Abstract
Long-chain fatty aldehydes are present in low concentrations in mammalian cells and serve as intermediates in the interconversion between fatty acids and fatty alcohols. The long-chain fatty aldehydes are generated by enzymatic hydrolysis of 1-alkyl-, and 1-alkenyl-glycerophospholipids by alkylglycerol monooxygenase, plasmalogenase or lysoplasmalogenase while hydrolysis of sphingosine-1-phosphate (S1P) by S1P lyase generates trans Δ2-hexadecenal (Δ2-HDE). Additionally, 2-chloro-, and 2-bromo- fatty aldehydes are produced from plasmalogens or lysoplasmalogens by hypochlorous, and hypobromous acid generated by activated neutrophils and eosinophils, respectively while 2-iodofatty aldehydes are produced by excess iodine in thyroid glands. The 2-halofatty aldehydes and Δ2-HDE activated JNK signaling, BAX, cytoskeletal reorganization and apoptosis in mammalian cells. Further, 2-chloro- and 2-bromo-fatty aldehydes formed GSH and protein adducts while Δ2-HDE formed adducts with GSH, deoxyguanosine in DNA and proteins such as HDAC1 in vitro. Δ2-HDE also modulated HDAC activity and stimulated H3 and H4 histone acetylation in vitro with lung epithelial cell nuclear preparations. The α-halo fatty aldehydes elicited endothelial dysfunction, cellular toxicity and tissue damage. Taken together, these investigations suggest a new role for long-chain fatty aldehydes as signaling lipids, ability to form adducts with GSH, proteins such as HDACs and regulate cellular functions.
Keywords: Long-chain fatty aldehydes, S1P Lyase, Plasmalogenase, lysoplasmalogenase, S1P, Hexadecenal
1. INTRODUCTION
Lipids comprising of phospholipids, sphingolipids and cholesterol are essential constituents of all biological membranes of eukaryotic cells. In addition to their structural role in the lipid bilayer membrane as a barrier between adjacent cells, the membrane lipids serve as substrates for lipid lipases, lipid kinases and lipid phosphatases, which generate several bioactive lipid mediators involved in intracellular and extracellular signaling events [1] [2] [3] [4]. Non-membrane lipids that are present in plasma and cytosol of cells are also subjected to hydrolysis and modifications to serve as signaling lipid molecules and ligands for G-protein coupled receptors on the cell plasma membrane. Bioactive lipids generated within the cell are metabolized, serve as precursor for de novo biosynthesis or transported outside by transporters such as ABC transporters [5] [6] [7] and SPNS2 [8] [9] [10]. For example, in response to extracellular stimuli, membrane lipids are hydrolyzed by specific phospholipases such as phospholipase (PL) A1, A2, C, and D to release fatty acid, diacylglycerol (DAG) or phosphatidic acid (PA), respectively. Polyunsaturated fatty acids such as arachidonic acid (AA), docosahexaenoic acid (DHA) or eicosapentanoic acid (EPA) are predominantly localized on the sn-2 position of phospholipids, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE) or phosphatidylserine (PS), and sprovide substrates for cyclooxygenase or lipoxygenase resulting in production of short lived prostanoids and hydroxy- and hydroperoxy-fatty acids that serve as signaling bioactive lipid second messengers in mammalian cells [2] [11] [12]. Lysophosphatidylcholine (LPC) generated by phospholipase A1 or A2 can be hydrolyzed by lysophospholipase D or autotaxin (ATX) to lysophosphatidic acid (LPA) [13] [14] that signals through LPA1–6 G-protein coupled receptors [15] [16]. PLD catalyzes the hydrolysis of PC, PE or PS to produce PA and the free polar head group [17] [18]. Further, PA generated by PLD can be dephosphorylated by PA phosphatases forming DAG [19]. Additionally, DAG can also be formed by hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) by PI-PLC [20] or hydrolysis of PC by PC-PLC [21, 22]
Although hydrolysis of PC by PC-PLC has been reported extensively [20], this is a controversial reaction as this enzyme has not been purified and characterized from mammalian cells. An alternate explanation for DAG formation from PC would be the synthesis of sphingomyelin (SM) by SM synthase 1 &2 where phosphocholine from PC is transferred to ceramide [23] [24] [25]. DAG is an endogenous activator of several PKC isoforms [26] and PA is a second-messenger in cells and also serves as substrate for PA-specific phosphatases to yield DAG [19]. PA is also hydrolyzed by PA-specific PLA1 or PLA2 to generate1-lyso-2-acyl- or 1-acyl-2-lyso-sn-glycero-3-phosphate (LPA) and free fatty acid [27] [28]. SM, the other major sphingophospholipid of biological membranes on hydrolysis by sphingomyelinases yields ceramide, an apoptotic sphingolipid [29] [30] [31], which subsequently produces sphingosine through ceramidases [32]. Sphingosine can be phosphorylated by sphingosine kinase (SPHK) 1 and 2 to sphingosine-1-phosphate (S1P) [33], a naturally occurring bioactive lipid mediator that signals via S1P1–5 G-protein coupled receptors [34] [35]. Generation of lipid mediators from phospholipids and sphingolipids by phospholipases, lipid kinases and lipid phosphate phosphatases is outlined in Figure 1.
Figure 1: Pathways involved in the degradation of phospholipids and sphingolipids.
(A) Phospholipases mediated hydrolysis of 1,2-diacyl-sn-glycero-3-phosphocholine (PC) by phospholipases and lysophospholipase D (lyso PLD) or autotaxin (ATX). Hydrolysis of 1,2-diacyl-sn-glycero-3-phosphocholine (PC) by phospholipase (PL) A1, or A2, releases fatty acid from carbon 1 or carbon 2 of PC and generates 2-lyso-1-acyl- or 1-lyso-2-acyl-sn-glycero-3phosphocholine (LPC). Hydrolysis of PC by PLC generates diacylglycerol (DAG) and phosphocholine. DAG kinase (DAGK) converts DAG to 1,2-diacyl-sn-glycero-3-phosphate (PA), which is acted by PA specific PLA1 or PLA2 to give rise to 2-acyl- or 1-acyl-sn-glycero-3-phosphate (LPA). PLD catalyzes the hydrolysis of PC to PA and choline. 2-lyso-1-acyl- or 1-lyso-2-acyl-sn-glycero-3-phosphocholine (LPC) generated by PLA1 or PLA2 can be hydrolyzed by lyso PLD or ATX to 2-acyl- or 1-acyl-sn-glycero-3-phosphate (LPA). PLA2 mediated hydrolysis of PC releases polyunsaturated fatty acids C20:4, C22:5 and C22:6 from carbon 2, which are subsequently converted to prostanoids, leukotrienes, hydroperoxyeicosatetraenoic acids (HETES) and hydroperoxyeicosatetraenoic acids (HPETES) mediated by cyclooxygenases, lipoxygenases, peroxidases and dehydrogenases. (B) Hydrolysis of sphingomyelin by sphingomyelinase, ceramidase and S1P lyase. Sphingomyelin (SM) is hydrolyzed by sphingomyelinase (SMase) to produce ceramide that is acted by ceramidase(s) to generate sphingosine. Sphingosine is converted to sphingosine-1-phosphate (S1P) by sphingosine kinases (SPHKs) 1 and 2. Sphingosine-1-phosphate, thus generated is converted back to sphingosine by sphingosine-1-phosphate phosphatases (SPPs) and lipid phosphate phosphatases (LPPs) or to trans Δ2-hexadecenal and ethanolamine phosphate by S1P lyase, a pyridoxal phosphate-dependent enzyme.
Enzymatic or non-enzymatic reactions on membrane lipids also generate highly reactive long-chain fatty aldehydes such as hexadecanal, octadecanal, and Δ2-hexadecenal, which have been shown to form adducts with proteins [36], and DNA [37]. Recent studies suggest that the long-chain fatty aldehydes can also signal and modulate cell functions [38] [39]. This article will primarily focus on various long-chain fatty aldehydes derived from phospholipids and sphingolipids in cellular signaling and function. Although 4-hydroxynonenal (4-HNE) is derived from membrane lipids by lipid peroxidation, this is not covered here as several excellent reviews have been published on biological and pathological roles of 4-HNE in human diseases [40] [41] [42]. Additionally, in this review, the role and biological properties of oxidized phospholipids such as 1-palmitoyl-2-glutaroyl- and 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine that have truncated aldehyde in the 2-carbon atom of the glycerol backbone has not been covered [43].
2. SOURCES OF LONG-CHAIN FATTY ALDEHYDES IN CELLS
Long-chain fatty aldehydes (C16–C24), either saturated or monounsaturated, play an important role in the metabolic pathways of both prokaryotic and eukaryotic organisms. Unsaturated long-chain aldehydes and alcohols are primary components of insect sex pheromones [44], and long-chain aldehydes have been described in the waxes which impregnate the matrix covering all organs of plants [45]. Long-chain aliphatic aldehydes with chain-length from C22 to C30 are present in virgin olive oil with hexacosanal (C26) being the most abundant aldehyde [46]. There are two major pathways in mammalian cells that generate long-chain fatty aldehydes. The first pathway is the de novo biosynthesis from long-chain fatty acids catalyzed by fatty acyl CoA reductase and the second is through metabolism of lipids including plasmalogens and ether lipids (alk-1-enyl and alkyl phospholipids). The second pathway involves long-chain fatty alcohols and branched-chain alcohols (phytol, farnesol and geranylgeraniol), sphingolipids(sphingosine-1-phosphate,dihydrosphingosine-1-phosphate and sphingosylphosphorylcholine), leukotriene B4 and wax esters. In addition to long-chain fatty aldehydes, some medium chain aldehydes such as hexanal, octanal and 4-HNE are generated via lipid peroxidation [47]. Further, partial lipid peroxidation of PC containing polyunsaturated fatty acids (C20:4, C22:5 and C22:6) also generate oxidized PC with short chain aldehyde attached to the sn-2 position of the glycerol backbone of the phospholipid [48].
2.1. Long-chain fatty aldehydes are intermediates in fatty acid and fatty alcohol metabolism:
The cellular concentrations of long-chain free fatty acids, fatty aldehydes and fatty alcohols are maintained at a low steady state level and interconversion of fatty acids and fatty alcohols proceeds through fatty aldehyde intermediate. Based on the requirement of long-chain fatty alcohols for the biosynthesis of ether lipids, plasmalogens and wax esters, saturated and monounsaturated free fatty acids of chain lengths (C16:0, C18:0, and C18:1) are activated to fatty acyl-CoA catalyzed by fatty acyl-CoA synthase and subsequently reduced to fatty alcohol via fatty aldehyde intermediate by fatty acyl-CoA reductase (FAR) [49] [50] [51]. Overall, this two-step process, consumes ATP in fatty acyl CoA generation, and peroxisomal FAR utilizes NADPH in reduction of the fatty acid to fatty alcohol. Fatty alcohols are substrates for the conversion of 1-acyl dihydroxyacetone phosphate (DHAP) to 1-alkyl DHAP catalyzed by 1-alkyl DHAP synthase and subsequent synthesis of ether glycerolipids and plasmalogens [50] [52] [53]. Fatty alcohols that are not utilized for ether lipid and plasmalogen biosynthesis are converted back to fatty acid, a reaction catalyzed by fatty alcohol: NAD oxidoreductase (FAO) [54], a membrane-bound multi-enzyme complex consisting of fatty alcohol dehydrogenase and fatty aldehyde dehydrogenase (FALDH) that act sequentially converting fatty alcohol to fatty acid via fatty aldehyde intermediate [55]. Interestingly, FALDH is necessary for FAO activity, and is involved in oxidation of fatty alcohols in several lipid pathways. The Sjögren-Larsson Syndrome (SLS), is caused by mutation in ALDH3A2 gene that encodes FALDH and SLS patients have deficiency in oxidation of fatty alcohol and fatty aldehydes [56] [57]. Thus, fatty alcohols and fatty aldehydes are likely candidates for causing the symptoms and SLS derived fibroblasts and keratinocytes are more susceptible to hexadecanal toxicity as compared to hexadecanol [58] [59].
2.2. Enzymatic cleavage of 1-O-alkylglycerolipids by alkylglycerol monooxygenase:
Ether lipids in mammalian cells are characterized by the presence of a long-chain alkyl group (C16:0; C18:0; C18:1) attached to the sn-1 carbon atom of the glycerol backbone instead of an ester bond that is present in most of the glycerophospholipids. The alkyl chain of C16:0/C18:0/C18:1 is derived from the corresponding long-chain fatty alcohols in a unique reaction wherein acyl group at sn-1 carbon of 1-acyl DHAP) is replaced with the fatty alcohol catalyzed by alkyl DHAP synthase that is predominantly localized in peroxisomes [60]. The 1alkyl DHAP is subsequently converted to 1-alkyl-2-acyl glycerophosphate and plasmalogens of the type PC, PE or PS in which the sn-1 position has a 1-O-alkenyl chain (vinyl ether bond). While the plasmalogens are enriched in erythrocytes, heart, brain, testis and tumors, the neutral ether glycerolipids such as 1-O-alkyl-2,3-diacylglycerol are more abundant in skin and are largely synthesized by sebaceous glands and secreted out as a component of sebum at the surface of the skin [61]. Cleavage of the 1-O-alkyl bond of ether lipids by microsomal alkyl-glycerol monooxygenase [62] releases the alkyl chain as fatty aldehyde, which is mostly oxidized to fatty acid by FALDH [63] or to fatty alcohol by fatty aldehyde reductase [36] (Figure 2).
