Abstract
Background
Agitation and aggression are commonly cited reasons for psychiatry consultation for individuals diagnosed with autism spectrum disorder (ASD). While risperidone and aripiprazole do not carry Health Canada approval for management of ASD-associated irritability, both are used for this indication but are not universally effective and carry substantial risk of adverse effects. This necessitates use of off-label medications to assist in management of behavioral dysregulation. Clonidine, an alpha-2 receptor agonist, is approved in Canada for treatment of hypertension. The evidence base also supports its use for attention deficit/hyperactivity disorder (ADHD) and for tics in Tourette’s disorder. This review focuses on examining the literature regarding clonidine as a treatment of challenging behaviours in the ASD population.
Method
Systematic search of MEDLINE, EMBASE, and PsycINFO databases resulted in 540 unique records. Ten publications were relevant to this review.
Results
Two cross-over studies, one open-label case series, and seven case reports were identified. One of two controlled studies suggested benefit from clonidine versus placebo. Caregivers typically noted improvement in behaviour with clonidine versus baseline. Clonidine was generally well-tolerated. Sedation was the most consistently reported adverse effect. Despite being an anti-hypertensive medication, few discontinued clonidine due to hypotension or bradycardia.
Conclusion
Clonidine has a limited evidence base for use in the management of behavioural problems in patients with ASD. Most evidence originates from case reports. Given the paucity of pharmacological options for addressing challenging behaviours in ASD patients, a clonidine trial may be an appropriate and cost-effective pharmaceutical option for this population.
Keywords: clonidine, irritability of autism, aggression, self-injurious behavior
Résumé
Contexte
L’agitation et l’agressivité sont des raisons justifiant fréquemment une consultation psychiatrique pour les personnes ayant reçu un diagnostic de trouble du spectre de l’autisme (TSA). Bien que la rispéridone et l’aripiprazole ne soient pas approuvés par Santé Canada pour la prise en charge de l’irritabilité associée au TSA, les deux médicaments sont utilisés à cette indication mais ne sont pas universellement efficaces et comportent un risque substantiel d’effets indésirables. Il faut donc utiliser des médicaments hors indications pour aider à la prise en charge des perturbations du comportement. La clonidine, un agoniste des récepteurs de type alpha-2, est approuvée au Canada pour le traitement de l’hypertension. L’ensemble des données probantes en soutient l’utilisation pour le trouble de déficit de l’attention avec hyperactivité (TDAH) et pour les tics du syndrome de Tourette. La présente revue se penche sur l’examen de la littérature en ce qui concerne la clonidine comme traitement des comportements difficiles dans la population du TSA.
Méthode
Une recherche systématique des bases de données MEDLINE, EMBASE, et PsycINFO a produit 540 documents uniques. Dix publications correspondaient à cette revue. Résultats: Deux études croisées, une série de cas ouverts, et sept études de cas ont été identifiées. L’une de deux études contrôlées suggérait un bénéfice de la clonidine contre un placebo. Les soignants notaient généralement une amélioration du comportement avec la clonidine par rapport au départ. La clonidine était généralement bien tolérée. La sédation était l’effet indésirable le plus constamment déclaré. Malgré que ce soit un médicament anti-hypertensif, peu interrompaient la clonidine en raison d’hypotension ou de bradycardie. Conclusion: Des données probantes limitées appuient l’utilisation de la clonidine pour la prise en charge des problèmes de comportement chez les patients souffrant de TSA. La plupart des données probantes sont issues d’études de cas. Étant donné la rareté des options pharmacologiques pour aborder les comportements difficiles chez les patients souffrant de TSA, un essai de clonidine peut constituer une option pharmaceutique appropriée et rentable pour cette population.
Mots clés: clonidine, irritabilité de l’autisme, agressivité, comportement d’automutilation
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by deficits in social communication and social interactions and by restricted, repetitive activities, behaviours or interests (APA, 2013). It encompasses diagnoses described in previous versions of the Diagnostic and Statistical Manual for Mental Disorders (DSM), including autistic disorder, Asperger Syndrome, childhood disintegrative disorder, Rett Syndrome, and pervasive developmental disorder not otherwise specified (PDD-NOS). Most recent population surveillance data estimate the prevalence of ASD in children at one in 58, with a ratio of about four affected males for every affected female (Baio et al., 2018).
Treatment of core symptoms of ASD consists of a multi-modal treatment approach which can include behaviour management, speech therapy, occupational and physical therapy, specialized educational supports and medications for comorbid conditions (Volkmar et al., 2014). While behavioural disturbances themselves are not core ASD symptoms, they often arise consequent to functional difficulties related to the condition’s core symptoms. For example, impairments in social communication can result in externalizing behaviours. Unfortunately, these can impair an individual’s ability to engage in educational and community activities, can create significant safety concerns for the individual, caregivers, and peers, and increases the likelihood of caregiver burnout. As a result, individuals with ASD are often referred for psychiatric consultation for concerns related to behavioural disturbances including aggression, agitation, self-injurious behaviours (SIB) and irritability (Weller, Rowan, Elia, & Weller, 1999).
