Abstract
Globally and in Africa specifically, female sex workers (FSWs) are at an extraordinarily high risk of contracting human immunodeficiency virus (HIV). Pre-exposure prophylaxis (PrEP) has emerged as an effective and ethical method with which to prevent HIV infection among FSWs. PrEP efficacy is, however, closely linked to adherence, and adherence to PrEP among FSWs is a complex and interrelated process that has been shown to be of importance to public health policies and HIV control and intervention programs. This comprehensive review categorizes barriers to and facilitators of adherence to HIV PrEP for FSWs, and describes five strategies for promoting PrEP adherence among FSWs. These strategies encompass 1) a long-term educational effort to decrease the stigma associated with sex work and PrEP use, 2) education on how PrEP works, 3) lifestyle modification, 4) research on next-generation PrEP products to address the inconvenience of taking daily pills, and 5) integration of PrEP into existing services, such as social services and routine primary care visits, to reduce the economic burden of seeking the medication. Our review is expected to be useful for the design of future PrEP intervention programs. Multidisciplinary intervention should be considered to promote PrEP adherence among FSWs in order to help control the HIV epidemic.
Keywords: Human immunodeficiency virus infection and acquired immune deficiency syndrome prevention, pre-exposure prophylaxis, drug adherence, female sex workers
INTRODUCTION
Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS) continue to be a major global public health issue.1 By the end of 2018, an estimated 37.9 million individuals worldwide were living with HIV/AIDS.2,3 However, not all population sub-categories face the same risk of acquiring HIV/AIDS. Globally and in Africa specifically, female sex workers (FSWs) are at an extraordinarily high risk of contracting HIV4 due to a complex intersection of multiple social, cultural, and economic factors: sex workers are defined as workers who provide and/or are engaged in sexual activities or acts in exchange for monetary, materialistic, or any type of support, favors, or gain.5,6 An estimated 29.3% of FSWs were living with HIV/AIDS in sub-Saharan Africa in 2014.7 Meanwhile, research has indicated that sex workers and clients of sex workers and other sexual partners account for 3% and 19% of new yearly HIV infections, respectively.2
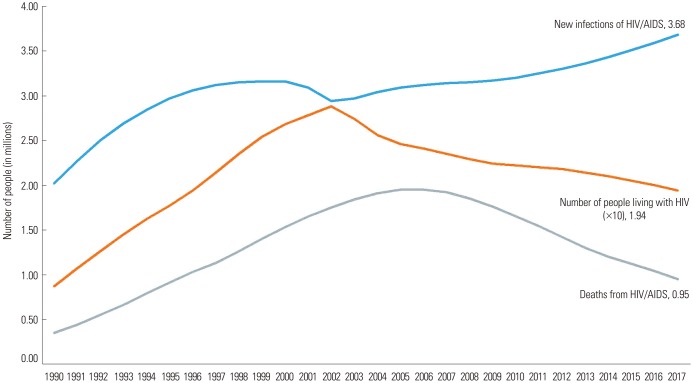
Globally, different approaches and public health efforts have been proposed and implemented to reduce HIV incidence rate. Traditionally, barrier methods, such as condom use, have been at the center of HIV protection efforts and have only proven partially efficient.8,9,10 Recently, newer protection methods have been developed, such as bio-medical, anti-retroviral, preexposure prophylaxis (PrEP). However, even with these newer approaches, the HIV/AIDS epidemic remains partially uncontrolled. In 2018, 1.7 million people globally became newly infected with HIV, an average of 5000 infections per day.2,3 Fig. 1 illustrates the 27-year global trend of new HIV infections, AIDS-related deaths, and people living with HIV.2 Even though the number of people living with HIV and HIV mortality are on the decline, they are far from null, and the number of HIV/AIDS infections are on the rise.
Fig. 1. Global number of AIDS-related deaths, new HIV infections, and people living with HIV (1990–2017). HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome. Data used in this Figure from https://www.unaids.org/en/resources/fact-sheet.
Studies of HIV/AIDS prevention programs have specifically targeted FSWs,8 for whom different approaches and interventions have been described and reported. Unlike counseling to promote condom use and conditional cash transfer for safer sex, PrEP has emerged as a more effective and ethical alternative option for HIV/AIDS prevention among FSWs. Designed for use as a preventative and prophylactic intervention, oral PrEP comprises the use of antiretroviral drugs prior to HIV exposure of healthy subjects in order to minimize and block HIV transmission and subsequent infection with the virus.11 Multiple studies have demonstrated that PrEP is highly effective with appropriate subject compliance and that the protective effect appears to be long-lasting.12 The Centers for Disease Control and Prevention (CDC) announced that daily PrEP reduces the risk of contracting HIV from sex by more than 90%, despite it being less effective and protective when taken inconsistently or erratically.13 Thus, adherence to taking PrEP as prescribed has become an element of extreme importance.
Although the use of and adherence to PrEP have already proven to be effective in preventing HIV infection among FSWs, few studies have looked at the use of PrEP among FSWs. Therefore, our comprehensive review focused on the barriers to and facilitators of adherence to HIV PrEP among FSWs. This review also suggests a roadmap for future PrEP intervention programs targeting this underserved and vulnerable sub-population.