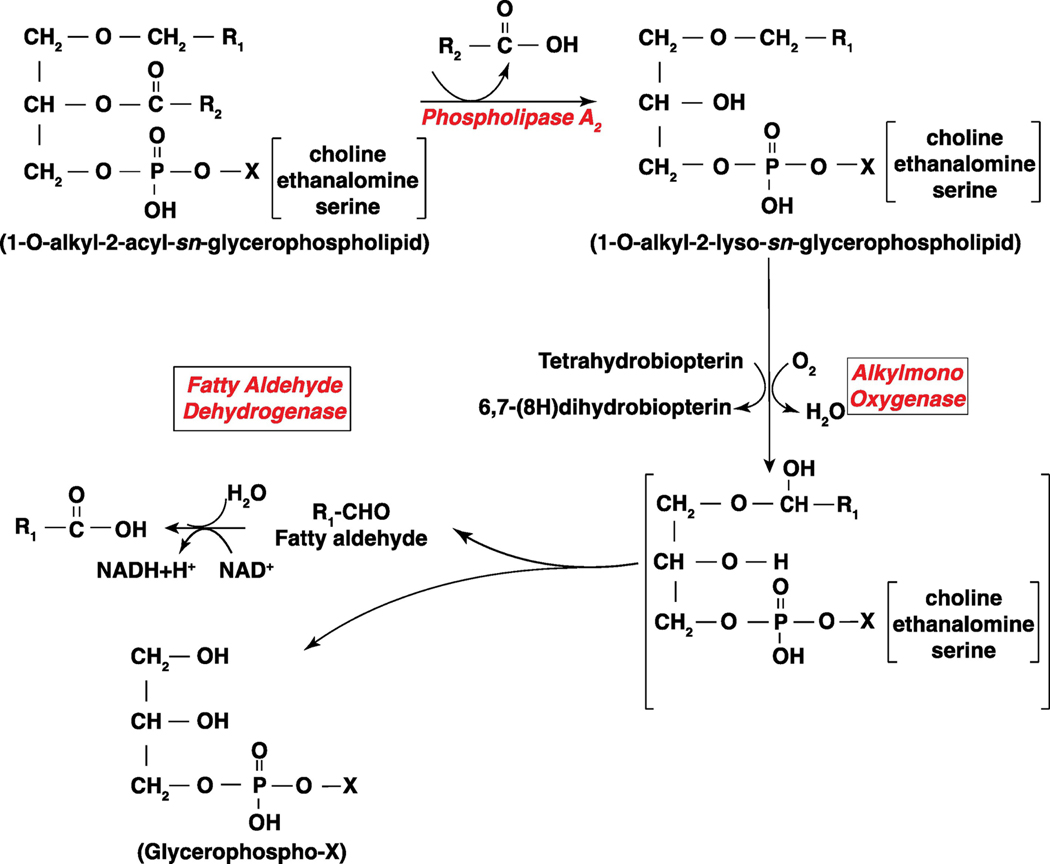
Figure 2: Pathway of long-chain fatty aldehyde production from 1-O- alkyl phospholipids by phospholipase A2 and alkylmono-oxygenase.
Phospholipase A2 dependent hydrolysis of 1-O-alkyl-2-acyl-sn-glycerophospholipid generates 1-O-alkyl-2-lyso-sn-glycerophospholipid (Alkyl- lysophospholipid). An alkylmono-oxygenase converts the alkyl-lysophospholipid to a long-chain fatty aldehyde, R1─CHO that gives rise to R1─COOH catalyzed by fatty aldehyde dehydrogenase. X denotes the polar head group, which could be choline, ethanolamine or serine.
2.3. Long-chain fatty aldehydes are derived from plasmalogen and lysoplasmalogen catalyzed by plasmalogenase (oxidized cytochrome c) and lysoplasmalogenase:
Plasmalogens, the term coined by Feulgen and Voigt in 1924 [64] describes an unidentified compound in plasma that produced an aldehyde after acid treatment. The unknown compound was identified and characterized as a glycerophospholipid containing an acid labile vinyl ether (1-O-alkenyl-) bond at the sn-1 position of the glycerol backbone [65] and enriched in AA (C20:4 ω-6), and DHA (C22:6 ω-3) at the sn-2 position of the glycerol backbone. Plasmalogens constitute ~20% of total phospholipids in cell membranes, with ≥ 50% of the glycerophosphoethanolamine fraction in brain, heart, neutrophils and eosinophils [66–68]. In addition to plasmenylethanolamine (1-O-alkenyl-2-acyl-sn-glycero-3phosphoethanolamine), cell membranes also contain plasmenylcholine (1-O-alkenyl-2-acylsn-glycero-3-phosphocholine) and plasmenylserine (1-O-alkenyl-2-acyl-sn-glycero-3-phosphoserine) but at a lower level compared to plasmenylethanolamine. Plasmalogens are also enriched in subcellular organelles of cells including nucleus, endoplasmic reticulum, Golgi and mitochondria. Ether phospholipids such as 1-O-alkyl-2-acyl-sn-glycero-3-phosphoethanolamine, choline or serine serve as the precursor lipid that is dehydrogenated at carbon1 position of the alkyl group by a cytochrome b5-dependent microsomal electron transport system and plasmenylethanolamine desaturase to form the 1-O-alkenyl- bond of plasmalogens.
The 1-O-alkenyl- bond of plasmalogens and lysoplasmalogens are susceptible to hydrolysis by enzymatic and non-enzymatic pathways that generate long-chain fatty aldehydes of C16:0, C18:0 and C18:1. Two enzymes, plasmalogenase and lysoplasmalogenase, have been described to hydrolyze the 1-O-alkenyl- or the vinyl ether bond of plasmalogens and lysoplasmalogens, respectively in mammalian cells. Plasmalogenase hydrolyzes 1-O-alkenyl-2-acyl-sn-glycero-3-phosphoethanolamine, (choline or serine) to 1-hydroxy-2-acyl-sn-glycero-3-phosphoethanolamine and 2-hydroxy-hexadecanal or pentadecanal (Figure 3). Interestingly, a recent study has reported that cytochrome c, which in the presence of cardiolipin (CL), O2 and H2O2 or oxidized CL and O2 , catalyzes the oxidation of vinyl ether bond of plasmalogen and promotes its hydrolysis to a lysophospholipid and highly reactive α-hydroxy fatty aldehydes [69]. Lysoplasmalogenase cDNA, which was cloned in 2011[70] catalyzes the hydrolysis of 1-O-alkenyl-2-hydroxy-sn-glycero-3-phosphoethanolamine or other lyso-plasmenyl phospholipids to glycerophosphoethanolamine and α-hydroxy fatty aldehydes (Figure 3). Oxidative stress seems to enhance plasmalogen degradation through increased ROS production, accumulation and disappearance of fatty aldehydes and alpha-hydroxy fatty aldehydes [71].
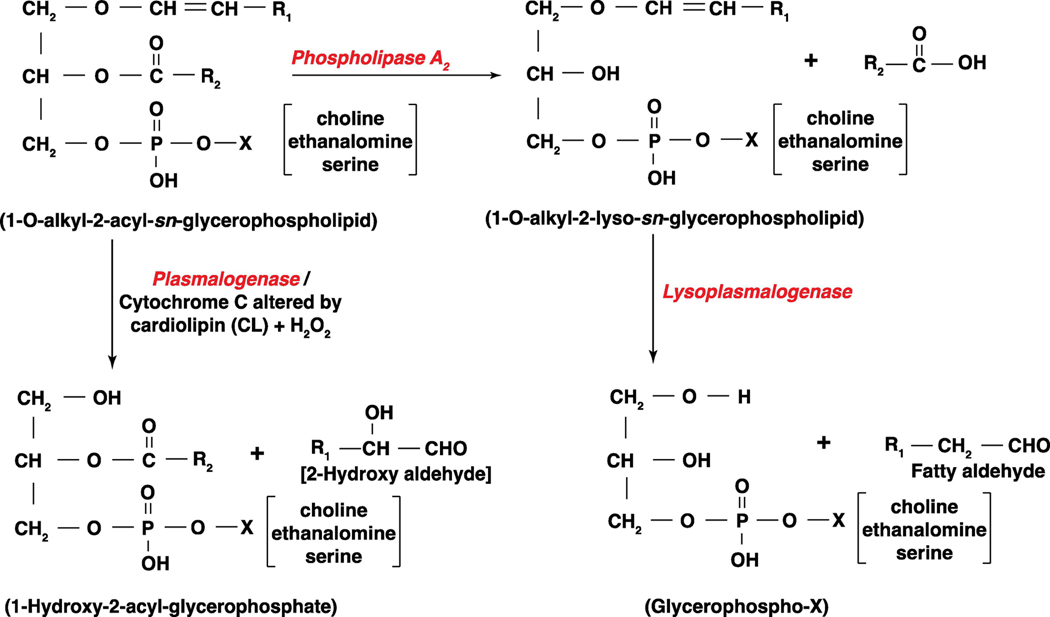
Figure 3: Pathways of plasmalogenase and lysoplasmalogenase in generating long-chain fatty aldehydes from 1-O-alkenyl phospholipids.
Plasmalogens are glycerophospholipids that have a 1-O-alkenyl- group linked to carbon 1 atom of the phospholipid, and lysoplasmalogens are derived from plasmalogens by the action of phospholipase A2. Plasmalogenase is cytochrome c that is activated by a cardiolipin (CL) and hydrogen peroxide (H2O2) in the mitochondria and releases 2-hydroxyl aldehyde and 1-lyso-2-acyl-sn-glycerophospholipid. Lysoplasmalogenase hydrolyzes 1-O-alkenyl-2-lyso-sn-glycerophospholipid generated from 1-O-alkenyl-2-acyl-sn-glycerophospholipids by PLA2 to fatty aldehyde and water soluble glycerophospho-X where X is choline, ethanolamine or serine.
2.4. 2-Halofatty aldehydes derived from plasmalogens:
Activation of neutrophils by bacterial endotoxin or N-formyl-Met-Leu-Phe (fMLP) increases the production of H2O2 with concomitant release of granules containing myeloperoxidase (MPO) while activation of eosinophils by allergens leads to increased release of eosinophil peroxidase (EPO), and H2O2. MPO amplifies the oxidant response by converting H2O2 to hypochlorous acid (HOCl) in the presence of Cl− ions while EPO in eosinophilic granules utilizes Br – ion and H2O2 to produce HOBr. Both HOCl and HOBr can target the 1-O-alkenyl- bond of plasmalogens to release lysophospholipid and 2-chlorofatty aldehyde (2-ClFALD) [72] or 2-bromofatty aldehyde (2-BrFALD), [73] respectively (Figure 4). Additionally, 2-Iodohexadecanal (2-IHDA) was identified and characterized as a major iodolipid from horse and dog thyroid gland in vitro and rat thyroid in vivo (74). The biosynthesis of 2-IHDA is unclear but might involve either hypoiodous acid (HOI) or iodine addition to the vinyl ether function of plasmalogens. It is reasonable to speculate that HOCl, HOBr and HOI could act on lysoplasmalogens to generate the 2-halo FALDs; however, this is yet to be demonstrated in biological systems.
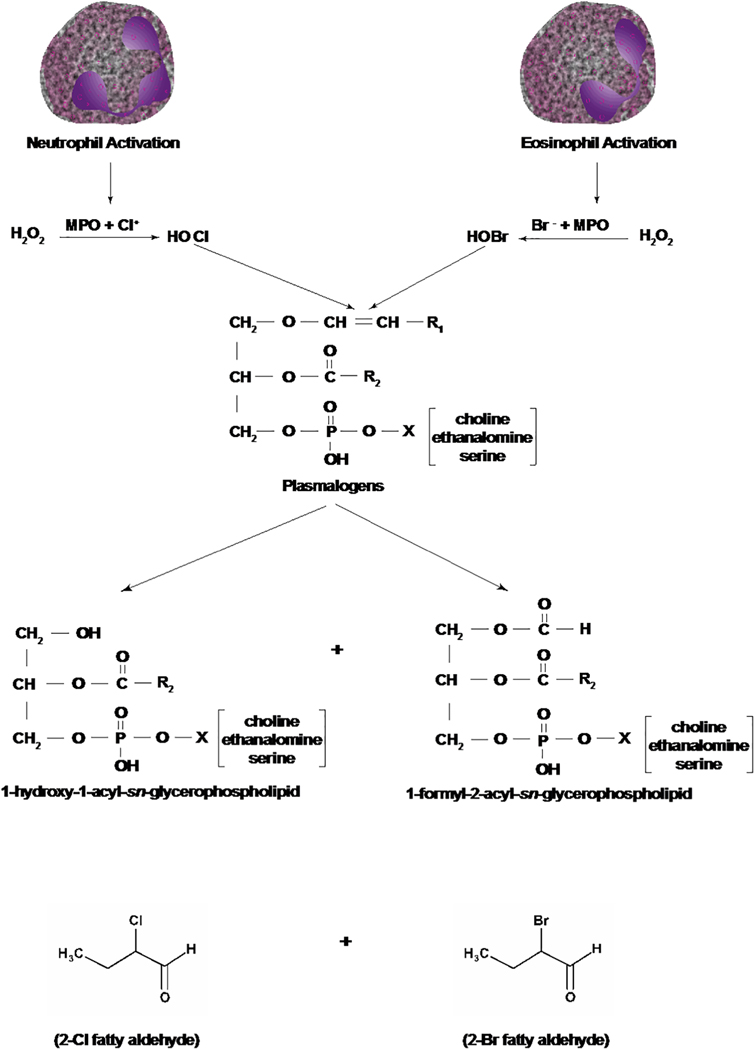
Figure 4: Pathways of generation of 2-chloro- and 2-bromo-fatty aldehydes from plasmalogens by hypochlorous and hypobromous acids.
