Abstract
Objective:
Although shared decision making (SDM) is a promising approach for improving outcomes for patients with chronic diseases, no evidence currently supports the use of SDM to delay asthma exacerbations. We evaluated the impact of an SDM intervention implemented by providers in a real-world setting on time to exacerbation in children with asthma.
Methods:
This study used a prospective cohort observed between 2011 and 2013 at five primary care practices that serve vulnerable populations (e.g., Medicaid and uninsured patients) in Charlotte, NC. Patients aged 2 to 17 receiving SDM were matched to those receiving usual care using propensity scores. Time to asthma exacerbation (asthma hospitalization, emergency department visit or oral steroid prescription in the outpatient setting) was compared between groups using Kaplan-Meier curves and conditional Cox proportional hazards models.
Results:
The cohort included 746 children, 60.5% male and 54.2% African American, with a mean age of 8.6 years. Of these, 625 received usual care and 121 received SDM. The final analysis included 100 matched pairs of children. Kaplan-Meier curves showed longer exacerbation-free time for patients in the SDM intervention compared to those in usual care (p = 0.005). The difference in risk of experiencing an exacerbation was marginally significant between the two groups (HR = 0.56, 95% C.I. = 0.29–1.08, p = 0.08).
Conclusions:
SDM was found to delay exacerbations among children with asthma. Clinicians should consider incorporating patient preferences in treatment decisions through SDM as a means for longer exacerbation-free time among children with poor asthma control.
Keywords: Emergency department, hospitalization, oral steroid, practice-based research, propensity score matching, survival analysis
Introduction
Asthma is a chronic airway disease that negatively impacts children as well as adults [1]. Approximately one in eight children in the United States will be diagnosed with asthma at some point in their life [2]. The economic burden of asthma is high, with average annual costs of $1,039 per child. Nearly 50% of children with asthma miss at least one day of school per year [3] and over 20% of children with the disease have an asthma-related emergency department (ED) visit annually [4]. While there is no cure for asthma, appropriate medication and lifestyle management can reduce exacerbations of this disease such as a hospitalization, an ED visit or the need to take an oral steroid medication.
Established in the mid-1990s, the chronic care model aims to provide better chronic illness care and to improve health outcomes through care delivery redesign, use of clinical information systems, coordination of healthcare and community resources, provision of self-management support and individualization of care based on patient needs and values [5, 6]. Shared decision making (SDM), a model of care in which patients and providers jointly participate in the treatment decision and agree on a treatment plan and targeted outcome based on individual patient needs, aligns with the goals of the chronic care model [7]. The U.S. Institute of Medicine recommends using SDM for management of chronic diseases like asthma in outpatient settings [8]. SDM is also advocated as an ideal model for decisions that impact daily management of chronic diseases [9]. The SDM process consists of negotiating and compromising between patients and providers on the proper treatment to benefit the patient. Providers offer expertise on the best evidence and treatment options for patients and their families, while patients share their culture, values and lifestyle to find the optimal treatment plan that will meet their goals [10].
SDM is particularly effective for management of chronic illnesses, including schizophrenic disorders, depression and cancer, where multiple potential evidence-based interventions exist [11]. Moreover, an SDM approach for patients with asthma has been shown to improve control, adherence to medications and quality of life [12–14], and to reduce exacerbations [15]. However, whether SDM delays exacerbations in asthma patients is unknown. The purpose of this study was to determine the impact of an SDM intervention on time to exacerbation in a cohort of patients attending primary care clinics.
Methods
Study setting and participants
This study included patients from five Carolinas HealthCare System primary care practices that serve a high-risk patient population (e.g., Patients with Medicaid, Medicare or who lack insurance) in Charlotte, NC. This study was part of a larger study implemented across 95 primary care practices that was designed to evaluate the effectiveness of three asthma interventions including (i) an integrated approach to care using the chronic care model; (ii) SDM, and (iii) a school-based care delivery model [16]. The chronic care model includes providing providers and patients with tools designed to optimize asthma care provision and patient self-management [6]. These tools were integrated into the practices including electronic medical record prompts for providers to assess patient symptoms, provide flu vaccination, and prescribe controller medication when appropriate. In addition, an asthma action plan was generated through either electronic or paper record that provided guidance for patients to manage their symptoms when outside of the clinical environment [17]. A subset of five practices (three family medicine clinics and two pediatrics clinics) from within the larger study implemented both the asthma management tools based on the chronic care model and the SDM intervention that was evaluated in this study. The study site selection was based on the population of asthma patients who are at the greatest risk for poor outcomes.