DEFINITIONS AND SCOPE OF THE REVIEW
Adherence is defined as the extent to which a person's behavior (e.g., taking medication, following a diet, and/or changing lifestyle) corresponds with agreed recommendations from a health worker.6 Use of antiretroviral drugs in our study refers to the use of medication for the prevention of HIV infection and includes drugs for preventing mother-to-child transmission, drugs to reduce the transmission among sero-discordant sexual partners, and drugs to prevent the acquisition of HIV after exposure. PrEP for HIV refers to the use of antiretroviral drugs by people who are HIV negative before coming into contact with HIV to reduce the risk of infection. Post-exposure prophylaxis (PEP) for HIV comprises the use of antiretroviral drugs among people who are HIV negative and came into contact with HIV (within the past 72 hours).11
Studies presenting quantitative data on PrEP for FSWs were reviewed, and those with strategies for PrEP adherence were included in this review. A comprehensive review was carried out for all 11 articles of interest. Findings and discussions on adherence to PrEP among FSWs were classified into different categories: social values and stigma, education on PrEP, lifestyle (substance use and coitus), inconvenience, and economic burden. The characteristics of the 11 studies used in our review are summarized in Table 1.
Table 1. Observational Studies of PrEP Adherence among FSW.
| Author, year | Geographical location | Intervention | Population | Sample size | Follow-up period | Design | Conclusion and key words | Categories |
|---|---|---|---|---|---|---|---|---|
| Mutua, et al., 201244 | Kenya | PrEP | MSW/FSW | 72 | 4 months | Clinical trial | Although adherence is lower than with daily regimens, intermittent PrEP dosing with a fixed regimen in this at-risk population is feasible | Inconvenience |
| Van der Elst, et al., 201335 | Kenya | PrEP | MSW/FSW | 51 | 4 months | Clinical trial | Most participants favored the intermittent dosing schedule; those in the intermittent group noted particular challenges in adhering to the post-coital dose. Culturally appropriate and consistent counseling addressing these issues may be critical for PrEP effectiveness | Lack of knowledge Inconvenience |
| Syvertsen, et al., 20144 | - | PrEP | FSW | - | - | Comprehensive | Involving social scientists in clinical and community-based research on PrEP is very important. Advocating for a shift away from a singular “re-medicalization” of the HIV epidemic to that of a “reintegration” of interdisciplinary approaches to prevention that could benefit FSW and other key populations at risk of acquiring HIV | Social values and stigma |
| Lack of knowledge | ||||||||
| Reynolds, et al., 201543 | USA | PrEP | MSM; heterosexual women and men; injection drug users | 1 | - | Grand rounds discussion | The patient’s risk for HIV transmission from their husband and from other partners, the magnitude of the risk reduction they would gain with PrEP, and nonpharmacologic alternatives to reduce the likelihood of contracting HIV infection | Lifestyle modification |
| Reza-Paul, et al., 201633 | India/Southern Kamataka | PrEP | FSW | 427 | Interview | Situating PrEP scale up within the trusted spaces of community-based organizations should be considered as a means of supporting PrEP roll-out | Social values and stigma | |
| Restar, et al., 201734 | Kenya/Monbasa | PrEP/PEP | MSW/FSW | 21/23 | - | Interview | Despite its availability, few knew about PEP and even fewer had used it, although most who had would use it again. Sex workers valued confidentiality, privacy, trustworthiness, and convenient location in health services and wanted thorough HIV/STI assessments | Lifestyle modification |
| Eakle, et al., 201740 | South Africa/Johannesburg, Pretoria | PrEP/early ART | FSW | 219/139 | 12 months | Part of a cohort study | PrEP and early ART can be aligned with existing health service programming (TAPS) for FSWs safely, without significant behavior change, with high rates of uptake for both interventions, and with expected cost reductions in routine settings at scale Economic burden | Lifestyle modification |
| Pines, et al., 201842 | Mexico-USA border region | PrEP (oral pill/vaginal gel) | FSW | 271 | 6 months | Survey | FSWs indicated a strong preference for oral pills; however, vaginal PrEP products may also facilitate uptake and ensure sufficient coverage | Lack of knowledge |
| Lifestyle modification | ||||||||
| Eakle, et al., 201839 | South Africa | PrEP (focus group discussion) | FSW | 69 | 1–2 hours | Interview | Through FGDs, PrEP became a positive and highly anticipated prevention option among the FSWs participants | Lack of knowledge |
| Eakle, et al., 201841 | South Africa | PrEP/early ART (formative research process) | FSW | - | - | Comprehensive | Formative research is critical in designing interventions, especially in new environments, but also in well-known contexts. Including intensive stakeholder engagement in formative research will help to ensure that interventions are designed with feasibility and relevance for populations in mind | Lack of knowledge |
| Pines, et al., 201959 | Mexico-USA border region | Tijuana vs. Ciudad (Vaginal/washing/Lubrication) | FSW | 145/150 | - | Survey | Vaginal PrEP product development and implementation should also consider the link between vaginal washing and lubrication (area difference) to ensure existing practices do not undermine vaginal PrEP product effectiveness | Lifestyle modification Inconvenience |
ART, antiviral therapy; PrEP, pre-exposure prophylaxis; PEP, post-exposure prophylaxis; MSW, male sex worker; MSM; men who have sex with men; FSW, female sex worker; HIV, human immunodeficiency virus; FGD, Focus Group Discussion; STI, Sexually Transmitted Infection; TAPS study, prospective observational cohort study with two groups (PrEP and early ART for FSWs).
