Abstract
Objectives.
This represents the first report on the development of metformin-containing dental resins. The objectives were to use the resin as a carrier to deliver metformin locally to stimulate dental cells for dental tissue regeneration, and to investigate the effects on odontogenic differentiation of dental pulp stem cells (DPSCs) and mineral synthesis.
Methods.
Metformin was incorporated into a resin at 20% by mass as a model system. DPSC proliferation attaching on resins was evaluated. Dentin sialophosphoprotein (DSPP), dentin matrix phosphoprotein 1 (DMP-1), alkaline phosphatase (ALP) and runt-related transcription factor 2 (Runx2) genes expressions were measured. ALP activity and alizarin red staining (ARS) of mineral synthesis by the DPSCs on resins were determined.
Results.
DPSCs on metformin-containing resin proliferated well (mean ± SD; n = 6), and the number of cells increased by 4 folds from 1 to 14 days (p > 0.1). DSPP, ALP and DMP-1 gene expressions of DPSCs on metformin resin were much higher than DPSCs on control resin without metformin (p < 0.05). ALP activity of metformin group was 70% higher than that without metformin at 14 days (p < 0.05). Mineral synthesis by DPSCs on metformin-containing resin at 21 days was 9 folds that without metformin (p < 0.05).
Significance.
A novel metformin-containing resin was developed, achieving substantial enhancement of odontoblastic differentiation of DPSCs and greater mineral synthesis. The metformin resin is promising for deep cavities and perforated cavities to stimulate DPSCs for tertiary dentin formation, for tooth root coatings with metformin release for periodontal regeneration, and for root canal fillings with apical lesions to stimulate bone regeneration.
Keywords: Dental resin, metformin, odontoblastic differentiation, dental pulp stem cells, mineral synthesis, tissue regeneration
1. Introduction
Dental caries is the most common infectious disease in humans worldwide [1]. Dental resins are widely used in tooth cavity restorations [2–7]. In cases of deep cavities, liners and bases are often used beneath a restoration, which include calcium hydroxide-based, glass ionomer-based, resin-based and zinc phosphate-based materials [8,9]. Liners and bases can help provide protection for the dental pulp, prevent microleakage under the restoration, inhibit bacterial growth, reduce postoperative sensitivity, and release fluoride into the tooth [8,10,11]. Besides the need for liners and bases, the success of deep carious cavity treatment also depends on the self-repairing ability of the host pulpal cells, especially odontoblasts [12].
Odontoblasts, as a layer of palisade cells lining the interface between the dental pulp and inner dentin, are specialized cells capable of synthesizing not only primary dentin during early tooth development, but also secondary dentin throughout the life span of the tooth [13]. They synthesize the matrix of type I collagen, and actively participate in its mineralization by secreting proteoglycans and non-collagenous proteins that are implicated in the nucleation and growth of the mineral phase [14]. They also participate in the maintenance of the pulp vitality by synthesizing tertiary dentin (reparative dentin). The primary odontoblasts could be destroyed when exposed to deep caries or severe dental injury [15]. In deep cavities with the remaining dentin thickness (RDT) < 0.25 mm, the number of odontoblasts decreased by 23% [12,16]. However, dental pulp stem cells (DPSCs), as precursor undifferentiated mesenchymal cells, can differentiate into odontoblasts and have the ability to regenerate dentin/pulp-like complexes in response to carious stimuli [17,18]. Therefore, in order to heal teeth and maintain a viable pulp, the development of bioactive liner/base materials that can promote odontoblastic differentiation and reparative dentin formation is beneficial.
Metformin, an anti-diabetic biguanide drug, is widely used to treat type 2 diabetes mellitus (T2DM) by controlling blood sugar levels [19]. The mechanism of action of metformin is not clearly understood, but is generally thought to be suppressing hepatic gluconeogenesis. The gut microbiota could serve as a major determinant of metformin action and metformin could alter the gut microbiome of individuals with T2DM [20]. Several studies indicated that metformin has an osteogenic effect by promoting the differentiation of mesenchymal stem cells (MSCs) and preosteoblasts [21–23]. Indeed, metformin induced the differentiation and mineralization of preosteoblasts into osteoblasts via activation of the AMP-activated kinase (AMPK) signaling pathway [24,25]. A recent study from our group showed that metformin promoted the osteogenic differentiation and mineralization of induced pluripotent stem cell-derived MSCs (iPSC-MSCs) via liver kinase B1 (LKB1)/AMPK pathway [26]. Hence metformin could help enhance bone and periodontal regeneration in diabetic patients. DPSCs share similar gene expression profiles and differentiation capabilities to other MSCs [27–29]. We have also recently demonstrated that metformin induced odontoblastic differentiation of DPSCs [30]. Therefore, it would be beneficial to develop a metformin-containing resin as a base or liner, to release metformin and stimulate DPSCs for mineral synthesis and dentin production. A literature and patent search revealed no report on metformin-containing dental resins.
Therefore, the objectives of this study were to develop a novel metformin-containing resin for dental applications, and investigate the effects of metformin incorporation on DPSC viability, proliferation and odontoblastic differentiation for the first time. It was hypothesized that: (1) Metformin-containing resin would not adversely affect DPSC attachment and viability compared to that without metformin; (2) Metformin-containing resin would substantially enhance the odontogenic gene expressions and alkaline phosphatase activity of DPSCs growing on resins, compared to control resin without metformin; (3) DPSCs on metformin-containing resin would synthesize much more mineral than that on the resin without metformin.
2. Material and methods
2.1. Preparation of experimental resin disks
The resin consisted of 44.5% (all mass fractions unless otherwise noted) pyromellitic glycerol dimethacrylate (PMGDM, Hampford, Stratford, CT), 39.5% ethoxylated bisphenol A dimethacrylate (EBPADMA, Sigma-Aldrich, St. Louis, MO), 10% 2-hydroxyethyl methacrylate (HEMA, Esstech, Essington, PA), and 5% bisphenol A glycidyl dimethacrylate (BisGMA, Esstech), following a previous study [31]. PMGDM and EBPADMA were selected because they had a low cytotoxicity similar to other dental methacrylates [32]. As an acidic adhesive monomer, PMGDM is beneficial to the adhesion between dentin and composite [33,34]. HEMA was added to improve the co-monomer diffusion into the demineralized dentin, according to previous studies [35,36]. BisGMA was chosen because of its functions in improving the crosslink of monomers [37,38]. 1 % of phenyl bis(2,4,6-trimethylbenzoyl) phosphine oxide (BAPO, Sigma-Aldrich) was added for photopolymerization. This resin matrix with PMGDM, EBPADMA, HEMA and BisGMA is referred to as PEHB. For mechanical reinforcement, barium boroaluminosilicate glass particles (Dentsply, Milford, DE) with a median size of 1.4 μm were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine, as previously described [32]. Then metformin (1,1-dimethylbiguanide hydrochloride, Sigma-Aldrich) was incorporated into the resin. Two groups were tested:
-
(1)
25% PEHB resin + 75% glass particles (Control group);
-
(2)
25% PEHB resin + 55% glass particles + 20% metformin (Metformin group).
