Abstract
The aim of the study was to investigate the relationship between the expression of the HER-2 membrane protein and other clinical-pathological parameters such as: histological size of the tumor, degree of the tumor’s differentiation, presence of vascular invasion and presence of metastases in regional lymph nodes, in cases of ductal infiltrative breast cancer. We have investigated 56 cases of ductal infiltrative breast cancer. In all patients a mastectomy with a dissection of axillary lymph nodes has been performed. All tissue samples, taken by biopsy, were embedded in the paraffin, stained by hematoxylin-eosin technique and screened, and evaluation was performed by using a semiquantitative method of the im-munohistochemical expression of the HER-2 protein. A decrease of the protein HER-2 expression was noticed in cases of an increase of the tumor’s diameter above 50 mm. Increased expression of the HER-2 protein was noticed in cases of moderate (grade II) and poor (grade III) differentiation of carcinoma, as well as in cases where there was no metastases in the regional lymph nodes. No relationship has been observed between the expression of HER-2 and occurrence of vascular invasion. In cases of ductal infiltrative breast cancer the expression of HER-2 protein is in correlation with the size and degree of tumor’s differentiation, as well as with the presence of metastases in regional lymph nodes.
Keywords: HER-2, immunohistochemistry breast cancer
A receptor for the human epidermal growth factor HER-2 is the cell membrane 185-kd oncoprotein coded by the c-erb-B2 gene located on the 17q12-21.32 chromosome (1). This receptor shows the activity of the tyrosine ki nase and has a key role in the regulation of growth, survivai and differentiation of lobular epithelial cells (1,2,3). Mutation (amplification) of the HER-2 gene leads to an increased expression of this protein (4). Excessive ex pression of the HER-2 protein causes an increased cell growth and their reproduction, whose end result is the development of cancer. Excessive expression of the HER2 protein on tumor cells’ membrane is part of the process of malignant transformation and tumor’s progression (5,6). Activation of this receptor stimulates the path of signal transduction which leads to elevated concentration level of intracellular Calcium and increased potential of plasma membrane, having a mitogenic effect (7). Beside classical clinical-pathological parameters (size of tumor, histological type, a degree of tumor’s differentiation, presence of local nodular and distant metastases), an independent prognostic factors such as the status of estrogen and progesterone receptors and expression of HER-2 protein are very important for the prognosis of the breast cancer. Excessive expression of the HER-2 is present in 40-60% of ductal carcinoma in situ cases, 2530% cases of invasive breast cancer, when its level can be 10-100 times higher compared to the surrounding normal breast tissue (8, 9, 10, 11). Increased HER-2 expression is in the correlation with an increased aggressiveness of the tumor and poorer prognosis (12,13). In the research of ductal infiltrative breast cancer, beside standard clinical-pathological (TNM) parameters, an immunohistochemical status of the HER-2 protein and its correlation with the above mentioned parameters have been investigated.
MATERIAL AND METHODS
The study included 56 patients whose age was ranging from 20 to 80 years (mean age 57 years). Due to breast cancer, in all patients a mastectomy with axillary dissection has been performed. The size of the tumor was measured in fresh surgical biopsies. Tissue samples were fixated in 10% neutral formalin, embedded in paraffin and, afterwards, sectioned in 3-5 microns slices, stained with standard hematoxylin-eosin (HE) method and immu-nohistochemically with Hercep Test (DAKO, K52049). Histological classification of the tumor was performed according to WHO criteria.
Microscopic study of the tumor included a determination of the following characteristics: size of the tumor (measuring in minimeters two biggest diameters), number of tubular formations (>75%, 10-75%, <10%), number of mitoses in 10 high magnification microscopic fields (<10/hpf, 11-20/hpf, >20/hpf), nuclear pleomorphism (slight, moderate, and severe). Ductal infiltrative breast cancers are graded according to the Bloom-Richardson scheme which, afterwards, has been modified by Elston and Ellis (well differentiated or grade I; moderately differentiated or grade II; poorly differentiated or grade III) (14); presence of vascular or lymphatic invasion and the analysis of the total number of axillary lymph nodes. Tumor’s status was determined according to the criteria for determination of breast cancer’s status given by the American Joint Committee (AJC) and International Union Against Cancer (IUCC) (15). In each axilla, a minimum of 14 lymph nodes was examined. Immuno-histochemical evaluation of the presence and intensity of staining of the HER-2 protein was determined on the base of the score system 0-3+ (0, +, ++, +++). The result was negative (0 or 1+) if there was no staining of the cell membranes at all, if the staining was of poor intensity and/or if the staining was incomplete in less than 10% of tumor’s cells. Slightly positive staining (2+) considered slight or moderate staining of the whole cell membrane in more than 10% of tumor’s cells. Strongly positive (3+) staining was considered when the whole cell membrane was intensively stained in more than 10% of tumor’s cells. Statistical analysis was performed by using a chi-square test. Statistical significance was defined for p<0.005.