Activation of neutrophils and eosinophils generates hypochlorous acid (HOCl) and hypobromous acid (HOBr), respectively. HOCl and HOBr by a non-enzymatic mechanism attacks the –CH=CH_ bond of plasmalogens generating 2-chloro- or 2-bromo- fatty aldehydes and 1-hydroxy (lyso)-2-acyl- and 1-formyl-2-acyl-sn-glycerophospholipid where X is choline, ethanolamine or serine. Neutrophil activation releases H2O2, which in the presence of myeloperoxidase (MPO) and chloride ion produces HOCl. Activation of eosinophils releases H2O2 plus eosinophil peroxidase that reacts with bromide ion to form HOBr.
2.5. Trans Δ2-Hexadecenal and hexadecanal derived by S1P lyase catalyzed hydrolysis of S1P and dihydro S1P:
S1P is a naturally occurring bioactive sphingolipid that is present in plasma (100 nM to 1μM) [75] [76] and generated in all mammalian cells by phosphorylation of sphingosine that is catalyzed by SPHK 1 and 2 [31] [33]. In contrast to plasma S1P levels, intracellular S1P levels are low in most of the mammalian cells except erythrocytes [77] and platelets [78], which are deficient in S1P lyase. S1P in cells is catabolized to sphingosine by lipid phosphate phosphatases [79] and S1P phosphatases [80]. Additionally, S1P lyase, a pyridoxal phosphate dependent enzyme, irreversibly hydrolyzes S1P to trans-Δ2-hexadecenal (Δ2-HDE) and ethanolamine phosphate [81] [82]. In addition to S1P, S1P lyase also hydrolyses dihydro S1P to hexadecanal and ethanolamine phosphate. S1P lyase is predominantly localized in the endoplasmic reticulum [83], but a recent study suggests that S1P lyase is also present in the nucleus of alveolar epithelial cells, lung endothelial cells and fibroblasts [84]. Pseudomonas aeruginosa infection of mouse and alveolar epithelial cells stimulated the phosphorylation of SPHK2 mediated by PKC δ, translocated the p-SPHK2 to the nucleus and generated nuclear S1P, which regulated pro-inflammatory cytokine(s) expression through HDAC1/2 activity and H3/H4 histone acetylation [85] [86]. In addition to increased nuclear S1P, Pseudomonas aeruginosa infection of lung alveolar epithelial cells also stimulated production of nuclear Δ2-HDE through S1P lyase localized in the nucleus of the cell [87] (Figure 5). In plasma, S1P, sphingosine and D-erythro-sphingosylphosphorylcholine (SPC) are associated with high-density lipoproteins (HDL) and MPO mediated generation of HOCl has been shown to attack HDL-associated SPC and S1P to generate Δ2-HDE [88]. However, it is not known if chlorinated hexadecenal is also generated by neutrophil MPO in plasma preparations. The Δ2-HDE is then oxidized to trans-2-hexadecenoic acid followed by CoA addition to generate trans-Δ2-hexadecenoyl CoA that is reduced to palmitoyl CoA by trans-2-enoyl-CoA reductase (TER) in mammalian cells [89]. However, a metabolism study with HepG2 cell lysates revealed that ~80% conversion of Δ2-HDE to its main oxidative metabolite trans- Δ2-hexadecenoic acid as assessed by LC-MS/MS quantification, implicating further possible metabolic routes of Δ2-HDE [90]. TER is a component of the fatty acid elongation system responsible for the production of very long-chain fatty acids (C24:0 and C24:1), which are predominantly used for sphingolipid synthesis and myelin lipids. Knockdown of TER in HeLa cells decreased SPHK activity, modulated sphingosine and dihydrosphingosine contribution to glycerophospholipid metabolism; however, molecular mechanisms underlying these changes are unclear [89]. Further studies are necessary to determine the regulatory mechanism linking fatty acid elongation by TER and S1P pathways involving Δ2-HDE formation by S1P lyase.
Figure 5: Degradation of sphingosine-1-phosphate (S1P).
Sphingosine-1-phosphate (S1P) generated from sphingosine by sphingosine kinases 1 and 2 (SPHK1 & 2) is irreversibly metabolized by S1P lyase, a pyridoxal phosphate dependent enzyme, to trans-Δ2-hexadecenal (Δ2-HDE) and ethanolamine phosphate. Δ2-HDE is further oxidized by fatty aldehyde dehydrogenase (FALDH) followed by coupling to coenzyme A (CoA) by acyl-CoA synthase. The product hexadecenoyl-CoA can be saturated by means of trans-2-enoyl-CoA reductase to form palmitoyl-CoA that can serve as building block for glycerolipid (and sphingolipid) synthesis.
2.6. ω-Oxidation of leukotriene B4:
In addition to β-oxidation of fatty acids, ω-oxidation represents an alternative pathway of fatty acid oxidation. In this pathway, the ω-terminal of the aliphatic chain (terminal CH3 group) is first hydroxylated by a cytochrome P450 ω-hydroxylase (CYP4) and subsequently oxidized to a carboxyl-group via an –CHO intermediate that is analogous to oxidation of long-chain fatty alcohols [46]. There is evidence for the participation of FALDH in ω-oxidation of certain type of fatty acids including leukotriene (LT) B4. LTs are oxygenated derivatives of polyunsaturated fatty acids that are generated by 5-lipooxygenase pathway. Among the various LTs, LTB4 is a potent mediator of allergic asthma and ω-hydroxylation of LTB4 and conversion to a carboxylic acid derivative renders it less potent. Thus ω-hydroxylation and ω-oxidation regulates the biological activity of LTB4. This defective LTB4 inactivation might play an important pathophysiological role in Sjögren-Larsson Syndrome (SLS), an autosomal recessively inherited disorder of lipid metabolism characterized by congenital ichthyosis, spastic di- or quadriplegia and mental retardation [91] [92].
3.0. FATTY ALDEHYDES AS SIGNALING LIPIDS
Short chain aldehydes such as formalin and acetaldehyde, and α, β-unsaturated aldehydes such as acrolein, crotonaldehyde and 4-HNE that are products of endogenous lipid peroxidation are unstable and highly reactive with cellular macromolecules. These highly reactive aldehydes exhibit genotoxic effects mediated via adduction of DNA, proteins, histones and lipids. Additionally, many of these aldehydes affect DNA methylation at C5 of cytosine leading to epigenetic gene regulation [93]. While generation of short-chain aldehyde adducts, including 4-HNE adducts, play important roles in several diseases characterized by increased oxidative stress; in certain chronic inflammatory and neuro-degenerative diseases, 4-HNE adducts can promote adaptive cell responses by stimulating intracellular GSH synthesis [94] [95] activating PLD, lipid signaling pathways [96] [97] [98 ] [99] [100], inducing HO-1 [101], and stimulating autophagy [102]. These studies suggest that many of the short-chain aldehydes can be toxic; however, they can also act as regulatory molecules involved in signaling pathways and cell metabolism. In contrast to short chain aldehydes, it is unclear if long-chain aldehydes can form adducts with DNA ; however, it has been shown that long-chain aldehydes can form adducts with albumin, and glutathione [36], phosphatidylethanolamine [71] and deoxyguanosine [37] in biological systems, suggesting their reactivity and ability to modify macromolecules. Recent studies show that exogenously added long-chain fatty aldehydes modulate signaling pathways related to cytoskeletal organization, adhesion, apoptosis, and epigenetic modification of histones, which will be summarized in detail.
3.1. Signaling and cellular functions of Trans Δ2-hexadecenal and hexadecanal:
Despite the importance of S1P lyase pathway in the degradation of S1P to Δ2-HDE or dihydro S1P to hexadecanal, only a few sparse sets of studies have addressed the signaling properties of these long-chain fatty aldehydes in mammalian cells [57] [103] [104] [105] . Further, as long-chain fatty aldehydes are not easily cell permeable, it is unclear if any of the described effects of Δ2-HDE or hexadecanal on cell signaling are related to perturbations and/or modifications of proteins and phospholipids on the cell plasma membrane.
3.1.1. Role of S1P lyase in LPS-induced lung inflammatory injury:
While it is difficult to determine a direct role of Δ2-HDE released from S1P by S1P lyase in various animal models of lung disorders, studies blocking S1P lyase have shown modulatory effects on lung inflammatory injury [103]. Intratracheal administration of lipopolysaccharide (LPS) enhanced S1P lyase expression in mouse lung with increased secretion of pro-inflammatory cytokines and blocking S1P lyase with 2-acetyl-4(5)-[1R,2(S),3R,4-tetrahydroxybutyl]-imidazole (THI) attenuated LPS-induced lung inflammatory injury and secretion of IL-6 [103]. In vitro, down-regulation of S1P lyase with siRNA in human lung microvascular endothelial cells attenuated LPS-mediated phosphorylation of p38 MAPK and I-kB, IL-6 secretion and endothelial barrier disruption via Rac1 activation [103] suggesting an inflammatory role for S1P degradation products in lung injury. However, a direct role for Δ2-HDE released from S1P by S1P lyase could not be established due to complexity of the in vivo animal model.
3.1.2. Trans Δ2-hexadedenal induces cytoskeletal reorganization and apoptosis:
In a pioneering study, Julie Saba and her group demonstrated, for the first time, that the S1P metabolite, Δ2-HDE, induced cellular effects in epithelial cells through reactive oxygen species (ROS)-dependent MAPK cell signaling. In this study (Figure 6A), exogenous addition of Δ2-HDE (25–50 μM) to HEK293T, NIH3T3 or HeLa cells resulted in cytoskeletal reorganization of stress fibers and apoptotic cell death after detachment, which was dependent on ROS mediated MLK3/JNK activation, whereas ERK, AKT and p-38 MAPK were unaffected [104]. In contrast to Δ2-HDE, similar concentrations of hexadecanoic acid had no effect on cellular ROS, JNK activation and apoptosis. Further, Δ2-HDE-induced apoptosis was followed by activation of downstream targets of JNK such as c-Jun phosphorylation, cytochrome c release, Bax activation, Bid cleavage and increased translocation of Bim into mitochondria. Inhibition of JNK with JNK inhibitor V attenuated Δ2HDE mediated cytoskeletal changes and apoptosis. Further investigation is necessary to delineate the source of ROS (mitochondrial vs. NOX family proteins) and mechanism(s) of signal transduction by Δ2-HDE from the plasma membrane to intracellular targets.
Figure 6. Trans Δ2-hexadecenal stimulates signaling pathways in HEK293 and C6 glioma cells.
(A) Trans-Δ2-hexadecenal (Δ2-HDE) stimulates cytoskeletal reorganization and apoptosis in HEK293/NIH3T3/HeLa cells via activation of MKK4/MKK7►MLK3►JNK signaling. Trans Δ2-HDE-induced apoptosis involved Jun N-terminal kinase (JNK) phosphorylation that was reactive oxygen species (ROS) dependent accompanied by Bax activation, translocation of Bax and Bim to mitochondria, and cytochrome release (104). (B) Exogenous addition of trans Δ2-HDE to C6 glioma cells activated p38-mitogen activated protein kinase (p38-MAPK), extracellular signal-related kinase (ERK) 1/2) and phosphatidylinositol 3-kinase (PI3K) signaling pathways that regulated glioma cell proliferation (105).
In a recent study (Figure 6B), Δ2-HDE (10–100 μM) treatment of C6 glioma cells reduced proliferation and mitotic indices without loss of cell viability while a higher Δ2-HDE (350 μM) concentration reduced survival of glioma cells by ~34% indicating necrosis of cells [105]. Addition of Δ2-HDE induced cytoskeletal rearrangement of F-actin redistribution and changes in cell morphology and apoptosis. Δ2-HDE also activated ERK1/2, p38 MAPK and JNK, but not PI3K pathways in C6 glioma cells. These data are in part consistent with the results shown by Kumar et al., in epithelial cells [104]. Both the studies utilized very high concentrations of Δ2-HDE to bring about cytoskeletal reorganization and apoptosis of cells. This suggests that activation of MAPKs and other pathways may not be mediated by specific long-chain fatty aldehyde receptors on the cell surface and to date no such receptors have been cloned and characterized. In contrast to short-chain aldehydes such as 4-HNE and acetaldehyde, Δ2-HDE and other long-chain fatty aldehydes are not readily cell permeable and it is not clear if they are transported inside the cell by active or passive transporters. It is conceivable that the long-chain fatty aldehydes, being highly reactive, can bind to and modify cell surface proteins by forming adducts that can lead to modification of membrane proteins and receptors involved in signal transduction. The ability of hexadecanal, octadecanal and other long-chain aldehydes to modulate cell signaling has not been thoroughly investigated and compared to Δ2-HDE. Exogenous addition of hexadecanal or ethanolamine phosphate (10 μM) to lung endothelial cells stimulated p38-MAPK and IKB phosphorylation; however, had no effect on LPS-mediated MAPK activation [103].
