moreover, the large majority of patients use antidepressants for these two disorders, which share similar guidelines for pharmacotherapy in terms of antidepressant classes used and duration of treatment.3,5,13 Information on ethnicity or income could have been valuable as potential factors associated with the chronicity of antidepressant use, but this information is not in our databases. However, in the previously cited Dutch study, socioeconomic status was not associated with long-term use of antidepressants.30 Moreover, antidepressant drugs are covered by the Italian public drug plan, and the proportion of patients purchasing them privately is negligible.67 Finally, certain drugs, such as trazodone, amitriptyline and mirtazapine, may be prescribed at daily doses lower than 1 DDD. Unfortunately, we cannot quantify this information bias, which is inherent to the use of the ATC-DDD system (which is, in turn, necessary since there no information about day’s supply in the BLHA databases). Nevertheless, this misclassification could have only underestimated actual chronic use.
Conclusion
Long-term and chronic use of antidepressants is common in the Bologna area, and this phenomenon may contribute to the increased prevalence and exposure level of antidepressants at a population level. Because of the administrative data we used, it is not possible to know if a long duration was clinically motivated, nor the proportion of unnecessary long-term use. Clinical guidelines indicate that durations of about 12–24 months should be targeted, with more prolonged treatments clinically motivated, mainly because of the lack of evidence on the risk/benefit ratio of long-term use. Reasons for a longer duration should be investigated to highlight the possible presence of potentially inappropriate prescription patterns. Risk factors for long-term and chronic use (older age, presence of other comorbidities, prescriptions made by a specialist, or the type of antidepressant prescribed) may suggest acceptable reasons for prolonged use. Specific antidepressants (ie, duloxetine) may be used for chronic pain, especially by women, presumably for longer periods than for depression. Similar considerations can be addressed for people with an anxiety disorder, for whom the clinician may find it more difficult to deprescribe antidepressants for fear of anxiety symptoms reappear. Specific subpopulations identified as being at higher risk for a long term and chronic use with this study should, therefore, be addressed for further research studies, and indications for antidepressant use should be investigated deeply.
Acknowledgments
We thank the LHA of Bologna for the extraction of the anonymized data of prescription claims for the purpose of this study. We wish to thank American Manuscript Editors for English language editing. The abstract of this paper was presented at the 2019 ISPOR Conference, May 18–22, 2019, New Orleans (“Long-term utilization of antidepressant drugs: prevalence and associated factors”; Lunghi C, Antonazzo IC, Poluzzi E, Burato S, Raschi E, Forcesi E, Sangiorgi E, Menchetti M, Roberge P.) and at the 34th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE), Prague Congress Centre, Prague, Czech Republic, August 22–26, 2018 (“The prevalence of chronic antidepressant drugs prescriptions in the province of Bologna, Italy”; Lunghi C, Roberge P, Forcesi E, Burato S, Sangiorgi E, Menchetti M, Antonazzo IC, Poluzzi E.) as a poster presentation with interim findings. The poster’s abstracts were published in the special issues of Pharmacoepidemiology and Drug Safety 2018; 27 (S2): p. 436 and of Value in Health 2019; 22 (S2): pS231.
Ethics
Ethics approval was obtained from the Ethical Committee of the Local Health Authority “Area Vasta Emilia Centro” (CE 18137).
Disclosure
The authors declare that they have no conflicts of interest regarding this article.