RESULTS
Clinical-pathological characteristics of the ductal infiltrative breast cancer in 56 studied cases are shown in Table 1. The majority of patients (96,4% of cases) were above 41-years-of-age. In the majority of cases (44,6%), cancers were moderately differentiated (grade II), and the majority of these tumors had 20-50 mm (pT2) in diameter with no signs of vascular invasion (60, 7% of cases) and no metastases in axillary lymph nodes (67, 8% of cases). The frequency of appearance of the HER-2 protein in 56 cases of ductal infiltrative breast cancer is presented in Table 2. In 39 cases, HER-2 protein was negative (35-7% = 0 and 33.9% = 1+). HER-2 protein was slightly positive (2+) (Figure 1.) in 8 patients (14.2%) and in 9 patients (16%) was strongly positive (3+) (Figure 2.). Results of the statistical analysis of the relation between the expression of HER-2 protein and individual characteristics of the tumor are presented in Graphs 1-4. Staining intensity of the HER-2 protein in 63.5% of cancers whose diameter was 2-5 cm (pT2) was of medium intensity (score 2+) and in 33.3% of cases of strong intensity (score 3+). With an increase of tumor’s size (>50 mm), a drop in the HER-2 expression was significant (p<0.05). With an increase of the degree of tumor’s differentiation (grade II and III), an increase of the HER-2 expression was significant (p<0.05). There was no statistically significant difference in the expression of HER-2 protein in cases with and without signs of vascular invasion. In cases with no metastases in axillary lymph nodes, expression of the HER-2 protein was significantly (p<0.05) higher in comparison to cases with no metastases.
TABLE 1.
Frequency of appearance of the HER-2 expression in 56 cases of ductal infiltrative breast cancer
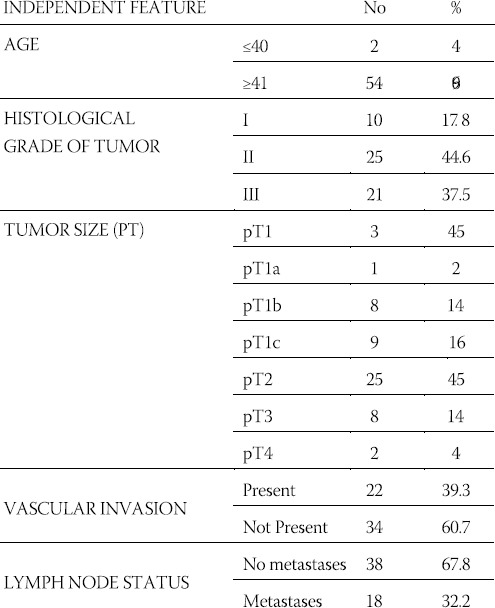
TABLE 2.
Frequency of appearance of the HER-2 expression in 56 cases of ductal infiltrative breast cancer
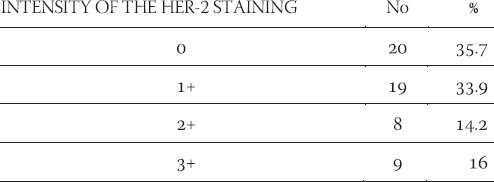
FIGURE 1.