HIV PRE-EXPOSURE PROPHYLAXIS
In July 2012, the Food and Drug Administration approved the combination of emtricitabine and tenofovir disoproxil fumarate as a PrEP formulation for HIV protection in both men and women.14,15 Since then, PrEP has been proven to be a highly plausible HIV preventive method, with strong evidence-based data on its safety and effectiveness.16
Pharmacokinetic studies suggest that adherence interventions are particularly important and impactful for women and should be carefully designed and implemented. Even though PrEP provides flexibility with regards to its method of administration [oral or topical (vaginal/rectal gel)], its use is subject to heterogeneity, as shown in our results. PrEP clinical trials have also revealed mixed efficacy results: BostwanaTDF217 PartnersPrEP,18 and CAPRISA19 reported efficacy of up to 72%. The VOICE20 and FEMPrEP21 trials were cut short over suboptimal efficacy concerns.4 In most of these trials, PrEP efficacy was strongly related to adherence to this bio-medical intervention, with evidence of non-efficaciousness corresponding with poor adherence and high efficacy with better adherence.17,22,23 Adherence to PrEP has been shown to be of extreme volatility and unpredictability, secondary to many FSWs' personal, external, and environmental factors and circumstances.
Adherence to PrEP has been quantified objectively in multiple studies. The drug is most effective when it reaches maximum intracellular concentration. However, its time window is highly variable and tissue-specific, ranging between 1 week for rectal tissue penetration and up to 20 days for serum and cervico-vaginal penetration.24 Subsequently, pharmacokinetic studies have suggested that protection of the cervico-vaginal organs against HIV requires a minimum of 85% adherence, corresponding to at least seven doses per week. Similarly, for colo-rectal organ protection, adherence should be in the range of 28%, corresponding to two to seven doses per week.25
PRE-EXPOUSRE PROPHYLAXIS AND FEMALE SEX WORKERS
Within different communities across a wide geographical distribution, PrEP has been shown to be an effective and sustainable option for HIV/AIDS prevention intervention among FSWs.26 The success of PrEP intervention, however, is heavily dependent on the user's willingness and compliance with the prescribed PrEP regimen, and a lack of awareness and insufficient knowledge regarding this technique by both healthcare providers and patients/subjects are major barriers to its optimal benefit. In order to ensure its ultimate effect in controlling the spread of the virus, adherence to the prescribed regimen is of utmost importance. Indeed, all clinical trials have concluded that PrEP efficacy is contingent upon regimen adherence.27 However, FSWs often find themselves in an underserved context of poverty, lacking significant opportunities for education or adequate healthcare.28,29 Moreover, they often face misogynist and gender-based obstacles, along with financial, societal, and cultural barriers,29,30 that limit large-scale implementation and efficient HIV/AIDS control among FSWs using the PrEP method.30
PrEP has been described as an empowering approach with which to ensure self-protection and health wellbeing among FSWs.31 This method allows FSWs to use PrEP without the need for consent or approval from their clients and partners. However, overcoming the challenges and barriers to adherence when implementing a PrEP intervention requires enormous effort and a multidisciplinary road map and actions from variable stakeholders.32
FACTORS AFFECTING THE ADHERENCE TO PRE-EXPOUSRE PROPHYLAXIS
A representative study on the feasibility of implementing PrEP interventions among FSWs in South India33 revealed major obstacles for adherence thereto. In the study, challenges to taking PrEP every day were surveyed among 424 FSWs. The top five reasons were fear of possible side effects (61.8%), social stigma secondary to PrEP use (47.2%), client or partner not giving permission or approval for PrEP use (39.4%), fear of being suspected/judged of carrying HIV (37.5%), and difficulties with daily tablet intake (33.0%): study participants were asked to choose all that applied. In addition to this report, in an attempt to identify factors affecting adherence to PrEP, we comprehensively reviewed the literature, and the results are summarized in Table 1.
Social values and stigma
Studies have pointed out the influence of social factors on PrEP adherence among FSWs. A study in Kenya found that attitudes among sex workers towards the use of PrEP were tied to positive personal and social values.34 A study of at-risk populations in Kenya highlighted a number of barriers and facilitators to PrEP regimens, including concerns around stigma and discrimination.35
Focus groups on experiences with PrEP among men who have sex with men (MSM) and FSWs in Kenya found that although the acceptance of oral PrEP was high, concerns around the social costs of PrEP (e.g., stigma, gossip, rumors) were pervasive. 4 Relationship factors were also associated with improved adherence to PrEP and, thus, greater effectiveness.34,36
A study on Nairobi-based FSWs reported that perceived stigma from other FSWs based on the notion that a person using PrEP is likely to be HIV-positive also emerged as one of the barriers to seeking PrEP,37 which is highly likely to be related to lower adherence. Similarly, one PEP reported fear of stigmatization from healthcare providers, especially upon repeated PEP requests, as a pertinent issue, since it would possibly imply that the FSW was not taking any measures to avoid the circumstances that led to previous PEP requests.37
Lack of education
In a study on FSWs in Baltimore, South Africa, PrEP education was suggested as one potential means through which to increase adherence to PrEP.38 Some sex workers were willing to use PrEP if it had limited side effects and could be used intermittently, suggesting the need to include PrEP education on known and unknown side effects, careful messaging about side effects, guidance on intermittent use, and proper counseling on medication adherence and management for those initiating PrEP.38