Only two medications, risperidone and aripiprazole, have a demonstrated evidence base for their use in ASD-related irritability in children (McCracken et al., 2002; Owen et al., 2009; Elbe & Lalani, 2012); neither medication has regulatory approval in Canada for this indication in children or adults with ASD. Furthermore, the adverse effect profile for these medications is not benign. Atypical antipsychotics are associated with development of metabolic syndrome (weight gain, dyslipidemia, insulin resistance), risk of QTc prolongation, potential for development of extrapyramidal symptoms (akathisia, dystonia, secondary parkinsonism) and tardive dyskinesia, and the rare but serious risk of neuroleptic malignant syndrome (Solmi et al., 2017). Both medications are prescribed off-label for a breadth of challenging behaviours. Unfortunately, in some cases neither are effective even in combination with non-pharmacological strategies. Given the limited pharmacological interventions available to aid in management of challenging behavioural symptoms associated with ASD, clonidine has been utilized off-label.
Clonidine, an alpha-2 receptor agonist, was first approved in the 1960s to treat hypertension (Riddle et al., 1999). It has since been used for other indications off-label. Clonidine is first-line treatment for tics in Tourette’s disorder and third-line pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) in children (Pringsheim et al., 2014; Canadian ADHD Resource Alliance [CADDRA], 2018). Clonidine has been reported, albeit in uncontrolled studies, to be effective in typically developing children with aggression and anger and in children with conduct disorder exhibiting aggression (Comings, Comings, Tacket, & Li, 1990; Chandran 1994; Kemph, DeVane, Levin, Jarecke, & Miller, 1993; Schvehla, Mandoki, & Sumner, 1994). Usual total daily doses for both Tourette’s disorder and ADHD are 0.1–0.4mg administered as three to four divided doses (American Academy of Pediatrics [AAP], 2019; Gormley, Turner, & Freeland, 2014; Qasaymeh & Mink, 2006). Caution is required with titration and tapering of clonidine due to its effects on heart rate and blood pressure (Riddle et al., 1999).
No systematic review has appraised the evidence supporting or refuting the effectiveness of clonidine for the indication of management of behavioural disturbance. Thus, the purpose of this review is to evaluate all evidence available describing the use and effectiveness of clonidine in children and adults with ASD for management of behavioural disturbances observed in ASD, Examples of behavioural disturbance in ASD may include one or more of aggression, agitation, hyperarousal, impulsivity, social difficulties (apart from core symptoms of ASD), mood disturbance, SIB and sleep disturbance.
Methods
The primary outcome of interest explored in this review was changes in behaviour compared to baseline in patients with ASD treated with and without clonidine. A secondary outcome explored was identification of adverse effects from clonidine.
Studies for inclusion in the systematic review required satisfaction of the following criteria: DSM or International Classification of Diseases (ICD) diagnosis of autism spectrum disorder, encompassing current and historical diagnostic terms including autistic disorder, childhood autism, Asperger syndrome, childhood disintegrative disorder, Rett Syndrome, and PDD-NOS; age and sex of the patient(s); use of clonidine for behavioural disturbance; and description of clinical response to use of clonidine using qualitative or quantitative means. Given the anticipated limited evidence base for the use of clonidine in a circumscribed patient population, inclusion criteria for study type were deliberately broad. Publications employing any study design were deemed eligible for review, including case reports in the form of letters to the editor. Non-English language publications were included if the publisher provided a full-text English translation. Years searched were based on the years spanned by the respective databases (MEDLINE: 1950-present, EMBASE: 1947-present, PSYCinfo: 1840-present).
Search Strategy
MEDLINE, EMBASE, and PSYCinfo database searches were completed in January 2019. ASD was searched using the following terms combined by Boolean operator “OR”: subject headings autism/, autism spectrum disorder/, autistic disorder/, child development disorders, pervasive/, infantile autism/, as well as (autism or autistic or Asperger*).mp, (pervasive adj2 development* disorder*).mp.
For clonidine, search terms included subject heading “clonidine/” and all clonidine name variations (including Canadian and international brand names and chemical names). Each term was searched using the “.mp” suffix and if applicable, any near identical terms were shortened using the “*” character to capture all appearances of the term. In total, 60 synonymous terms for clonidine were searched using the Boolean operator “OR”. Hits obtained for all ASD and all clonidine terms were combined using Boolean operator “AND.” Appendix 1 shows an example of the complete search strategy for one database (EMBASE), including the list of clonidine synonyms.
Article Extraction and Compilation
Publications were screened by title and abstract to identify potentially relevant articles. In rare instances where an abstract was unavailable via database record then the actual publication was located for full review. The full-text of each short-listed publication was reviewed to ascertain if it met selection criteria. The short-listed publications’ references were screened for additional studies.
Data Extraction
The following items were extracted from identified studies: study design, number of patients, age, sex, patient diagnosis, treatment setting, intellectual functioning, adaptive functioning, psychiatric and medical comorbidities, clonidine formulation, clonidine dose range, duration of treatment, dose changes or discontinuation with reasons if provided, comparator treatment, concomitant medications taken with doses if provided, targeted behavioural disturbance(s), type of outcome measure used (scales, observations), behaviour outcomes (qualitative or quantitative), caregiver and clinician qualitative and quantitative impressions regarding behaviour change, and medication adverse effects. In addition to the above items, for randomized trials the number of study drop-outs, randomization methods, and blinding were documented. All data were collated in table format.