The SDM intervention implementation occurred between March 2011 and September 2013, and patient outcomes were examined through December 2014. The SDM intervention involved the use of an evidence-based asthma SDM toolkit (available at https://asthma.carolinashealthcare.org), with step-by-step instructions including an assessment of asthma control, a review of treatment goals and medication preferences, and education on asthma medications, inhaler technique and triggers. During a specialized asthma visit, the patient (and/or caregiver) met with a health coach (a specially trained pharmacist, nurse or patient educator involved in asthma care at the practice) to utilize the toolkit to discuss goals and preferences prior to meeting with their provider to negotiate a treatment plan. Practice-level adoption of the SDM intervention was reinforced through monthly meetings with representatives from participating practices and a refresher training at the end of year one [17]. Additional details of the intervention implementation and roll-out process, which involved a 12-week training period at each practice location, is described elsewhere [15].
Patients were recruited into the SDM intervention via provider referral, which was facilitated by asthma patient population reports that the research team developed by querying the electronic medical record using ICD-9-CM codes (493.xx). Patients had to meet one of the following criteria in the prior 18 months to be included in the patient population reports: (1) two outpatient asthma visits; (2) one outpatient asthma visit and one asthma ED visit; or (3) one outpatient asthma visit and one asthma hospitalization visit. Providers used the report to identify patients with poor asthma control or recent history of asthma exacerbations. Referred patients were scheduled for an upcoming SDM intervention visit. The intervention targeted children aged 2 to 17 with poor asthma control (i.e., history of exacerbations or symptoms) determined by the referring provider. Similar patients who met the inclusion criteria but did not receive the intervention formed the usual care group (comparison). This study was approved by the Carolinas HealthCare System Institutional Review Board.
Measures
Asthma exacerbation was defined as an asthma-related (i.e., with a primary diagnosis of asthma, ICD-9-CM: 493.xx) hospitalization, ED visit or outpatient visit where an oral steroid prescription (prednisone, prednisolone, methylprednisolone, dexamethasone) was provided. The primary outcome was time from the first SDM visit to asthma exacerbation measured in months. For patients in the comparison group, time to exacerbation was measured from the patients’ first clinic visit during the study period. Additional measures in the study included patient age, gender, race/ethnicity, insurance status, comorbidities and neighborhood characteristics. Patients were categorized into four racial/ethnic categories: Caucasian, African American, Hispanic/Latino and other. Although more than 80% of patients were insured with Medicaid, patients with commercial insurance generally have different demographics than patients with other health insurance. Therefore, insurance was defined as a binary variable indicating commercial or Medicaid/other insurance (Medicaid, other public insurance and uninsured). Comorbidities including obesity, upper respiratory infection and rhinitis were defined as binary variables. Information about these measures was obtained from Carolinas HealthCare System billing data and electronic medical records.
Because of the lack of information regarding patient-level socioeconomic status, area level poverty and education were used as proxy measures. Patients’ street addresses were geocoded to the census tract level and corresponding census tract measures for percentage of residents living in poverty and percentage of residents with less than a high school education were used to represent neighborhood income and education levels, respectively. Census tract data were obtained from the 2005–2009 American Community Survey (U.S. Census Bureau).
Analysis
Sample characteristics were reported as means or percent-ages as appropriate. Propensity score matching was used to achieve balance between the SDM intervention and usual care groups. Propensity scores were calculated as the probability of receiving the SDM intervention based on age, race/ethnicity, gender, insurance, comorbidities, index month, baseline exacerbations and neighborhood characteristics using a generalized estimating equation model. Propensity score matching was conducted using an established macro [18] with nearest neighbor matching algorithm (distance = 0.05). Standardized differences were calculated to assess the balance between the SDM and usual care groups, with values greater than 0.2 indicating imbalance. All variables were balanced between the matched SDM intervention and usual care groups and were not included in the further analysis. Time to asthma exacerbation was assessed using Kaplan-Meier curves. Risk of asthma exacerbation over time for comparison vs. intervention patients was computed using conditional Cox proportional hazards regression. p-values less than 0.05 were considered statistically significant. All analyses were performed using SAS® version 9.4 (SAS Institute Inc., Cary, NC).
Results
The baseline sample included 746 children, of which 625 (83.8%) received usual care and 121 (16.2%) received the SDM toolkit intervention (Table 1). The mean age was 8.6 (±4.2) years, 60.5% were male, 54.2% were African Americans, 13.1% were commercially insured and 9.5% were obese. Race/ethnicity, obesity and baseline ED visits were not balanced between the usual care group and SDM toolkit intervention group at baseline (p < 0.001, p = 0.035, and p = 0.005, respectively). However, the groups were balanced after propensity score matching (Table 2), which resulted in 100 pairs of matched children in the SDM and usual care groups (Figure 1). The mean age of the matched sample was 9.0 (±4.5) years. Characteristics of the matched sample mirrored those of the larger sample with the majority being male (56.0%), and African American (59.0%), and 8.0% being commercially insured.
Table 1.