Regarding education, research has indicated that one effective strategy is to focus on PrEP within existing curricula, campaigns, and interventions for antiviral therapy and PEP, while framing it as another modality of contraception/prevention.39 Awareness and understanding were important themes when exploring PrEP acceptability. PrEP motivations included the availability of choice, taking into account PrEP challenges and barriers; and, de-stigmatizing and empowering PrEP delivery.39 Participant discussions and proposal also highlighted the central role of developing clear education and messaging to precisely convey the concept of PrEP and intervention integration into supportive and individulaized services.4
In a study on FSWs in South Africa, the importance of adherence was emphasized after 1 month of initiation of PrEP during 1-month check-ins;40 however, the authors did not evaluate whether it was associated with higher adherence. FSWs who initiated PrEP were scheduled for an initial 1-month follow-up to assess safety and/or adherence issues, after which they were scheduled for quarterly clinical testing and safety monitoring visits.40 The importance of consistent and high adherence was emphasized for PrEP use; however, because participants could cycle on and off medication during periods of lower risk as desired and remain in the study,40 there were no data on women who did not return to the study. Another short-coming of this study was that adherence and sexual behavior data were self-reported, increasing the possibility of recall and social desirability biases.40
In a study on PrEP and early HIV treatment interventions for FSWs in South Africa, messaging and education were emphasized to promote adherence.41 The authors of the study mentioned that it is important to focus on strategies for and the philosophy around supporting PrEP adherence and condom use, both in early messaging and in on-going education and monitoring for sex workers.41
LIFESTYLE MODIFICATION
Complexities of daily life pose challenges to PrEP adherence.35 A study that examined vaginal washing and vaginal lubrication among FSWs in Tijuana and Ciudad Juarez, Mexico documented moderate (39%) and high (54%) prevalences of vaginal washing and lubrication in the past month, respectively, suggesting that vaginal PrEP products formulated as douches or gels may be acceptable HIV prevention methods to many FSWs in the Mexico-US border region.42 The authors of the study also identified several predictors of each vaginal practice, providing insight into the characteristics of FSWs for whom these vaginal PrEP products may be most acceptable.42
Lifestyle factors, such as substance use, can affect PrEP adherence. One study found that combined use of illicit drugs and tenofovir disoproxil fumarate can be nephrotoxic. Thus, guidelines call for quarterly visits that should include HIV testing, STI assessment, adherence counseling, and risk reduction counseling.43 In an at risk-population in Kenya, researchers noted that substance use was a barrier to adherence: alcohol use was a reason for not taking pills on time or not taking them altogether.35 In the same study, the authors identified the frequency of sex as another lifestyle issue that affected lower PrEP adherence. Intermittent dosing, especially after sexual coitus, may not be appropriate for populations at-risk with relatively high rates of transactional sex work.Travel was also mentioned as one lifestyle factor that interfered with proper medication intake.40 Another study on African MSMs and FSWs reported that adherence to any post-coital doses was 26%.44
Inconvenience
When using PrEP, tablets should be taken daily, posing inconvenience for the user. A study among FSWs in India reported that 40 survey participants (33%) expressed daily intake as a challenge to PrEP use and pointed to frequent travel and unpredictable work schedules as obstacles towards adherence.32 A study among FSWs in the Mexico-US border region reported that the study participants preferred monthly product use to daily and on-demand use, with which they may have perceived as less burdensome and easier to comply.42
Economic burden
Research has indicated that the extreme poverty and material insecurity that typically drives sex work in sub-Saharan Africa may render HIV prevention and PrEP adherence a lower priority than meeting basic needs (e.g., food, shelter) for many women.4 Although 80% of surveyed South Indian FSWs indicated that they would be willing to pay for PrEP, it was also clear that adherence might be a challenge if women were expected to assume the cost of medication.34 However, considering that most FSWs live in poverty, it is likely that imposing an economic burden would negatively impact PrEP adherence.
PRE-EXPOUSRE PROPHYLAXIS AMONG FEMALE SEX WORKERS: BARRIERS AND STRATEGIES TO INCREASE ADHERENCE
All of the evidence above highlights the complexity of PrEP adherence among FSWs. Proactive educational intervention, lifestyle modifications, concerns for convenience and FSWs preferences, and finally, alleviation of economic burden have been shown to play an important role in PrEP adherence and subsequent HIV control outcomes. Although multiple clinical trials have indicated that FSWs are interested in using PrEPs,38,45,46 this willingness is not always translated into actual PrEP use or adherence.
Social values and stigma are difficult to address and modify, and long-term educational efforts to change notions, judgment, and stigmas in local communities towards PrEP use are needed. Church-based efforts to promote screening and modify health behaviors have been successful, and thus, working in partnership with religious communities may potentially help shift attitudes towards HIV/AIDS and PrEP. Indeed, faith leaders have been shown to hold the potential to play a pivotal role in HIV de-stigmatization and in increasing collective acceptance of efforts to control HIV, such as PrEP usage, in the community.47,48
Brothel leadership and their faciliatory role in condom use has been shown to limit HIV exposure among FSWs and their clients.49 Similarly, cooperation from brothel owners may also be relevant to promoting PrEP use. With support, it is likely that FSWs will be more inclined to adhere to PrEP intervention.