A total filler level of 75% mass fraction was blended with the resin, and the mixed paste was cohesive, not too dry, nor too liquidous. The samples used for cell experiments were prepared following a previous study [26]. Briefly, the cover of a sterile 96-well plate (Costar, Corning Inc., NY) was used as molds to make resin disks with approximately 8 mm in diameter and 1 mm in thickness, which were photo-cured for 1 minute. The cured resin disks were immersed in distilled water at 37 °C with stirring for 24 h to remove any unpolymerized monomers, and then sterilized by ultraviolet ray before use.
2.2. Metformin release from resin
Metformin release from the resin disks was measured. Disks (n = 3) of each group were placed in the wells of 24-well plates. Each well was filled with 1 mL of deionized water. The disks were transferred to new wells every 24 h, from the first day to the 28th day. The water in each well on days 1, 2, 3, 4, 5, 6, 7, 14, 21 and 28 was collected for the measurement of metformin release. The measurement was based on high performance liquid chromatography and mass spectrometry (HPLC-MS/MS) [39], using Surveyor Plus HPLC System, triple stage quadrupole (TSQ) Quantum Ultra mass spectrometry (Thermo Fisher Scientific, CA, USA),which was equipped with an autosampler, electrospray ionization (ESI) source, and TSQ mass analyzer.
The chromatographic separation of HPLC was achieved on a Hypersil Gold C18 analytical column (150 mm x 2.1 mm, 5 μm) (Thermo Fisher Scientific,CA) under isocratic conditions. The mobile phase consisted of a mixture based on 0.1% aqueous formic acid/methanol (95/5, v/v). The column temperature was 25 °C and the flow rate was 0.2 mL/min. The mass spectrometer operating conditions were: positive ion mode for ESI, multiple reaction monitoring (MRM) modes for quantification, spray voltage 4000 V, sheath gas pressure 30 (arbitrary units), aux gas pressure 10 (arbitrary units), capillary temperature 350 °C, collision pressure 1.5 mTorr, parent ion 130.1 m/z, quantum product ion 71.1 m/z, collision energy 23 ev, and tube lens 57 ev. Data acquisition and processing were performed with LCQUAN quantitative software, and the calculations were made using a series of standard metformin solutions with concentration of 1, 2, 5, 25 and 50 μg/mL.
2.3. Cells culture
Before DPSCs collection, clinically healthy dental pulp tissues were obtained from human adult third molars that were removed from individuals who had their teeth extracted due to orthodontic treatment. The procedure was approved by the Institutional Review Board of the University of Maryland Baltimore. Then DPSCs were isolated and characterized as described in our previous study [40]. Briefly, pulp tissues were minced and digested in a solution of 3 mg/mL of collagenase type I and 4 mg/mL dispase for 30–60 min at 37 °C. Then cell suspension was obtained by passing the digested tissue through a 70-μm cell strainer.
After that, cells were pelleted and seeded into culture dishes, and incubated with Dulbecco’s Modified Eagle’s medium (DMEM, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 1% penicillin/streptomycin at 37 °C with 5% CO2 [41]. After 48 h, non-adherent cells were removed and the medium was replaced every 2 days. After approximately 1–2 weeks, the culture became subconfluent, and the cells were collected by trypsinization and subcultured into new medium at 5,000 cells/cm2. A previous study [42] confirmed that DPSCs generated from this method expressed surface markers characteristic of MSCs (CD29, CD44, CD166, CD73), and were negative for typical hematopoietic (CD34, CD45, CD14). The 4th passage DPSCs were used in the following experiments.
Each resin disk was placed into a well of a 48-well plate with culture medium for 2 h at 37°C. Then DPSCs were seeded with different initial concentrations in 1 mL medium in each well for the different experiments, which are described in the following sections. Then the well plates were cultured in a CO2 incubator at 37 °C.
2.4. Cell viability assay
To evaluate whether mixing metformin into resin would harm the attached DPSCs, cell viability on the resin disks with or without metformin was assessed using the cell counting kit-8 (CCK-8, Endo Life Sciences, Farmingdale, NY), according to the manufacturer’s instructions. CCK-8 is based on the water-soluble tetrazolium salt, WST-8 reaction that could produce an orange water-soluble formazan dye in an amount that is directly related to the number of viable cells. Firstly, each well with a resin disk was seeded with 1 mL of DPSC suspension at a seeding density of 5000 cells/well [26]. The medium was replaced every two days. Cell proliferation at 1, 4, 7 and 14 days was measured using the cell counting kit. The resin disks with DSPCs were rinsed with phosphate buffered saline (PBS) and transferred into a new 48-well plate, then 300 μL CCK-8 dye was added into each well and placed in a CO2 incubator for 4 h. Finally, the number of viable cells was determined by measuring the absorbance of the orange colored formazan at optical density of 450 nm (OD450nm) with a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). Six disks were tested for each group at each time point.
2.5. Live dead staining
Separate resin disks were seeded with cells and cultured for live/dead staining to examine the growth of DPSCs on resins with or without metformin, as described previously. At each time points (1, 4, 7 or 14 day), the resin disks were taken out from each well of 48-well plate, washed by PBS and immersed at 37 °C for 15 min in a live/dead staining solution (Sigma-Aldrich) containing 2 μM Calcein AM and 2 μM Propidium Iodide [40]. Then the samples were observed at an inverted fluorescence microscope (Eclipse TE-2000S, Nikon, Japan) equipped with a digital camera. Three random locations of each disk were imaged, with 4 disks yielding 12 images for each group at time point.
2.6. Real time quantitative PCR (qPCR)
Quantitative real-time reverse transcription polymerase chain reaction (qPCR) was used to measure the gene expression of odontoblastic differentiation in DPSCs after culturing on resins with or without metformin. The total RNA was extracted using the PureLink RNA Mini Kit (Invitrogen), and then reverse-transcribed into cDNA by a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). The expressions of odontogenic differentiation genes markers, including dentin sialophosphoprotein (DSPP), dentin matrix phosphoprotein-1 (DMP-1), alkaline phosphatase (ALP) and runt-related transcription factor 2 (Runx2), were determined by qPCR using SYBR Green PCR Master Mix (Applied Biosystems), as previously described [26,43]. The housekeeping gene GAPDH was used as an internal control to normalize the expression levels of different genes [44]. The sequences of human specific primers used for the amplification of the indicated genes were synthesized commercially (Sigma-Aldrich) and are listed in Table 1. qPCR data collection and analyses were performed using an Applied Biosystems Prism 7000 Sequence Detection System, and relative expression was evaluated using the 2-ΔΔCt method and normalized by the cycle threshold (Ct) values of GAPDH, according to a previous study [26]. Ct values of DPSCs on resin disks of control group at day 1 served as the calibrator (n = 6).
Table 1.
