INTRODUCTION
Many medications commonly used in the perioperative setting have well-known pharmacogenomic associations and interindividual variability in responses can lead to decreased efficacy and/or adverse drug events (ADEs), resulting in devastating patient morbidity and mortality. We describe our institutional project, “The ImPreSS Trial”, which tests a model of whether preemptive pharmacogenomic genotyping and clinical implementation of results into the perioperative workflow will aid perioperative medication decision-making, and ultimately improve the high-stakes drug responses of this critical healthcare setting.
PROJECT OVERVIEW
Pharmacogenomic implementation approaches have been successfully studied in a growing number of clinical programs1–2; however, to the best of our knowledge, pharmacogenomics has not been widely applied in the perioperative setting. This is despite the many high-profile examples of pharmacogenomic associations for key medications used in perioperative care (e.g., volatile anesthetics/succinylcholine, muscle relaxants, antihypertensives, opioids). Our objective was to establish a model to test whether preemptive genotyping and clinically implementing pharmacogenomic information into the current perioperative workflow will aid prescribing and govern decision-making, and ultimately improve drug responses and reduce ADEs. Our primary objectives are:
to explore the feasibility and utility of implementing broad preemptive pharmacogenomic testing in the perioperative setting by determining the frequency of use of our institutional pharmacogenomic results portal (the Genomic Prescribing System; GPS)3 by anesthesiologists, critical care and pain medicine physicians, and associated providers during the perioperative period; and
to determine the rate of use of high-risk drugs (i.e., genomically discordant by GPS designated red or yellow light pharmacogenomic risk, defined as increased risk of toxicity or non-response with use of a medication) in the group of patients for whom pharmacogenomic results are available compared to their rate of use in the control arm (without provider knowledge of pharmacogenomic risk designation).
Our secondary objectives are: 1) to determine the rate of use of genomically concordant drugs (i.e., GPS designated green light by pharmacogenomic risk, defined as improved chance of benefit or decreased risk of toxicity with a medication) in both arms; 2) to determine the occurrence of pharmacogenomically-associated ADEs (Table S1) in both arms; and 3) to explore the effects of pharmacogenomics results on pain scores between both arms among patients receiving dedicated pain management service consultations.
PARTICIPANTS AND STUDY DESIGN
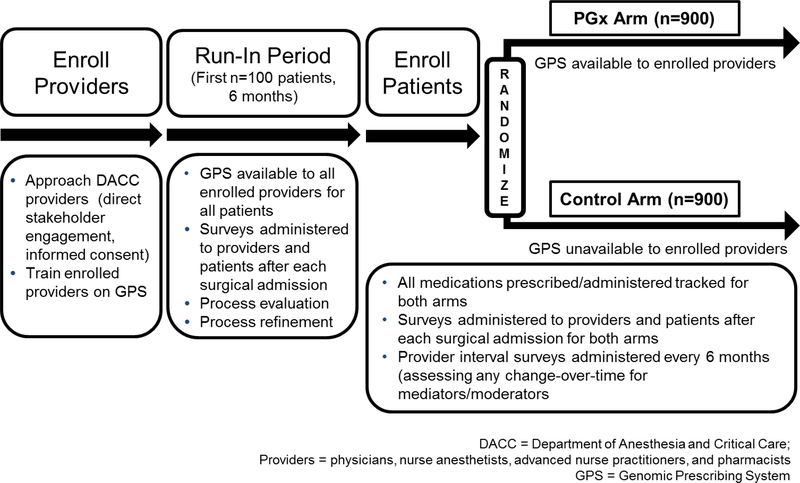
This project is an institutional review board-approved prospective study open at The University of Chicago (ClinicalTrials.gov NCT03729180), consisting of an initial run-in phase and a second randomized phase (Figure 1). The study is targeting anesthesiologists, critical care and pain management physicians and associated providers (nurse anesthetists, pharmacists, and physician assistants) within the Department of Anesthesia and Critical Care (DACC). Providers are approached for enrollment through a process of direct stakeholder engagement and informed consent. The eligible patient population is comprised of adults with inpatient or outpatient elective surgical procedures. Patients will be approached for recruitment during their pre-operative clinic visit within the Anesthesia Perioperative Medicine Clinic (APMC), as shown in Figure 2.
Figure 1.