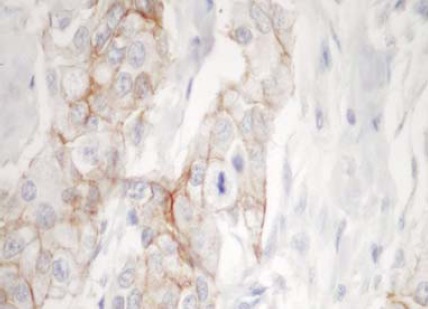
More then 90% of the ductal carcinoma cells are stained for HER2, but the staining intensity is moderate. The Hercept Test TM score is 2+. (250X)
FIGURE 2.
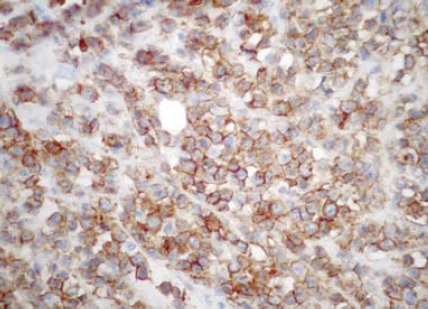
More than 90% of the ductal carcinoma cells are strongly stained for HER2 in the entire membrane. The Hercept Test™ score is 3+. (250X)
GRAPH 1.
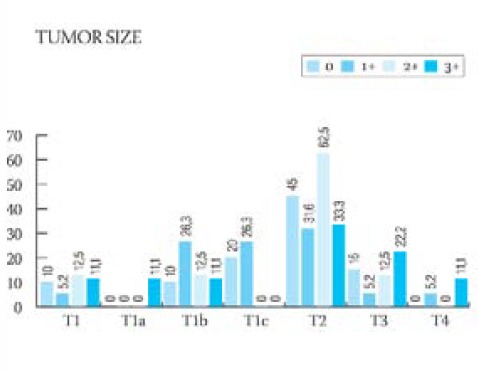
Graphic presentation of the semiquantitative analysis of HER-2 protein in relation to the histological grade of the tumor
GRAPH 2.
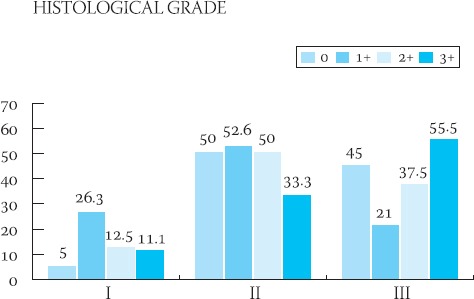
Graphic presentation of the semiquantitative analysis of HER-2 protein in relation to the histological grade of the tumor
GRAPH 3.
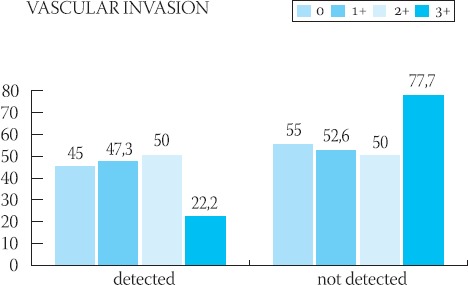
Graphic presentation of the semiquantitative analysis of HER-2 protein in relation to the presence of vascular invasion with tumor cells
GRAPH 4.