Synthesis of Studies
The Cochrane Collaboration Assessing Risk of Bias in Crossover Trials Tool (Higgins et al., 2016) was used to comment on the quality of randomized studies identified for review and to assist in decision making regarding appropriateness of meta-analysis. For the remainder of studies, any methodology employed to demonstrate objectivity and minimize bias despite inherent study design limitations was highlighted.
Results
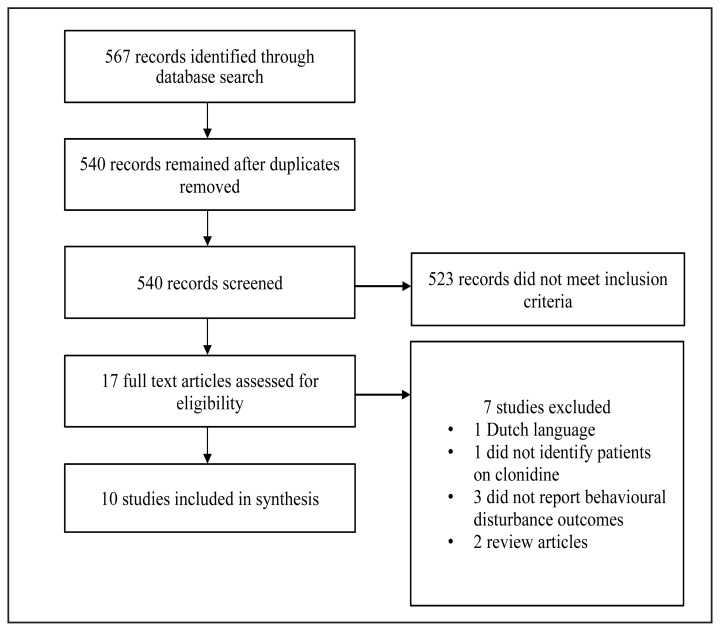
Figure 1 is a PRISMA flow diagram summarizing the publications retrieved, reviewed, and reasons for exclusion. The search of MEDLINE, EMBASE, and PSYCinfo databases yielded 567 publications. After adjusting for duplicates, 540 unique hits remained for screening. A subset of 27 publications were assessed with 17 discarded due to not meeting eligibility criteria. One article was discarded as it was only available in the Dutch language. Three studies did not report outcomes related to behavioural disturbance with clonidine use. One chart review study was rejected as it did not identify which patients used clonidine for behavioural disturbance nor outcomes related to clonidine use. Two articles were excluded because they were research reviews. No articles were identified for inclusion from reference list screening. In total, ten studies met inclusion criteria.
Figure 1.
The PRISMA flow chart summarizes the search, selection, and exclusion of articles related to clonidine use for behavioural disturbances in ASD.
Randomized Studies
Table 1 provides an overview of notable findings from both crossover studies.
Table 1.
Comparison of Crossover Studies Examining Use of Clonidine in ASD.
| Study | # Patients | Age Range (years) | Sex | Clonidine Preparation | Dose Range | Treatment Duration | Notable Outcomes with Clonidine Use |
|---|---|---|---|---|---|---|---|
| Jaselskis et al., 1992 | 8 | 5–13 | male | tablet (TID dosing) | 0.15–0.2mg daily | 6 weeks | CPTQ: decrease* in hyperactive, inattentive, impulsive behaviours observed by parents ABC: decrease* on irritability and hyperactivity subscales observed by teachers CGAS, CPRS, CGI-I: No significant difference observed by clinicians |
| Fankhauser et al., 1992 | 9 | 5–33 | male | transdermal patch (weekly dosing) | 0.1–0.3mg daily | 4 weeks | CPTQ: no difference observed by parents Parent Likert Rating Scale: improvement* in behaviour observed by parents RLRS: decrease* in sensory responses (agitated by noise, stereotypy) CGI-I: improvement* in global impression subscale noted by clinicians |
denotes statistically significant finding. CPTQ: Conners Parent Teacher Questionnaire. ABC: Aberrant Behaviour Checklist. CGAS: Children’s Global Assessment Scale. CPRS: Children’s Psychiatric Rating Scales. CGI-I; Clinical Global Impression- Improvement. RLRS: Ritvo-Freeman Real Life Rating Scale.
The only randomized studies employing clonidine as an intervention for behavioural disturbance in the ASD population were both crossover trials. The patient population in one study (Fankhauser, Karumanchi, German, Yates, & Karumanchi, 1992) consisted of eight males with ASD between the ages of five and 33 years, while the other study (Jaselskis, Cook, Fletcher, & Leventhal, 1992) studied effects of clonidine in eight males with ASD between ages five and 13 years. All patients were treated on an outpatient basis. Intellectual functioning varied between studies, with one study including patients with intelligence quotient (IQ) 30–75 (severe to mild intellectual disability) while the other included males with intellectual functioning between “moderate mental retardation” (spanning approximately IQ 40–100) to “normal” (Fankhauser et al., 1992; Jaselskis et al., 1992). Co-morbidities in the respective study populations differed; notably, two patients in one group (Fankhauser et al., 1992) had seizure disorder whereas in the other there were no reports of seizure disorder but all patients had hyperactivity, distractibility, and impulsivity excessive for their developmental level which impaired their function in multiple environments (Jaselskis et al., 1992).