3.1.3. Nuclear generation of Δ2-hexadecenal by S1P lyase and modulation of HDAC1/2 activity:
S1P lyase is a pyridoxal-phosphate dependent enzyme [39] [78], which catalyzes the hydrolysis of S1P and dihydro S1P to Δ2-HDE and hexadecanal, respectively, in mammalian cells [81] [82] [83]. It is predominantly localized in the endoplasmic reticulum (ER) inner membrane; however, recent studies with lung endothelial and epithelial cells, as well as lung fibroblasts, have shown nuclear localization of S1P lyase after release of the ER membranes from the nuclear outer membrane by mild detergent [84]. Further, the nuclear fractions isolated from lung endothelial and epithelial cells showed no contamination of ER membranes as determined by ER and nuclear markers and electron microscopy [84]. Pseudomonas aeruginosa infection of mouse lung epithelial cells elevated nuclear S1P [85] and nuclear Δ2-HDE levels [84] as measured by LC-MS/MS [106]. Inhibition of S1P lyase by 4-deoxypyridoxine enhanced nuclear S1P levels under basal condition and attenuated Pseudomonas aeruginosa-induced Δ2-HDE production in mouse lung epithelial cells [84], confirming the role of nuclear S1P lyase in generating Δ2-HDE in the nucleus. In a recent study a critical role for nuclear SPHK2/S1P signaling in regulation of Pseudomonas aeruginosa-mediated acetylation of H3 and H4 histones has been shown [85] and inhibition of S1P lyase by 4-deoxypyridoxine reduced Pseudomonas aeruginosa-induced H3 and H4 histone acetylation indicating a potential role for Δ2-HDE released from S1P to modulate HDAC1/2 activity [86] [87] [107] [108]. Additionally, pre-treatment of lung epithelial cells with phloretin, a nonspecific fatty acid uptake inhibitor [109], and a chelator of fatty aldehydes, attenuated Pseudomonas aeruginosa-induced H3 and H4 histone acetylation [87] confirming modulation of HDAC activity by Δ2-HDE released in the nucleus (Figure 7).
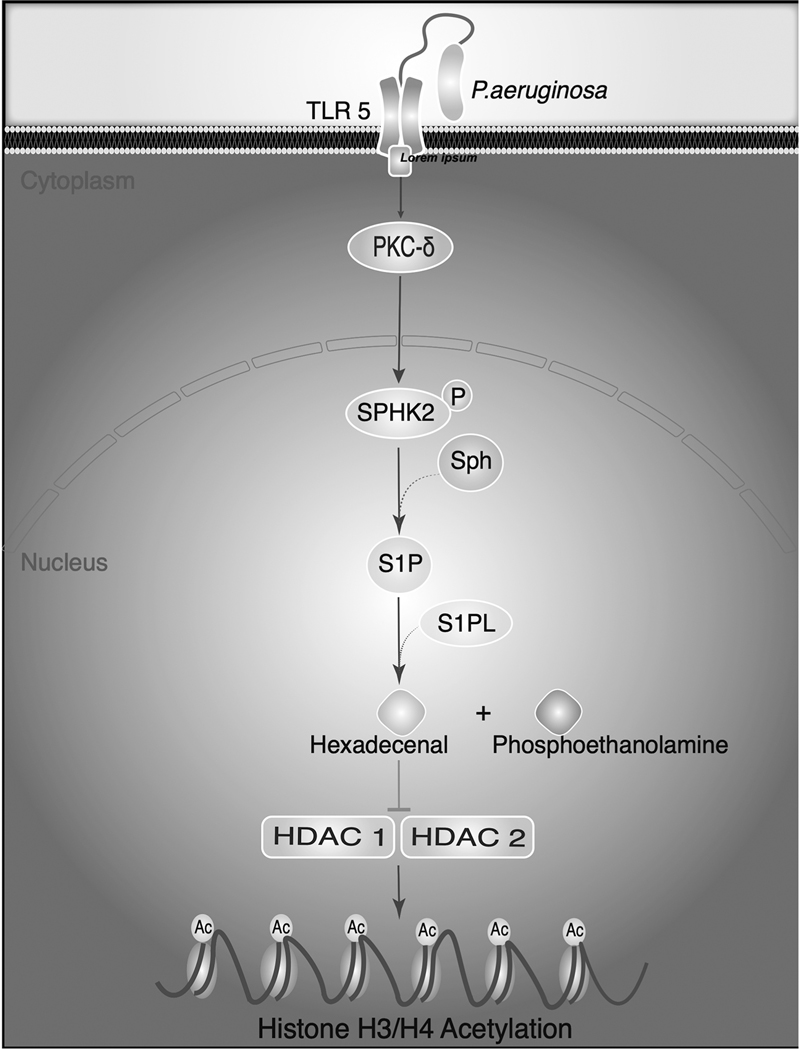
Figure 7: Nuclear S1P lyase generated trans Δ2-hexadecenal from nuclear S1P modulates HDAC activity and histone acetylation.
Pseudomonas aeruginosa infection of mouse lung and lung epithelial cells in vitro stimulates phosphorylation of sphingosine kinase 2 (SPHK2) in the cytoplasm mediated by protein kinase C (PKC)-δ. Activated SPHK2 is translocated to the nucleus of the epithelial cell where it converts sphingosine to sphingosine-1-phosphate (S1P). Although S1P lyase is predominantly localized in the endoplasmic reticulum (ER), presence of S1P lyase in the nuclear preparations was detected by Western blotting and purity of the nuclear preparations from ER was verified by electron microscopy and immunostaining of the preparations with specific markers for ER, Golgi, cytoplasm and nuclear membrane. S1P generated in the nucleus by nuclear SPHK2 is hydrolyzed by S1P lyase to generate trans Δ2-hexadecenal (Δ2-HDE) and ethanolamine phosphate. S1P or Δ2-HDE generated from S1P modulates HDAC1/2 activity and H3/H4 histone acetylation pattern in lung epithelial cells (85, 86).
3.2. Signaling and biological effects of 2-halofatty aldehydes:
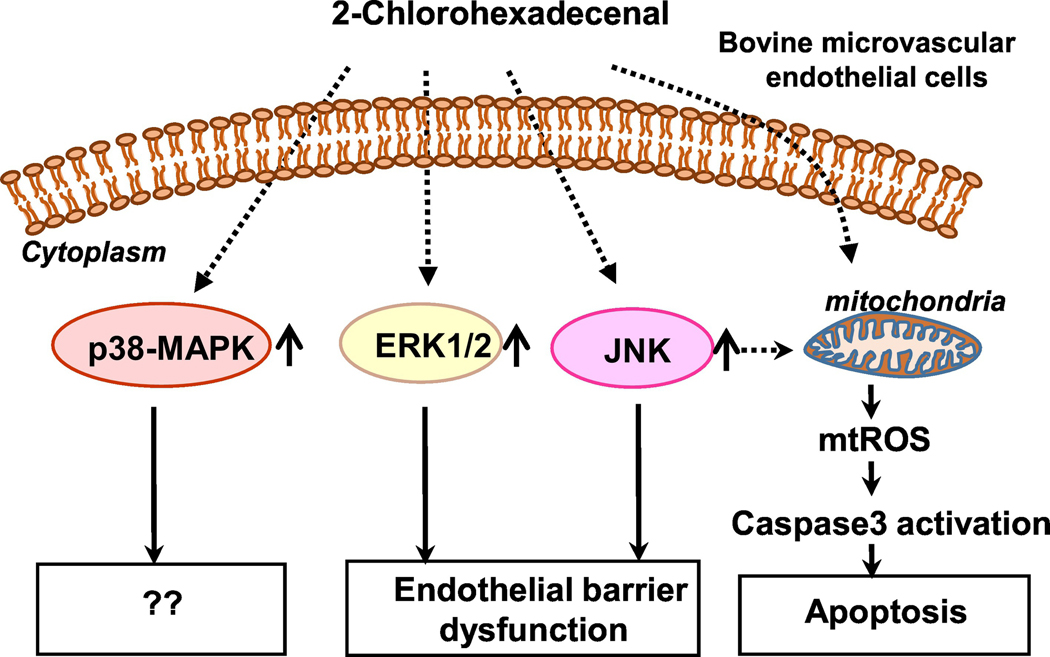
Myeloperoxidase-derived HOCl from neutrophils, HOBr from eosinophils or HOI from thyroid targets the sn-1 alkenyl (vinyl ether) bond of choline, ethanolamine or serine plasmalogens liberating a lysophospholipid and 2-chloro- [65], 2-bromo- [66] or 2-iodo fatty aldehyde [74]. These halofatty aldehydes have been quantified by negative ion chemical ionization detection and gas chromatography-mass spectrometry methods. In unstimulated human neutrophils and monocytes, the 2-ClFALD level was very low (<0.5 pmol/106 cells) and stimulation with phorbol ester enhanced the level ~5 to 10-fold [110] [111]. MPO-derived 2-ClFALD has been implicated in a number of biological processes including endothelial barrier function, neutrophil chemotaxis and biology, monocyte apoptosis, vascular tone and inflammation [112]. 2-Chlorohexadecanal (2-ClHDA) is a potent chemo attractant for neutrophils [110], accumulates in post-ischemic tissues [113] [114], which might contribute to ischemia/perfusion injury, hemorrhagic shock and sepsis. The role of 2-ClHDA in eliciting endothelial dysfunction have been investigated at the blood-brain barrier (BBB) level and endothelial cells in culture. In vivo, in a mouse model of LPS-induced systemic inflammation, MPO deficiency significantly reduced LPS-mediated BBB dysfunction as compared to wild-type littermates [115]. In vitro, exogenous addition of 2-ClHDA to brain microvascular endothelial cells (BMVECs) induced loss of barrier function, apoptosis via activation of caspase 3, mitochondrial dysfunction and altered intracellular redox balance, which were completely abrogated by phloretin [116]. These results suggest that activation of neutrophils and subsequent release of 2-ClHDA could contribute to BBB dysfunction under pathological conditions and new pharmacotherapeutical strategies such as phloretin treatment could ameliorate the fatty aldehyde mediated BBB dysfunction. The 2-ClHDA-induced endothelial barrier dysfunction was shown to involve activation of MAPK pathway (Figure 8). 2-ClHDA, in a dose-dependent manner, enhanced phosphorylation of ERK1/2, p38 MAPK and JNK in BMVECs and inhibition of ERK1/2 and JNK, but not p38 MAPK, with specific pharmacological inhibitors provided partial rescue against 2-ClHDA-induced endothelial barrier dysfunction, including morphological changes of continuous distribution of junctional proteins to “frizzy-like” structures and the transformation from spindle to a more rounded cell shape [115]. The MPO derived 2-ClHDA is converted to either 2-chlorohexadecanoic acid (2-ClHA) or 2chlorohexadecanol (2-ClHDL), which can also affect neutrophil chemotaxis and inflammatory responses. In rat mesentery, 2-ClHDA induced a number of pro-inflammatory responses in vivo and in vitro. 2-ClHDA increased leukocyte-endothelial cell interactions, platelet-endothelial cell adhesion, and neutrophil infiltration into the tissues, mast cell activation, ROS production and disrupted endothelial barrier function [117]. Interestingly the non-chlorinated long fatty aldehydes, such as hexadecanal and hexadecanoic acid, did not produce these responses in vivo. Similar pro-inflammatory responses were observed in response to 2ClHDA and 2-ClHA in cultured mesenteric micro-vascular endothelial cells in vitro [117].
Figure 8: 2-Chlorohexadecanal signaling and endothelial barrier dysfunction.
Exogenous addition of 2-chlorohexadecanal (2-ClHDA) to brain microvascular endothelial cells stimulated extracellular signal-related kinase1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38- MAPK) and Jun N-terminal kinase (JNK) signaling pathways and blocking ERK1/2 and JNK, but not p38 MAPK, attenuated 2-ClHDA-mediated endothelial dysfunction. 2-ClHDA also caused induced mitochondrial dysfunction including increased mitochondrial reactive oxygen species (mtROS), and apoptosis via activation of caspase 3.
Two groups of iodolipids have been identified to be generated in thyroid gland by peroxidases in the presence of excess iodide. One of them is the iodinated derivatives of arachidonic acid formed when the gland is exposed to exogenous arachidonic acid [118] [119] and the other is 2-iodoalkanals derived from iodination of plasmalogens [74]. The biological effects of 2-IHDA have been investigated using human thyroid membranes and dog thyroid cells in culture. 2-IHDA inhibited adenylate cyclase activity in thyroid membranes, increased inositol phosphates, stimulated carbachol-induced formation of H2O2 generation in dog thyroid cells [120] and thyroid NADPH-oxidase in cell-free system [121]. As 2-IHDA also inhibited thyroid peroxidase [121], this iodinated lipid could regulate the metabolism of iodide in thyrocytes.
3.2.1. Biological role of 2-chlorofatty acids derived from 2-chlorofatty aldehydes:
2-ClHDA derived from sn-1 alkenyl- bond of plasmalogens by MPO/HOCl is readily oxidized by cellular FADH to 2-ClHA [122], which can be exported out of neutrophils, and monocytes; however, very little is known on its role in cell function. Recent studies suggest that 2-ClHA also elicits pro-inflammatory responses in mesenteric vascular endothelial cells in vitro. 2-ClHA, similar to 2-ClHDA, displayed increased platelet and neutrophil adherence that was associated with elevated expression of ECAMs and increased permeability [117]. In vivo, 2ClHA induced mast cell activation, enhanced ROS production and albumin leakage in post-capillary venules of rat mesentery, and enhanced MPO expression in jejunal submucosa [117].