List of primer sequences used in qPCR
| Gene | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) | |
|---|---|---|---|
| DSPP | ATGACAGTGATAGCACATCAGA | ATTGTTACCATTGCCATTACTG | |
| DMP-1 | CAGTGAGGAAGATGGCCA | CTTGGCAGTCATTGTCATCTT | |
| ALP | CGGATCCTGACCAAAAACC | TCATGATGTCCGTGGTCAAT | |
| Runx2 | GACTGTGGTTACCGTCATGGC | ACTTGGTTTTTCATAACAGCGGA | |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
2.7. Alkaline phosphatase (ALP) activity
DPSCs were seeded onto resin disks in 48-well plates at a density of 104 cells/well [45]. After 24 h for the cells to attach to the resin disks, the medium was replaced by an osteogenic medium, which consisted of DMEM growth medium, 10% FBS plus 100 nM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid, and 10 nM 1ɑ, 25-dihydroxyvitamin D3 (Sigma-Aldrich). At 7 and 14 days, the ALP activity of DPSCs was measured using an QuantiChrom ALP assay kit (BioAssay Systems, Cambridge, MA). Briefly, resin disks with DPSCs were washed by cold PBS. The attached cells were scraped and re-suspended into the lysis buffer for 30 min, then sonicated in an ice bath and centrifuged at 1500 rpm for 5 min. Then, the ALP activity of the supernatant was measured using an ALP working solution containing 200 μL assay buffer, 5 μL Mg Acetate (final 5 mM) and 2 μL pNPP liquid substrate (10 mM), with a ratio of 20 μL sample supernatant/180 μL working solution. After mixing, the mixtures were detected shortly the absorbance at OD405nm, and again after 4 min using a microplate reader (SpectraMax M5), following the manufacturer’s protocol. Finally, the ALP activity was normalized via the protein concentrations [26]. The protein concentration was quantified using a Micro BCA protein assay kit (Thermo Scientific, Rockford, IL), following the manufacture’s protocol. Briefly, the cells lysis supernatants were mixed with the working reagent in the kit, consisting of Reagent A and Reagent B (50:1, Reagent A:B), at a volume ratio of 1:19. Then the colorimetric reaction mixtures were used for the absorbance measurements at OD562nm using a microplate reader (SpectraMax M5).Standard curves were prepared by albumin standard ampule (BSA) with different concentrations of 0, 25, 125, 250, 500, 750, 1000, 2000 μg/mL, which was used to obtain the corresponding protein concentrations.
2.8. Alizarin red staining of minerals synthesized by the cells
DPSCs were seeded onto resin disks in 48-well plates at a seeding density of 104 cells/well [45], and cultured for 1, 7, 14 and 21 days in the osteogenic medium. Six disks were tested for each group at each time period for mineral synthesis evaluation (n = 6). Then the mineral deposit by DPSCs on resin disks were examined via alizarin red staining (ARS, Millipore, Billerica, MA), following the manufacturer’s instruction. The cells on resin disks were fixed with 1% formalin for 10 min and stained for 30 min by 2% ARS solution, which could stain calcium-rich deposits made by the cells into a dark red color [46]. Then the ARS solution was removed, the disks were washed with PBS to remove any loose alizarin red, and the disks were imaged. For quantification, the ARS-stained cells on resin disks were de-stained in 10% cetylpryidinium chloride (Sigma-Aldrich) for 15 min and the solutions were measured at OD652nm using a microplate reader (SpectraMax M5). The results were expressed by the folds of increases, with the OD value of control group on day 1 as the reference standard.
2.9. Statistical analysis
The SPSS21.0 (SPSS Inc., Chicago, IL) software was used for the statistical analysis. Two-way analyses of variance (ANOVA) were performed to detect the significant effects of variables. All data were expressed as mean value ± standard deviation (SD). The level of statistical significance was set at p < 0.05.
3. Results
3.1. Metformin release from resin
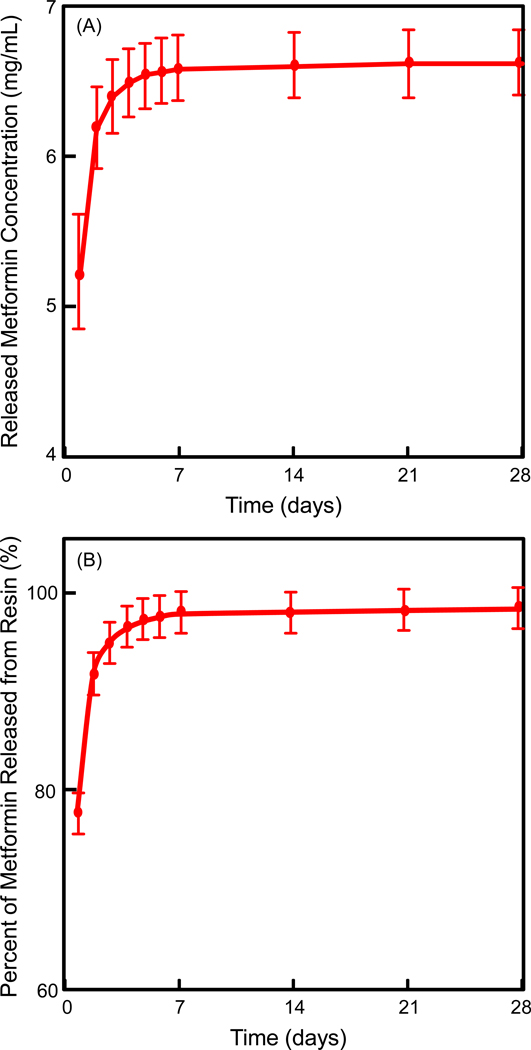
The release results of metformin from resin are plotted in Fig. 1: (A) Cumulative concentration of the released metformin, and (B) Percentage of metformin released from the resin disks (mean ± SD; n = 3). In (A), the release of metformin from the resin lasted for about a week. The metformin concentration reached a plateau from 7 to 28 days. In (B), approximately all the metformin in the resin was exhausted in the first week, with little metformin left in the resin after 7 days.
Figure 1.
Release profile of metformin from resin disks after immersion in water for 28 days: (A) Cumulative concentration of the released metformin, and (B) Percentage of metformin released from resin. The data represent the mean of three independent disks ± standard deviation (mean ± SD; n = 3).