Regarding the experimental intervention, one study examined use of weekly-applied transdermal clonidine patch (Fankhauser et al., 1992) whereas the other utilized oral clonidine (Jaselskis et al., 1992). Both studies utilized similar dosage ranges: an estimated 0.1–0.3mg total daily dose via transdermal patch and 0.15–0.2 mg total daily dose via tablets over similar treatment durations preceded by at least a two-week medication-free period. Experimental designs differed in that one study lacked a washout period before crossover to the alternate treatment, risking the potential for effects of the initial treatment to persist during the alternate treatment period (Jaselskis et al., 1992).
In terms of behavioural disturbances examined, both crossover studies examined changes in level of hyperactivity with clonidine use. However, one study also examined changes in inattention, impulsivity, and irritability (Jaselskis et al., 1992) while the other study focused their observations on reductions in “hyperarousal” (defined to include stereotypy, self-stimulatory behaviours, hypervigilance and hyperactivity) (Fankhauser et al., 1992). Jaselskis et al. (1992) demonstrated a statistically significant decrease in hyperactivity identified by parents via Conners Parent-Teacher Questionnaire (CPTQ) and by teachers via the Aberrant Behaviour Checklist (ABC). Of note, teachers noted significant decrease in irritability subscale of the ABC with use of clonidine. Interestingly, clinicians’ scores on Children’s Global Assessment Scale (CGAS), modified Children’s Psychiatric Rating Scale (CPRS), and Clinical Global Impressions-Improvement (CGI-I) scale did not reflect any changes in behaviour disturbance between clonidine and placebo (Jaselskis et al., 1992).
In Fankhauser et al., (1992), parent ratings via CPTQ did not reflect any statistically significant difference in hyperactivity, impulsivity, or inattention with clonidine use (Fankhauser et al., 1992). An author-created and parent-rated “autistic rating scale” to assess core symptoms of ASD also did not show any significant differences with clonidine. However, parents did report a significant improvement on a Likert scale assessing noticeable changes in behaviour at different time points during the study which corresponded with placebo versus several weeks of clonidine treatment (Fankhauser et al., 1992). Clinician ratings reflected significant improvement on the CGI-I “global improvement score” as well as significant decrease in the “sensory responses” symptom domain assessed via Ritvo-Freeman Real Life Rating Scale (RLRS) (Fankhauser et al., 1992).
Both randomized studies shared similar drop-out rates (two and one respectively) for reported excess sedation, moving away from trial location, and medication non-compliance (Fankhauser et al., 1992; Jaselskis et al., 1992).
Risk of bias of the crossover studies was evaluated using the Cochrane Risk of Bias (RoB) tool. One study (Jaselskis et al., 1992) was evaluated at high risk of bias and the other was found to pose some concern for bias (Fankhauser et al., 1992). Areas of greatest concern of bias related to randomization and deviation from intended intervention (Jaselskis et al., 1992), while unavailability of information in several RoB tool domains increased the concern for bias in the other study. Meta-analysis was not conducted based on assessment of risk of bias, compounded by a very small patient pool (N=15). The assessed RoB of the individual studies also differed. Further, each study used a different formulation of clonidine (transdermal patch versus oral tablets) in two different populations (all ages versus young children).
Case Studies
Table 2 summarizes relevant observations made by caregivers, teachers, and/or clinicians with regards to the impact of clonidine treatment on patient behaviour.
Table 2.
Comparison of Findings Across Case Studies Examining Use of Clonidine in ASD.
| Study | # Patients | Age range (years) | Sex | Clonidine Preparation | Dose range | Treatment duration | Notable outcomes with clonidine use |
|---|---|---|---|---|---|---|---|
| Blew, Luiselli, & Thibadeau, 1999 | 1 | 9 | female | tablet | 0.3–0.4mg daily | 3.5 years |
|
| Cocchi, 1996 | 1 | 3 | female | tablet | 0.05–0.1mg daily | 3 months |
|
| Dillon, 1990 | 1 | 7.5 | female | transdermal patch | 0.5mg weekly | 18 months |
|
| Dowben, Grant, & Keltner, 2011 | 3 | 18–30 | 2 males 1 female | tablet | 0.05–0.15mg daily | unclear |
|
| Ghaziuddin, Tsai, & Ghaziuddin, 1992 | 7 | 5–13 | 5 males 2 females | tablet | 0.05–0.25mg day | 3 months |
|
| Koshes & Rock, 1994 | 1 | 21 | female | tablet followed by transdermal patch | 0.4–0.6mg daily | 4 weeks |
|
| McCracken & Martin, 1997 | 1 | 8 | male | tablet | 0.2mg daily | unclear |
|
| Ming et al., 2008 | 19 | 4–16 | 14 males 5 females | tablet | 0.05–0.1mg day | 6–24 months |
|
SIB: Self-injurious Behaviours
The remaining eight publications eligible for review were either single case studies or case series representing a total of 34 patients, of which 23 were males with ASD. One case series contributed 19 cases (Ming, Gordon, Kang, & Wagner, 2008). Demographically, the patients in the case studies were a heterogeneous group. Age spanned between three to 30 years. Intellectual functioning was described as severe intellectual disability to average or higher IQ; intellectual functioning was not clearly defined in seven cases. Co-morbid conditions varied substantially to include one or more of ADHD, anxiety, borderline personality disorder, epilepsy, intermittent explosive disorder, mood disorder, schizoid personality disorder, sleep difficulties, tic disorder, Tourette Syndrome, and gastrointestinal dysfunction. Thirty patients were treated in the outpatient setting. Twenty patients were taking at least one psychotropic medication in addition to clonidine. The most commonly prescribed medication class was antipsychotics.