Baseline characteristics of the study sample before matching.
| Total | Usual care | SDM toolkit | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p-value† | |
| Total | 746 | 625 | 83.8 | 121 | 16.2 | ||
| Age, mean (SD) | 8.55 | 4.2 | 8.52 | 4.1 | 8.74 | 4.5 | 0.596 |
| Race/ethnicity | <0.001 | ||||||
| Caucasian | 58 | 7.8 | 55 | 8.8 | 3 | 2.5 | |
| African American | 404 | 54.2 | 338 | 54.1 | 66 | 54.5 | |
| Hispanic/latino | 158 | 21.2 | 113 | 18.1 | 45 | 37.2 | |
| Other | 126 | 16.9 | 119 | 19.0 | 7 | 5.8 | |
| Insurance | 0.105 | ||||||
| Commercial | 98 | 13.1 | 88 | 14.1 | 10 | 8.3 | |
| Medicaid/other‡ | 648 | 86.9 | 537 | 85.9 | 111 | 91.7 | |
| Gender | 0.295 | ||||||
| Male | 451 | 60.5 | 383 | 61.3 | 68 | 56.2 | |
| Female | 295 | 39.5 | 242 | 38.7 | 53 | 43.8 | |
| Comorbidities | |||||||
| Obesity | 71 | 9.5 | 55 | 8.8 | 16 | 13.2 | 0.035 |
| Development delay | 9 | 1.2 | 8 | 1.3 | 1 | 0.8 | 1.000 |
| Upper respiratory tract infection | 23 | 3.1 | 17 | 2.7 | 6 | 5.0 | 0.243 |
| Rhinitis | 27 | 3.6 | 24 | 3.8 | 3 | 2.5 | 0.601 |
| Baseline ED visits, mean (SD) | 0.23 | 0.6 | 0.20 | 0.6 | 0.39 | 0.7 | 0.005 |
| Baseline inpatient visits, mean (SD) | 0.14 | 0.5 | 0.14 | 0.5 | 0.13 | 0.4 | 0.821 |
| Baseline oral steroid use, mean (SD) | 0.43 | 0.9 | 0.42 | 0.9 | 0.50 | 0.9 | 0.336 |
| Percentage of patients with poverty, mean (SD) | 0.24 | 0.1 | 0.23 | 0.1 | 0.24 | 0.1 | 0.654 |
| Percentage of patients with education less than high school, mean (SD) | 0.21 | 0.1 | 0.21 | 0.1 | 0.22 | 0.1 | 0.258 |
| Median follow-up (month) | 30.45 | 31.60 | 27.10 | <0.001 | |||
Note.
Based on Student’s t-test, Wilcoxon rank sum test, and Chi-square/Fisher’s exact test.
Other includes other public insurance and uninsured.
Table 2.
Matched children sample characteristics by intervention*.
| Total | Usual care | SDM toolkit | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | Standardized difference† | |
| Total | 200 | 100 | 50.0 | 100 | 50.0 | ||
| Age, mean (SD) | 8.96 | 4.5 | 9.03 | 4.2 | 8.89 | 4.7 | 0.03 |
| Race/ethnicity | 0.00 | ||||||
| Caucasian | 6 | 3.0 | 3 | 3.0 | 3 | 3.0 | |
| African American | 118 | 59.0 | 62 | 62.0 | 56 | 56.0 | |
| Hispanic/latino | 62 | 31.0 | 27 | 27.0 | 35 | 35.0 | |
| Other | 14 | 7.0 | 8 | 8.0 | 6 | 6.0 | |
| Insurance | 0.07 | ||||||
| Commercial | 16 | 8.0 | 7 | 7.0 | 9 | 9.0 | |
| Medicaid/other‡ | 184 | 92.0 | 93 | 93.0 | 91 | 91.0 | |
| Gender | 0.04 | ||||||
| Male | 112 | 56.0 | 57 | 57.0 | 55 | 55.0 | |
| Female | 88 | 44.0 | 43 | 43.0 | 45 | 45.0 | |
| Comorbidities | |||||||
| Obesity | 32 | 16.0 | 18 | 18.0 | 14 | 14.0 | 0.11 |
| Upper respiratory tract infection | 7 | 3.5 | 3 | 3.0 | 4 | 4.0 | 0.05 |
| Rhinitis | 5 | 2.5 | 2 | 2.0 | 3 | 3.0 | 0.06 |
| Baseline ED visits, mean (SD) | 0.36 | 0.7 | 0.35 | 0.8 | 0.36 | 0.6 | 0.01 |
| Baseline inpatient visits, mean (SD) | 0.09 | 0.3 | 0.08 | 0.3 | 0.10 | 0.4 | 0.06 |
| Baseline oral steroid use, mean (SD) | 0.53 | 1.0 | 0.54 | 1.1 | 0.51 | 0.9 | 0.03 |
| Percentage of patients with poverty, mean (SD) | 0.24 | 0.1 | 0.24 | 0.1 | 0.24 | 0.1 | 0.06 |
| Percentage of patients with education less than high school, mean (SD) | 0.23 | 0.1 | 0.23 | 0.1 | 0.22 | 0.1 | 0.08 |
| Median follow-up (months) | 27.98 | 29.35 | 27.65 | 0.05 | |||
Note.