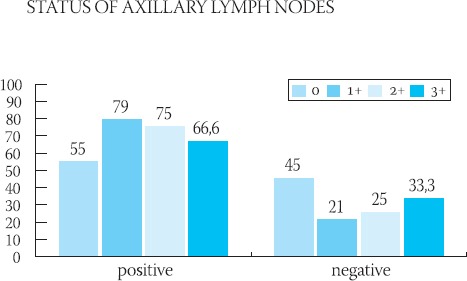
Graphic presentation of the semiquantitative analysis of HER-2 protein in relation to the status of axillary lymph nodes
DISCUSSION
During the investigation of the frequency of the occurrence of an independent prognostic factor HER-2 protein and its relation to the classical clinical-pathological parameters in 56 cases of ductal infiltrative breast cancer, it was noticed that the protein expression is in the correlation with the size and degree of tumor’s differentiation, as well as the absence of metastases in regional lymph nodes. Clinical behavior and metastatic potential, even in the cases of same pathological status of the breast cancer, are often very different (16). That is why this search for parameters that would point out more precisely the biological potential of the cancer continues. Some researchers believe that the HER-2 status, beside the nodal and menopausal, is the only significant and independent prognostic parameter of the breast cancer’s behavior. Protooncogene HER-2/new (HER-2 or p185 HER-2) which codes the same protein is located on the surface of the cell membrane and which acts as a receptor for the growth factor, is involved in the malignant transfermation of many tissue types - breast, ovaries, stomach, lungs (5,17,18,19). In breast cancer, HER-2 expression is a marker of poor prognosis (4). It is generally accepted that the expression of this oncogene on tumor cells is related to a short-time survival, low levels of estrogen and higher histological grade of tumor (4,5,20). It was not found that the increased expression of the c-erb-2 oncogene is related to patient’s age, size of tumor, occurrence of positive lymph nodes or histological type of tumor (21). Prognostic influence of the HER-2 presence is smaller in cases of negative axillary lymph nodes, compared to the cases with positive lymph nodes (4). In our research, we noticed that the increased expression of the HER-2 protein is in correlation with the size of the tumor, which is in accordance with researches performed by other authors (22,23). Statistically significant decrease of the expression of HER-2 protein was noticed when tumor’s size exceeded 50 mm and it was most intensive in tumors whose diameter ranged from 20 to 50 mm. Since it is the correlation occurring in early phase of the disease, it is possible that the expression of HER-2 protein represents a step in the process of tumor’s evolution. It is possible that the mutation of the HER-2 gene in cases of ductal infiltrative breast cancer is responsible for the increased proliferation of the cell mass only up to a certain point, when other things happen on some other genes. The fall of the expression can be a final act in the process of tumor’s progression, when tumor cells cease to be dependent of this protein. It was noticed that, with an increase of cell differentiation, there is a significant increase in the amount of protein on cell’s membrane which, too, is in accordance with results of some other researchers (4). This would present another confirmation of the HER-2’s true relation with early genesis of the tumor. Contrary to some quotations from literature which speak about non-existence of the correlation between the occurrence of metastases in axillary lymph nodes and expression of the HER-2 (21), in our study, the occurrence of positive nodes was followed by a decrease in the expression of HER-2 protein, which can be explained by the involvement of some other genetic anomalies that are far more potent in causing the spreading of the cancer. The occurrence of HER-2 in tumors which did not metastasize in the nodes, according to some researches, increases the risk of recurrent disease, compared to cases which showed normal HER-2 expression (6,24). Abner (25) believes that the significance of positive HER-2 in cases with no nodal metastases is not clear, i.e. it is controversial. Future investigations will, for sure, put some light on the relation between the HER-2 expression and occurrence, i.e. absence, of nodal metastases. There was no statistically significant relation between the occurrence of vascular invasion and expression of the HER-2 protein. Regulation of the HER-2 expression would be independent from the occurrence of vascular invasion. It can be concluded that, in ductal infiltrative breast cancer, the oncogenic effects of the HER-2 protein are potent and associated with some pathological parameters of poor prognosis. It is possible that there are multiple levels of the control and regulation factors which dictate the occurrence and intensity of the expression of HER-2 protein, which would be the goal for some researches to come since all aspects of progression and spreading of cancer are not completely explained still.