Apart from two cases examining the use of clonidine for behavioural disturbance prospectively (Blew, Luiselli, & Thibadeau, 1999; Cocchi 1996), the experimental design, duration of treatment, and means of measuring outcomes were inconsistent across case studies. Clonidine was always administered open-label, total daily dose between 0.05mg to 0.4mg, and most frequently in tablet form (25 patients). Seven patients transitioned from transdermal patch to tablet due to patch intolerance. One exception was a 26-year-old receiving a daily dose of 0.6mg administered by weekly transdermal patch (Koshes & Rock, 1994).
The primary objective of all the case studies was to describe the effect of clonidine on a subset of behaviours. These subsets varied across cases, however included one or more of the following: aggression, hyperactivity, impulsivity, mood disturbance, poor self-care, reactivity, SIB, sleep disturbance, and tics. Special note should be made of one case report that employed a prospective design examining the effect of clonidine on frequency and severity of SIB in a patient with PDD-NOS with severe and treatment-resistant SIB (Blew et al., 1999). Several trained observers completed 12 weeks of daily baseline SIB monitoring followed by 18 weeks of daily SIB monitoring while the patient was on clonidine treatment. Ongoing monitoring, albeit at fewer intervals, continued for the next 24 months to definitively establish ongoing efficacy and benefit of clonidine treatment for severe behavioural disturbance.
Only two other case studies utilized quantitative measures to estimate changes in behavioural disturbance with clonidine treatment: the Conners scale to detect changes in ADHD and other related symptomatology (Ghaziuddin, Tsai, & Ghaziuddin, 1992) and an author-created Likert scale for parents to rate presence and absence of behavioural disturbances (Ming et al., 2008). Qualitative observations of clonidine effects were regularly described from caregiver, teacher, and/or clinician perspective. Seven of eight reports were largely favorable towards clonidine’s impact on the targeted behavioural disturbances.
Adverse Effects
Adverse effects of clonidine were reported in both crossover trials and six case studies. The most commonly reported adverse effects described were drowsiness, sedation, lethargy/fatigue, and hypotension. One patient discontinued treatment due to excess sedation (Fankhauser et al., 1992). Sedation and fatigue were observed in several cases during the initial treatment period but abated within weeks and were inconsistently reported to worsen with dose titration. With the transdermal formulation, seven patients discontinued transdermal clonidine in favour of oral clonidine due to redness and irritation at the patch skin site. As expected with an anti-hypertensive medication, in several studies hypotension was identified but patients remained asymptomatic. Hypotension was not reported as a reason for clonidine discontinuation in any study.
Irritability was inconsistently reported as a side effect, however in one case report clonidine administration for aggression led to development of “severe syndromal depression” associated with increased aggressive behaviour despite initial observed improvement of historical aggressive behaviours. Withdrawal of clonidine reversed the depression and severity of aggression and SIB (McCracken & Martin, 1997).
A single case report stated that clonidine withdrawal precipitated increased aggression and impulsive self-destructive acts (suffocation) and with reinstitution of clonidine both the new and original behavioural disturbances abated (Dillon, 1990).
Discussion
The primary outcome of interest explored in this review was changes in behaviour in patients with ASD treated with clonidine, while the secondary outcome explored was adverse effects from clonidine. To capture all available evidence for clonidine use in this population, behavioural disturbance was broadly defined to include one or more of the following: aggression, agitation, hyperarousal, impulsivity, social difficulties, mood disturbance, SIB, and sleep disturbance.
The two studies comprising the highest level of evidence for clonidine use for behavioural disturbance in ASD were conducted on two small cohorts of patients. Meta-analysis was not deemed appropriate for synthesis of results for several reasons. Bias appraisal using the RoB tool revealed moderate to high potential for bias. While both studies shared evaluation of some similar outcomes with CPTQ, the studies demonstrated opposite effects which could be a function of substantially different study cohorts. Specifically, one study clearly selected a subset of young males with ASD with significant symptomatology suggestive of ADHD (Jaselskis et al., 1992). The other study cohort’s symptomatology was depicted as more heterogeneous, supported by the lack of significant difference in CPTQ scores with clonidine use (Fankhauser et al., 1992). Therefore, the pooling of such disparate cohorts with a very small pooled N of 15 would have been unlikely to generate any meaningful conclusion and, as a result, a narrative synthesis was deemed more appropriate.
