Abstract
Gentamicin is still widely used in clinical practice in spite of its renal toxicity. The role of nitric oxide (NO) in that process is not completely elucidated. The aim of this study was to investigate the relationship between plasma level of NO and the histopathological changes of kidney in acute tubular necrosis (ATN) induced by gentamicin in rats. Study was carried out in Albino-Wistar rats, both sexes (n=16), average body weight 200-250 g. divided in two equal groups: control and gentamicin group. The control group was injected with 0,9 % NaCl i.p. and gentamicin group was injected with gentamicin in the dose of 80 mg/kg/day i.p. in a period of 5 consecutive days. NO plasma level was determined by the production of nitrates and nitrites using classical colorimetrical Griess reaction. Kidney specimens were stained with hematoxylin-eosin (H-E) and Periodic acid-Schiff (PAS) stain. Semiquantitative histological analysis was used for the evaluation of the level of kidney damage. Both, the plasma NO level and the level of kidney damage were statistically higher in rats with gentamicin-induced ATN in comparison to the control group. In spite of that the correlation between plasma NO level and the level of kidney damage was not found. The rise of plasma level NO in gentamicin induced ATN in rats could possibly indicate on the role of NO in renal damage caused by gentamicin.
Keywords: nitric oxide, gentamicin, acute tubular necrosis
INTRODUCTION
Aminoglycosides, including gentamicin, are still widely used in the treatment of gram negative infections. However, marked nephrotoxicity limits the use of these drugs. The gentamicin renal toxicity is apparently related to its accumulation in the proximal convolute tubules. Cell death may be due to lysosomal dysfunction, interference with mitochondrial respiration or damage to cell membrane ionic pumps. It is not known, however, whether the gentamicin induced some cytotoxic products and/or factors resulting from exposure to gentamicin modulate ATN (1). Nitric oxide (NO) could be involved in this process. NO diffuse rapidly across membranes and transmit a signal over many cells (2). NO is involved in the regulation of many physiological processes, as well as in the pathophysiology of a number of diseases (3). NO is synthesized enzymatically from L-arginine in numerous tissues and cells by three structurally distinct isoforms of the enzyme, nitric oxide synthase (NOS). The special role has inducible (iNOS) isoform. When it is induced by endotoxine and/or cytokines it generates high, sustained levels of NO. These elevated levels of NO can cause cellular cytotoxicity and tissue damage (4). In the last few years, a series of in vivo and in vitro studies have begun to reveal a close relationship between NO and proximal renal tubules and a significant role of NO in proximal tubule physiology and pathophysi-ology. It is still controversial whether the proximal tubules produce NO under basal conditions. However, numerous evidences suggest that the proximal tubules be constantly exposed to NO, which might include NO from non-proximale tubule sources. When challenged with variety of stimuli, including toxic agents and hypoxia, the structures of the proximal tubule are able to produce large quantities of NO (5). A wide range of insults including ischemia, sepsis and neph-rotoxic agents (gentamicin) can lead to the renal failure. Results of numerous studies have shown that NO plays important role, in development of acute renal failure (ARF) as well as chronic renal failure (CRF) (2). Pharmacological studies of NO’s role in renal failure have produced confusing and contradictory results (6, 7, 8, 9). Because of that the aim of this study was to compare the plasma level of NO and the histopatho-logical changes of kidney tissue in Wistar rats with gentamicin-induced acute tubular necrosis.
MATERIALS AND METHODS
ANIMALS
Study was carried out in Albino-Wistar rats, both sexes (n=16), average body weight 200-250 g. Experiments were performed with the permission of the local Ethic Committee. All animals were allowed one week of adaptation period before beginning of the experiment. Standard rat chow and tap water were given ad libitum. Animals were divided in two equal groups: control and gentamicin group and housed in standard cages.
EXPERIMENTAL PROTOCOL
The control group (n=8) was injected intraperitone-ally (i.p.) with 0,9 % NaCl for a period of 5 consecutive days. The gentamicin group was injected intraperitone-ally with gentamicin in the dose of 80 mg/kg/day in a same period. The injections were carried out between 9.00 and 9.30 a.m. to minimize the circadian variation seen in gentamicin-induced nephrotoxicity (10).
SURGICAL PROCEDURE
The animals were sacrificed 24 hours after the last injection of gentamicin. The animals were anesthetized with ether and the front wall of the abdominal cavity was removed. Blood for the plasma NO level measurement was collected from the bifurcation of the aorta. The kidneys were removed immediately after that, vertically divided into two sections and fixed in 10% formalin and then embedded in paraffin wax.
NO
The plasma level of NO was determined by the production of nitrates and nitrites using classical colorimetrical Griess reaction (11). Absorbency was measured at 546 nm. The results were expressed as μmol/dms.