Matched with age, race/ethnicity, gender, insurance, comorbidities (developmental delay, obesity, upper respiratory infection and rhinitis), baseline exacerbations (asthma-related inpatient and ED visits and oral steroid prescription orders during 12 months prior to intervention date), and neighborhood characteristics (poverty and education level).
Standardized difference = difference in means or proportions divided by standard error; imbalance defined as value greater than 0.20 (small effect size).
Other includes other public insurance and uninsured.
SDM, shared decision making; SD, standard deviation.
Figure 1.
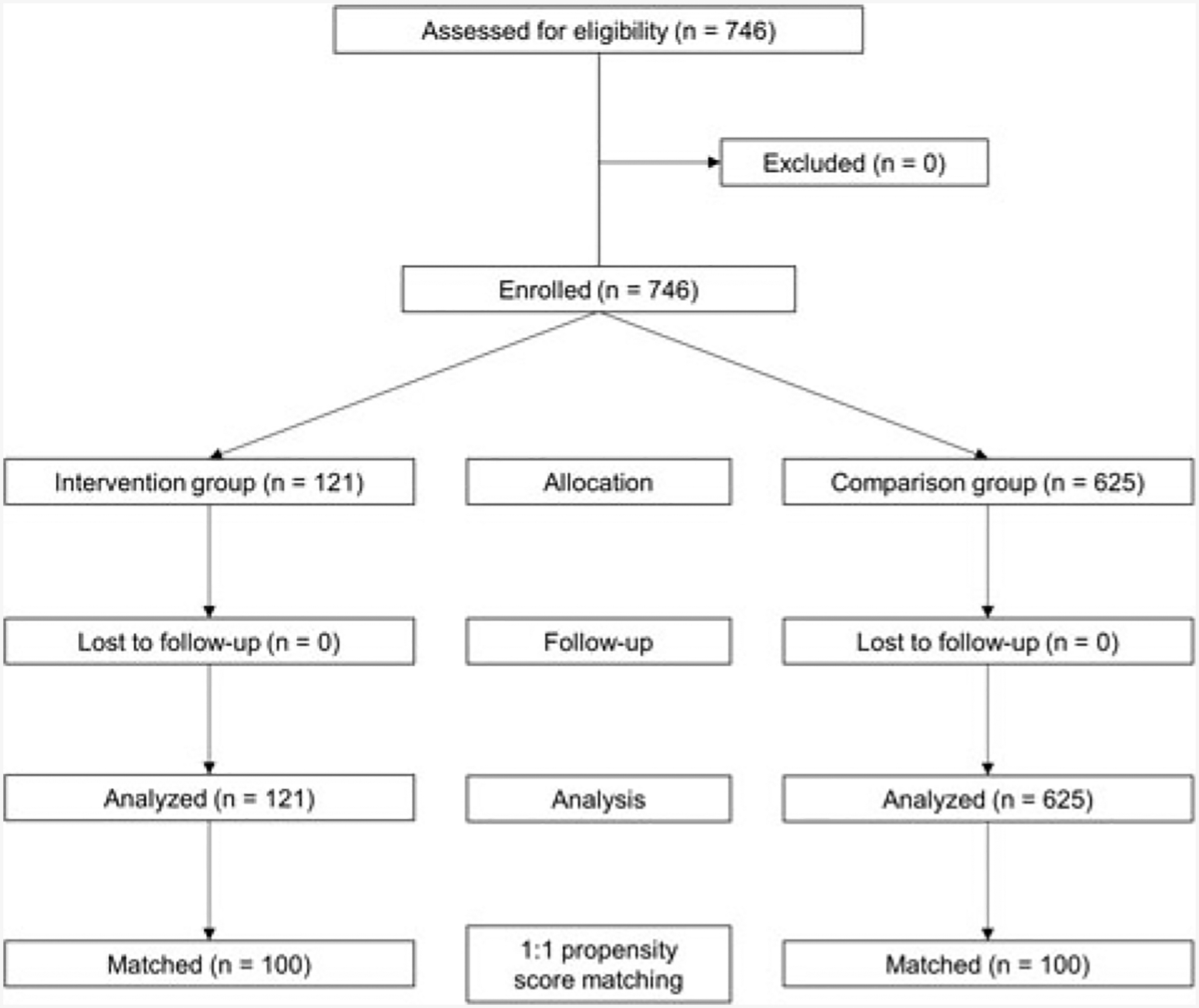
Patients inclusion criteria for propensity scoring
Almost one-fourth (22.5%) of children in the matched sample had an asthma exacerbation in the follow-up period, with higher rates in the usual care group (31.0%) than in SDM toolkit group (14.0%) (Figure 2). Children in usual care had twice as many oral steroid prescription orders than children receiving SDM (30.0% and 14.0%, respectively, standardized difference = 0.39). The median time to exacerbation was 27.98 months (27.65 months in the SDM group; 29.35 months in the usual care group, p = 0.59). Results from Kaplan-Meier curves showed that SDM was associated with delayed incidence of exacerbations with significantly lower probability of experiencing an exacerbation in patients who received SDM compared to those who received usual care over time (p-value = 0.005). Results from the conditional Cox proportional hazards model showed a lower risk of exacerbation in patients receiving SDM over time (hazard ratio [HR] = 0.56, 95% confidence interval [CI]: 0.29–1.08, p-value = 0.08) (Figure 3), which was marginally significant.