The biological effects of 2-ClHDA and 2-ClHA have been evaluated by exogenous addition of these chlorinated lipids on cells while in reality these are generated inside the cell via neutrophil derived MPO and generation of HOCl that acts on plasmalogens enriched in ER, Golgi and other membranes. Hence, to study the intracellular role of 2-ClHA, a synthetic ‘clickable’ analog of 2-ClHA, 2-chlorohexadec-15-ynoic acid (2-ClHyA) that phenocopied the biological activity of the parent 2-ClHA was utilized. Exposure of human brain microvascular EC line (hCMEC/D3) to 2-ClHyA revealed its accumulation in the ER and mitochondria and 2ClHyA interfered with protein palmitoylation [123]. 2-ClHA, which was added exogenously, induced ER stress markers, reduced ER ATP levels and activated transcription and secretion of IL-6 and IL-8 [123]; however, the effect of 2-ClHyA on ER stress markers, mitochondrial membrane potential, apoptosis and IL-6/IL-8 secretion was not investigated. Inhibition of protein kinase R-like ER Kinase (PERK) with inhibitor GSK2606414 suppressed 2-ClHAmediated activating transcription factor 4 synthesis and IL-6/IL-8 secretion without affecting endothelial barrier dysfunction and cleavage of procaspase-3 [123]. Further, exposure of human coronary artery ECs to ‘clickable’ 2-ClHyA revealed localization to Weibel-Palade bodies and promoted release of P-Selectin, von Willebrand factor, and angiopoietin-2. Further, functionally, 2-ClHyA and 2-ClHA caused neutrophils to adhere to platelets and aggregate on the endothelium as well as increase endothelial permeability [124]. These findings suggest a broader implication of 2-ClHDA and 2-ClHA in promoting endothelial dysfunction that leads to inflammation, thrombosis and vessel wall stability.
3.3. Cellular targets of Trans Δ2-hexadecenal, hexadecanal, and 2-chloro- and 2-bromo- hexadecanal:
Protein modifications by reactive and chemically diverse electrophilic bioactive lipids such as α,β-unsaturated aldehydes, epoxides and eicosanoids have been recognized as an important post-translational modification to modulate cell signaling, redox homeostasis, adaptive responses to environmental toxins and inflammatory injury. The chemical reactivity of electrophilic lipid oxidation products with cellular protein nucleophiles such as cysteine, histidine and lysine lead to formation of 1,4-Michael or Schiff’s base adducts. It has been postulated that electrophiles prefer to coordinate with nucleophiles of close softness and hardness and the most stable adducts can be formed between soft nucleophiles and electrophiles and weaker adducts result from the interaction of hard nucleophiles and electrophiles [125] [126]. Among reactive aldehydes formed during lipid peroxidation, 4-HNE reacts with both low-molecular weight compounds such as glutathione and macromolecules such as proteins and DNA and 4-HNE adducts have been well characterized in several human pathologies including cancer, neurodegenerative, inflammatory and autoimmune diseases [42]. Studies pertaining to formation of adducts between long-chain fatty aldehydes and proteins, glutathione or DNA adducts in vivo and in vitro are limited and advancement in LC-MS/MS with availability of stable isotopically labeled reference materials should advance the nature and type of adducts formed under normal and pathological conditions.
3.3.1. Trans Δ2-hexadecenal forms protein adducts with HDACs and glutathione conjugates in vitro:
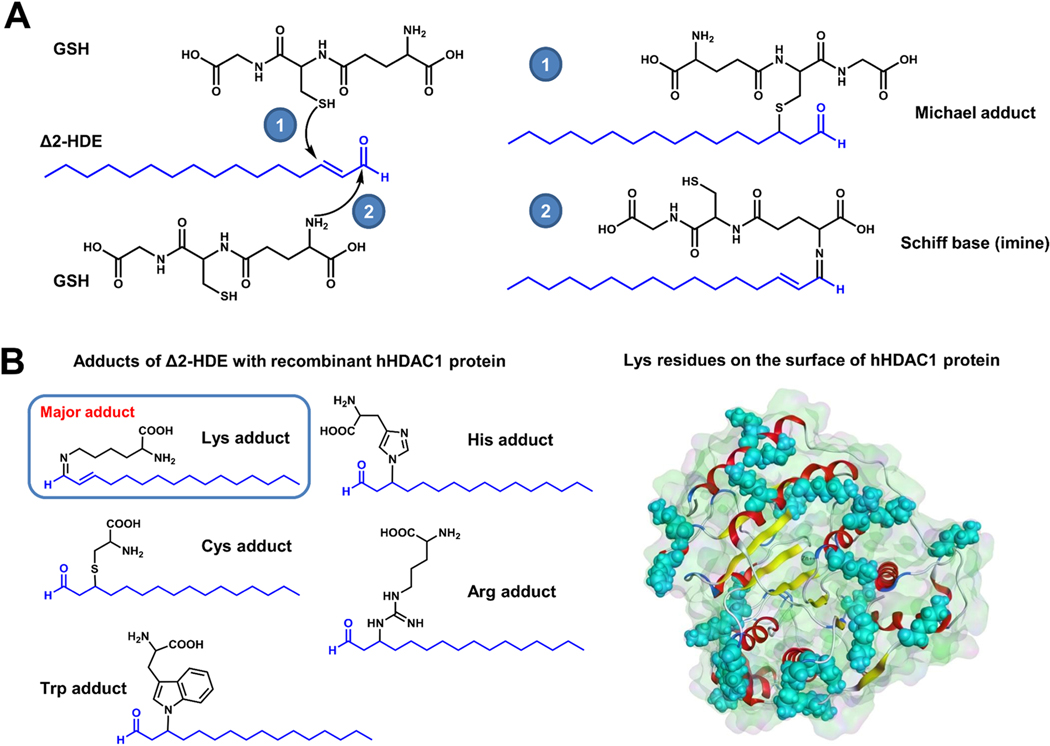
Δ2-HDE, due to its α,β-unsaturated carbonyl function, possesses two electrophilic centers at C-1 and C-3 carbon atoms within the molecule which are susceptible to nucleophilic substitutions. The β carbon atom (C-3 carbon) is a soft electrophile acceptor that produces soft Michael adducts with nucleophiles of comparable softness (thiols). The carbonyl carbon (C-1 carbon), which is a much stronger electrophile, can react with primary amino group of phosphatidylethanolamine and proteins to generate Schiff bases. In a cell-free system, a total of two GSH conjugates and seven L-amino acid adducts were identified and characterized using LC-MS/MS and stable isotopically labeled internal standards [36].Similarly, incubation of HepG2 cell lysates with Δ2-HDE resulted in identification of two GSH conjugates. Δ2-HDE also formed Michael adducts mainly with L-histidine of BSA and proteins extracted from HepG2 cell lysates. Michael adducts with tryptophan, cysteine and Schiff base adducts with lysine were also detected and inhibition of oxidative degradation of Δ2-HDE resulted in increased levels of GSH conjugates and protein adducts in HepG2 cell lysates (Figure 9) [36]. Δ2-HDE is generated in the nucleus by nuclear S1P lyase in response to Pseudomonas aeruginosa infection of lung epithelial cells and modulated HDAC 1/2 activity in vitro [84] [87]. There is a possibility of potential adduct formation between Δ2-HDE and HDAC1. Incubation of Δ2-HDE with recombinant HDAC1 in vitro generated five different hexadecenal adducts as determined by LC-MS/MS. The Schiff base (imine) of Lys and Δ2HDE was by far the most prominent Michael adduct while adducts of cysteine, histidine, arginine and tryptophan were also detected at much lower levels (Figure 9). This fits well to the high amount of Lys residues in the sequence of HDAC1 and their exposed localization on the protein’s surface [84] . Formation of adducts between Δ2-HDE and HDACs 1 & 2 in the nucleus of lung epithelial and endothelial cells with or without Pseudomonas aeruginosa infection needs to be established in vivo.
Figure 9: Trans-Δ2-hexadecenal forms adducts with glutathione, and HDAC1 in vitro.
(A) Incubation of Δ2-HDE with glutathione (GSH) in vitro resulted in formation of Michael adducts (1), and Schiff’s base adducts (2) as determined by LC-MS/MS. (B) Incubation of Δ2-HDE with recombinant hHDAC1 in vitro generated five different adducts (left panel) with the Schiff’s base (imine) of lysine and Δ2-HDE by far was the most predominant species generated as detected by LC-MS/MS. This observation is consistent with the high amount of Lys residues in the sequence of hHDAC1 (40x) and their exposed localization on the protein’s surface (shown in cyan, right panel).
3.3.2. Clickable analogues of trans Δ2-hexadecenal as probes for cellular targets:
Δ2HDE added exogenously cannot easily penetrate the cell making it very difficult to determine the potential intracellular adducts formed. To overcome this difficulty, use of clickable analogues of Δ2-HDE, hexadec-15-ynal (Probe 1, control) and (E)-hexadec-2-en-15-ynal (Probe 2, experimental) were developed [127], and labeling experiments carried out with Probe 1 and Probe 2 showed that Probe 2 labeled more than 500 endogenous proteins in HEK293A cells compared to the saturated aldehyde (Probe 1). The identified protein targets included BAX, mTOR, CNOT1, BASP1, ABCB7, PPID and ATAD3A. Interestingly, Δ2-HDE covalently modified the conserved Cys62 of BAX and modification of Cys62 compromises BAX activation [127]. It is unclear if other cysteine residues in BAX are also modified by Δ2-HDE.
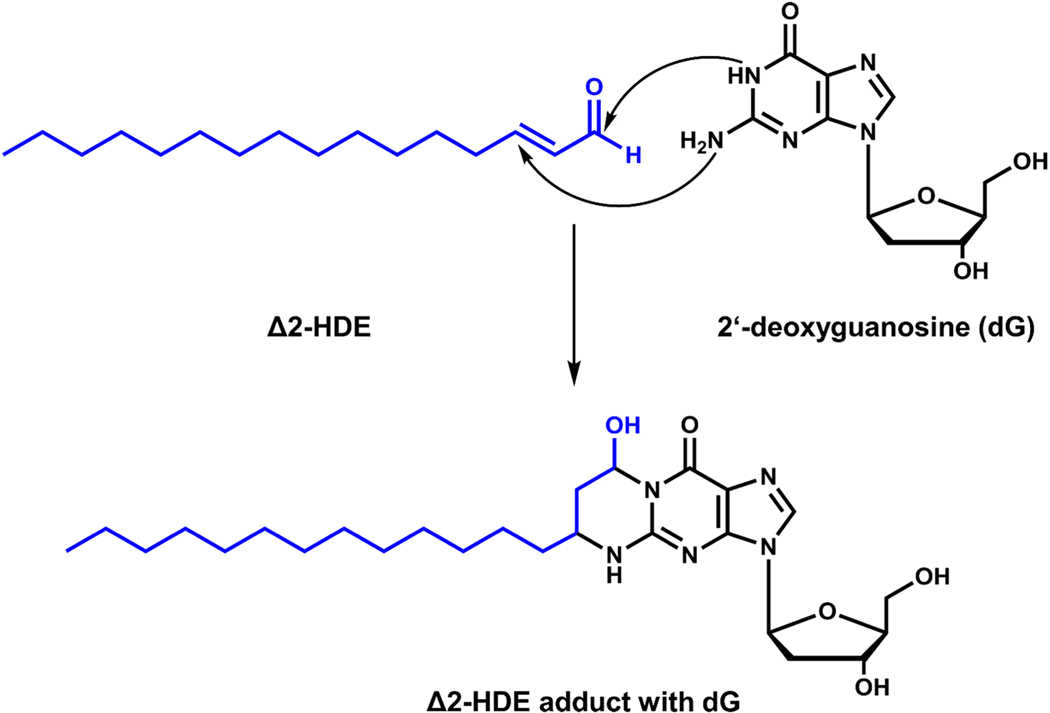
3.3.3. Trans Δ2-hexadecenal forms cyclic adducts with 2’-deoxyguanosine:
α,β-unsaturated aldehydes such as acetaldehyde, crotonaldehyde and 4-HNE that are highly electrophilic have been shown to alkylate deoxyguanosine and deoxycytosine residues in DNA, forming 1 and 3, N4 DNA adducts respectively in vivo [128] and in vitro [129], which are cytotoxic, genotoxic, mutagenic and clastogenic and play an important role in carcinogenesis [130]. The 1, N2-deoxyguanosine adducts also undergo reversible cyclization yielding 1,N2-deoxyguanosine exocyclic products [131]. Similarly, Δ2-HDE reacted with deoxyguanosine and DNA in vitro to form diasterometric cyclic 1,N2-deoxyguanosine adducts 3-(2-deoxy-βDerythro-pentafuranosyl)-5,6,7,8-tetrahydro-8R-hydroxy-6R-tridecylpyrimido [1,2-α]purine-10 (3H)one and 3-(2-deoxy-β-D-erythro-pentafuranosyl)-5,6,7,8-tetrahydro-8S-hydroxy-6S-tridecylpyrimido[1,2–]purine-10 (3H)one [37] (Figure 10). To determine the significance of in vitro adduct of Δ2-HDE with deoxyguanosine and DNA, in vivo formation of these adducts needs to be demonstrated under normal and pathological conditions, such as Sjögren-Larsson syndrome, wherein the deficiency of ALDH3A2 affects the conversion of hexadecenal to hexadecanoic acid [57]. It is yet to be established if there is an increase in Δ2-HDE containing DNA adducts in any human pathology.