References
- 1.Poluzzi E, Piccinni C, Sangiorgi E, et al. Trend in SSRI-SNRI antidepressants prescription over a 6-year period and predictors of poor adherence. Eur J Clin Pharmacol. 2013;69(12):2095–2101. doi: 10.1007/s00228-013-1567-8 [DOI] [PubMed] [Google Scholar]
- 2.Aarts N, Noordam R, Hofman A, Tiemeier H, Stricker BH, Visser LE. Utilization patterns of antidepressants between 1991 and 2011 in a population-based cohort of middle-aged and elderly. Eur Psychiatry. 2014;29(6):365–370. doi: 10.1016/j.eurpsy.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Wong J, Motulsky A, Eguale T, Buckeridge DL, Abrahamowicz M, Tamblyn R. Treatment indications for antidepressants prescribed in primary care in Quebec, Canada, 2006–2015. JAMA. 2016;315(20):2230–2232. doi: 10.1001/jama.2016.3445 [DOI] [PubMed] [Google Scholar]
- 4.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81 [DOI] [PubMed] [Google Scholar]
- 5.Patten SB, Esposito E, Carter B. Reasons for antidepressant prescriptions in Canada. Pharmacoepidemiol Drug Saf. 2007;16(7):746–752. doi: 10.1002/pds.1385 [DOI] [PubMed] [Google Scholar]
- 6.Wesselhoeft R, Jensen PB, Talati A, et al. Trends in antidepressant use among children and adolescents: a Scandinavian drug utilization study. Acta Psychiatr Scand. 2020;141(1):34–42. doi: 10.1111/acps.13116 [DOI] [PubMed] [Google Scholar]
- 7.Mars B, Heron J, Kessler D, et al. Influences on antidepressant prescribing trends in the UK: 1995–2011. Soc Psychiatry Psychiatr Epidemiol. 2017;52(2):193–200. doi: 10.1007/s00127-016-1306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Lopez MC, Rodriguez-Lopez CM, Parron-Carreno T, Luna JD, Del Pozo E. Trends in the dispensation of antidepressant drugs over the past decade (2000–2010) in Andalusia, Spain. Soc Psychiatry Psychiatr Epidemiol. 2015;50(5):705–712. doi: 10.1007/s00127-014-0995-9 [DOI] [PubMed] [Google Scholar]
- 9.Morkem R, Barber D, Williamson T, Patten SB. A Canadian primary care sentinel surveillance network study evaluating antidepressant prescribing in Canada from 2006 to 2012. Can J Psychiatry. 2015;60(12):564–570. doi: 10.1177/070674371506001207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercier A, Auger-Aubin I, Lebeau JP, et al. Evidence of prescription of antidepressants for non-psychiatric conditions in primary care: an analysis of guidelines and systematic reviews. BMC Fam Pract. 2013;14(1):55. doi: 10.1186/1471-2296-14-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. doi: 10.1136/bmj.j603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry. 2004;65(3):395–404. doi: 10.4088/JCP.v65n0316 [DOI] [PubMed] [Google Scholar]
- 14.Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009;339:b3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. national health and nutrition examination survey. J Clin Psychiatry. 2014;75(2):169–177. doi: 10.4088/JCP.13m08443 [DOI] [PubMed] [Google Scholar]
- 16.Piek E, van der Meer K, Nolen WA. Guideline recommendations for long-term treatment of depression with antidepressants in primary care—a critical review. Eur J Gen Pract. 2010;16(2):106–112. doi: 10.3109/13814781003692463 [DOI] [PubMed] [Google Scholar]
- 17.NICE, NIfHaCE. Depression in adults: recognition and management. Clinical guideline [CG90]; 2009. Available from: https://www.nice.org.uk/guidance/CG90. Accessed April15, 2020.
- 18.Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14 Suppl 1(Suppl1):S1. doi: 10.1186/1471-244X-14-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright C, Gibson K, Read J, Cowan O, Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401–1407. doi: 10.2147/PPA.S110632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–121. doi: 10.1016/j.addbeh.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 22.Hengartner MP. Methodological flaws, conflicts of interest, and scientific fallacies: implications for the evaluation of antidepressants’ efficacy and harm. Front Psychiatry. 2017;8:275. doi: 10.3389/fpsyt.2017.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henssler J, Kurschus M, Franklin J, Bschor T, Baethge C. Trajectories of acute antidepressant efficacy: how long to wait for response? A systematic review and meta-analysis of long-term, placebo-controlled acute treatment trials. J Clin Psychiatry. 2018;79(3). doi: 10.4088/JCP.su17023ah3c [DOI] [PubMed] [Google Scholar]
- 24.Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: current status of research. Psychother Psychosom. 2010;79(5):267–279. doi: 10.1159/000318293 [DOI] [PubMed] [Google Scholar]
- 25.D’Addona D, Carotenuto A, Novellino E, et al. Novel sst5-selective somatostatin dicarba-analogues: synthesis and conformation-affinity relationships. J Med Chem. 2008;51(3):512–520. doi: 10.1021/jm070886i [DOI] [PubMed] [Google Scholar]
- 26.Petty DR, House A, Knapp P, Raynor T, Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing. 2006;35(5):523–526. doi: 10.1093/ageing/afl023 [DOI] [PubMed] [Google Scholar]
- 27.Lockhart P, Guthrie B. Trends in primary care antidepressant prescribing 1995–2007: a longitudinal population database analysis. Br J Gen Pract. 2011;61(590):e565–e72. doi: 10.3399/bjgp11X593848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343(aug02 1):d4551. doi: 10.1136/bmj.d4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951. doi: 10.1136/bmj.k1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huijbregts KM, Hoogendoorn A, Slottje P, van Balkom A, Batelaan NM. Long-term and short-term antidepressant use in general practice: data from a large cohort in the Netherlands. Psychother Psychosom. 2017;86(6):362–369. doi: 10.1159/000480456 [DOI] [PubMed] [Google Scholar]
- 31.WHO Collaborating centre for drug statistics methodology. DDD - definition and general considerations; 2018. Available from: https://www.whocc.no/ddd/definition_and_general_considera/. Accessed April15, 2020.