Educational initiatives at the individual and community level should be administered regularly to promote adherence. These efforts should be directed towards healthcare workers in local communities, FSWs, and different stakeholders, including religious figures and brothel owners. Multiple studies have shown that innovative educational training strategies result in better HIV risk assessment and can overcome many barriers to PrEP prescription.50,51
Motivational interviews have proven to be effective in promoting PrEP use among men having sex with men.52,53 For FSWs, motivational interviewing may facilitate assessing the pros and cons of taking PrEP and explaining them in lay terms to FSWs, allowing FSWs to understand the very low risk and great benefits of such a prophylactic approach. Discussions and/or consultations should be centered and focused on FSWs, empowering them to choose PrEP and to adhere to the prescribed regimen. To reinforce PrEP adherence, healthcare workers should consider FSW preferences, needs, and context. Indeed, better HIV and PrEP knowledge and education have been shown to lead to protection seeking behavior among FSWs, encouraging them to better adhere to the PrEP regimen.15,35
One special sub-group of FSWs worth mentioning is female workers of a younger age. Special attention should be given to younger FSWs as previous studies have expressed concerns of lower adherence in this population. Evidence from wide scaling of the treatment points out that younger people find it more challenging to adhere to treatment than older individuals,54 and many may need increased adherence encourengment tailored to their age group and lifestyle.55 In a phase III trial on dapivirine vaginal ring,56 adherence was lowest in the younger age group: women aged 18–21 years had lower adherence than those aged over 21 years.54 This subsequently led to a major decrease in the efficacy of the intervention.56,57
Almost all oral PrEP prescriptions consist of daily pills, which is an important hurdle and barrier to adherence. Nevertheless, the inconvenience of daily pills might be overcome with research on next-generation PrEP products. In the meantime, there is a need to consider existing vaginal practices to ensure the development of safe and effective vaginal PrEP products.58,59 Implementation of various PrEP product formulations, including behaviorally-congruent vaginal gels and douches, have been shown to enhance future PrEP uptake and adherence and to ultimately reduce the burden of HIV among FSWs.59 However, reports have warned of a potential interaction or dilution of PrEP effectiveness with concomitant use of vaginal washing or lubrication with vaginal gel- or douche-based PrEP products.60 Some options that have been proposed to avert the daily usage of PrEP is an intermittent protocol. Notwithstanding, major concerns have been raised regarding the efficiency of intermittent dosage. A mathematical model in fact proved that the highest HIV protection is directly correlated with the exact number of PrEP doses per week and is not dependent on the dosage interval.61 Furthermore, intermittent PrEP dosing has not been universally adopted as a safe alternative to daily use, because references could not agree on a single definition nor frequency for intermittent protocols.62 Newer and promising PrEP formulations, including weekly controlled slow-release dosages and long-acting oral pills, may provide a potential solution to poor adherence with currently available regimens.63
In the last two decades, major international and local governmental/non-governmental agencies have worked relentlessly to limit HIV through preventative efforts.64 Controlling HIV has proven to improve economic growth and productivity, prosperity, and quality of life indices, especially in middle- and low-income countries.65 Focusing on high-risk groups for HIV prevention, such as FSWs, has been reported to be cost-effective:66 targeted intervention for FSWs has proven to result in a 47% reduction in HIV prevalence and 36% reduction in cumulative HIV cases.67 Interestingly, among the FSW population specifically, PrEP has been shown to have limited cost-effectiveness in HIV prevention.68 This is probably due to the economic and financial vulnerabilities that FSW populations face, which can make additional PrEP costs a major challenge to adherence. In order to overcome the economic burden of PrEP, advocates should fight to have PrEP covered by health insurance.39
Facilitators of and barriers to efficient PrEP adherence are summarized in Table 2. Some high-yield facilitators for PrEP adherence include educational and motivational interventions, social support, and making sure healthcare is guaranteed. All these interventions occur at the individual, social, and structural level, respectively.
Table 2. Facilitators of and Barriers to Efficient PrEP Adherence Protocols at the Individual, Social, and Structural Level among Female Sex Workers62,63,64,65.
| High-yield facilitators of PrEP usage adherence | Major barriers to PrEP usage and adherence | |
|---|---|---|
| Individual level | PrEP education and motivation to maintain good health | Poor knowledge of PrEP, doubts about its effectiveness, fear of side effects, low perception of HIV risk, and the need to adhere to multiple medications |
| Social level | Partner, peer, and family support | Anticipated stigma from peers, partners, and family members related to sexual orientation, PrEP, and/or HIV status |
| Structural level | Presence of and good out-reach from healthcare workers and establishments, financial support, and patient privacy | Concerns regarding attitudes of healthcare providers, quality assurance, data protection, and cost |
PrEP, pre-exposure prophylaxis; HIV, human immunodeficiency virus.
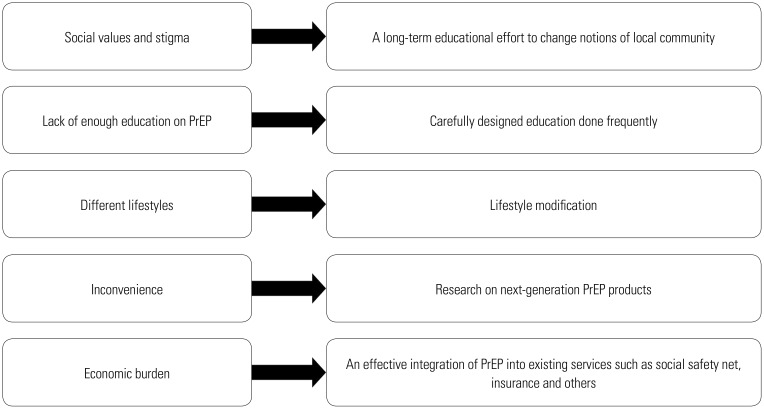
A summary of barriers to PrEP adherence among FSWs and potential ways to overcome them are presented in Fig. 2. Adherence to PrEP faces multiple barriers at the individual, social, structural, and economic level. Multiple stakeholders must coordinate and act in synergy to help overcome these barriers.
Fig. 2. Summary of barriers to PrEP adherence among female sex workers and ways to overcome them. PrEP, pre-exposure prophylaxis.
