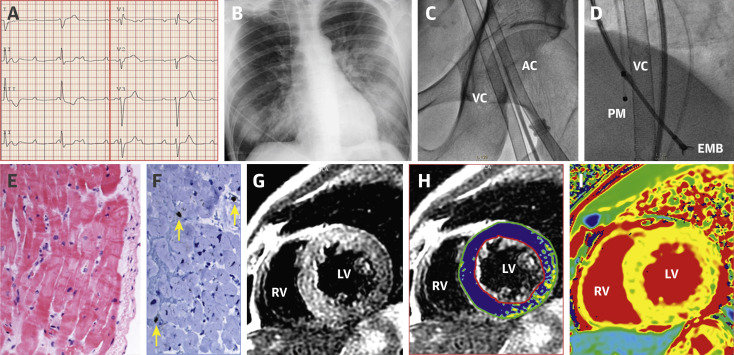
A previously healthy 44-year-old man was admitted to our hospital for severe dyspnea and syncope on March 25, 2020. Seven days before, during the escalating coronavirus disease-2019 (COVID-19) pandemic in our country, he presented at the Emergency Department with fever, dry cough, diarrhea, and myalgia, being diagnosed as presumed COVID-19 infection. He was discharged home with symptomatic therapy and isolation measures. However, symptoms worsened over the following days and finally he came back with severe bradycardia, hypotension, and signs of peripheral hypoperfusion. The electrocardiogram (ECG) showed a third-degree atrioventricular block (Figure 1A ) and an echocardiogram revealed a nondilated but globally and severely dysfunctional left ventricle (left ventricular ejection fraction [LVEF] ∼15%) (Video 1). A temporary pacemaker was implanted and both dobutamine and norepinephrine perfusions were initiated but, eventually, intubation and mechanical ventilation were required. High-sensitive troponin T peak was 745 ng/l, creatine-kinase isoenzyme MB was 30 U/l, and N-terminal pro–B-type natriuretic peptide increased to 24,167 pg/ml. Nasopharyngeal and oropharyngeal swabs for polymerase chain reaction test of COVID-19 and other respiratory viral infections were obtained. Only SARS-CoV-2 had positive results, whereas influenza A virus, influenza A H1N1, influenza A H3N2, bocavirus, adenovirus, rhinovirus, parainfluenza, metapneumovirus, influenza B virus, other common coronaviruses, and respiratory syncytial virus were negative. Legionella pneumophilla, Mycoplasma pneumoniae, and Chlamydophila pneumonia serological test results were negative. Chest X-ray showed signs of bilateral pneumonia (Figure 1B). In spite of increasing doses of vasoactive drugs, hemodynamic derangement ensued and in this dramatic clinical scenario urgent coronary angiography revealed normal coronary arteries. Venous-arterial extracorporeal membrane oxygenation (Figure 1C) and an intra-aortic balloon pump were implanted through femoral cannulation with drastic improvement of the hemodynamic condition. Several endomyocardial biopsy samples were obtained (Figure 1D). A working diagnosis of “fulminant myocarditis” was made, and a 1,000-mg bolus of methylprednisolone was administered followed by treatment with tocilizumab, hydroxychloroquine, azithromycin, and lopinavir-ritonavir. Blood test results showed abnormal values of D-dimer (3.17 μg/ml), ferritin (1,135 ng/ml), and circulating interleukin-6 (121.71 pg/l). Myocardial samples showed no significant inflammatory infiltrates, even after CD3, CD20, and CD68 staining (Figures 1E and 1F) and steroid therapy was withheld. Clinical status improved during the following days, with a rapid reduction of lactate levels to normal values, normalization of kidney and liver functions, and progressive recovery in left ventricular systolic function (Video 2). Blood test results showed reduction of high-sensitive troponin T levels to 221 ng/l and N-terminal pro–B-type natriuretic peptide to 7,624 pg/ml. Venous-arterial extracorporeal membrane oxygenation and the intra-aortic balloon could be successfully withdrawn 6 days after implantation and the patient could be eventually weaned from ventilation 2 days later. On day 14 from admission, cardiac magnetic resonance imaging was performed. A nondilated left ventricle without regional wall motion abnormalities was seen (LVEF ∼75%) (Video 3). Native T1 (mean, 1,120 ms), T2 signal intensity ratio (myocardium to serratus anterior muscle on T2 images processed using a signal intensity correction algorithm), and extracellular volume (mean, 36%) were diffusely increased with slightly less involvement of the inferolateral wall (Figures 1G to 1I). Late gadolinium enhancement was negative (Supplemental Figure 1). These findings were suggestive of diffuse edema without macroscopic necrosis. Subsequent clinical course was uneventful with a striking complete recovery of left ventricular systolic function (LVEF ∼70%) on echocardiography.
Figure 1.