Figure 10: Adduct formation between trans-Δ2-hexadecenal and 2’-deoxyguanosine.
Scheme depicts the adduct formation between trans-Δ2-hexadecenal (Δ2-HDE) and DNA nucleoside 2’-deoxyguanosine (dG) in vitro as detected by LC-MS/MS.
3.3.4. 2-Chloro- and 2-bromofatty aldehydes form adduct with glutathione and proteins:
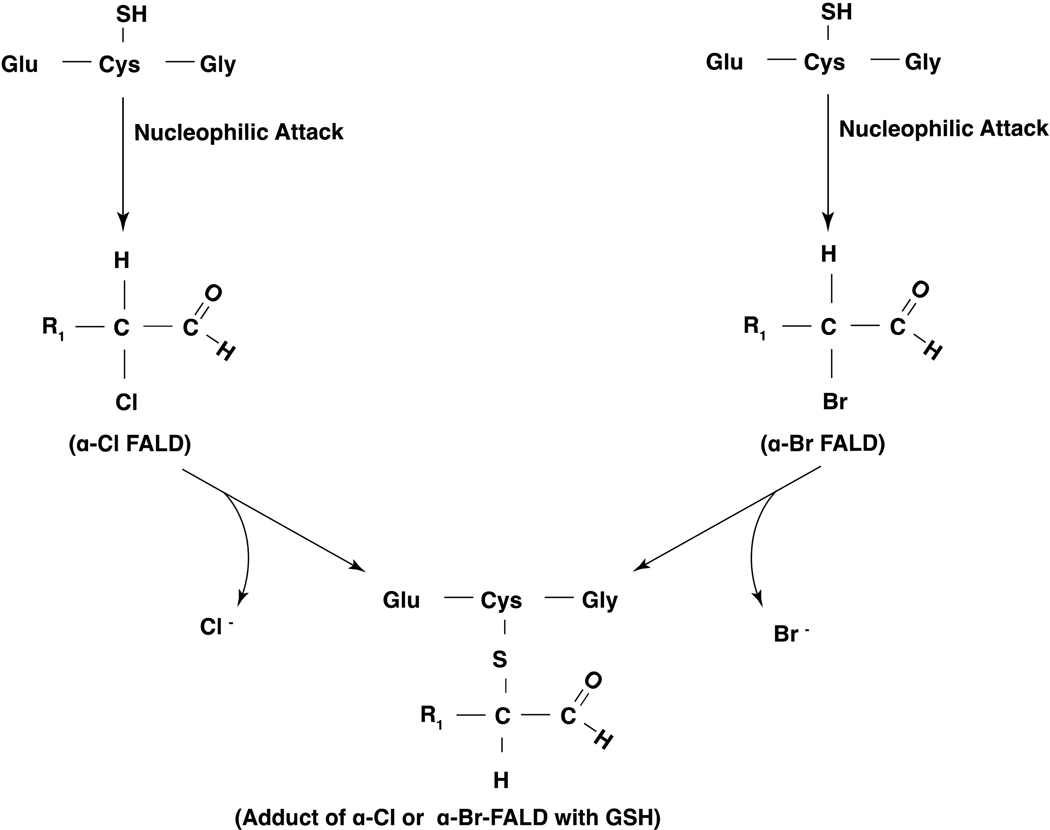
2-ClFALDs are produced from plasmalogens by HOCl during phagocyte activation [110] [111] while 2-BrFALDs are generated by HOBr reacting with plasmalogens in activated eosinophils [132]. The 2-chlorinated or 2-brominated carbon atom of either 2-ClFALD or 2-BrFALD is a stronger electrophile and would be attacked by nucleophiles to form adducts. Incubation of 2-ClHDA and 2-BrHDA with GSH resulted in formation of adduct as characterized by ESI/MS/MS that stained positive with ninhydrin (stains for amine of GSH but does not stain 2-ClHDA or 2-BrHDA) and the reaction product also stained by dinitrophenyl hydrazine (stains aldehydes but not GSH) supporting the structure of the adduct with the nucleophilic attack of cysteine residue of GSH at the α-carbon atom of α-ClHDA or α-BrHDA (Figure 11) [133] [134]. RAW 264.7 cells treated with exogenous 2-ClHDA or 2-BrHDA formed HAD-GSH adduct in a dose- and time-dependent fashion. Similarly, stimulation of human neutrophils or eosinophils with phorbol ester produced the FALD-GSH molecular species, HDA-GSH and octadecanal-GSH (ODA-GSH), and the adduct formation was inhibited by sodium azide in eosinophils [133] [134]. Interestingly, production of 2-ClFALD and 2-BrFALD and FALD-GSH were elevated in lungs of chlorine or bromine gas exposed mice compared to controls. Further, in a K/B×N mouse model of arthritis, mediated in part by activated neutrophils, elevation in plasma FALD-GSH and 2-ClHA, and 2chlorooctadecanoic acid (2-ClODA) was observed [133]. Additionally, the role of thiol modification of 2-ClFALD and 2-BrFALD reactivity with proteins using clickable alkyne analogs, 2-ClHDya, 2-BrHDyA and HDyA demonstrated that both 2-ClHDyA and 2-BrHDyA formed protein adducts while HDyA adducts were barely detectable [134]. The order of protein adduct formation was 2-BrHDyA > 2-ClHDyA > HDyA. These studies clearly show elevated production of the α-halofatty aldehydes in mouse lung and plasma and in phorbol ester stimulated neutrophils, providing a relevance to pathophysiological situations such as arthritis and exposure to toxic chlorine or bromine gas.
Figure 11: Glutathione adducts of 2-chlorofatty aldehydes and 2-bromofatty aldehydes.
2-Chlorofatty aldehydes (2-ClFALDs) and 2-bromofatty aldehydes (2-BrFALDs) generated by hypochlorous acid (HOCl) or hypobromous acid (HOBr) react with a nucleophile such as glutathione (GSH) and form FALD-GSH adducts in vitro and in animals exposed to either chlorine or bromine gas.
4. CONCLUSIONS AND FUTURE PERSPECTIVES
There is compelling evidence demonstrating biological roles for long-chain fatty aldehydes (C16:0, C18:0 and C18:1), 2-halofatty aldehydes derived from plasmalogens and Δ2-HDE from S1P as signaling lipids in mammalian cells. Further, long-chain aldehydes and 2-halofatty aldehydes forms Michael adduct and Schiff’s base in vivo and in vitro with GSH, intracellular proteins and other reactive nucleophiles. Similarly, Δ2-HDE also forms adducts with GSH, HDAC1, DNA and deoxyguanosine, modulates HDAC1/2 activity and H3/H4 histone acetylation in vitro suggesting an epigenetic role for the S1P metabolite in mammalian cells. The use of clickable analogues of 2-halofatty aldehydes and Δ2-HDE has identified several intracellular protein targets, including BAX, further confirming the biological activity of long-chain aldehydes and Δ2-HDE with macromolecular cellular targets. Future studies need to address several relevant and interesting areas such as: (i) formation and characterization of new intracellular adducts of fatty aldehydes from plasmalogens and Δ2-HDE from S1P in mammalian cells and tissues; (ii) physiological and pathophysiological role of fatty aldehyde adducts of GSH, proteins, HDACs and DNA in cell signaling and epigenetic regulation; (iii) effects of intracellularly released fatty aldehydes from plasmalogens and Δ2HDE from S1P in modulating apoptosis, mitochondrial function, ER stress/unfolded protein response, cytoskeletal reorganization, ROS production signaling pathways, and gene expression; (iv) circulating long-chain fatty aldehydes, 2-halofatty aldehydes and Δ2-HDE as potential biomarkers in human pathologies including sepsis, Alzheimer’s disease and Sjögren-Larsson Syndrome and (v) potential function of 2-halofatty aldehydes and Δ2-HDE as pro- or anti-inflammatory mediators. The Sjögren-Larsson Syndrome resulting from a mutation in the ALDH3A2 gene [135] and the recently identified S1P lyase insufficiency syndrome (SPLIS), an inborn error of sphingolipid metabolism [136] are excellent model systems to explore the contributions of S1P metabolism and hexadecenal in the development of human pathologies. As extracellular long-chain fatty aldehydes and Δ2-HDE modulate intracellular pathways of MAPKs and BAX, it will be important to determine if these aldehydes signal through any low-affinity receptors similar to fatty acid signaling via fatty acid receptors [137], and if their binding/interaction with plasma membrane proteins include cell surface receptors such as G-protein coupled receptors and tyrosine kinase receptors. The observation that TER is involved not only in very long-chain fatty acid synthesis but also regulates sphingosine degradation within the sphingolipids [89] warrants future studies linking the interdependency of S1P degradation by S1P lyase, TER mediated conversion of Δ2-HDE to palmitoyl CoA and the glycerophospholipid metabolism.
Highlights.
Enzymatic or non-enzymatic reactions on membrane lipids generate highly reactive long-chain fatty aldehydes including 2-halofatty aldehydes from plasmalogens, and Δ2-hexadecenal from sphingosine-1-phosphate.
Long-chain fatty aldehydes, Δ2-hexadecenal and 2-chlorofatty aldehydes form adducts with proteins, glutathione and DNA.
Δ2-Hexadecenal stimulates mitochondrial reactive oxygen species via JNK, induces cytoskeletal reorganization and apoptosis, and endothelial barrier dysfunction.
Nuclear generation of Δ2-hexadecenal by sphingosine-1-phosphate lyase modulates HDAC1/2 activity.
Δ2-Hexadecenal forms protein adducts with HDACs in vitro.
Lon-chain aldehydes, 2-halofatty aldehydes and Δ2-hexadecenal signal and modulate cell functions.
6. Acknowledgments
This manuscript was supported from funds from the National Institutes of Health grant HLBI P01 HL 060678 (Project 4) and P01 HL 126609 (Project 3) to V.N. We are thankful to Dr. Evgeny Berdyshev, PhD for mass spectrometry measurement of hexadecenal from biological samples at National Jewish Hospitals, Denver, Colorado and the Lipidomics facility at UIC.