Study schema for the The ImPreSS Trial: Implementation of Point-of-Care Decision Support in Perioperative Setting. The DACC has approximately 130 clinical personnel who will be targeted for participation, and we anticipate being able to successfully enroll 70% of these providers. Therefore, we expect to enroll approximately 90 providers and approximately 20 patients per provider during the randomization phase [n=1800 total patients who will be randomly assigned to the control (n=900) or the PGx arm (n=900)]. The randomization process will allow us to compare the frequency of GPS use by DACC providers in both arms and to determine the rate of use of high-risk drugs in the group of patients for whom pharmacogenomic results are available compared to their rate of use in the control arm (without provider knowledge of pharmacogenomic risk designation). We will use a mixed effects model and include provider as a random effect to allow for correlation of responses among patients treated by the same provider. All medications prescribed/administered will be tracked for both arms. Providers and patients will be asked to complete a survey after each surgical admission. Providers will also be asked to complete a questionnaire every six months thereafter to assess knowledge and attitudes towards pharmacogenomics. For the pain sub-analysis, we anticipate that 20% of the randomized patients will receive pain consultations after each surgery (n=180 in each arm). Pain will be assessed using a 11-point numeric rating scale, and differences between groups will be compared using a two-sample t-test. Assuming a pooled standard deviation of 2.5 (range of 10/4), the study sample size of 360 (n=180 for each group) will achieve 85% power and a level of significance of 5% (p<0.05) for detecting a true mean difference of 0.8 between the groups.
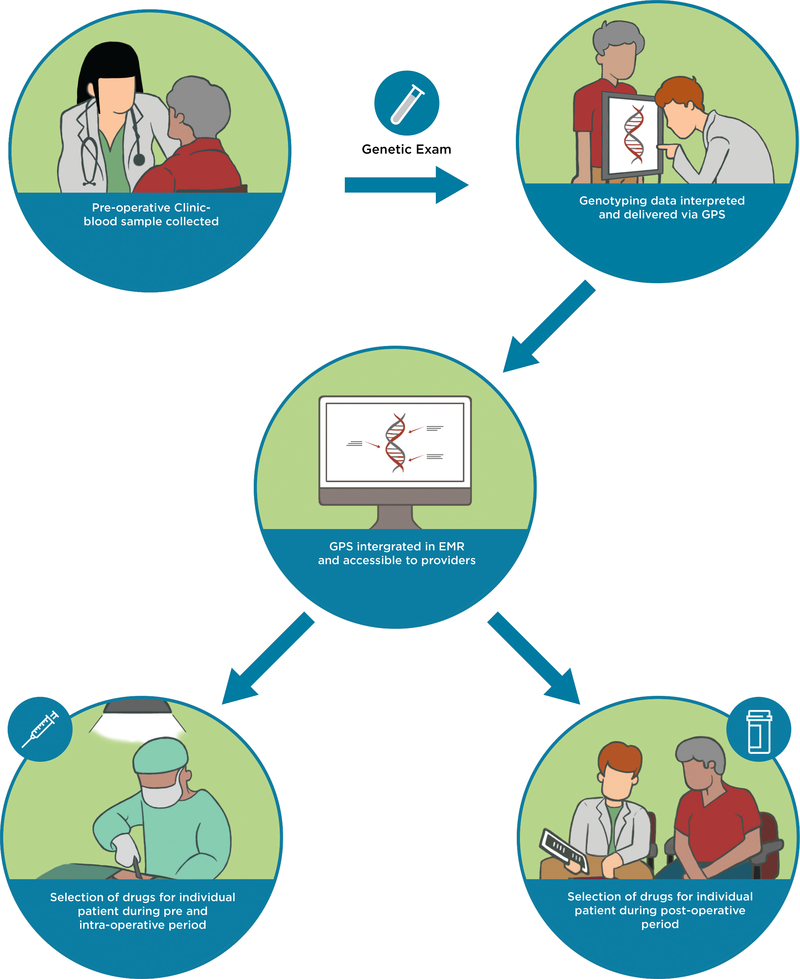
Figure 2.
A model for implementing pharmacogenomics into the current perioperative workflow. Patient exclusion criteria include 1) patients who have undergone, or are being actively considered for, liver or kidney transplantation; 2) patients with known active or prior leukemia; and 3) patients who are unable to understand and give informed consent to participate. During the patients’ pre-operative clinic visit at the APMC, a sample of whole blood will be collected in conjunction with standard-of-care phlebotomy. Genotyping will be carried out in the CLIA-certified and CAP-accredited Clinical Pharmacogenomics Laboratory at The University of Chicago. Raw genotyping data will be interpreted and delivered to providers as clinical decision support summaries through our clinical portal, the GPS, which is available as a link-out within our institutional EMR. Participating providers can use the GPS to guide selection of drugs pre-operatively, intra-operatively, and post-operatively. A clinical research coordinator from the study team will be embedded in the APMC to actively recruit eligible patients. The research coordinator will also be readily available throughout the perioperative period to alert providers about enrolled patients upon admission as well as to help them navigate GPS. This will allow us to integrate ourselves within the perioperative workflow and help us determine key points during the perioperative workflow that need to improve for our implementation program. APMC = Anesthesia Perioperative Medicine Clinic; CLIA = Clinical Laboratory Improvement Amendments; CAP = College of American Pathologists; GPS = Genomic Prescribing System; EMR = electronic medical record.