Figure 2.
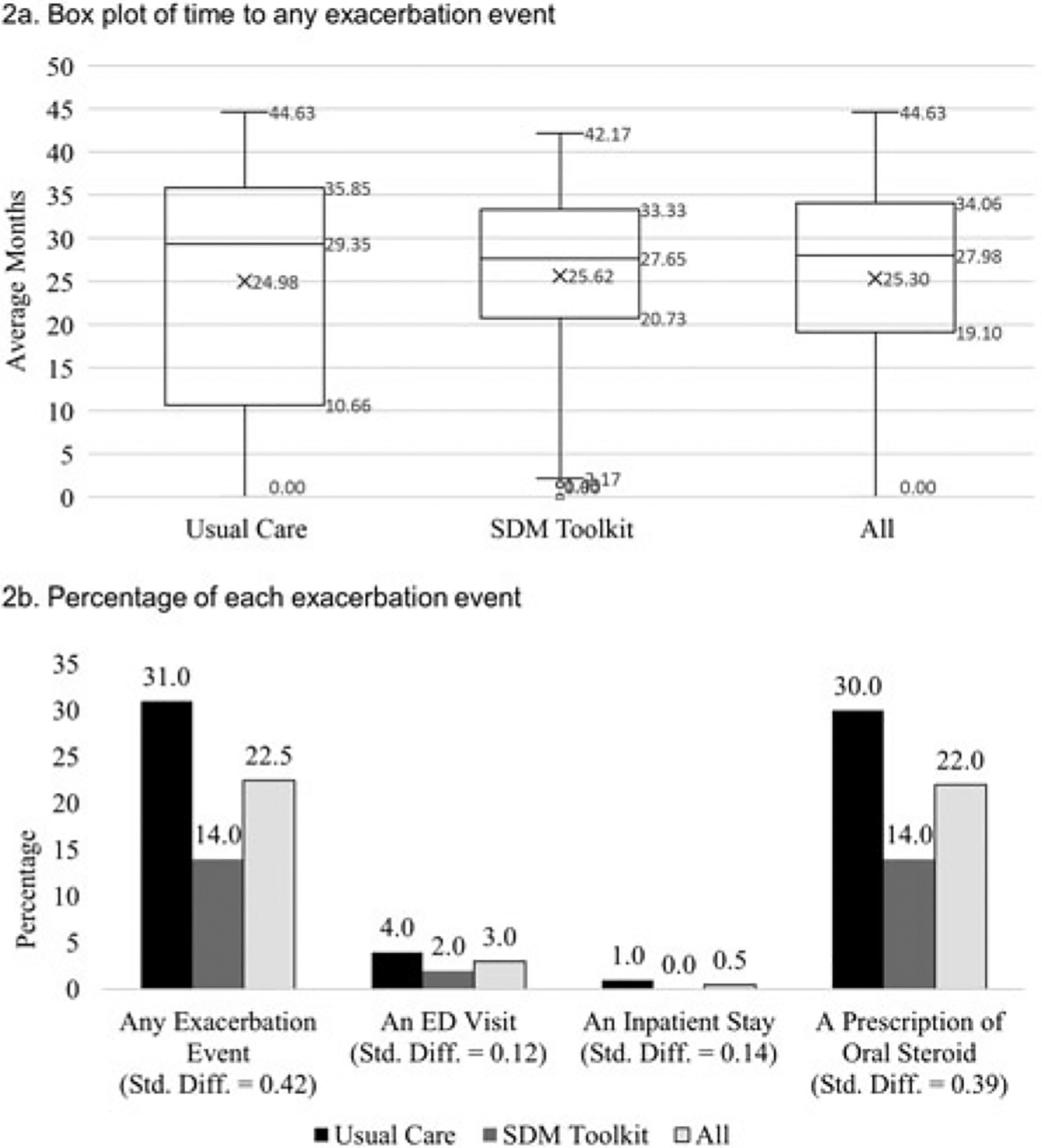
Box plot of time to any asthma exacerbation (2a) and percentage of each asthma exacerbation event (2b) among matched sample of children with asthma (n = 200).
Figure 3.