3.2. Evaluation of cell viability
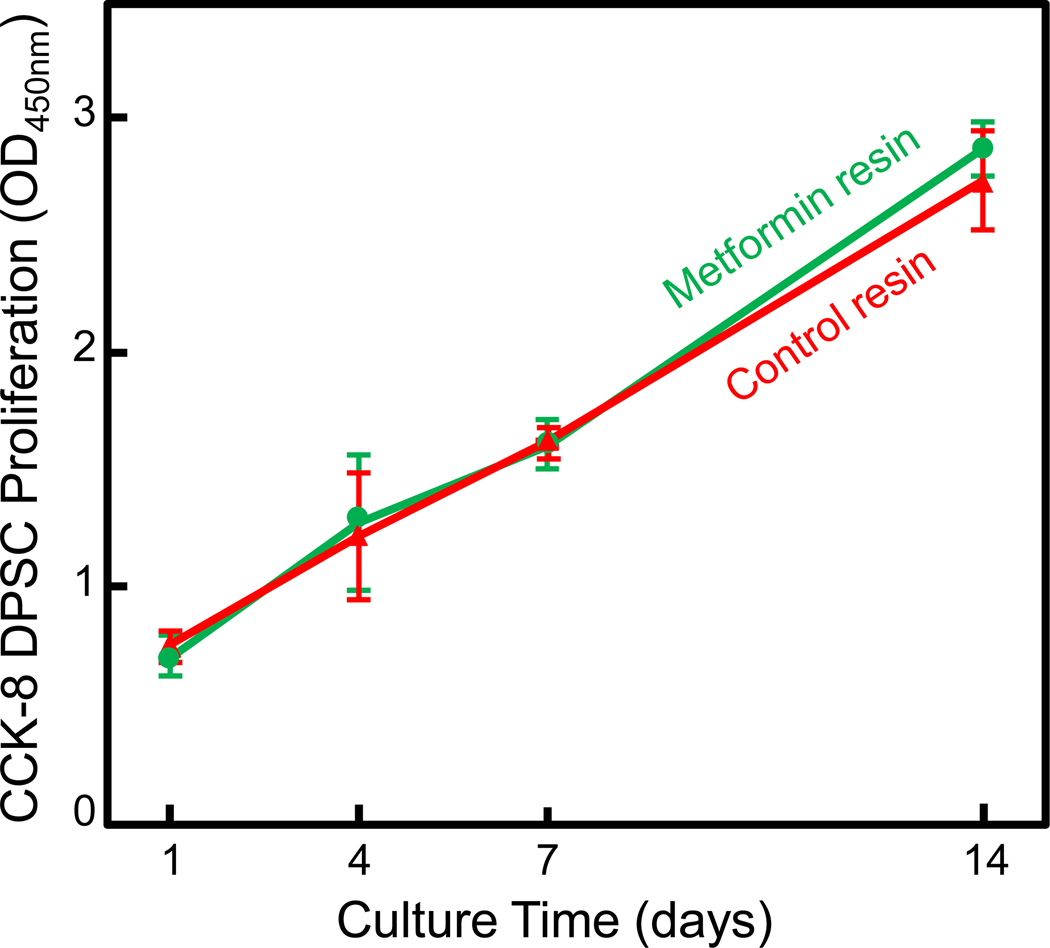
The metformin particle sizes were measured (mean ± SD; n = 28) to be (5.10±1.9) μm. When metformin particles were incorporated in the resin, in order to detect any cell cytotoxicity of the metformin-containing resin, CCK-8 test was performed for DSPCs on resin at 1, 4, 7 and 14 days (mean ± SD; n = 6). Cell viability and proliferation were not adversely affected by the addition of metformin (Fig. 2). CCK-8 assay showed that the growth of the attached DPSCs on metformin-containing resin was similar to control without metformin (p > 0.1). The number of cells on resin disks increased by approximately 4 times from 1 to 14 days; both groups had the same proliferation rate (p > 0.1).
Figure 2.
Evaluation of cell proliferation of DPSCs attaching to dental resins (mean ± sd; n = 6). The cell amount increased with time due to proliferation. Control group and metformin resin group had similar cell proliferation (p > 0.1), indicating that metformin did not adversely affect cell attachment and proliferation.
3.3. Live/dead staining
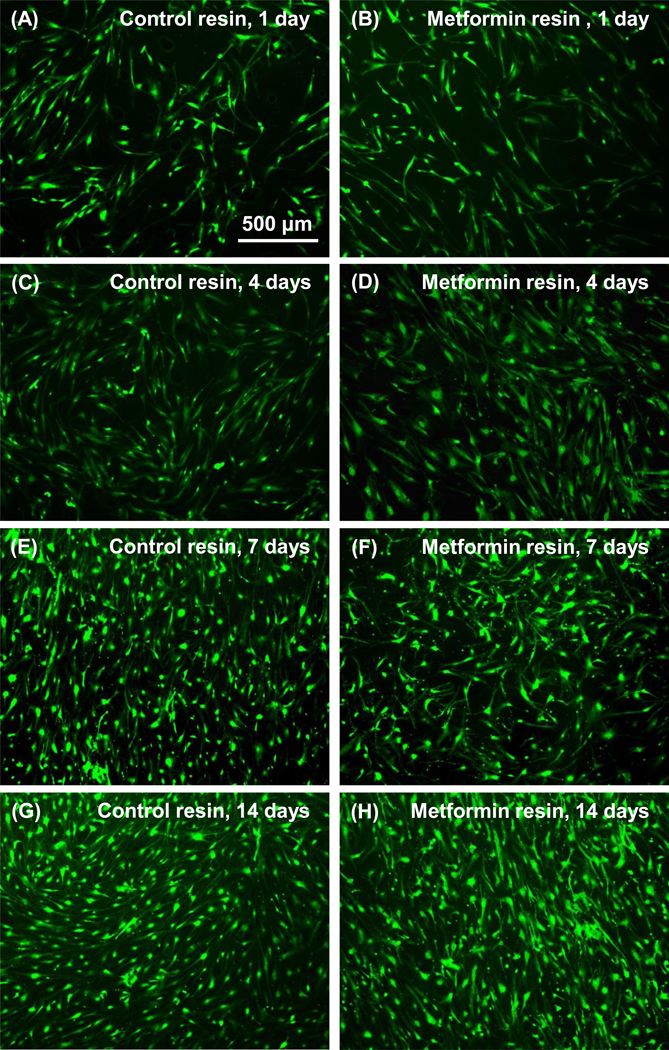
The DPSCs are relatively uniformly attached to resin in both control and metformin groups (Fig. 3). Live cells are numerous on the resin surfaces, with very few scattered red dots in the pictures. The live cell numbers increased steadily with increasing time for both groups. These results demonstrate that the resins with or without metformin both had similarly good cell compatibility, enabled cell attachment, and supported cell proliferation.
Figure 3.
Representative live/dead cell fluorescence staining images of DPSCs on resins with or without metformin. There was no noticeable difference between the two groups.