Other interventions have also been described in the literature among other high-risk groups for HIV to increase PrEP adherence. The CDC, in 2014, included in their guideline algorithms regular PrEP adherence counseling actions for adherence optimization.50,51 A weekly counseling session delivered by a dedicated nurse has been found to result in 84% adherence to the PrEP regimen at 6 months after the intervention for MSM.51 Kar, et al.69 coined the acronym EMPOWER as an approach to improve adherence, consisting of the following actions and interventions: “Education/leadership development, Media/Advocacy, Public education/Participation, Organizing associations/Unions, Work training/micro-enterprise, Enabling services/Assistance, and Rights protection/Promotion.”69 Even though this approach is comprehensive, its realistic application among FSWs can be extremely challenging and unrealistic in some instances.
CONCLUSION
FSWs are a vulnerable population and frequently subjected to violence, stigma, marginalization, economic, and social hardship. All of these factors pose barriers to sexual reproductive health and HIV preventative interventions. Research has repeatedly shown that low PrEP adherence accounts for the lack of efficiency with this intervention in protecting against HIV. Therefore, PrEP adherence strategies should be developed and designed as a holistic approach, acknowledging the contextual factors of FSWs. Targeted interventions taking into account socio-cultural, economic, and individual preferences have the potential to assure the highest adherence level. To achieve HIV/sexually transmitted infection PrEP intervention goals, a model among multiple stakeholders should intervene at the structural, environmental, community, and personal/individual level in order to achieve the maximum protection and to ensure FSW wellbeing. Combined interventions that are designed to build self-efficacy, empowerment, and social cohesion with evidencebased individualized adherence support are likely to be most effective (Table 2).70,71,72 Designing tailored approaches to PrEP delivery and adherence support, based on each individual participant's needs and preferences,14 is necessary for future interventions. A conceptual framework73 that can be used to locate where the adherence interventions lie in the greater picture of HIV/AIDS intervention and its impact is needed.
Ultimately, more research should be conducted in real-world settings to help design and implement creative and original interventions with the purpose of maximizing HIV/AIDS protection among FSWs. Adopting such targeted and population-specific approaches will ultimately contribute to efforts aiming to control the HIV/AIDS epidemic.
ACKNOWLEDGEMENTS
The views expressed in the submitted article are of those of the authors themselves and not an official position of their affiliated institutions and organizations.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sung Hwi Hong and Ramy Abou Ghayda.
- Data curation: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Formal analysis: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Investigation: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Methodology: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Project administration: Sung Hwi Hong and Jae Il Shin.
- Resources: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Software: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Supervision: Jae Il Shin.
- Validation: Sung Hwi Hong, Ramy Abou Ghayda, Jae Won Yang, and Jae Il Shin.
- Visualization: Sung Hwi Hong and Jae Won Yang.
- Writing—original draft: Sung Hwi Hong, Ramy Abou Ghayda, and Jae Won Yang.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
References
- 1.Ghoma Linguissi LS, Lucaccioni V, Bates M, Zumla A, Ntoumi F. Achieving sustainable development goals for HIV/AIDS in the Republic of the Congo-Progress, obstacles and challenges in HIV/AIDS health services. Int J Infect Dis. 2018;77:107–112. doi: 10.1016/j.ijid.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Joint United Nations Program on HIV/AIDS (UNAIDS) UNAIDS data 2019. [accessed on 2020 April 1]. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf.
- 3.Joint United Nations Program on HIV/AIDS (UNAIDS) UNAIDS data 2018. [accessed on 2020 April 1]. Available at: https://www.aidsdatahub.org/sites/default/files/publication/UNAIDS_Data_2018.pdf.
- 4.Syvertsen JL, Robertson Bazzi AM, Scheibe A, Adebajo S, Strathdee SA, Wechsberg WM. The promise and peril of pre-exposure prophylaxis (PrEP): using social science to inform prep interventions among female sex workers. Afr J Reprod Health. 2014;18(3 Spec No):74–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Scorgie F, Chersich MF, Ntaganira I, Gerbase A, Lule F, Lo YR. Socio-demographic characteristics and behavioral risk factors of female sex workers in sub-saharan Africa: a systematic review. AIDS Behav. 2012;16:920–933. doi: 10.1007/s10461-011-9985-z. [DOI] [PubMed] [Google Scholar]
- 6.Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 7.Beyrer C, Crago AL, Bekker LG, Butler J, Shannon K, Kerrigan D, et al. An action agenda for HIV and sex workers. Lancet. 2015;385:287–301. doi: 10.1016/S0140-6736(14)60933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson D. HIV Programs for sex workers: lessons and challenges for developing and delivering programs. PLoS Med. 2015;12:e1001808. doi: 10.1371/journal.pmed.1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;(1):CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 10.Hallett TB, Aberle-Grasse J, Bello G, Boulos LM, Cayemittes MP, Cheluget B, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm Infect. 2006;82 Suppl 1:i1–i8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 12.Marrazzo JM. HIV prevention: opportunities and challenges. Top Antivir Med. 2017;24:123–126. [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. PrEP: pre-exposure prophylaxis. [accessed on 2020 April 1]. Available at: https://www.cdc.gov/hiv/basics/prep.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Factagainstaids%2Fbasics%2Fprep.html.