During the initial six month run-in period a small cohort of patients (n=100) will have their pharmacogenomic results made available to participating treating DACC providers. The purpose of this run-in is to allow for process evaluation and refinement of pharmacogenomic information delivery. After the run-in, genotyped patients will be prospectively randomized in a 1:1 ratio at the time of surgical admission to one of two treatment arms. In the PGx arm (n=900), treating providers will be given access to GPS (Figure S1) and patient-specific pharmacogenomic information throughout the patient’s evaluable surgical admission, whereas in the control arm (n=900), genotype information will be withheld and providers will not have access to the GPS (approximating current standard of care). Although all patients including those in the control arm will have their blood collected and genotyped, the laboratory staff and clinical research team will be blinded to the randomization assignments until the time of data analysis. A computer algorithm based on random group assignment will “release” the genotyping results for patients in the PGx arm to treating providers, but will securely store genotyping results for release at a 6-month unblinding time point for those in the control group. High value actionable results control patients will be systematically identified and reported to an appropriate longitudinal provider (and to the patient) at the 6-month unblinding time point. This is designed so that there is no bias introduced into genotype interpretations and turn-around-time assessments during reporting, and importantly, so that measurements of outcomes among investigators and study staff are unbiased.
Enrolled providers will give permission for their medical decisions to be followed and analyzed, but they will never be instructed on how to practice or prescribe; the use of the GPS is not mandatory. For both arms, all medications prescribed/administered will be tracked and patients will be monitored through the course of their perioperative care to determine the occurrence of pharmacogenomically-related drug toxicity and efficacy through medical record review. Details about decision-making regarding medication choices will be ascertained through analysis of GPS click-log records, provider documentation in electronic medical records (EMR), through longitudinal tracking of the medications being used by the same providers when treating control-arm patients (we will formally assess for possible “spillover” effects), and through real-time surveys given to providers.
Opioids, when used as part of multi-modal analgesia, are the cornerstone therapy for the management of postoperative pain, but wide interindividual variability in responses can lead to inadequate analgesia, significant ADEs, and possibly even opioid dependence. Our institutional Data and Analytics Core will be utilized for a key sub-analysis to collect pain care quality data and assess pain management services for both arms, including pain assessment (patient reported pain scores using a 11-point numeric scale; determined from EMR), pain therapy administration, and rate of opioid-induced ADEs (i.e., nausea/vomiting, urinary retention, constipation, pruritus, sedation, respiratory depression). Reported ADEs will be reviewed to determine whether they were due to prescribed or administered medications during the admission, and whether these medication choices were potentially the result of pharmacogenomics related decision-support delivered to providers in the context of this study.
PREEMPTIVE GENOTYPING
During the patients’ APMC visit, a sample of blood will be collected and genotyping will be carried out in the Clinical Laboratory Improvement Amendments (CLIA)-certified and College of American Pathologists (CAP)-accredited Clinical Pharmacogenomics Laboratory at The University of Chicago. DNA will be extracted from patient blood samples and tested (using an OpenArray platform from Thermofisher) across a panel of actionable germline variants recognized as affecting drug disposition, response, and/or toxicity. Patient DNA will also be tested using our custom CYP2D6 panel using the Invader technology from Hologic4, in addition to assessing copy number variants for this gene using the Taqman method.5 It will take approximately one week for results to be available in the GPS for providers to access before the patient’s surgical admission.
THE GENOMIC PRESCRIBING SYSTEM
The development of our protected-access portal, the GPS, has been previously described (Figure S1).3 A resourceful feature of the GPS is that it allows physicians to acquire information about medications they might be considering prescribing. This is especially useful in the perioperative setting as providers from different phases of care may access the GPS for their patient during the evaluable surgical admission (i.e., during creation of the preoperative anesthesia plan, before intraoperative medication administrations, postoperatively in the intensive care unit (ICU), and during postoperative pain management) (Figure S1).
IMPLEMENTATION SCIENCE
Our conceptual model for this study combines elements of the Self-Regulation Theory of Health Behavior and Rogers’ Diffusion of Innovation Theory, a dissemination and implementation model. Our integrated model hypothesizes that our intervention—deployment of the GPS—will impact provider knowledge and attitudes/perceptions about specific prescribing choices in a way that changes outcome expectancy about specific medications, and that this change will be the primary mediator of prescribing behavior.
We initiated the process of direct stakeholder engagement by including key physician champions from the DACC, who are highly valuable because they will lead the adoption of pharmacogenomics in clinical practice as well as provide important insights into the very process of implementation. Departmental engagement and interest were brokered through presentations at grand rounds, departmental faculty meetings, and through a series of various clinical group meetings. We will also personally engage those providers not captured through the above mechanisms in order to saturate provider enrollment within the department.