3.4. Gene expressions of DPSCs on resins
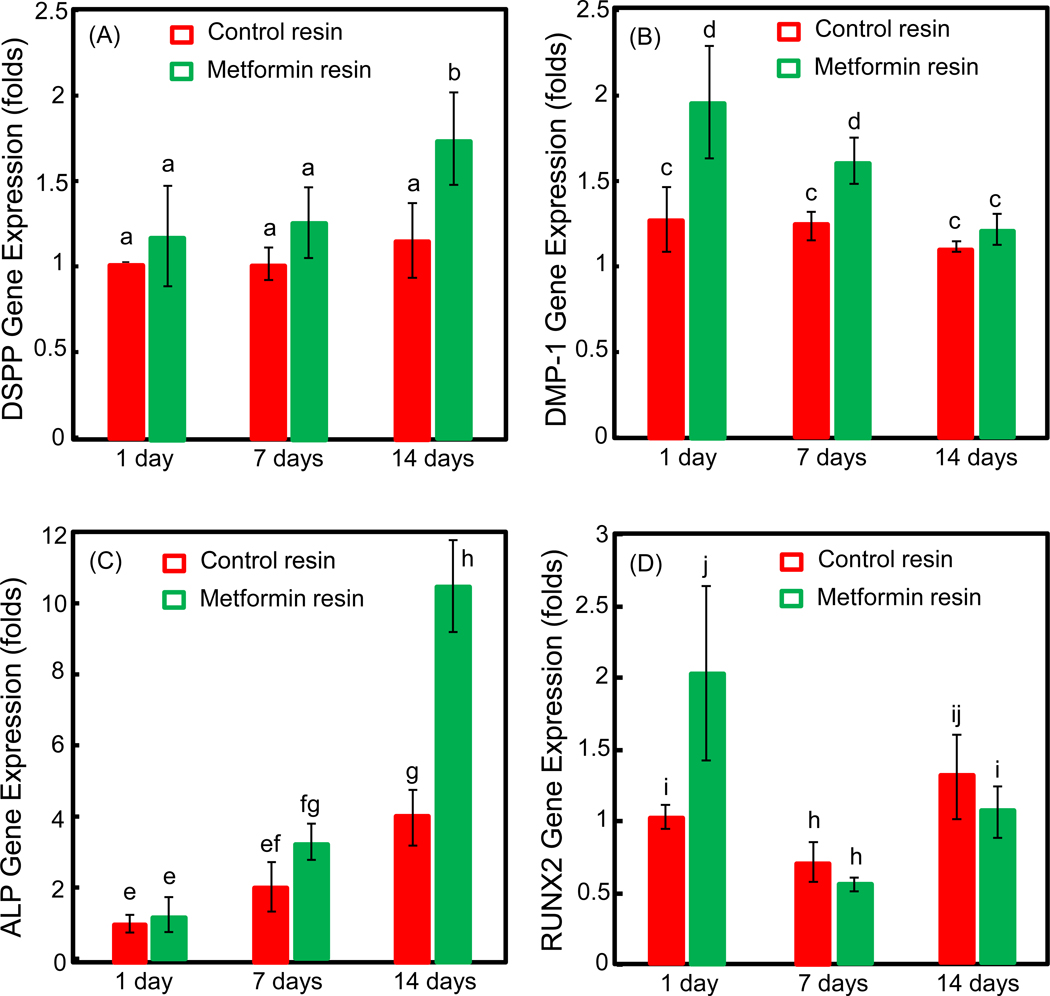
The expressions of odontoblast-related genes (DSPP and DMP-1) and mineralization-related genes (ALP and Runx2) are shown in Fig. 4 (mean ± SD; n = 6). Two-way ANOVA showed significant effects of culture time (p < 0.05) and different groups (p < 0.05), with a significant interaction between the two variables (p < 0.05). DSPP and ALP expressions were higher at day 7 and day 14, compared to control group (p < 0.05). The expression of DMP-1 gene was significantly enhanced by the addition of metformin in resin at day 1 and day 7 (p < 0.05). These qPCR results demonstrate that metformin in resin up-regulated the odontoblast- and mineralization-related genes of DPSCs on resin surfaces.
Figure 4.
qPCR results for odontoblast differentiation of DPSCs on resins with or without metformin (mean ± sd; n = 6). The gene expressions of DSPP (A), DMP-1 (B), ALP (C) and Runx2 (D) were analyzed at 1, 7 and 14 days. Compared to control resin, there was significant up-regulation of odontoblast-related genes: DSPP (A) at day 7 and day 14, and DMP-1(B) at day 1 and day 7. Mineral-related gene expression of ALP (C) at 7 and 14 days were also higher than that of DPSCs on control resin (p < 0.05). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
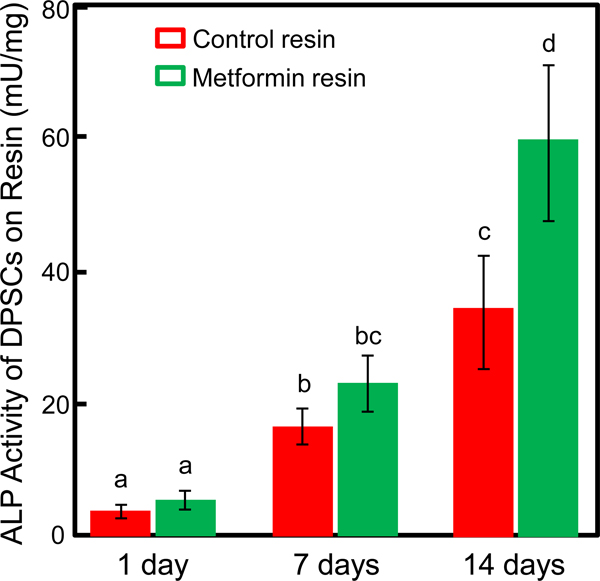
3.5. Alkaline phosphatase (ALP) activity
The ALP activity of DPSCs in metformin group and control group both increased with time, as the osteogenic medium was used for both groups (Fig. 5). Two-way ANOVA showed significant effects of culture time (p < 0.05) and different groups (p < 0.05), with a significant interaction between the two variables (p < 0.05). Remarkable differences were observed between the two groups at 7 and 14 days, with significantly higher ALP activity for metformin group than control group (p < 0.05). At 14 days, the ALP activity of the metformin group was 30 folds that at 1 day (p < 0.05). The ALP activity of the metformin group was and 70% higher than that of control at 14 days (p < 0.05).
Figure 5.
ALP activity of DPSCs on resins with or without metformin (mean ± sd; n = 6). Greater ALP activity values in metformin group were obtained than those of control group at day 14 and day 21 (p < 0.05). Values with dissimilar letters are significantly different from each other (p < 0.05).
3.6. Alizarin red staining
Representative ARS images of DPSC mineral synthesis are shown in Fig. 6A. DPSCs formed abundant mineral nodules, which were stained red. The resin disks at 1 day were stained red because of the non-specific exogenous coloring from the ARS dye; however, no mineral nodules appeared at 1 day. For resin disks with metformin, mineral nodules started to appear at 7 days and increased at 14 days; these mineral nodules showed as particle deposits on the resin which made the surface rough. With increasing time, by day 14, the disks were covered by a layer of new mineralized matrix synthesized by DPSCs, which became thicker and more abundant at day 21. In contrast, there were much less mineral nodules on control resin disks. Fig. 6B plots the quantitative mineral synthesis results (mean ± SD; n = 6). Two-way ANOVA showed significant effects of culture time (p < 0.05) and different groups (p < 0.05), with a significant interaction between the two variables (p < 0.05). Mineral synthesis amount by DPSCs on metformin-resin disks greatly increased with culture time. The mineral synthesis value of metformin group at day 14 was nearly 5 folds that without metformin (p < 0.05). At 21 days, mineral of metformin group was 9 folds that without metformin (p < 0.05).
Figure 6.
Alizarin red staining (ARS) of mineral synthesis by DPSCs on resins with or without metformin. (A) Representative ARS staining images of DPSC-seeded resin disks. (B) DPSC mineral synthesis quantification (mean ± sd; n = 6). The amount of mineral synthesis by DPSCs on metformin resin was greatly increased with culture time, and at day 14 and day 21 was much more than that on control resin (p < 0.05). Values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
The present study incorporated metformin into a dental resin for the first time, which greatly enhanced odontoblastic differentiation of DPSCs and increased their mineral synthesis, with potential applications to stimulate pulp cells for new dentin synthesis and bridge formation. There was no cytotoxicity from the metformin-containing resin, with the proliferation of DPSCs similar to that without metformin. Upregulation of odontoblastic gene expressions as well as mineralization-related gene expressions of DPSCs were higher on metformin resin than control resin. In addition, ALP protein synthesis by DPSCs on metformin resin was substantially enhanced. Mineral formation by DPSCs on metformin resin was 7–9 folds of that on resin without metformin. Therefore, novel metformin-containing base and liner resins are promising for deep cavities and pulp-capping applications to greatly increase tertiary dentin formation and promote pulpal repair to protect the dental pulp.