REFERENCES
- 1.Popescu N.C, King CR, Kraus M.H. Location of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics. 1989:362–366. doi: 10.1016/0888-7543(89)90343-1. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann C, Hung M.C, Weinber RA. Theneu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 3.King CR, Kraus M.H, Aaronson S. A. Amplification of novel V-erb B-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 4.Menard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a Prognostic Factor in Breast Cancer. Oncology. 2001;61:57–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D.J, Godolphin W, Jones L.A, et al. Studies of the HER-2/neu protooncogenie in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 6.Press MT, Pike MC, Chazin V.R, et al. Her-2/neu expression in node-negative breast cancer:Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of current disease. Cancer res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 7.Hynes NT, Stem D. E. The biology of erbB-q/neu/HER-q and its role in cancer. Biophym. Biophys. Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 8.Hanna W, Kahn H.J, Trudeau M. Evaluationog Her-2/neu (erbB-2) status ij breast cancerTrom bench to bedside. Mod Pathol. 1999;12:827–834. [PubMed] [Google Scholar]
- 9.Bianchi S, Paglierani M, Zampi G, et al. Prognostic significance ef cerbB-2 expression in node negative breast cancer. Br J Cancer. 1993;67:625–629. doi: 10.1038/bjc.1993.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borg A, Tandon A.K, Sigurdsson H, et al. HER-2/neu amplification predict poor survival in node positive breast cancer. Cancer Res. 1990;50:4332–4337. [PubMed] [Google Scholar]
- 11.Tetu B, Brisson J. Prognosticsignificance of HER-2/neu onco-protein expression in node-positive breast cancerThe influence of the pattern of immunosteining and adjuvant therapy. Cancer. 1994;73:2359–2365. doi: 10.1002/1097-0142(19940501)73:9<2359::aid-cncr2820730919>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Persons D.L, Borrelli K.A, Hsu PH. Quantificationof Her-2/neu and c-myc gene amplification in breast carcinoma using flúores-cence in situ hybridisation. Mod Pathol. 1997;10:720–727. [PubMed] [Google Scholar]
- 13.Jacobs T.W, Gown A.M, Yazdii H, et al. Her-2/neu protein exšression in breast cancer evaluated by immunohistochemistry. Am J Clin Pathol. 2000;3:251–258. doi: 10.1309/980M-E24R-V19K-595D. [DOI] [PubMed] [Google Scholar]
- 14.Robbins P, Pinder S, de Klerk N, et al. Histological grading of breast carcinomas;a study of interobserver agreement. Hum Pathol. 1995;28:873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. Manual for Staging for Breast Carcinomas. 3rd end. Philadelphia: Lippincott; 1989. [Google Scholar]
- 16.Gasparini G, Pozza F, Harris AT. Evaluationthe potential usefulness of new prognostic and predictive indicators in node-negative breast cancer patients. J Nat Cancer Inst. 1993;85:1206–1219. doi: 10.1093/jnci/85.15.1206. [DOI] [PubMed] [Google Scholar]
- 17.Pegram M. D, Finn R.S, Arzoo K, et al. The effect of HER-2/neu overexpression on chemotherapeutic drug sensivity in human breast and ovarian cancer cell. Oncogene. 1997;15:537–547. doi: 10.1038/sj.onc.1201222. [DOI] [PubMed] [Google Scholar]
- 18.Yonemura Y, Ninomiya I, Yamaguchi Α, et al. Evaluation of immunoreactivity for erb-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991;51:1034–1038. [PubMed] [Google Scholar]
- 19.Kern JA, Torney L, Weiner D, et al. Inhibition of human lung cancer cell line ggrowth by an anti-p185 HER2 antibody. Am J Respir Cell Mol Biol. 1993;9:448–454. doi: 10.1165/ajrcmb/9.4.448. [DOI] [PubMed] [Google Scholar]
- 20.Narita M, Nakao K, Ogino N, et al. Indenpendent prognostic factors in breast cancer patients. Am J Surg. 1998;175:73–75. doi: 10.1016/s0002-9610(97)00225-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosen P.P, Lesser MT, Arrogo CD, Cranor M, Borgen N, Norton L. Cancer. 1995;1575(6):1320–1326. doi: 10.1002/1097-0142(19950315)75:6<1320::aid-cncr2820750614>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Weisener B, Hauser-Kronberger C.E, Zipperer E, et al. P34 cdc2 in invasive breast cancer. relationship to DNA content, Ki67 index and c-erbB-2 expression. Histopathology. 1998;33:522–530. doi: 10.1046/j.1365-2559.1998.00500.x. [DOI] [PubMed] [Google Scholar]
- 23.Quenel N, Waifflart J, Bonichom F, et al. The prognostic value of c-erbB-2 in primary breast carcinomas. A study on 942 cases. Breast Cancer Res Treat. 1995;35:283–291. doi: 10.1007/BF00665980. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi S, Paglierani M, Zampi G, et al. Prognostic significance of c-erbB-2 expression in node negative breast cancer. Br J Cancer. 1993;67:625–629. doi: 10.1038/bjc.1993.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abner AT, Collins L, Peiro G, et al. Correlation on tumor size and axillary node involvement with prognosis in patients with T1 breast carcinoma. Cancer. 2001;83(12):2502–250. [PubMed] [Google Scholar]