- 14.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 15.Ye L, Wei S, Zou Y, Yang X, Abdullah AS, Zhong X, et al. HIV pre-exposure prophylaxis interest among female sex workers in Guangxi, China. PLoS One. 2014;9:e86200. doi: 10.1371/journal.pone.0086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaron E, Blum C, Seidman D, Hoyt MJ, Simone J, Sullivan M, et al. Optimizing delivery of HIV preexposure prophylaxis for women in the United States. AIDS Patient Care STDS. 2018;32:16–23. doi: 10.1089/apc.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 18.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute of Allergy and Infectious Diseases (NIAID) NIAID Statement: NIH modifies ‘VOICE’ HIV prevention study in women. [accessed on 2020 April 1]. Available at: https://www.nih.gov/news-events/news-releases/niaid-statement-nih-modifies-voice-hiv-prevention-study-women.
- 21.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–F19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 23.Galea JT, Kinsler JJ, Salazar X, Lee SJ, Giron M, Sayles JN, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22:256–262. doi: 10.1258/ijsa.2009.009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States–2017: a clinical practice guideline. [accessed on 2020 April 1]. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf.
- 25.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483–1493. doi: 10.3851/IMP2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell JD, Herbst JH, Koppenhaver RT, Smith DK. Antiretroviral prophylaxis for sexual and injection drug use acquisition of HIV. Am J Prev Med. 2013;44(1 Suppl 2):S63–S69. doi: 10.1016/j.amepre.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Camlin CS, Kwena ZA, Dworkin SL. Jaboya vs. jakambi: Status, negotiation, and HIV risks among female migrants in the “sex for fish” economy in Nyanza Province, Kenya. AIDS Educ Prev. 2013;25:216–231. doi: 10.1521/aeap.2013.25.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsberg WM, Luseno WK, Lam WK. Violence against substance-abusing South African sex workers: intersection with culture and HIV risk. AIDS Care. 2005;17 Suppl 1:S55–S64. doi: 10.1080/09540120500120419. [DOI] [PubMed] [Google Scholar]
- 30.Shannon K, Goldenberg SM, Deering KN, Strathdee SA. HIV infection among female sex workers in concentrated and high prevalence epidemics: why a structural determinants framework is needed. Curr Opin HIV AIDS. 2014;9:174–182. doi: 10.1097/COH.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdool Karim Q, Humphries H, Stein Z. Empowering women in human immunodeficiency virus prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:487–493. doi: 10.1016/j.bpobgyn.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Cohen MS, Baden LR. Preexposure prophylaxis for HIV--where do we go from here? N Engl J Med. 2012;367:459–461. doi: 10.1056/NEJMe1207438. [DOI] [PubMed] [Google Scholar]
- 33.Reza-Paul S, Lazarus L, Doshi M, Hafeez Ur Rahman S, Ramaiah M, Maiya R, et al. Prioritizing risk in preparation for a demonstration project: a mixed methods feasibility study of oral pre-exposure prophylaxis (PREP) among female sex workers in South India. PLoS One. 2016;11:e0166889. doi: 10.1371/journal.pone.0166889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Restar AJ, Tocco JU, Mantell JE, Lafort Y, Gichangi P, Masvawure TB, et al. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in Mombasa, Kenya: implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29:141–153. doi: 10.1521/aeap.2017.29.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Elst EM, Mbogua J, Operario D, Mutua G, Kuo C, Mugo P, et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav. 2013;17:2162–2172. doi: 10.1007/s10461-012-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izulla P, McKinnon LR, Munyao J, Ireri N, Nagelkerke N, Gakii G, et al. Repeat use of post-exposure prophylaxis for HIV among nairobi-based female sex workers following sexual exposure. AIDS Behav. 2016;20:1549–1555. doi: 10.1007/s10461-015-1091-1. [DOI] [PubMed] [Google Scholar]
- 38.Eakle R, Manthata G, Stadler J, Mbogua J, Sibanyoni M, Venter WDF, et al. Preparing for PrEP & immediate treatment: focus group discussions in advance of a demonstration project in South Africa. AIDS Res Hum Retroviruses. 2014;30:A269–A270. [Google Scholar]
- 39.Eakle R, Bourne A, Mbogua J, Mutanha N, Rees H. Exploring acceptability of oral PrEP prior to implementation among female sex workers in South Africa. J Int AIDS Soc. 2018;21:e25081. doi: 10.1002/jia2.25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eakle R, Gomez GB, Naicker N, Bothma R, Mbogua J, Cabrera Escobar MA, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med. 2017;14:e1002444. doi: 10.1371/journal.pmed.1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eakle R, Mutanha N, Mbogua J, Sibanyoni M, Bourne A, Gomez G, et al. Designing PrEP and early HIV treatment interventions for implementation among female sex workers in South Africa: developing and learning from a formative research process. BMJ Open. 2018;8:e019292. doi: 10.1136/bmjopen-2017-019292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pines HA, Semple SJ, Strathdee SA, Hendrix CW, Harvey-Vera A, Gorbach PM, et al. Vaginal washing and lubrication among female sex workers in the Mexico-US border region: implications for the development of vaginal PrEP for HIV prevention. BMC Public Health. 2018;18:1009. doi: 10.1186/s12889-018-5946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds EE, Libman H, Mayer KH. Preexposure prophylaxis for HIV prevention: grand rounds discussion from Beth Israel deaconess medical center. Ann Intern Med. 2015;163:941–948. doi: 10.7326/M15-1993. [DOI] [PubMed] [Google Scholar]