Footnotes
Declaration of Interest Statement
The authors declare no conflict of interest and no financial obligations
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- [1].O’Donnell VB, Rossjohn J, Wakelam MJ, Phospholipid signaling in innate immune cells, J Clin Invest, 128 (2018) 2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aloulou A, Rahier R, Arhab Y, Noiriel A, Abousalham A, Phospholipases: An Overview, Methods Mol. Biol, 1835 (2018) 69–105. [DOI] [PubMed] [Google Scholar]
- [3].Chap H, Forty five years with membrane phospholipids, phospholipases and lipid mediators: A historical perspective, Biochimie, 125 (2016) 234–249. [DOI] [PubMed] [Google Scholar]
- [4].Eyster KM, The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist, Adv Physiol Educ, 31 (2007) 5–16. [DOI] [PubMed] [Google Scholar]
- [5].Phillips MC, Is ABCA1 a lipid transfer protein? J. Lipid Res, 59 (2018) 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chai AB, Ammit AJ, Gelissen IC, Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation, Respir. Res, 18 (2017) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Neumann J, Rose-Sperling D, Hellmich UA, Diverse relations between ABC transporters and lipids: An overview, Biochim Biophys Acta Biomembr, 1859 (2017) 605–618. [DOI] [PubMed] [Google Scholar]
- [8].Zhu X, Ren K, Zeng Y-Z, Zheng Z, Yi G-H, Biological function of SPNS2: From zebrafish to human, Mol. Immunol, 103 (2018) 55–62. [DOI] [PubMed] [Google Scholar]
- [9].Nishi T, Kobayashi N, Hisano Y, Kawahara A, Yamaguchi A, Molecular and physiological functions of sphingosine 1-phosphate transporters, Biochim. Biophys. Acta, 1841 (2014) 759–765. [DOI] [PubMed] [Google Scholar]
- [10].Samaha D, Hamdo HH, Wilde M, Prause K, Arenz C, Sphingolipid-Transporting Proteins as Cancer Therapeutic Targets, Int J Mol Sci, 20 (2019) 3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Astudillo AM, Balboa MA, Balsinde J, Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization, Biochim Biophys Acta Mol Cell Biol Lipids, 1864 (2019) 772–783. [DOI] [PubMed] [Google Scholar]
- [12].Funk CD, Prostaglandins and leukotrienes: advances in eicosanoid biology, Science, 294 (2001) 1871–1875. [DOI] [PubMed] [Google Scholar]
- [13].Perrakis A, Moolenaar WH, Autotaxin: structure-function and signaling, J. Lipid Res, 55 (2014) 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okudaira S, Yukiura H, Aoki J, Biological roles of lysophosphatidic acid signaling through its production by autotaxin, Biochimie, 92 (2010) 698–706. [DOI] [PubMed] [Google Scholar]
- [15].Yung YC, Stoddard NC, Chun J, LPA receptor signaling: pharmacology, physiology, and pathophysiology, J. Lipid Res, 55 (2014) 1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao Y, Natarajan V, Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling, Biochim. Biophys. Acta, 1831 (2013) 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vines CM, Phospholipase C, Adv. Exp. Med. Biol, 740 (2012) 235–254. [DOI] [PubMed] [Google Scholar]
- [17].Nelson RK, Frohman MA, Physiological and pathophysiological roles for phospholipase D, J. Lipid Res, 56 (2015) 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cummings R, Parinandi N, Wang L, Usatyuk P, Natarajan V, Phospholipase D/phosphatidic acid signal transduction: Role and physiological significance in lung, Molecular and Cellular Biochemistry, 234–235 (2002) 99–109. [PubMed] [Google Scholar]
- [19].Brindley DN, Pilquil C, Sariahmetoglu M, Reue K, Phosphatidate degradation: Phosphatidate phosphatases (lipins) and lipid phosphate phosphatases, Biochim Biophys Acta Mol Cell Biol Lipids, 1791 (2009) 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rhee SG, Regulation of phosphoinositide specific phospholipase C., Annu. Rev. Biochem, 70 (2001) 281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Augert G, Bocckino SB, Blackmore PF, Exton JH, Hormonal stimulation of diacylglycerol formation in hepatocytes. Evidence for phosphatidylcholine breakdown. J. Biol. Chem, 264 (1989) 21689–21698. [PubMed] [Google Scholar]
- [22].Sakai T, Sugiyama T, Banno Y, Kato Y, Nozawa Y, Involvement of phosphatidylcholine hydrolysis by phospholipase C in prostaglandin F2α-induced 1,2-diacylglycerol formation in osteoblast-like MC3T3-E1 cells, J. Bone Miner. Metab, 22 (2004) 198–206. [DOI] [PubMed] [Google Scholar]
- [23].Eppler CM, Malewicz B, Jenkin HM, Baumann WJ, Phosphatidylcholine as a choline donor in sphingomyelin synthesis, Lipids, 22 (1987) 351–357. [DOI] [PubMed] [Google Scholar]
- [24].Li Z, Hailemariam TK, Zhou H, Li Y, Duckworth DC, Peake D.a., Zhang Y, Kuo MS, cao G, Jiang XC, Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization, Biochim. Biophys. Acta, 1771 (2007) 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding T, Li Z, Hailemariam TK, Mukherjee S, Maxfield FR, Wu MP, Jiang XC, SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis, J. Lipid Res, 49 (2008) 376–385. [DOI] [PubMed] [Google Scholar]
- [26].Sasaki Y, Asaoka Y, Nishizuka Y, Potentiation of Diacylglycerol-Induced Activation of Protein-Kinase-C by Lysophospholipids - Subspecies Difference, FEBS Letters, 320 (1993) 47–51. [DOI] [PubMed] [Google Scholar]
- [27]. Higgs HN, Glomset JA, Identification of a phosphatidic acid-preferring phospholipase A1 from bovine brain and testis, Proc. Natl. Acad. Sci. U.S.a, 91 (1994) 9574–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ito M, Tchoua U, Okamoto M, Tojo H, Purification and properties of a phospholipase A2/lipase preferring phosphatidic acid, bis(monoacylglycerol) phosphate, and monoacylglycerol from rat testis, J. Biol. Chem, 277 (2002) 43674–43681. [DOI] [PubMed] [Google Scholar]
- [29].Airola MV, Hannun YA, Sphingolipid metabolism and neutral sphingomyelinases, Handb Exp Pharmacol, 215 (2013) 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gault CR, Obeid LM, Hannun YA, An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown, Adv. Exp. Med. Biol, 688 (2010) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y, Garcia JGN, Chun J, Natarajan V, Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice, Am. J. Respir. Cell Mol. Biol, 49 (2013) 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coant N, Sakamoto W, Mao C, Hannun YA, Ceramidases, roles in sphingolipid metabolism and in health and disease, Adv Biol Regul, 63 (2017) 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pitson SM, Regulation of sphingosine kinase and sphingolipid signaling, Trends Biochem. Sci, 36 (2011) 97–107. [DOI] [PubMed] [Google Scholar]
- [34].Blaho VA, Hla T, An update on the biology of sphingosine 1-phosphate receptors, J. Lipid Res, 55 (2014) 1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MBA, Sphingosine-1-phosphate and its receptors: structure, signaling, and influence, Annu. Rev. Biochem, 82 (2013) 637–662. [DOI] [PubMed] [Google Scholar]
- [36].Schumacher F, Neuber C, Finke H, Nieschalke K, Baesler J, Gulbins E, Kleuser B, The sphingosine 1-phosphate breakdown product, (2E)-hexadecenal, forms protein adducts and glutathione conjugates in vitro, J. Lipid Res, 58 (2017) 1648–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Upadhyaya P, Kumar A, Byun H-S, Bittman R, Saba JD, Hecht SS, The sphingolipid degradation product trans-2-hexadecenal forms adducts with DNA, Biochem. Biophys. Res. Commun, 424 (2012) 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rizzo WB, Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function, Biochim. Biophys. Acta, 1841 (2014) 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saba JD, Fifty years of lyase and a moment of truth: sphingosine phosphate lyase from discovery to disease, J. Lipid Res, 60 (2019) 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barrera G, Pizzimenti S, Ciamporcero ES, Daga M, Ullio C, Arcaro A, Cetrangolo GP, Ferretti C, Dianzani C, Lepore A, Gentile F, Role of 4-hydroxynonenal-protein adducts in human diseases, Antioxid. Redox Signal, 22 (2015) 1681–1702. [DOI] [PubMed] [Google Scholar]
- [41].Mali VR, Palaniyandi SS, Regulation and therapeutic strategies of 4-hydroxy-2nonenal metabolism in heart diseases, Free Radical Research, 48 (2014) 251–263. [DOI] [PubMed] [Google Scholar]
- [42].Csala M, Kardon T, Legeza B, Lizak, Mandl J, Margittai E, Puskas F, Szaraz P, Szelenyi P, Banhegyi G, On the role of 4-hydroxynonenal in health and disease, Biochim. Biophys. Acta 1852 (2015) 826–838. [DOI] [PubMed] [Google Scholar]
- [43].Karki P, Birukov KG, Lipid mediators in the regulation of endothelial barriers, Tissue Barriers, 6 (2018) e1385573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Riendeau D, Meighen E, Enzymatic reduction of fatty acids and acyl-CoAs to long chain aldehydes and alcohols, Experientia, 41 (1985) 707–713. [DOI] [PubMed] [Google Scholar]
- [45].Vermeer CP, Nastold P, Jetter R, Homologous very-long-chain 1,3-alkanediols and 3-hydroxyaldehydes in leaf cuticular waxes of Ricinus communis L, Phytochemistry, 62 (2003) 433–438. [DOI] [PubMed] [Google Scholar]
- [46].Pérez-Camino MC, Gómez-Coca RB, Moreda W, Waxy fraction containing long-chain aliphatic aldehydes in virgin olive oils, Food Chem, 132 (2012) 1451–1456. [DOI] [PubMed] [Google Scholar]
- [47].Fritz KS, Petersen DR, An overview of the chemistry and biology of reactive aldehydes, Free Radic. Biol. Med, 59 (2013) 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Greig FH, Kennedy S, Spickett CM, Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation, Free Radic. Biol. Med, 52 (2012) 266–280. [DOI] [PubMed] [Google Scholar]
- [49].Natarajan V, Sastry PS, Enzymatic acylation of 1-alkyl, 1-alkenyl- and 1-acyl glycero-3-phosphorylethanolamine in developing rat brains, Journal of Neurochemistry, 23 (1974) 187–192. [DOI] [PubMed] [Google Scholar]
- [50].Natarajan V, Schmid HH, Substrate specificities in ether lipid biosynthesis Metabolism of polyunsaturated fatty acids and alcohols by rat brain microsomes, Biochem. Biophys. Res. Commun, 79 (1977) 411–416. [DOI] [PubMed] [Google Scholar]
- [51].Bishop JE, Hajra AK, Mechanism and specificity of formation of long chain alcohols by developing rat brain, J. Biol. Chem, 256 (1981) 9542–9550. [PubMed] [Google Scholar]
- [52].Natarajan V, Sastry PS, Studies on the biosynthesis of ether linked ethanolamine phospholipids in developing rat brain, Indian Journal of Biochemistry and Biophysics, 12 (1975) 340–350. [Google Scholar]
- [53].Hardwick JP, Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases, Biochem. Pharmacol, 75 (2008) 2263–2275. [DOI] [PubMed] [Google Scholar]
- [54].Lee T, Characterization of fatty alcohol:NAD+ oxidoreductase from rat liver, J. Biol. Chem, 254 (1979) 2892–2896. [PubMed] [Google Scholar]
- [55].Ichihara K, Kusunose E, Noda Y, Kusunose M, Some properties of the fatty alcohol oxidation system and reconstitution of microsomal oxidation activity in intestinal mucosa, Biochim. Biophys. Acta, 878 (1986) 412–418. [DOI] [PubMed] [Google Scholar]
- [56].Rizzo WB, Craft DA, Sjögren-Larsson syndrome Deficient activity of the fatty aldehyde dehydrogenase component of fatty alcohol:NAD+ oxidoreductase in cultured fibroblasts, J Clin Invest, 88 (1991) 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nakahara K, Ohkuni A, Kitamura T, Abe K, Naganuma T, Ohno Y, Zoeller RA, Kihara A, The Sjögren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway, Mol. Cell, 46 (2012) 461–471. [DOI] [PubMed] [Google Scholar]
- [58].James PF, Zoeller RA, Isolation of animal cell mutants defective in long-chain fatty aldehyde dehydrogenase Sensitivity to fatty aldehydes and Schiff’s base modification of phospholipids: implications for Sj-ogren-Larsson syndrome, J. Biol. Chem, 272 (1997) 23532–23539. [DOI] [PubMed] [Google Scholar]
- [59].Haug S, Braun-Falco M, Restoration of fatty aldehyde dehydrogenase deficiency in Sjögren-Larsson syndrome, Gene Ther, 13 (2006) 1021–1026. [DOI] [PubMed] [Google Scholar]
- [60].van den Bosch H, de Vet EC, Alkyl-dihydroxyacetonephosphate synthase, Biochim. Biophys. Acta, 1348 (1997) 35–44. [DOI] [PubMed] [Google Scholar]
- [61].Oku H, Shudo J, Mimura K, Haratake A, Nagata J, Chinen I, 1-O-alkyl-2,3-diacylglycerols in the skin surface lipids of the hairless mouse, Lipids, 30 (1995) 169–172. [DOI] [PubMed] [Google Scholar]
- [62].Taguchi H, Armarego WL, Glyceryl-ether monooxygenase [EC 114165] A microsomal enzyme of ether lipid metabolism, Med Res Rev, 18 (1998) 43–89. [DOI] [PubMed] [Google Scholar]
- [63].Rizzo WB, Heinz E, Simon M, Craft DA, Microsomal fatty aldehyde dehydrogenase catalyzes the oxidation of aliphatic aldehyde derived from ether glycerolipid catabolism: implications for Sjögren-Larsson syndrome, Biochim. Biophys. Acta, 1535 (2000) 1–9. [DOI] [PubMed] [Google Scholar]
- [64].Snyder F, The ether lipid trail: a historical perspective, 1999. [DOI] [PubMed] [Google Scholar]
- [65].Marinetti GV, Erbland J, The structure of pig heart plasmalogen, Biochim. Biophys. Acta, 26 (1957) 429–430. [DOI] [PubMed] [Google Scholar]
- [66].Panganamala RV, Horrocks LA, Geer JC, Cornwell DG, Positions of double bonds in the monounsaturated alk-1-enyl groups from the plasmalogens of human heart and brain, Chem. Phys. Lipids, 6 (1971) 97–102. [DOI] [PubMed] [Google Scholar]
- [67].Rapport MM, Lerner B, The structure of plsmalogens IV Lipids in normal and neoplastic tissues of man and in normal tissues of rabbit and rat, Biochim. Biophys. Acta, 33 (1959) 319–325. [DOI] [PubMed] [Google Scholar]
- [68].Braverman NE, Moser AB, Functions of plasmalogen lipids in health and disease, Biochim. Biophys. Acta, 1822 (2012) 1442–1452. [DOI] [PubMed] [Google Scholar]