ECG, Radiographic, Angiographic, Myocardial Biopsy, and Cardiac Magnetic Resonance Findings
(A) ECG showing complete atrioventricular block. (B) Chest X-ray depicting diffuse bilateral infiltrates. (C) Femoral access VA-ECMO. (D) Radiological image showing the EMB forces. (E) EMB without necrosis, inflammation, or fibrosis (HEx200). (F) Isolated intersticial infiltrate with lymphocytes CD3+ (yellow arrows). (G,H) T2-weighted and T2 signal intensity ratio mapping images (blue indicates a ratio more than 2) showing diffuse edema with slightly less involvement of the inferolateral wall. (I) T1 mapping with diffuse increase of native T1 (septal T1 = 1,120 ms) following the pattern of edema. AC = arterial cannula; ECG = electrocardiogram; EMB = endomyocardial biopsy; LV = left ventricle; PM = pacemaker; RV = right ventricle; VA-ECMO = veno-arterial extracorporeal membrane oxygenation; VC = venous cannula.
The year 2020 will be remembered for the world pandemic due to COVID-19 infection. COVID-19 morbidity and mortality are mainly associated with lung involvement. However, underlying cardiovascular conditions play a major role in clinical outcomes (1,2). Moreover, recent studies suggest that cardiac injury has important prognostic implications. Elevations in cardiac troponin levels are frequently seen with clinically evident myocardial damage demonstrated in most severe cases (1,2). However, so far, only a few cases of COVID-19–related myocarditis have been described (3, 4, 5). Unspecific pathological findings have been described in isolated reports with only 1 necropsy study reporting mild inflammatory infiltrate (5). However, we report the successful treatment of cardiogenic shock with temporary mechanical circulatory support in a patient with a clear diagnosis of COVID-19 infection presenting as fulminant myocarditis. Takotsubo cardiomyopathy remains a potential differential diagnosis considering the stressful situation, the myocardial edema, and the transient left ventricular dysfunction. A wide phenotypic presentation of myocardial damage appears to exist in patients with COVID-19, ranging from mild myocardial injury (asymptomatic troponin elevation) to severe forms of myocarditis (likely secondary to the cytokine storm). The absence of scar might be a clinical marker of myocardial recovery. The fact that cardiac function fully recovered after a few days of mechanical circulatory support is of major interest, opening new avenues for the management of patients critically ill with COVID-19 with fulminant myocarditis because complete recovery of the myocardial function can be expected.
The patient provided informed consent to publish his data but institutional review board approval was not requested considering that this was a clinical observation obtained in a single patient who was strictly treated according to standard clinical practice.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imagingauthor instructions page.
Appendix
For a supplemental figure and videos, please see the online version of this paper.
Appendix
Supplemental Figure 1.
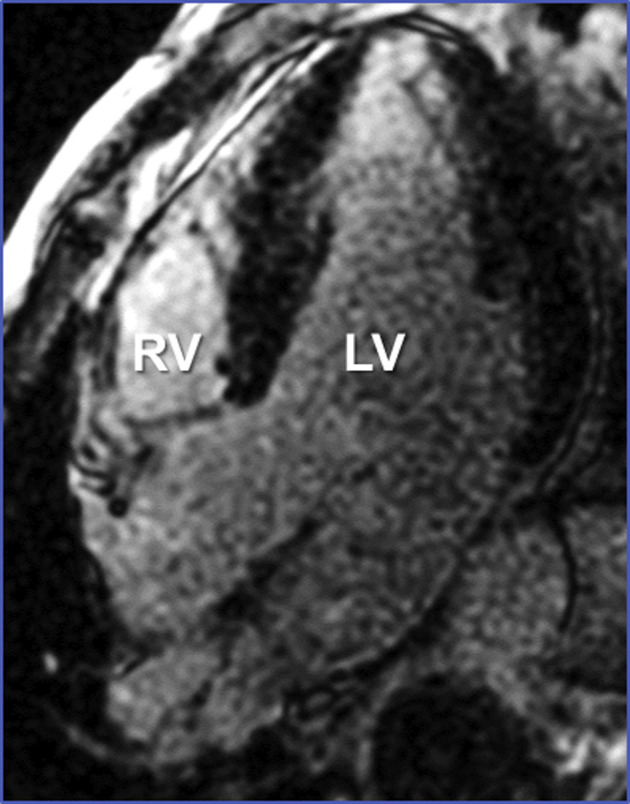
Late Gadolinium Enhancement Without Macroscopic Necrosis.
Pre-ECMO echocardiographic subxiphoid view.Video shows a non-dilated but globally and severely dysfunctional left ventricle.
Post-ECMO echocardiographic subxiphoid view. Video illustrates complete recovery in left ventricular systolic function.
Post-ECMO cardiac magnetic resonance 4-chamber view cine. Video shows a nondilated left ventricle with normal systolic function.
References
- 1.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmerov A., Marban E. COVID-19 and the Heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng J.H., Liu Y.X., Yuan J. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pre-ECMO echocardiographic subxiphoid view.Video shows a non-dilated but globally and severely dysfunctional left ventricle.
Post-ECMO echocardiographic subxiphoid view. Video illustrates complete recovery in left ventricular systolic function.
Post-ECMO cardiac magnetic resonance 4-chamber view cine. Video shows a nondilated left ventricle with normal systolic function.