HISTOPATHOLOGY
Sections of kidney were cut and stained with hema-toxylin-eosin (H-E) and Periodic acid-Schiff (PAS). The light microscopic of the kidney sections was done according to Houghton et al. (12). The changes were limited to the tubulointerstitial areas and were graded as follows: 0=normal; 1= areas of focal granulovacuolar epithelial cell degeneration and granular debris in the tubular lumina with or without evidence of desquamation in small foci (< 1% of total tubule population involved by desquamation); 2= tubular epithelial necrosis and desquamation easily seen but involving less than half of cortical tubules; 3= more than half of the proximal tubules showing necrosis and desquama tion, but intact tubules are easily identified and 4=com-plete or almost complete proximal tubular necrosis.
STATISTICAL ANALYSIS
Values are presented as mean ± SEM. Differences between the groups were tested by Student’s t-test. Differences were considered significant for p<0,05.
RESULTS
NO
The plasma level of NO in rats with gentamicin-in-duced ATN and in control group are showed in Figure 1. Gentamicin group had significantly higher plasma NO level compared with the control group (p < 0,002).
FIGURE 1.
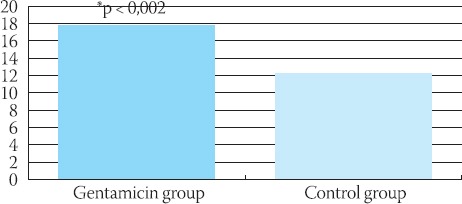
Plasma levels of NO (nmol/dm3) in gentamicin and control group of rats. The values are express as mean ± SEM. “p<0,002 compared with control
HISTOPATHOLOGY
The specimens of kidney taken from the gentamicin administrated rats showed extensive tubular damage (Figure 2, A and B). The necrotic areas were observed particularly in the superficial cortex. Desquamated proximal tubular epithelial cells were widely observed in these necrotic areas. Brush-border membranes of almost all cells were disrupted. In the intact proximal tubular epithelial cells the presence of vacuolas were observed. Kidney specimens from control animals showed the normal structure of healthy tubules with abundant luminal brush-border membranes (Figure 3, A and B).
FIGURE 2.
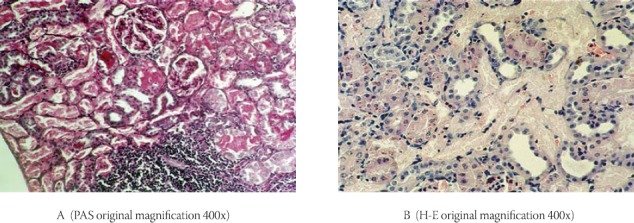
Histopathology of kidney in the gentamicin group
FIGURE 3.

Histopathology of kidney in the control group
SEMIQUANTITATIVE HISTOLOGY
Kidney tissue sections were scored in a blinded semiquantitative manner based on histological changes (12) (Figure 4). In the gentamicin group, three were grade 4, four were grade 3 and one was grade 2. In the control group all specimens of kidney tissue were not changed (grade 0).
FIGURE 4.

Histopathologic injury score Renal tissue injury was assessed in PAS and H-E stained sections. Sections were scored in a blinded, semiquantitative manner (12)
DISCUSSION
The numerous studies (13,14,15,16) describe experimental toxic ATN caused by uranyl-nitrate, mercury chloride, radiocontrast or nephrotoxic agents such as aminoglicosides. These studies were conducted with the purpose for clarification of underlying pathophysiological mechanisms and for identification of new therapeutic strategies. Acute renal tubular damage induced by toxic agents leads to different morphological changes in certain structures of kidney. Proximal tubules are the part of the nephron in which gentamicin induced the primary damage of various degrees. The histopathological changes could be less marked in intensity and limited only to smaller areas with focal granulovacuolar degeneration and granular debris within tubule’s lumen or can also be manifested by completely necrotic proximal tubule (14). Most of the studies so far, have shown that, in the pathogenesis of toxic renal injury, NO could has certain role. It is not still, completely certain how, and to what extent, this gas contributes to the development of tubular damage (2). Our results showed that the plasma level of NO risen significantly in rats with ATN caused by gentamicin. The gentamicin dose in our study was 80mg/kg/day for 5 consecutive days and it was sufficient for induced ATN although previous reports suggested higher doses of gentamicin (14,15,17). The presence of ATN was confirmed by histological findings of kidney in gentamicin group of animals. Morphological changes in kidney specimens in our study were similar to the ones observed by Erdem et al. (14,15) who investigated protective effects of different substances in gentamicin-induced acute tubular necrosis in rats. Results of our histopathological analysis have shown marked necrosis of cortical tubules in the gentamicin group. Proximal tubular cells were transferred into an amorphous mass. In certain distal tubules smaller quantity of amorphous content was observed, while in others that amorphous content filled out whole lumen of the tubules. Epithelium of distal tubules was affected by early dystrophic -necrobiotic changes (swelled cells, and around the nuclei was observed light ring). Certain proximal tubules in cortico-medullary zone had normal epithelium and cellular nuclei, while in adjunct tubules multiple PAS positive granules were observed within the cytoplasm. Some of the proximal tubules, located closer to corticomedullar zone, had partially damaged brush border. Kidney specimens from control animals showed the normal structure of healthy tubules with abundant luminal brush-border membranes. The observed rise of NO plasma level is in accordance with results of Yanagisawe and co-workers (13), who reported increased NO plasma level in rats with mercury-chloride induced ATN. Similar results were obtained by Chatterjee co-workers (18), who also observed significant increase of plasma NO level in renal ischemia /reperfusion induced ATN. These findings could indicate that increased production of nitric oxide by different kidney cells and structures might also play a role in the progression of gentamicin-induced acute tubular necrosis through the exacerbation of proximal and partially distal tubule epithelial cell damage. Orida and Lai (2) have stated that iNOS is expressed constitutively along the renal tubule, with the greatest expression around the medullary thick ascending loop of Henle and also within cells of proximal tubules. Our hypothesis is that gentamicin as an toxic agent in our study model have triggered the iNOS expression, so that increased plasma concentration of NO and consequent renal cells damage and necrosis might be mainly due to induced expression of iNOS and excessive production of NO under that conditions. We believe that the use of inhibitors of iNOS in our further studies will offer explanation for possible role of NO in renal damage caused by gentamicin.