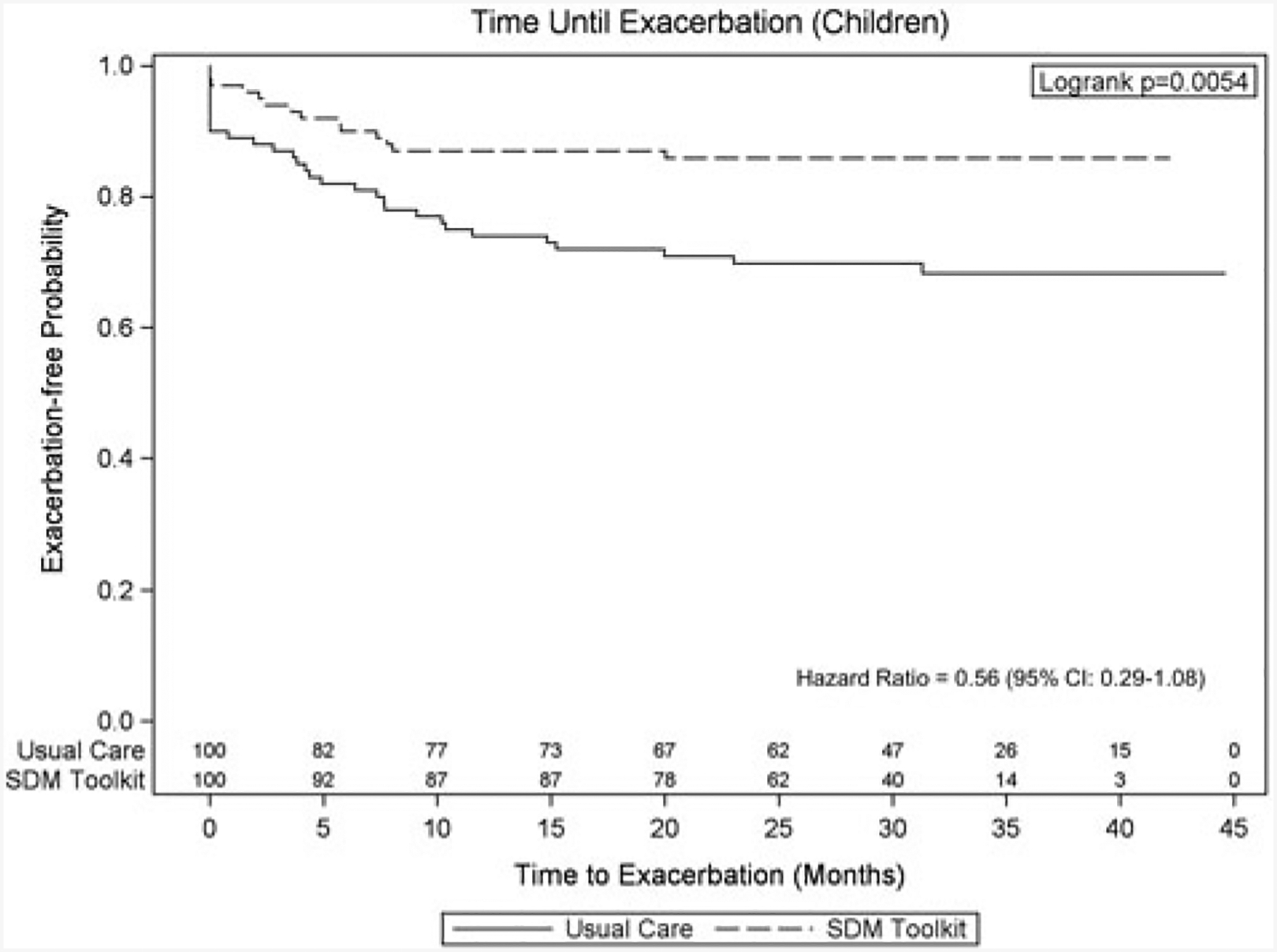
Survival time until exacerbation among children patients with asthma using Kaplan-Meier curve.
Discussion
This study evaluated the impact of an SDM intervention on the time to exacerbation in children with asthma in a real-world setting. Our study found that SDM is associated with a delay in experiencing acute exacerbations among children with asthma. Children with persistent symptoms have a higher risk of having severe exacerbations [19]. By affirming the results of previous studies that found that SDM interventions may improve adherence to asthma pharmacotherapy [12,20], self-management, clinical outcomes [15,21] and quality of life [12,13], our findings provide further support for incorporating SDM in asthma management. The results from our study add to the evidence regarding the effectiveness of implementing SDM among pediatric patients with asthma to address this increasingly prevalent disease.
SDM has been widely used to enhance patient satisfaction, treatment adherence and health outcomes among patients with chronic conditions, such as schizophrenic disorders, depression, cancer, and asthma [11,12,15,21,22]. Aside from those studies targeting adult asthma patients [12,22], two studies of children with asthma also examined healthcare utilization outcomes including ED visits, hospitalization and oral steroids [15,21]. SDM has also been found to be associated with reduced number of exacerbations and reduced prevalence of exacerbations [15]. Our results extend these previous findings by demonstrating that SDM can delay these deteriorating outcomes.