Stem cell research has been one of the most promising fields of biology due to its promising therapeutic implications [47]. Adult MSCs derived from dental tissues, including dental pulp and periodontal ligament could be used for dental tissue engineering and regenerative medicine applications [48,49]. Among these cells, DPSCs are able to differentiate into various cell types, including osteoblasts, adipocytes, and chondrocytes, which could be sources for cell-based therapy to regenerate pulp/dentin tissues [50]. Several studies of pulp regeneration using DPSCs have reported positive results [51,52]. For example, in vivo study demonstrated the ability of DPSCs to generate functional dental tissue in forms of dentine/pulp-like complexes [53]. Even DPSCs from inflamed pulps maintained the capability of tissue regeneration [42]. Therefore, DPSC-mediated dental pulp regeneration is considered a promising method for the treatment of deep caries with pulpitis. Indeed, the present study showed DPSCs growing on resins and synthesizing minerals indicating the promise of DPSCs in dental tissue regeneration.
For deep cavities and cavities with exposed pulps, it is beneficial for the resin to carry bioactive agents to be released into the pulp to enhance DPSCs for repair and regeneration. Dental liner or base materials that are placed between a restoration and the exposed dentin within a deep cavity following removal of the carious lesion can serve this purpose. Their primary functions include dentinal sealing, pulpal protection, thermal insulation, and stimulation of the formation of secondary (tertiary) dentin formation [54]. The ideal liner/base material should possess several chemical and mechanical properties, including adhesion to the dentin substrate, antibacterial activity, and copolymerization with the restorative resin-based material [55,56]. Liners and bases are available in different forms, compositions and functions, including calcium hydroxide, zinc oxide eugenol, resin-modified glass ionomer and resins [57,58]. In the clinical situation, the liners and bases in deep cavities are covered under a composite, without direct exposure to drinks and oral saliva. Therefore, the amount of metformin released from the resin and entered the pulp may be less than that released into the culture medium with the resin being fully immersed, as in the present study. In such a clinical situation, the metformin release may be slower and lasting a longer time. Further study is needed to investigate metformin release into the pulp from a liner in a clinically relevant manner. In previous studies on liner and base materials, remineralizing resin-based Ca-PO4 cement was made as a restoration-supporting liner/base material and could remineralize carious dentin tissues [59]. Amorphous calcium phosphate (ACP)-containing composite, with the ability to release Ca and PO4 ions, was shown to have mechanical properties suitable for use as base and lining materials [60]. While there has been no report of these materials’ interactions with DPSCs, the biocompatibility of bases/liners has been investigated. One report showed that resin-modified glass ionomer cement used in deep caries cavities caused a mild initial pulp damage, which subsided over time, thus indicating an acceptable biocompatibility [61]. Another study evaluated the biocompatibility of resin-based materials applied as liners in deep cavities prepared in human teeth in vivo, showing that resin-based cements Vitrebond™ (3M ESPE) and Ultra-Blend Plus (Untradent) had acceptable biocompatibility when applied in deep cavities [55]. However, a bioactive base/liner that can substantially promote DPSC regeneration capabilities is needed. To date, there has been no report of metformin applications in dental resins.
In the present study, the metformin in resin displayed a continuous release for about a week. This duration appeared to be sufficient to substantially increase the DPSC differentiation. However, further study is needed to develop a method to prolong the metformin release from resin, and potentially to further enhance the DPSC differentiation. The addition of metformin into resin did not disturb DPSC growth and proliferation, displaying a good biocompatibility. Moreover, both odontoblastic and osteoblastic differentiations of the DPSCs were greatly enhanced by the metformin resin. Therefore, the results indicated that the novel metformin-containing resin had an excellent biocompatibility.
Metformin is a commonly prescribed antidiabetic drug [62]. However, this biguanide compound is also finding new and wider applications in other fields; therefore, its potential repurposing independently of its glucose-lowering effects is very attractive. For example, in vitro and in vivo studies demonstrated that metformin could stimulate osteoblast differentiation of MSCs [21,24], which could be applied in tissue regeneration. Recent clinical evidence indicated that locally-delivered metformin significantly improved the clinical and radiological parameters of chronic periodontitis [63–66]. As an AMPK pathway activator that plays a role in glucose energy metabolism and cell protection, the mechanism of action for metformin on MSCs was related to its role on AMPK activation [66]. Similarly, a recent study showed that metformin enhanced the odontoblastic differentiation of DPSCs by activating AMPK signaling [30]. Another study investigated four small bioactive compounds (phenamil, purmorphamine, genistein, and metformin) that induced reparative dentin formation by enhancing the odontogenic differentiation and mineralization of DPSCs [67]. However, literature and patent searches revealed no report on incorporating metformin in dental resin to be filled in tooth cavity restorations to stimulate DPSCs. Hence, the present study indicated a new potential application of metformin in dentistry.
The present study showed that the metformin-incorporated resin could deliver metformin to be locally released to stimulate DPSCs. The results are consistent with a previous study using free metformin without using resin as the delivery vehicle for dental application; that study reported significant enhancement on cell differentiation and mineralization [30,67]. Regarding the odontoblast- and mineralization-related gene expressions, the present study showed some genes were elevated in the early stage, while others were elevated in the later stage, of the odontoblastic differentiation and mineral synthesis process. For example, DMP-1 and RUNX2 gene expressions were elevated by metformin at 1 day. DSPP and ALP genes were promoted by metformin at 14 days (Fig. 4). ALP protein synthesis (Fig. 5) was also highly promoted by metformin at 14 days, consistent with this usually occurring in the later stage of the cell differentiation process. Mineral synthesis (Fig. 6B) was moderately promoted at 14 days, and highly promoted at 21 days, by metformin. This is also consistent with mineral synthesis happening near the final stage of the cell differentiation process.
The group with 20% metformin and 55% glass filler level is expected to be slightly weaker mechanically than the control group with 75% glass filler level. In deep cavities, liners and bases are often used beneath a restoration. The metformin-containing resin could be used as a base or liner for deep cavities and perforated cavities to release metformin into the pulp to stimulate DPSCs for tertiary dentin formation. The liner or base is not exposed on the occlusal surface and, instead, is covered by a mechanically-strong restoration such as a composite which supports the chewing forces. Therefore, mechanical properties are not critically important for liners and bases. In addition, the material with 55% glass fillers still expected to be relatively strong mechanically. Further study is needed to optimize the metformin and glass filler levels and determine the mechanical and physical properties.