The primary outcomes examined by both crossover studies related to ADHD symptomatology, however both studies examined additional relevant outcomes. From a qualitative perspective, the crossover studies are inconsistent with respect to the observed impact of clonidine on behavioural disturbances in ASD. Fankhauser and colleagues (1992) concluded that clonidine dosed between 0.1 to 0.3mg daily demonstrated greatest benefit in management of hyperarousal associated with autism. Specifically, the authors noted that clinicians observed improvement in the participants’ sensory responses to the environment as evidenced by less agitation to noise and new activities, less repetitive behaviours and vocalizations, and less spinning of objects (Fankhauser et al., 1992). Both caregivers and clinicians reported “overall improvement” with clonidine treatment as assessed by an author-created Likert global improvement scale and the CGI-I respectively (Fankhauser et al., 1992). In comparison, the study by Jaselskis and colleagues (1992) reported that only teachers identified a significant decrease in irritability when children with ASD were administered clonidine 0.15–0.2mg daily in three divided doses. These inconsistent observations across studies likely reflect the heterogeneity of symptoms across individuals with ASD and between cohorts utilizing only children versus children and adults with ASD. Still, both studies support clonidine use for decreasing hyperarousal and ameliorating irritability in ASD.
Table 3.
Reported Adverse Effects with Clonidine Use in Children and Adults with ASD*
| Study | Adverse Effects with Clonidine Use |
|---|---|
| Blew, Luiselli, & Thibadeau, 1999 | Lethargy, asymptomatic hypotension |
| Dowben, Grant, & Keltner, 2011 | Weight gain of 5–6 lbs (2.3–2.6kg) |
| Fankhauser et al., 1992 | Sedation, fatigue, irritation at transdermal patch site |
| Ghaziuddin, Tsai, & Ghaziuddin, 1992 | Sedation, asymptomatic hypotension |
| Jaselskis et al., 1992 | Drowsiness, decreased level of activity, asymptomatic hypotension, irritability |
| Koshes & Rock, 1994 | Transdermal patch losing adhesiveness |
| McCracken & Martin, 1997 | “Severe syndromal depression”, self-injurious behaviours, worsened aggression |
| Ming, et al., 2008 | Sedation, lethargy, asymptomatic hypotension, tachycardia, skin irritation at transdermal patch site |
Two case reports were not included as neither reported any adverse effects.
Of the identified case studies, all but one study endorsed at least some benefit from treatment with clonidine in children and young adults of both sexes with ASD. As first demonstrated several decades ago by Hunt, Minderaa, and Cohen (1985), symptoms most frequently responding to clonidine are those associated with ADHD (hyperactivity and impulsivity). Still, about two-thirds of the cases across eight studies reported at least partial improvement with initiation of clonidine of one or more non-ADHD-related behavioural disturbances (Blew et al., 1999; Cocchi, 1996; Dillon, 1993; Dowben et al., 2011; Ghaziuddin et al., 1992; Grant, & Keltner, 2011; Koshes & Rock, 1994; Ming et al., 2008). The behaviours most commonly reported to improve with clonidine administration included sleep disturbance, aggression, and SIB. As shown in Table 2, no single behavioural disturbance was consistently assessed across all case reports. In those individuals for whom clonidine was particularly effective for aggression or SIB, the decreased intensity, frequency, or (in some instances) full extinguishment of the behavioural disturbance was reported to result in improved patient engagement in school, home or community activities (Blew et al., 1999; Cocchi, 1996; Dillon, 1993; Koshes & Rock, 1994). Furthermore, the positive effects attributed to clonidine were reported to persist, in some cases, for 18 months or longer (Blew et al., 1999; Dillon, 1993). Clonidine responsiveness did not consistently appear to increase if patients were already prescribed one or more concomitant psychotropic agents; however, the potential for a synergistic effect cannot be ruled in or out based on the available data.
Several reports reinforced that behavioural improvements were exclusive of the known sedative effect of clonidine. Notably, one case series highlighted the use of clonidine for sleep difficulties in ASD, with partial to full resolution of sleep difficulties for all patients plus the added benefit of partial or greater improvement of behavioural difficulties, mood problems, and ADHD symptoms for more than half of those patients (Ming et al., 2008). It remains unclear whether clonidine itself or the improvement in overall sleep quality alone provided the nidus for the decrease in behavioural disturbances.
Despite the overall positive endorsement for clonidine in ASD, a single case report cautioned against its whole-hearted recommendation. The case described a dramatic increase in aggressive behaviour, new-onset depression and suicidal gestures during clonidine use in a young child with ASD which reversed upon clonidine discontinuation (McCracken & Martin, 1997). While it is impossible to ascertain causation by clonidine for the significant behaviour change, this case serves as a reminder for prescribers to vigilantly monitor for paradoxical effects, particularly when prescribing for off-label indications.
Apart from the significant adverse effect described above, clonidine was well tolerated, with only one patient discontinuing secondary to excess sedation (Fankhauser et al., 1992). Adherence may be a greater issue if the transdermal formulation (which is unavailable in Canada) is used, secondary to the drug delivery mechanism rather than to clonidine adverse effects (Ming et al., 2008). Hypotension, bradycardia, and rebound hypertension and tachycardia are known clonidine adverse effects that can largely be avoided with careful monitoring and slow dosage adjustment (Riddle et al., 1999).