- 44.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7:e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PLoS One. 2012;7:e28238. doi: 10.1371/journal.pone.0028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robertson AM, Syvertsen JL, Martinez G, Rangel MG, Palinkas LA, Stockman JK, et al. Acceptability of vaginal microbicides among female sex workers and their intimate male partners in two Mexico-US border cities: a mixed methods analysis. Glob Public Health. 2013;8:619–633. doi: 10.1080/17441692.2012.762412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ransome Y, Bogart LM, Nunn AS, Mayer KH, Sadler KR, Ojikutu BO. Faith leaders' messaging is essential to enhance HIV prevention among black Americans: results from the 2016 National Survey on HIV in the black community (NSHBC) BMC Public Health. 2018;18:1392. doi: 10.1186/s12889-018-6301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunn A, Parker S, McCoy K, Monger M, Bender M, Poceta J, et al. African American clergy perspectives about the HIV care continuum: results from a qualitative study in Jackson, Mississippi. Ethn Dis. 2018;28:85–92. doi: 10.18865/ed.28.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okafor U, Crutzen R, Okekearu I, Adebajo S, Uzoh A, Awo EA, et al. Using brothel leadership to promote condom use among brothelbased female sex workers in Abuja, Nigeria: study protocol for a cluster randomized pilot trial. Pilot Feasibility Stud. 2017;3:10. doi: 10.1186/s40814-017-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS. 2012;7:593–599. doi: 10.1097/COH.0b013e3283590446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silapaswan A, Krakower D, Mayer KH. Pre-exposure prophylaxis: a narrative review of provider behavior and interventions to increase PrEP implementation in primary care. J Gen Intern Med. 2017;32:192–198. doi: 10.1007/s11606-016-3899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moitra E, van den Berg JJ, Sowemimo-Coker G, Chau S, Nunn A, Chan PA. Open pilot trial of a brief motivational interviewing-based HIV pre-exposure prophylaxis intervention for men who have sex with men: preliminary effects, and evidence of feasibility and acceptability. AIDS Care. 2020;32:406–410. doi: 10.1080/09540121.2019.1622644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starks TJ, Robles G, Pawson M, Jimenez RH, Gandhi M, Parsons JT, et al. Motivational interviewing to reduce drug use and HIV incidence among young men who have sex with men in relationships and are high priority for pre-exposure prophylaxis (Project PARTNER): randomized controlled trial protocol. JMIR Res Protoc. 2019;8:e13015. doi: 10.2196/13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hensen B, Hargreaves JR, Chiyaka T, Chabata S, Mushati P, Floyd S, et al. Evaluating the impact of DREAMS on HIV incidence among young women who sell sex: protocol for a non-randomised study in Zimbabwe. BMC Public Health. 2018;18:203. doi: 10.1186/s12889-018-5085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montgomery ET, van der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017;31:1159–1167. doi: 10.1097/QAD.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilber AM, Chersich MF, van de Wijgert JH, Rees H, Temmerman M. Vaginal practices, microbicides and HIV: what do we need to know? Sex Transm Infect. 2007;83:505–508. doi: 10.1136/sti.2007.028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pines HA, Strathdee SA, Hendrix CW, Bristow CC, Harvey-Vera A, Magis-Rodríguez C, et al. Oral and vaginal HIV pre-exposure prophylaxis product attribute preferences among female sex workers in the Mexico-US border region. Int J STD AIDS. 2019;30:45–55. doi: 10.1177/0956462418793038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sidebottom D, Ekström AM, Strömdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV-how can we improve uptake and adherence? BMC Infect Dis. 2018;18:581. doi: 10.1186/s12879-018-3463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mugo PM, Sanders EJ, Mutua G, van der Elst E, Anzala O, Barin B, et al. Understanding adherence to daily and intermittent regimens of oral HIV pre-exposure prophylaxis among men who have sex with men in Kenya. AIDS Behav. 2015;19:794–801. doi: 10.1007/s10461-014-0958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selinger C, Kirtane A, Abouzid O, Langer R, Traverso CG, Bershteyn A. Anticipated adherence, efficacy, and impact of weekly oral preexposure prophylaxis [Abstract 1035]; Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA. [Google Scholar]
- 64.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82 Suppl 3:iii45–iii50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dixon S, McDonald S, Roberts J. The impact of HIV and AIDS on Africa's economic development. BMJ. 2002;324:232–234. doi: 10.1136/bmj.324.7331.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen DA, Wu SY, Farley TA. Comparing the cost-effectiveness of HIV prevention interventions. J Acquir Immune Defic Syndr. 2004;37:1404–1414. doi: 10.1097/01.qai.0000123271.76723.96. [DOI] [PubMed] [Google Scholar]
- 67.Prinja S, Bahuguna P, Rudra S, Gupta I, Kaur M, Mehendale SM, et al. Cost effectiveness of targeted HIV prevention interventions for female sex workers in India. Sex Transm Infect. 2011;87:354–361. doi: 10.1136/sti.2010.047829. [DOI] [PubMed] [Google Scholar]
- 68.Rinaldi G, Kiadaliri AA, Haghparast-Bidgoli H. Cost effectiveness of HIV and sexual reproductive health interventions targeting sex workers: a systematic review. Cost Eff Resour Alloc. 2018;16:63. doi: 10.1186/s12962-018-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kar SB, Pascual CA, Chickering KL. Empowerment of women for health promotion: a meta-analysis. Soc Sci Med. 1999;49:1431–1460. doi: 10.1016/s0277-9536(99)00200-2. [DOI] [PubMed] [Google Scholar]
- 70.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–834. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7:44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;(3):CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awungafac G, Delvaux T, Vuylsteke B. Systematic review of sex work interventions in sub-Saharan Africa: examining combination prevention approaches. Trop Med Int Health. 2017;22:971–993. doi: 10.1111/tmi.12890. [DOI] [PubMed] [Google Scholar]