Incorporating metformin into resins to be placed in tooth restorations could have several advantages over administering metformin systemically or delivering metformin without a resin carrier. First, the metformin-containing resin could enable metformin to be locally-delivered to avoid systemic effects where it is not needed. Second, the resin could slowly release metformin to diffuse into the pulp to have a sustained stimulation on the pulpal cells. Third, for perforated pulp chambers where the resin surface directly covers the exposed pulp, the metformin-containing resin could allow cell attachment, proliferation and differentiation to synthesize mineralized new dentin tissues on the resin, to facilitate dentin bridge formation. Therefore, novel metformin-containing base and liner resins for deep cavities and pulp-capping applications are promising to greatly increase tertiary dentin formation and promote pulpal repair and protection. Forth, metformin-containing resins could have other potential applications, including tooth root coating with an adhesive resin containing metformin for patients with periodontal diseases and alveolar bone resorption. The resin would then release metformin into the periodontal area to stimulate bone and cementum regeneration by the local stem cells. Fifth, after root canal treatment in patients with apical lesions and bone resorption caused by endodontic infections, a metformin-containing resin could be used to fill the root canal, to release metformin into the apical region to stimulate the host cells for bone regeneration to heal the apical areas and support the tooth. Sixth, it could be feasible to add antibacterial monomers into the metformin-containing resin, such as dimethylaminohexadecyl methacrylate (DMAHDM) to co-polymerize with the resin, to kill bacteria and combat infections [68,69]. Bacterial inhibition is critically important for base/liner/pulp-capping uses, periodontal applications, and endodontic treatments. Seventh, remineralization agents such as nanoparticles of amorphous calcium phosphate (NACP) [69,70] could be potentially incorporated into the metformin-containing resins to enhance hard tissue regeneration.
Metformin is widely used by millions of diabetic patients as the first-line treatment for T2DM. Several studies reported that metformin had osteogenic effects by promoting the differentiation of stem cells, such as MSCs and DPSCs [21–23,25,26,71]. In addition, clinical studies indicated that locally-delivered metformin appeared to improve the clinical and radiographic parameters of chronic periodontitis [63–65,72,73]. However, to take metformin orally for a systemic treatment for the purpose of dentin regeneration, it would be difficult for the metformin to reach the DPSCs residing inside the tooth pulp enclosed by tooth structures. Therefore, delivering metformin locally, by adding it into a liner or a base for perforated pulps or deep cavities, would facilitate the metformin to reach the DPSCs thereby enhancing dentin synthesis by DPSCs. Regarding any side effects of metformin, the liner or base is clinically used under a composite without direct exposure to the oral saliva. Even though the released metformin would reach the DPSCs in the dental pulp, because this is a local delivery with a very small quantity into the enclosed tooth pulp, the final metformin concentration that reaches the systemic circulation would be extremely small and negligible. Furthermore, as the widely-used first-line diabetic treatment drug, metformin is known to be safe, non-toxic and well-tolerated. In addition, it should be noted that the present study has several limitations. For example, the mechanistic pathway of metformin in DPSCs was not investigated, and a systematic study on the effects of metformin mass fraction in the resin was not performed. Furthermore, the present study did not evaluate the combination of antibacterial agent or amorphous calcium phosphate together with metformin for multifunctional and synergistic effects. In addition, other dental applications of metformin, such as stimulating the periodontal regeneration, and enhancing the repair of apical bone lesions, remain to be evaluated.
5. Conclusion
This study developed a novel metformin-containing resin and determined its substantial enhancements on odontoblastic differentiation of DPSCs and their secretion of ALP protein and synthesis of mineral for the first time. The metformin-containing resin did not compromise the DPSC attachment and cell viability compared to that without metformin, and it greatly promoted the odontogenic gene expressions and alkaline phosphatase activity of DPSCs growing on the resin. DPSCs on the metformin-containing resin synthesized mineral that was 7–9 folds that without metformin. Therefore, the novel metformin-containing resins are promising for a wide range of dental applications, including filing in deep cavities and cavities with perforated pulps to stimulate DPSCs to synthesize new dentin and protect the pulp.
Acknowledgments
We are gratefully to Drs. Bing Song, Wei Qin and Laurence C. Chow for discussions and experimental help. This work was supported by University of Maryland School of Dentistry Bridging Fund (HX) and University of Maryland Seed Grant (HX), National Institutes of Health/National Institute of Dental and Craniofacial Research Grant R01 DE023578 (AS), and International Science and Technology Cooperation Program of Sichuan Province 2017HH0008 (LC).
Footnotes
Conflict of interest
There is no conflict of interest.
Reference
- 1.Farges JC, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, et al. Dental pulp defence and repair mechanisms in dental caries. Mediators Inflamm. 2015;2015:230251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27(1):29–38. [DOI] [PubMed] [Google Scholar]
- 3.Lynch CD, McConnell RJ, Wilson NH. Posterior composites: the future for restoring posterior teeth? Prim Dent J. 2014;3(2):49–53. [DOI] [PubMed] [Google Scholar]
- 4.Blum IR, Lynch CD, Wilson NH. Factors influencing repair of dental restorations with resin composite. Clin Cosmet Investig Dent. 2014;17(6):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19(6):449–57. [DOI] [PubMed] [Google Scholar]
- 6.Maas MS, Alania Y, Natale LC, Rodrigues MC, Watts DC, Braga RR. Trends in restorative composites research: what is in the future? Braz Oral Res. 2017;31(suppl 1): e55. [DOI] [PubMed] [Google Scholar]
- 7.Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent Mater. 2008;24(10):1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mickenautsch S, Yengopal V, Banerjee A. Pulp response to resin-modified glass ionomer and calcium hydroxide cements in deep cavities: A quantitative systematic review. Dent Mater. 2010;26(8):61–70 [DOI] [PubMed] [Google Scholar]
- 9.Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. Effect of biomaterials on angiogenesis during vital pulp therapy. Dent Mater J. 2016; 35(5):701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McComb D, Ericson D. Antimicrobial action of new, proprietary lining cements. J Dent Res. 1987; 66(5):1025–8. [DOI] [PubMed] [Google Scholar]
- 11.Davis HB, Gwinner F, Mitchell JC, Ferracane JL.Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater. 2014; 30(10):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.About I, Murray PE, Franquin JC, Remusat M, Smith AJ. The effect of cavity restoration variables on odontoblast cell numbers and dental repair. J Dent. 2001; 29(2):109–17. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Yelick PC. Vital pulp therapy-current progress of dental pulp regeneration and revascularization. Int J Dent. 2010;2010:856087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima N, Okiji T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom(Kyoto). 2016;56(4):144–53. [DOI] [PubMed] [Google Scholar]
- 15.Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Human odontoblast cell numbers after dental injury. J Dent. 2000; 28(4):277–85. [DOI] [PubMed] [Google Scholar]
- 16.Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. 2002;15(1):41–6. [PubMed] [Google Scholar]
- 17.Umemura N, Ohkoshi E, Tajima M, Kikuchi H, Katayama T, Sakagami H. Hyaluronan induces odontoblastic differentiation of dental pulp stem cells via CD44. Stem Cell Res. 2016;7(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tecles O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005; 50(2):103–8. [DOI] [PubMed] [Google Scholar]