Asthma exacerbations also impose significant economic burden. Ivanova and colleagues [23] found higher total healthcare costs and asthma-related medical costs among asthma patients with exacerbations than patients with well-managed asthma [23]. Our study found that SDM delays exacerbations among children, which can result in savings for families and payers in terms of both direct and indirect costs. However, the only study that followed patients for more than 1 year found that medication adherence increased in the first year of follow-up after an SDM intervention and decreased in the second year of follow-up, which may potentially increase the risk of exacerbation [12]. In addition, whether SDM improves long-term outcomes and decreases long-term risk on exacerbation is unknown. Future longitudinal time-to-event studies should investigate these questions.
Despite the positive health impact of SDM [9], it has been reported that SDM is underused among children, particularly in non-metropolitan areas. One study used 2009–2010 National Survey of Children with Special HealthCare Needs found that prevalence of SDM utilization was only 10% or less in non-metropolitan areas, especially among minority groups and lower income families [24]. Another study conducted in five pediatric practices in non-urban North Carolina found that physicians obtained children’s input in only 6% of encounters, and caregivers’ input in only 10% of the visits [25]. Strategies aimed at increasing the adoption of SDM in pediatric clinical practice need to be planned and implemented.
Although satisfaction with the SDM intervention was not measured in this study, a prior process evaluation study found that satisfaction with the SDM intervention was universally positive and that adoption of the intervention was good, with all practices planning to continue the intervention beyond the study period [17]. In addition, survey data showed that nearly 90% of SDM visits resulted in a shared decision between the patient and the provider [15]. Lack of resources, time constraints and other barriers to the implementation of SDM in clinical practice may be addressed using a participatory approach that engages providers, patients and clinical staff in the implementation process [17]. Benefits of SDM among children including decreased healthcare use and improved health outcomes [12,15,26] suggest that consideration of these and other approaches to incorporating SDM into clinical practice may be useful.
There were a few limitations in this study. First, selection bias may have occurred in this non-randomized study design, as patients exposed to SDM may have been more likely to be engaged in preventative care. To address this issue, we used propensity score matching to control for the observable differences in terms of demographics, insurance, comorbidities and neighborhood characteristics between the intervention and comparison groups. Although there may still be unobservable factors that could not be assessed, this approach minimized potential biases. Second, children with other respiratory illnesses might have been misdiagnosed with asthma. However, any potential misclassification should be non-differential between the intervention and comparison groups. Third, our sample size was reduced after the propensity score matching process. The result of our conditional Cox proportional hazard model was at the margins of statistical significance, suggesting that the results may be underestimated because of the smaller sample size. Last, our study was conducted in an urban setting in Charlotte, North Carolina, among patients who were primarily disadvantaged with low incomes and low education levels and results may not be generalizable to other practice settings.
Conclusion
The implementation of an evidence-based SDM intervention was found to delay exacerbations among children with asthma. This is the first study examining the association between SDM and time to asthma exacerbation. In the context of other evidence, it is clear that incorporating patient preferences in treatment decisions through SDM is a helpful strategy to improve outcomes in children with asthma. Additional research is still needed to examine different modes and settings whereby SDM could be effectively implemented as well as the potential impact of SDM on asthma-related health disparities.
Funding
All phases of this study were supported by the Agency for Healthcare Research and Quality Grant Number 1R18HS019946-01.
Footnotes
Declaration of interest
Dr Blanchette reports being employed and receiving consulting fee from Precision Health Economics, receiving consulting fee from Grifols and United Therapeutics, and receiving research fund as principal investigator at Teva Pharmaceuticals and Novartis Pharmaceuticals. Dr Dulin reports receiving consulting fee from Tresata. The authors alone are responsible for the content and writing of the paper.
References
- 1.The Global Asthma Network. The global asthma report 2014 [Internet]. Auckland, New Zealand: 2014. Available from: http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf [cited 26 September 2017]. [Google Scholar]
- 2.NHIS. 2014. National Health Interview Survey (NHIS) data - lifetime asthma prevalence percents [Internet]. Nat Health Interview Surv Nat Cent Health Statist CDC. 2016. Available from: http://www.cdc.gov/asthma/nhis/2014/table2-1.htm [cited 5 December 2016], p. 1.
- 3.Centers For Disease Control and Prevention. Asthma stats: Asthma-related missed school days among children aged 5–17 years [Internet]. Nat Cent Environ Health. 2015. Available from: https://www.cdc.gov/asthma/asthma_stats/aststatchild_missed_school_days.pdf [cited 3 February 2017], p. 1.