- 32.Sirois C, Domingues NS, Laroche ML, Zongo A, Lunghi C, Guenette L, Kroger E, Emond V. Polypharmacy definitions for multimorbid older adults need stronger foundations to guide research, clinical practice and public healht. Pharmacy (Basel) 2019;7(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassanin H, Harbi A, Saif A, Davis J, Easa D, Harrigan R. Changes in antidepressant medications prescribing trends in children and adolescents in Hawai’i following the FDA black box warning. Hawaii Med J. 2010;69(1):17–19. [PMC free article] [PubMed] [Google Scholar]
- 35.Katz LY, Kozyrskyj AL, Prior HJ, Enns MW, Cox BJ, Sareen J. Effect of regulatory warnings on antidepressant prescription rates, use of health services and outcomes among children, adolescents and young adults. CMAJ. 2008;178(8):1005–1011. doi: 10.1503/cmaj.071265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinert C, Hofmann M, Kruse J, Leichsenring F. The prospective long-term course of adult depression in general practice and the community. A systematic literature review. J Affect Disord. 2014;152:65–75. doi: 10.1016/j.jad.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 37.Ten Have M, Penninx B, Tuithof M, et al. Duration of major and minor depressive episodes and associated risk indicators in a psychiatric epidemiological cohort study of the general population. Acta Psychiatr Scand. 2017;136(3):300–312. doi: 10.1111/acps.12753 [DOI] [PubMed] [Google Scholar]
- 38.Strawn JR, Geracioti L, Rajdev N, Clemenza K, Levine A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057–1070. doi: 10.1080/14656566.2018.1491966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014;44(11):2363–2374. doi: 10.1017/S0033291713003243 [DOI] [PubMed] [Google Scholar]
- 40.Batelaan NM, Bosman RC, Muntingh A, Scholten WD, Huijbregts KM, van Balkom A. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. doi: 10.1136/bmj.j3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eveleigh R, Muskens E, Lucassen P, et al. Withdrawal of unnecessary antidepressant medication: a randomised controlled trial in primary care. BJGP Open. 2018;1(4):bjgpopen17X101265. doi: 10.3399/bjgpopen17X101265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leydon GM, Rodgers L, Kendrick T. A qualitative study of patient views on discontinuing long-term selective serotonin reuptake inhibitors. Fam Pract. 2007;24(6):570–575. doi: 10.1093/fampra/cmm069 [DOI] [PubMed] [Google Scholar]
- 43.Verbeek-Heida PM, Mathot EF. Better safe than sorry–why patients prefer to stop using selective serotonin reuptake inhibitor (SSRI) antidepressants but are afraid to do so: results of a qualitative study. Chronic Illn. 2006;2(2):133–142. doi: 10.1177/17423953060020020801 [DOI] [PubMed] [Google Scholar]
- 44.Baldwin DS, Waldman S, Allgulander C. Evidence-based pharmacological treatment of generalized anxiety disorder. Int J Neuropsychopharmacol. 2011;14(5):697–710. doi: 10.1017/S1461145710001434 [DOI] [PubMed] [Google Scholar]
- 45.Rubio JM, Markowitz JC, Alegría A, et al. Epidemiology of chronic and nonchronic major depressive disorder: results from the national epidemiologic survey on alcohol and related conditions. Depress Anxiety. 2011;28(8):622–631. doi: 10.1002/da.20864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 47.Lunghi C, Moisan J, Gregoire JP, Guenette L. Incidence of depression and associated factors in patients with type 2 diabetes in Quebec, Canada: a population-based cohort study. Medicine. 2016;95(21):e3514. doi: 10.1097/MD.0000000000003514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: a systematic review and meta-analysis. J Affect Disord. 2017;221:36–46. doi: 10.1016/j.jad.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 49.Qato DM, Ozenberger K, Olfson M. Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA. 2018;319(22):2289–2298. doi: 10.1001/jama.2018.6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers D, Pies R. General medical with depression drugs associated. Psychiatry. 2008;5(12):28–41. [PMC free article] [PubMed] [Google Scholar]
- 51.Patten SB, Love EJ. Drug-induced depression. Psychother Psychosom. 1997;66(2):63–73. doi: 10.1159/000289110 [DOI] [PubMed] [Google Scholar]