Limitations
Because study designs, participant demographics, additional interventions, behavioural disturbance descriptions and reported outcome measures varied markedly between publications, this review focused on collation and comparison of qualitative descriptions. Limitations in interpretation of the available data are largely due to lack of consistent use of validated tools to evaluate response to clonidine treatment. Further, only two reports examined the effectiveness of clonidine in adults (Dowben et al., 2011; Koshes & Rock, 1994). Whether this indicates limited clonidine effectiveness in adults with ASD or reflects that studies have not yet been undertaken in this population is unclear. Nevertheless, this limits the ability to comment on clonidine’s usefulness in the adult ASD population.
Most available data regarding clonidine use in ASD originated from case reports; half of these were letters to the editor. With the likelihood that only letters reporting overtly positive findings regarding clonidine use in ASD would be chosen for publication, such findings may indicate publication bias. Despite relative lenience of inclusion criteria, the restriction to English language publications resulted in at least one additional case report being excluded.
Conclusion
Clonidine may be an effective and low-cost pharmacological option for individuals with ASD and behavioural disturbances for whom little or no benefit has been derived from evidence-based pharmacological interventions combined with robust behaviour management strategies delivered by interdisciplinary teams. The available data, while supportive of clonidine, arose from a small collection of case reports and two crossover trials; thus, we can only support a weak recommendation for use of clonidine for behavioural disturbances in ASD. Youth with ASD who struggle with hyperactivity, hyperarousal, impulsivity, sleep difficulties, aggression and/or SIB are most likely to benefit. Further research is required before any clear recommendation can be made regarding clonidine for adults with ASD and behaviour disturbances.
Given the paucity of available data, additional studies, ideally of a randomized design and involving the adult ASD population, would be paramount in establishing clonidine’s role in the management of this complex, phenotypically diverse, and often challenging treatment population.
Acknowledgements / Conflicts of Interest
The authors have no financial relationships to disclose.
Appendix 1. Full search terms for Embase database search
clonidine/
catapres*.mp.
chlophazolin*.mp.
clofelin*.mp.
clofenil*.mp.
“clonidine dihydrochloride”.mp.
“clonidine hydrochloride”.mp.
“clonidine monohydrobromide”.mp.
“clonidine monohydrochloride”.mp.
clophelin*.mp.
“dihydrochloride, clonidine”.mp.
dixarit*.mp.
gemiton*.mp.
hemiton*.mp.
“hydrochloride, clonidine”.mp.
isoglaucon.mp.
klofelin.mp.
klofenil.mp.
(m adj2 5041t).mp.
monohydrobromide, clonidine.mp.
“monohydrobromide, clonidine”.mp.
monohydrochloride, clonidine.mp.
“monohydrochloride, clonidine”.mp.
adesipress*.mp.
adesipress tts2.mp.
“adesipress tts2”.mp.
arkamin.mp.
atensina.mp.
caprysin.mp.
catasan.mp.
chlofazolin.mp.
chlophazolin.mp.
clinidine.mp.
clofelin*.mp.
clomidine.mp.
clondine.mp.
clonicel.mp.
clonidin.mp.
“clonidine chlorhydrate”.mp.
“clonidine hydrochloride”.mp.
clonidine tts*.mp.
clonipresan.mp.
clonistada*.mp.
clonnirit.mp.
daipres.mp.
dcai.mp.
dichlorophenylaminoimidazoline*.mp.
duraclon.mp.
haemiton.mp.
hemiton.mp.
huma-clonidine.mp.
hypodine.mp.
jenloga.mp.
kapvay*.mp.
melzin.mp.
normopres*.mp.
paracefan.mp.
(st adj2 “155”).mp.
sulmidine.mp.
taitecin.mp.
(tenso adj 2 timelets).mp.
autism/
autism spectrum disorder/
asperger syndrome/
autistic disorder/
child development disorders, pervasive/
(autism or autistic or asperger*).mp.
(pervasive adj2 development* disorder*). mp.