- 4.Centers for Disease Control and Prevention. Asthma facts-CDc’s national asthma control program grantees. Atlanta, GA: US Dep Heal Hum Serv Centers Dis Control Prev; 2013, p. 16 [Google Scholar]
- 5.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Aff 2009;28(1):75–85. Available from: 10.1377/hlthaff.28.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Aff 2001;20(6):64–78. 10.1377/hlthaff.20.6.64 [DOI] [PubMed] [Google Scholar]
- 7.Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns 2014;96(3):295–301. 10.1016/j.pec.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Crossing the quality chasm: A new health system for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 9.Alston C, Berger ZD, Brownlee S, Elwyn G, Fowler FJ Jr, Hall LK, et al. Shared decision-making strategies for best care: Patient decision aids [Internet]. Inst Med 2014;1–49. Available from: https://nam.edu/wp-content/uploads/2015/06/SDMforBestCare2.pdf [cited 23 February 2017]. [Google Scholar]
- 10.Rivera-Spoljaric K, Halley M, Wilson SR. Shared clinician-patient decision-making about treatment of pediatric asthma: What do we know and how can we use it? Curr Opin Allergy Clin Immunol 2014;14(2):161–167. 10.1097/ACI.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 11.Joosten EAG, DeFuentes-Merillas L, De Weert GH, Sensky T, Van Der Staak CPF, De Jong CAJ. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychothe Psychosom 2008;77(4):219–226. 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM, Better Outcomes of Asthma Treatment (BOAT) Study Group. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010;181(6):566–577. 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi PK, Kenzik KM, Thompson LA, DeWalt DA, Revicki DA, Shenkman EA, Huang IC. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir Res 2013;14(1):26 10.1186/1465-9921-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor YJ, Tapp H, Kuhn L, Liu T-L, Mowrer L, Dulin MF. Impact of shared decision making on asthma quality of life and asthma control in children. J Asthma 2017; In press. 10.1080/02770903.2017.1362423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapp H, Shade L, Mahabaleshwarkar R, Taylor YJ, Ludden T, Dulin MF. Results from a pragmatic prospective cohort study: Shared decision making improves outcomes for children with asthma. J Asthma 2017;54(4):392–402. 10.1080/02770903.2016.1227333. [DOI] [PubMed] [Google Scholar]
- 16.Tapp H, Hebert L, Dulin M. Comparative effectiveness of asthma interventions within a practice based research network. BMC Health Serv Res. BioMed Central Ltd. 2011;11(1):188 10.1186/1472-6963-11-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapp H, Kuhn L, Alkhazraji T, Steuerwald M, Ludden T, Wilson S, Mowrer L, Mohanan S, Dulin MF. Adapting community based participatory research (CBPR) methods to the implementation of an asthma shared decision making intervention in ambulatory practices. J Asthma 2014;51(4):380–390. 10.3109/02770903.2013.876430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox. Boston, MA: The Division of Pharmacoepidemiology & Pharmacoeconomics at the Brigham and Women’s Hospital; 2013. Available from: http://www.drugepi.org/dope-downloads/. [cited 5 December 2016]. [Google Scholar]
- 19.Wu AC, Tantisira K, Li N, Schuemann B, Weiss ST, Fuhlbrigge AL. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest 2011;140(1):100–107. 10.1378/chest.10-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan CL, Hogan MB, Tien KJ, Chorney JM, Zettler MD, Koven L, Koven L, Wilson NW, Dinakar C, Portnoy J. Efficacy of a parent - youth teamwork intervention to promote adherence in pediatric asthma. J Pediatr Psychol 2013;38(6):617–628. 10.1093/jpepsy/jss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiks AG, Mayne SL, Karavite DJ, Suh A, O’Hara R, Localio AR, Ross M, Grundmeier RW. Parent-reported outcomes of a shared decision-making portal in asthma: A practice-based RCT. Pediatrics 2015;135(4):e965–e973. 10.1542/peds.2014-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peek ME, Drum M, Cooper LA. The association of patient chronic disease burden and self-management requirements with shared decision making in primary care visits. Heal Serv Res Manag Epidemiol 2014;1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–1235. 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Smalley LP, Kenney MK, Denboba D, Strickland B. Family perceptions of shared decision-making with health care providers: Results of the national survey of children with special health care needs, 2009–2010. Matern Child Health J 2014;18(6):1316–1327. 10.1007/s10995-013-1365-z. [DOI] [PubMed] [Google Scholar]
- 25.Sleath BL, Carpenter DM, Sayner R, Ayala GX, Williams D, Davis S, Tudor G, Yeatts K. Child and caregiver involvement and shared decision-making during asthma pediatric visits. J Asthma 2011;48(10):1022–1031. 10.3109/02770903.2011.626482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiks AG, Mayne S, Localio AR, Alessandrini EA, Guevara JP. Shared decision-making and health care expenditures among children with special health care needs. Pediatrics 2012;129(1):99–107. 10.1542/peds.2011-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]