REFERENCES
- 1.Bennett W.M. Drugand the kidney. In: Whitworth J.A, Lawrence J.R, Kincaid-Smith P, editors. In Textbook of renal disease. 2nd ed. New York: Churchill Livingston; 1994. pp. 325–342. [Google Scholar]
- 2.Orida N.K, Lai Ch S. Nitric oxide and renal patient. Dial. Transplant. 2000;29(4):174–185. [Google Scholar]
- 3.Moncada S, Palmer R.M. J, Higgs E.A. Nitricoxide physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 4.Hobss A, Higgs A, Moncada S. Inhibitionof nitric oxide synthase as a pontential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 5.Liang M, Knox F.G. Production and functional roles of nitric oxide in the proximal tubule. Am. J. Physiol. 2000;278(5):R1117–1124. doi: 10.1152/ajpregu.2000.278.5.R1117. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Gengaro P.E, Niedergerger M, et al. Nitric oxide. A mediator in rat tubular hypoxia/reoxygenation injury. Proc. Natl. Acad. Sci. USA. 1994;91:1691–1695. doi: 10.1073/pnas.91.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agmon Y, Pegel H, Greenfeld Z, et al. Nitric oxide and pros-tanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J. Clin. Invest. 1994;94:1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrario R, Takahashi K, Fogo A, et al. Consequences of acute nitric oxide synthesis inhibition in experimental glomerulone-phritis. J. Am. Soc. Nephrol. 1994;4:1847–1854. doi: 10.1681/ASN.V4111847. [DOI] [PubMed] [Google Scholar]
- 9.Sogawa K, Numayama-Tsuruta K, Ema M, et al. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc. Natl. Acad. Sci. USA, 1998;95:7368–7373. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pariat C, Courtois P, Cambar J, Prior A, Bouquet S. Cicardianvariation in the renal toxicity of gentamicin in rats. Toxicol. Lett. 1988;40:175–182. doi: 10.1016/0378-4274(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 11.Green L.C, Wagner D.A, Glogowski J, et al. Analysis of nitrate, nitrite and 14N nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Houghton D.C, Plamp C.E, DeFehr J.M, et al. Gentamicin and tobramycin nephrotoxicity. a morphologic and functional comparison in the rat. Am J. Pathol. 1978;93:137–151. [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagisawa H, Nodera M, Wada O. Induciblenitric oxide syn-thase expression in mercury chloride-induce acute tubular necrosis. Ind. Health. 1998;36(4):324–330. doi: 10.2486/indhealth.36.324. [DOI] [PubMed] [Google Scholar]
- 14.Erdem A, Gündogan N.U, Usubütün A, et al. The antioxidant action of N-acetylcystein on gentamicin-induced acute tubular necrosis in rats. Gazi Med. J. 1999;10:21–28. [Google Scholar]
- 15.Erdem A, Gündogan N.U, Usubütün A, et al. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rat. Nephrol. Dial. Transplant. 2000;15:1175–1182. doi: 10.1093/ndt/15.8.1175. [DOI] [PubMed] [Google Scholar]
- 16.Yanagisawa H. HgCl2-induced acute renal failure and its patho-physiology. Nippon Eiseigaku Zasshi. 1998;52(4):618–623. doi: 10.1265/jjh.52.618. Review. Japanese. [DOI] [PubMed] [Google Scholar]
- 17.Walker P.D, Shah S.V. Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J. Clin. Invest. 1988;81:334–341. doi: 10.1172/JCI113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee P.C, Patel N.S. A, Kvale E.O, et al. Inhibition of induc-ible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–871. doi: 10.1046/j.1523-1755.2002.00234.x. [DOI] [PubMed] [Google Scholar]