We will be continually assessing the processes of implementation throughout the study to help us optimize the method of information dissemination. In particular, we will be assessing different moderators that can affect physician behavior and the adoption of pharmacogenomics. These moderators might include, but are not limited to, the practice setting and available resources, patient desires and influences, clinical factors (disease or procedure-specific), and other physicians’ behaviors as perceived through organizational communication. This latter potential moderator may evolve during the course of the project, since peer behaviors might dramatically influence genomic adoption and may be particularly important in the setting of perioperative care where multiple DACC clinicians will often provide care to any one patient during a single operative admission. These learnings will allow process enhancement and similar outcomes gathered from interval provider surveys throughout the study will be interpreted alongside the primary study endpoints to help build an eventual model that can effectively disseminate pharmacogenomic information in routine clinical care. We aim 1) to determine providers’ knowledge and perceptions of prescribing decisions; and 2) to determine whether differences in patient-reported satisfaction and adherence likelihood are observable for patients whose providers had access to pharmacogenomic information.
CONCLUSIONS
This study will be the first of its kind to implement pharmacogenomic information across the perioperative care setting. Our project seeks to demonstrate the potential benefit of using broad preemptive pharmacogenomic testing to provide individualized therapy throughout the perioperative period. If successful, and when combined with emerging evidence in other clinical settings, greater consideration for the inclusion of pharmacogenomic information into current standardized health-care models will be warranted to guide decision-making in a manner that optimizes patient outcomes.
Supplementary Material
Figure S1. The GPS provides instantaneous patient-specific interpretations of the genomic data for all clinically actionable drugs, refined into concise 30-second summaries that providers can read in real-time. Raw genotyping results for each patient will be delivered as drug-centered summaries of drug-gene pairs, comprising a synopsis of the patient’s results, an interpretation, prescribing recommendation with a list of alternative pharmacogenomic drugs, and literature references. Each summary will employ our traffic light iconography - red “warning”, yellow “caution”, and green “favorable” lights - to provide physicians patient-specific recommendations of each drug-gene pair.
Table S1. List of drug-gene pairs of interest for genotyping in this study, with their pharmacogenomicallyassociated adverse drug events (ADEs).
ACKNOWLEDGMENTS
We thank Ms. Brittany A. Borden for her contributions in reviewing the study design descriptions for this project, and Dr. Rebecca Gerlach for her assistance with considering clinical workflows in the APMC.
FUNDING
This work is supported by National Institutes of Health (NIH) grants 5T32GM007019-41 (T.M.T.) and 1R01HG009938-01A1 (P.H.O.), and the Benjamin McAllister Research Fellowship (T.M.T.).
Footnotes
CONFLICT OF INTEREST
Drs. Ratain and O’Donnell and Keith Danahey are named as co-inventors on a pending patent for the Genomic Prescribing System. Dr. Ratain is a co-inventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping. All other authors declared no competing interests for this work.
REFERENCES
- 1.Weitzel KW, Elsey AR, Langaee TY, Burkley B, Nessl DR, Obeng AO, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am. J. Med. Genet. C. Semin. Med. Genet 2014;166C(1):56–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luzum JA, Pakyz RE, Elsey AR, Haidari C, Peterson JF, Whirl-Carrillo M, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin. Pharmacol. Ther 2017;102(3):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danahey K, Borden BA, Furner B, Ykman P, Hussain S, Saner D, et al. Simplifying the use of pharmacogenomics in clinical practice: Building the genomic prescribing system. J. Biomed. Info 2017(75): 110–121. [DOI] [PubMed] [Google Scholar]
- 4.Fang H, Liu X, Ramirez J, Choudhury N, Kubo M, Im HK, et al. Establishment of CYP2D6 reference samples by multiple validated genotyping platforms. Pharmacogenomics J. 2014;14(6):564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung EKY, Agolini E, Pei X, Melis R, McMillin GA, Friedman PN, et al. Validation of an extensive CYP2D6 assay panel based on Invader and Taqman copy number assays. J. App. Lab. Med 2017:471–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The GPS provides instantaneous patient-specific interpretations of the genomic data for all clinically actionable drugs, refined into concise 30-second summaries that providers can read in real-time. Raw genotyping results for each patient will be delivered as drug-centered summaries of drug-gene pairs, comprising a synopsis of the patient’s results, an interpretation, prescribing recommendation with a list of alternative pharmacogenomic drugs, and literature references. Each summary will employ our traffic light iconography - red “warning”, yellow “caution”, and green “favorable” lights - to provide physicians patient-specific recommendations of each drug-gene pair.
Table S1. List of drug-gene pairs of interest for genotyping in this study, with their pharmacogenomicallyassociated adverse drug events (ADEs).