infantile autism/
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61
62 or 63 or 64 or 65 or 66 or 67 or 68 or 69
References
- American Academy of Pediatrics. Clonidine – Catapres (IR) 2019. Retrieved June 5, 2019, from https://www.aap.org/en-us/professional-resources/Psychopharmacology/Pages/Clonidine-Catapres-IR.aspx.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Dowling NF. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report Surveillance Summary. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blew P, Luiselli JK, Thibadeau S. Beneficial effects of clonidine on severe self-injurious behavior in a 9-year-old girl with pervasive developmental disorder. Journal of Child and Adolescent Psychopharmacology. 1999;9(4):285–291. doi: 10.1089/cap.1999.9.285. [DOI] [PubMed] [Google Scholar]
- Canadian ADHD Resource Alliance. Canadian ADHD Practice Guidelines. 4th ed. Toronto, ON: CADDRA; 2018. [Google Scholar]
- Chandran KS. ECG and clonidine. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(9):1351–1352. doi: 10.1097/00004583-199411000-00023. [DOI] [PubMed] [Google Scholar]
- Cocchi R. A work in progress on drug therapy of an autistic child aged three (at first consultation): 2. The second six-months’ therapy. Italian Journal of Intellective Impairment. 1996;9(1):31–102. [Google Scholar]
- Comings DE, Comings BG, Tacket T, Li SZ. The clonidine patch and behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29(4):667–668. doi: 10.1097/00004583-199007000-00026. [DOI] [PubMed] [Google Scholar]
- Dillon JE. Self-injurious behavior associated with clonidine withdrawal in a child with Tourette’s disorder. Journal of Child Neurology. 1990;5(4):308–310. doi: 10.1177/088307389000500406. [DOI] [PubMed] [Google Scholar]
- Dowben JS, Grant JS, Keltner NL. Clonidine: Diverse Use in Pharmacologic Management. Perspectives in Psychiatric Care. 2011;47(2):105–108. doi: 10.1111/j.1744-6163.2011.00302.x. [DOI] [PubMed] [Google Scholar]
- Elbe D, Lalani Z. Review of the pharmacotherapy of irritability in autism. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2012;21(2):130–146. [PMC free article] [PubMed] [Google Scholar]
- Fankhauser MP, Karumanchi VC, German ML, Yates A, Karumanchi SD. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. The Journal of Clinical Psychiatry. 1992;53(3):77–82. [PubMed] [Google Scholar]
- Ghaziuddin M, Tsai L, Ghaziuddin N. Clonidine for autism. Journal of Child and Adolescent Psychopharmacology. 1992;2(4):239–240. [Google Scholar]
- Gormley L, Turner A, Freeland K. Clonidine and guanfacine IR vs ER: Old drugs with “new” formulations. Mental Health Clinician. 2014;4(1):22–26. [Google Scholar]
- Gunning WB, Ferdinand RF, de Vrijer JC, Minderaa RB. The application of clonidine in child and adolescent psychiatry. Tijdschrift voor Psychiatrie. 1990;32(7):462–472. [Google Scholar]
- Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Eldridge S. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. 2016. (Original work published Cochrane Database of Systematic Reviews, 10(Suppl 1).) [Google Scholar]
- Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: Report of a double-blind placebo-crossover therapeutic trial. Journal of the American Academy of Child Psychiatry. 1985;24(5):617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- Jaselskis CA, Cook EH, Jr, Fletcher KE, Leventhal BL. Clonidine treatment of hyperactive and impulsive children with autistic disorder. Journal of Clinical Psychopharmacology. 1992;12(5):322–327. [PubMed] [Google Scholar]
- Kemph JP, DeVane CL, Levin GM, Jarecke R, Miller RL. Treatment of aggressive children with clonidine: Results of an open pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(3):577–581. doi: 10.1097/00004583-199305000-00013. [DOI] [PubMed] [Google Scholar]
- Koshes RJ, Rock NL. Use of clonidine for behavioral control in an adult patient with autism. The American Journal of Psychiatry. 1994;151(11):1714. doi: 10.1176/ajp.151.11.1714b. [DOI] [PubMed] [Google Scholar]
- McCracken JT, Martin W. Clonidine side effect. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):160–161. doi: 10.1097/00004583-199702000-00002. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Stigler KA, Posey DJ. Treatment of aggression in children and adolescents with autism and conduct disorder. Journal of Clinical Psychiatry. 2003;64(SUPPL 4):16–25. [PubMed] [Google Scholar]
- Ming X, Gordon E, Kang N, Wagner GC. Use of clonidine in children with autism spectrum disorders. Brain and Development. 2008;30(7):454–460. doi: 10.1016/j.braindev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Owen R, Sikich L, Marcus RN, Corey-Lisle P, Manos G, McQuade RD, …Findling RL. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Doja A, McKinlay D, Billinghurst L, Carroll A, Dion Y, …Sandor P. Canadian guidelines for the evidence-based treatment of tic disorders: Pharmacotherapy. Canadian Journal of Psychiatry. 2012;57(3):133–143. doi: 10.1177/070674371205700302. [DOI] [PubMed] [Google Scholar]
- Qasaymeh MM, Mink JW. New treatments for tic disorders. Current Treatment Options in Neurology. 2006;8(6):465–473. doi: 10.1007/s11940-006-0036-4. [DOI] [PubMed] [Google Scholar]
- Riddle MA, Bernstein GA, Cook EH, Leonard HL, March JS, Swanson JM. Anxiolytics, adrenergic agents, and naltrexone. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(5):546–556. doi: 10.1097/00004583-199905000-00016. [DOI] [PubMed] [Google Scholar]
- Schvehla TJ, Mandoki MW, Sumner GS. Clonidine therapy for comorbid attention deficit hyperactivity disorder and conduct disorder: Preliminary findings in a children’s inpatient unit. Southern Medical Journal. 1994;87(7):692–695. doi: 10.1097/00007611-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, Carvalho AF. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: A state-of-the-art clinical review. Therapeutics and Clinical Risk Management. 2017;2017(13):757–777. doi: 10.2147/TCRM.S117321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J, State M the American Academy of Child and Adolescent Psychiatry Committee on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(2):237–257. doi: 10.1016/j.jaac.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Weller EB, Rowan A, Elia J, Weller RA. Aggressive behavior in patients with attention-deficit/hyperactivity disorder. 1999 [PubMed] [Google Scholar]