- [69].Jenkins CM, Yang K, Liu G, Moon SH, Dilthey BG, Gross RW, Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage, J. Biol. Chem, 293 (2018) 8693–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu L-C, Pfeiffer DR, Calhoon EA, Madiai F, Marcucci G, Liu S, Jurkowitz MS, Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen, J. Biol. Chem, 286 (2011) 24916–24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stadelmann-Ingrand S, Favreliere S, Fauconneau B, Mauco G, Tallineau C, Plasmalogen degradation by oxidative stress: production and disappearance of specific fatty aldehydes and fatty alpha-hydroxyaldehydes, Free Radic. Biol. Med, 31 (2001) 1263–1271. [DOI] [PubMed] [Google Scholar]
- [72].Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA, Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal, J. Biol. Chem, 276 (2001) 23733–23741. [DOI] [PubMed] [Google Scholar]
- [73].Albert CJ, Crowley JR, Hsu F-F, Thukkani AK, Ford DA, Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes, J. Biol. Chem, 277 (2002) 4694–4703. [DOI] [PubMed] [Google Scholar]
- [74].Pereira A, Braekman JC, Dumont JE, Boeynaems JM, Identification of a major iodolipid from the horse thyroid gland as 2-iodohexadecanal, J. Biol. Chem, 265 (1990) 17018–17025. [PubMed] [Google Scholar]
- [75].Yatomi Y, Plasma sphingosine 1-phosphate metabolism and analysis, Biochimica Et Biophysica Acta-General Subjects, 1780 (2008) 606–611. [DOI] [PubMed] [Google Scholar]
- [76].Venkataraman K, Lee Y-M, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T, Vascular endothelium as a contributor of plasma sphingosine 1-phosphate, Circulation Research, 102 (2008) 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Haenel P, Andreani P, Graeler MH, Erythrocytes store and release sphingosine 1-phosphate in blood, Faseb J, 21 (2007) 1202–1209. [DOI] [PubMed] [Google Scholar]
- [78].Tani M, Sano T, Ito M, Igarashi Y, Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets, J. Lipid Res, 46 (2005) 2458–2467. [DOI] [PubMed] [Google Scholar]
- [79].Brindley DN, Pilquil C, Lipid phosphate phosphatases and signaling, J. Lipid Res, 50 (2009) S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ogawa C, Kihara A, Gokoh M, Igarashi Y, Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2, J. Biol. Chem, 278 (2003) 1268–1272. [DOI] [PubMed] [Google Scholar]
- [81].Stoffel W, LeKim D, Sticht G, Distribution and properties of dihydrosphingosine-1-phosphate aldolase (sphinganine-1-phosphate alkanal-lyase), Hoppe-Seyler’s Z. Physiol. Chem, 350 (1969) 1233–1241. [DOI] [PubMed] [Google Scholar]
- [82].Serra M, Saba JD, Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function, Adv. Enzyme Regul, 50 (2010) 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Van Veldhoven PP, Mannaerts GP, Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver, J. Biol. Chem, 266 (1991) 12502–12507. [PubMed] [Google Scholar]
- [84].Ebenezer DL, Fu P, Mangio LA, Berdyshev E, Schumacher F, Kleuser B, Van Veldhoven PP, Natarajan V, Δ−2 Hexadecenal Generated from S1P by Nuclear S1P Lyase Is a Regulator of HDAC1/2 Activity and Histone Acetylation in Lung Epithelial Cells, Faseb J, 33 (2019) 489.3–489.3. [Google Scholar]
- [85].Ebenezer DL, Berdyshev EV, Bronova IA, Liu Y, Tiruppathi C, Komarova Y, Benevolenskaya EV, Suryadevara V, Ha AW, Harijith A, Tuder RM, Natarajan V, Fu P, Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury, Thorax, 74 (2019) 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fu P, Ebenezer DL, Ha AW, Suryadevara V, Harijith A, Natarajan V, Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression, J. Cell. Biochem, 119 (2018) 6337–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ebenezer D, Fu P, Berdyshev E, Natarajan V, Nuclear S1P Lyase Regulates Histone Acetylation In Pseudomonas aeruginosa-Induced Lung Inflammation, Faseb J, 29 (2015) 863.26. [Google Scholar]
- [88].Brahmbhatt VV, Hsu F-F, Kao JL-F, Frank EC, Ford DA, Novel carbonyl and nitrile products from reactive chlorinating species attack of lysosphingolipid, Chem. Phys. Lipids, 145 (2007) 72–84. [DOI] [PubMed] [Google Scholar]
- [89].Wakashima T, Abe K, Kihara A, Dual functions of the trans-2-enoyl-CoA reductase TER in the sphingosine 1-phosphate metabolic pathway and in fatty acid elongation, J. Biol. Chem, 289 (2014) 24736–24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Neuber C, Schumacher F, Gulbins E, Kleuser B, Method to simultaneously determine the sphingosine 1-phosphate breakdown product (2E)-hexadecenal and its fatty acid derivatives using isotope-dilution HPLC-electrospray ionization-quadrupole/time-of-flight mass spectrometry, Anal. Chem, 86 (2014) 9065–9073. [DOI] [PubMed] [Google Scholar]
- [91].Sjogren T, Larsson T, Oligophrenia in combination with congenital ichthyosis and spastic disorders; a clinical and genetic study, Acta Psychiatr Neurol Scand Suppl, 113 (1957) 1–112. [PubMed] [Google Scholar]
- [92].Rizzo WB, Sjögren-Larsson syndrome, Semin Dermatol, 12 (1993) 210–218. [PubMed] [Google Scholar]
- [93].Goll MG, Bestor TH, Eukaryotic cytosine methyltransferases, Annu. Rev. Biochem, 74 (2005) 481–514. [DOI] [PubMed] [Google Scholar]
- [94].Backos DS, Fritz KS, Roede JR, Petersen DR, Franklin CC, Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal, Free Radic. Biol. Med, 50 (2011) 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ji C, Kozak KR, Marnett LJ, IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal, J. Biol. Chem, 276 (2001) 18223–18228. [DOI] [PubMed] [Google Scholar]
- [96].Natarajan V, Scribner WM, Taher MM, 4-hydroxynonenal, a metabolite of lipid peroxidation, activates phospholipase D in vascular endothelial cells, Free Radic. Biol. Med, 15 (1993) 365–375. [DOI] [PubMed] [Google Scholar]
- [97].Natarajan V, Scribner WM, Vepa S, Phosphatase Inhibitors Potentiate 4-Hydroxynonenal-induced Phospholipase D Activation in Vascular Endothelial Cells, Am. J. Respir. Cell Mol. Biol, 17 (2012) 251–259. [DOI] [PubMed] [Google Scholar]
- [98].Usatyuk PV, Natarajan V, Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells, J. Biol. Chem, 279 (2004) 11789–11797. [DOI] [PubMed] [Google Scholar]
- [99].Usatyuk PV, Parinandi NL, Natarajan V, Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins, J. Biol. Chem, 281 (2006) 35554–35566. [DOI] [PubMed] [Google Scholar]
- [100].Usatyuk PV, Natarajan V, Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function, Microvascular Research, 83 (2012) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA, Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment, Free Radic. Biol. Med, 52 (2012) 2292–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Haberzettl P, Hill BG, Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response, Redox Biol, 1 (2013) 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhao Y, Gorshkova IA, Berdyshev E, He D, Fu P, Ma W, Su Y, Usatyuk PV, Pendyala S, Oskouian B, Saba JD, Garcia JGN, Natarajan V, Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression, Am. J. Respir. Cell Mol. Biol, 45 (2011) 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kumar A, Byun H-S, Bittman R, Saba JD, The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner, Cell. Signal, 23 (2011) 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Amaegberi NV, Semenkova GN, Kvacheva ZB, Lisovskaya AG, Pinchuk SV, Shadyro OI, 2-Hexadecenal inhibits growth of C6 glioma cells, Cell Biochem. Funct, 37 (2019) 281–289. [DOI] [PubMed] [Google Scholar]
- [106].Berdyshev EV, Goya J, Gorshkova I, Prestwich GD, Byun H-S, Bittman R, Natarajan V, Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization–liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal, Analytical Biochemistry, 408 (2011) 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ebenezer DL, Fu P, Ha AW-Z, Natarajan V, PKC-δ Mediates Sphk2 Activation And Histone Acetylation In Pseudomonas aeruginosa-Induced Lung Inflammation, Faseb J, 31 (2017) 629.27–629.27. [Google Scholar]
- [108].Ebenezer DL, Fu P, Suryadevara V, Zhao Y, Natarajan V, Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase, Adv Biol Regul, 63 (2017) 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mitchell RW, Edmundson CL, Miller DW, Hatch GM, On the mechanism of oleate transport across human brain microvessel endothelial cells, J. Neurochem, 110 (2009) 1049–1057. [DOI] [PubMed] [Google Scholar]
- [110].Thukkani AK, Hsu F-F, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA, Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal, J. Biol. Chem, 277 (2002) 3842–3849. [DOI] [PubMed] [Google Scholar]
- [111].Thukkani AK, Albert CJ, Wildsmith KR, Messner MC, Martinson BD, Hsu F-F, Ford DA, Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein, J. Biol. Chem, 278 (2003) 36365–36372. [DOI] [PubMed] [Google Scholar]
- [112].Palladino END, Hartman CL, Albert CJ, Ford DA, The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: Metabolism, clearance, and biological properties, Arch. Biochem. Biophys, 641 (2018) 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA, Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury, Am. J. Physiol. Heart Circ. Physiol, 288 (2005) H2955–64. [DOI] [PubMed] [Google Scholar]
- [114].Anbukumar DS, Shornick LP, Albert CJ, Steward MM, Zoeller RA, Neumann WL, Ford DA, Chlorinated lipid species in activated human neutrophils: lipid metabolites of 2-chlorohexadecanal, J. Lipid Res, 51 (2010) 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, Hammer A, Kratky D, Heinemann A, Holzer P, Malle E, Sattler W, Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo, PLoS ONE, 8 (2013) e64034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Üllen A, Fauler G, Bernhart E, Nusshold C, Reicher H, Leis H-J, Malle E, Sattler W, Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro, Free Radic. Biol. Med, 53 (2012) 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yu H, Wang M, Wang D, Kalogeris TJ, McHowat J, Ford DA, Korthuis RJ, Chlorinated Lipids Elicit Inflammatory Responses in vitro and in vivo, Shock, 51 (2019) 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Boeynaems JM, Hubbard WC, Transformation of arachidonic acid into an iodolactone by the rat thyroid, J. Biol. Chem, 255 (1980) 9001–9004. [PubMed] [Google Scholar]
- [119].Dugrillon A, Bechtner G, Uedelhoven WM, Weber PC, Gärtner R, Evidence that an iodolactone mediates the inhibitory effect of iodide on thyroid cell proliferation but not on adenosine 3“,5-”monophosphate formation, Endocrinology, 127 (1990) 337–343. [DOI] [PubMed] [Google Scholar]
- [120].Panneels V, Van den Bergen H, Jacoby C, Braekman JC, Van Sande J, Dumont JE, Boeynaems JM, Inhibition of H2O2 production by iodoaldehydes in cultured dog thyroid cells, Mol. Cell. Endocrinol, 102 (1994) 167–176. [DOI] [PubMed] [Google Scholar]
- [121].Ohayon R, Boeynaems JM, Braekman JC, Van den Bergen H, Gorin Y, Virion A, Inhibition of thyroid NADPH-oxidase by 2-iodohexadecanal in a cell-free system, Mol. Cell. Endocrinol, 99 (1994) 133–141. [DOI] [PubMed] [Google Scholar]
- [122].Wildsmith KR, Albert CJ, Anbukumar DS, Ford DA, Metabolism of myeloperoxidase-derived 2-chlorohexadecanal, J. Biol. Chem, 281 (2006) 16849–16860. [DOI] [PubMed] [Google Scholar]
- [123].Bernhart E, Kogelnik N, Prasch J, Gottschalk B, Goeritzer M, Depaoli MR, Reicher H, Nusshold C, Plastira I, Hammer A, Fauler G, Malli R, Graier WF, Malle E, Sattler W, 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells, Redox Biol, 15 (2018) 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hartman CL, Duerr MA, Albert CJ, Neumann WL, McHowat J, Ford DA, 2-Chlorofatty acids induce Weibel-Palade body mobilization, J. Lipid Res, 59 (2018) 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].LoPachin RM, Gavin T, Petersen DR, Barber DS, Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation, Chem. Res. Toxicol, 22 (2009) 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Pearson RG, Hard and Soft Acids and Bases - the Evolution of a Chemical Concept, Coordination Chemistry Reviews, 100 (1990) 403–425. [Google Scholar]
- [127].Jarugumilli GK, Choi J-R, Chan P, Yu M, Sun Y, Chen B, Niu J, DeRan M, Zheng B, Zoeller R, Lin C, Wu X, Chemical Probe to Identify the Cellular Targets of the Reactive Lipid Metabolite 2- trans-Hexadecenal, ACS Chem. Biol, 13 (2018) 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP, Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal, Chem. Res. Toxicol, 22 (2009) 759–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chung FL, Young R, Hecht SS, Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde, Cancer Res, 44 (1984) 990–995. [PubMed] [Google Scholar]
- [130].Sapkota M, Wyatt TA, Alcohol, Aldehydes, Adducts and Airways, Biomolecules, 5 (2015) 2987–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Winter CK, Segall HJ, Haddon WF, Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro, Cancer Res, 46 (1986) 5682–5686. [PubMed] [Google Scholar]
- [132].Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA, Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde, J. Biol. Chem, 278 (2003) 8942–8950. [DOI] [PubMed] [Google Scholar]
- [133].Duerr MA, Aurora R, Ford DA, Identification of glutathione adducts of α-chlorofatty aldehydes produced in activated neutrophils, J. Lipid Res, 56 (2015) 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA, Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein, J. Lipid Res, 59 (2018) 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, Markova N, Rizzo WB, Sjögren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene, Nat. Genet, 12 (1996) 52–57. [DOI] [PubMed] [Google Scholar]
- [136].Choi Y-J, Saba JD, Sphingosine phosphate lyase insufficiency syndrome (SPLIS): A novel inborn error of sphingolipid metabolism, Adv Biol Regul, 71 (2019) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Falomir-Lockhart LJ, Cavazzutti GF, Giménez E, Toscani AM, Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors, Front. Cell. Neurosci, 13 (2019) 396. [DOI] [PMC free article] [PubMed] [Google Scholar]