- 52.Menchetti M, Sighinolfi C, Di Michele V, et al. Effectiveness of collaborative care for depression in Italy. Randomized Controlled Trial Gen Hosp Psychiatry. 2013;35(6):579–586. doi: 10.1016/j.genhosppsych.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 53.Milea D, Guelfucci F, Bent-Ennakhil N, Toumi M, Auray J-P. Antidepressant monotherapy: a claims database analysis of treatment changes and treatment duration. Clin Ther. 2010;32(12):2057–2072. doi: 10.1016/j.clinthera.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 54.Lunghi C, Zongo A, Grégoire J-P, Moisan J, Guénette L. Factors associated with antidiabetic medication non-adherence in patients with incident comorbid depression. J Diabetes Complicat. 2017;31(7):1200-1206. [DOI] [PubMed] [Google Scholar]
- 55.Guenette L, Moisan J, Breton MC, Sirois C, Gregoire JP. Difficulty adhering to antidiabetic treatment: factors associated with persistence and compliance. Diabetes Metab. 2013;39(3):250–257. doi: 10.1016/j.diabet.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 56.Kuramoto-Crawford SJ, Han B, Jacobus-Kantor L, Mojtabai R. Differences in patients’ perceived helpfulness of depression treatment provided by general medical providers and specialty mental health providers. Gen Hosp Psychiatry. 2015;37(4):340–346. doi: 10.1016/j.genhosppsych.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 57.Tournier M, Cougnard A, Boutouaba-Combe S, Verdoux H. Duration of antidepressant drug treatment and its determinants in France. Encephale. 2011;37(Suppl 1):S36–S41. doi: 10.1016/j.encep.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 58.Araya R, Zitko P, Markkula N, Rai D, Jones K. Determinants of access to health care for depression in 49 countries: a multilevel analysis. J Affect Disord. 2018;234:80–88. doi: 10.1016/j.jad.2018.02.092 [DOI] [PubMed] [Google Scholar]
- 59.Berardi D, Ferrannini L, Menchetti M, Vaggi M. Primary care psychiatry in Italy. J Nerv Ment Dis. 2014;202(6):460–463. doi: 10.1097/NMD.0000000000000145 [DOI] [PubMed] [Google Scholar]
- 60.Renoux C, Vahey S, Dell’Aniello S, Boivin JF. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. 2017;74(2):173–180. doi: 10.1001/jamaneurol.2016.4529 [DOI] [PubMed] [Google Scholar]
- 61.De Picker L, Van Den Eede F, Dumont G, Moorkens G, Sabbe BG. Antidepressants and the risk of hyponatremia: a class-by-class review of literature. Psychosomatics. 2014;55(6):536–547. doi: 10.1016/j.psym.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 62.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. doi: 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 63.Wright A, Luedtke KE, Vandenberg C. Duloxetine in the treatment of chronic pain due to fibromyalgia and diabetic neuropathy. J Pain Res. 2010;4:1–10. doi: 10.2147/JPR.S12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naldi I, Piccinni C, Mostacci B, et al. Prescription patterns of antiepileptic drugs in young women: development of a tool to distinguish between epilepsy and psychiatric disorders. Pharmacoepidemiol Drug Saf. 2016;25(7):763–769. doi: 10.1002/pds.3984 [DOI] [PubMed] [Google Scholar]
- 65.Charlton RA, Neville AJ, Jordan S, et al. Healthcare databases in Europe for studying medicine use and safety during pregnancy. Pharmacoepidemiol Drug Saf. 2014;23(6):586–594. doi: 10.1002/pds.3613 [DOI] [PubMed] [Google Scholar]
- 66.Gini R, Schuemie MJ, Francesconi P, et al. Can Italian healthcare administrative databases be used to compare regions with respect to compliance with standards of care for chronic diseases? PLoS One. 2014;9(5):e95419. doi: 10.1371/journal.pone.0095419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Istituto Superiore di Sanità. L’uso dei Farmaci in Italia. Rapporto nazionale anno 2011 Rome2012 Available from: http://www.agenziafarmaco.gov.it/sites/default/files/1_-_rapporto_osmed_2011.pdf. Accessed April15, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- NICE, NIfHaCE. Depression in adults: recognition and management. Clinical guideline [CG90]; 2009. Available from: https://www.nice.org.uk/guidance/CG90. Accessed April15, 2020.
- WHO Collaborating centre for drug statistics methodology. DDD - definition and general considerations; 2018. Available from: https://www.whocc.no/ddd/definition_and_general_considera/. Accessed April15, 2020.
- Istituto Superiore di Sanità. L’uso dei Farmaci in Italia. Rapporto nazionale anno 2011 Rome2012 Available from: http://www.agenziafarmaco.gov.it/sites/default/files/1_-_rapporto_osmed_2011.pdf. Accessed April15, 2020.