- 19.Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012; 122(6):253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7): 850–8. [DOI] [PubMed] [Google Scholar]
- 21.Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48(4):885–93. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Xue J, Li X, Jia Y, Hu J. Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol. 2008;60(12): 1695–700. [DOI] [PubMed] [Google Scholar]
- 23.Cortizo AM, Sedlinsky C, McCarthy AD, Blanco A, Schurman L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol. 2006;536(1–2):38–46. [DOI] [PubMed] [Google Scholar]
- 24.Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, et al. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25(2):211–21. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun. 2008;375(3):414–9. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Ma T, Guo D, Hu K, Shu Y, Xu HH, et al. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2017;12(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25): 13625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan M, Yu Y, Zhang G, Tang C, Yu J. A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev. 2011;7(1):161–71. [DOI] [PubMed] [Google Scholar]
- 29.Kolar MK, Itte VN, Kingham PJ. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7(1):12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin W, Gao X, Ma T, Weir MD, Zou J, Song B, et al. Metformin enhances the differentiation of dental pulp stem cells into odontoblasts in vitro by activating AMPK signaling. J Endodontics. 2017; doi: 10.1016/j.joen.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Weir MD, Hack G, Fouad AF, Xu HH. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release. J Dent. 2015; 43(12):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Weir MD, Chow LC, Antonucci JM, Chen J, Xu HH. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 2016;32(2):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venz S, Dickens B. Modified surface-active monomers for adhesive bonding to dentin. J Dent Res. 1993;72(3):582–6. [DOI] [PubMed] [Google Scholar]
- 34.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dent Mater. 2011;27:997–1002. [DOI] [PubMed] [Google Scholar]
- 35.Tauscher S, Angermann J, Catel Y, Moszner N. Evaluation of alternative monomers to HEMA for dental applications. Dent Mater. 2017;33(7): 857–65. [DOI] [PubMed] [Google Scholar]
- 36.Imazato S, McCabe JF. Influence of incorporation of antibacterial monomer on curing behavior of a dental composite. J Dent Res. 1994;73:1641–5. [DOI] [PubMed] [Google Scholar]
- 37.Atai M, Ahmadi M, Babanzadeh S, Watts DC. Synthesis, characterization, shrinkage and curing kinetics of a new low-shrinkage urethane dimethacrylate monomer for dental applications. Dent Mater. 2007;23(8):1030–41. [DOI] [PubMed] [Google Scholar]
- 38.Braga RR, Ballester RY, Ferracane JL.Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21(10):962–70. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Wang X, Vernikovskaya DI, Fokina VM, Nanovskaya TN, Hankins GDV, et al. Quantitative determination of metformin, glyburide and its metabolites in plasma and urine of pregnant patients by LC-MS/MS. Biomed Chromatogr. 2015;29(4):560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Zhang C, Li C, Weir MD, Wang P, Reynolds MA, et al. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater Sci Eng C Mater Biol Appl. 2016; 69:1125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu LN, Watson D, Thames K, Primus CM, Bergeron BE, Jiao K, et al. Effects of a discoloration-resistant calcium aluminosilicate cement on the viability and proliferation of undifferentiated human dental pulp stem cells. Sci Rep. 2015;5: 17177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5(4):617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eid AA, Hussein KA, Niu LN, Li GH, Watanabe I, Mohamed Al-Shabrawey M, et al. Effects of tricalcium silicate cements on osteogenic differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Acta Biomater. 2014; 10(7):3327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang GQ, Wimpenny I, Dey RE, Zhong X, Youle PJ, Downes S, et al. The unique calcium chelation property of poly(vinyl phosphonic acid-co-acrylic acid) and effects on osteogenesis in vitro. J Biomed Mater Res A. 2018;106(1):168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayobian-Markazi N, Fourootan T, Kharazifar MJ. Comparison of cell viability and morphology of a human osteoblast-like cell line (SaOS-2) seeded on various bone substitute materials: An in vitro study. Dent Res J. 2012;9(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Zhou H, Weir MD, Bao C, Xu HH. Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012;8(6):2297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen AH, Yelick PC. Dental tissue regeneration-a mini review. Gerontology. 2011; 57(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015; 9(11):1205–16. [DOI] [PubMed] [Google Scholar]
- 49.Yi Q, Liu O, Yan F, Lin X, Diao S, Wang L, et al. Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs. 2017;203(1):1–11. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramirez MD, Meseguer L, et al. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal. 2012;17(6):e1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mooney DJ, Powell C, Piana J, Rutherford B. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12(6):865–8. [DOI] [PubMed] [Google Scholar]
- 52.Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20(12):715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. [DOI] [PubMed] [Google Scholar]
- 54.Weiner R. Liners and bases in general dentistry. Aust Dent J. 2011;56 Suppl 1:11–22. [DOI] [PubMed] [Google Scholar]
- 55.de Souza Costa CA, Teixeira HM, Lopes do Nascimento AB, Hebling J. Biocompatibility of resin-based dental materials applied as liners in deep cavities prepared in human teeth. J Biomed Mater Res B Appl Biomater. 2007;81(1):175–84. [DOI] [PubMed] [Google Scholar]
- 56.Imazato S, Chen JH, Ma S, Izutani N, Li F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn Dent Sci Rev. 2012;48(2):115–25. [Google Scholar]
- 57.Duque C, Negrini Tde C, Sacono NT, Spolidorio DM, de Souza Costa CA, Hebling J, Clinical and microbiological performance of resin-modified glass-ionomer liners after incomplete dentine caries removal. Clin Oral Investig. 2009;13(4):465–71. [DOI] [PubMed] [Google Scholar]
- 58.Mitra SB. In vitro fluoride release from a light-cured glass-ionomer liner/base. J Dent Res. 1991;70(1):75–8. [DOI] [PubMed] [Google Scholar]
- 59.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19(6):558–66. [DOI] [PubMed] [Google Scholar]
- 60.Park MS, Eanes ED, Antonucci JM, Skrtic D. Mechanical properties of bioactive amorphous calcium phosphate/methacrylate composites. Dent Mater. 1998; 14(2):137–41. [DOI] [PubMed] [Google Scholar]
- 61.Costa CA, Ribeiro AP, Giro EM, Randall RC, Hebling J. Pulp response after application of two resin modified glass ionomer cements (RMGICs) in deep cavities of prepared human teeth. Dent Mater. 2011;27(7): e158–70. [DOI] [PubMed] [Google Scholar]
- 62.Hajjar J, Habra MA, Naing A. Metformin: an old drug with new potential. Expert Opin Investig Drugs. 2013;22(12):1511–7. [DOI] [PubMed] [Google Scholar]
- 63.Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2015;86(6): 729–37. [DOI] [PubMed] [Google Scholar]
- 64.Pradeep AR, Patnaik K, Nagpal K, Karvekar S, Guruprasad CN, Kumaraswamy KM. Efficacy of 1% metformin gel in patients with moderate and severe chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2017; 88(10):1023–9. [DOI] [PubMed] [Google Scholar]
- 65.Pradeep AR, Patnaik K, Nagpal K, Karvekar S, Ramamurthy BL, Naik SB, et al. Efficacy of locally-delivered 1% metformin gel in the treatment of intrabony defects in patients with chronic periodontitis: a randomized, controlled clinical trial. J Investig Clin Dent. 2016;7(3):239–45. [DOI] [PubMed] [Google Scholar]
- 66.Ahn MJ, Cho GW. Metformin promotes neuronal differentiation and neurite outgrowth through AMPK activation in human bone marrow-mesenchymal stem cells. Biotechnol Appl Biochem. 2017;64(6):836–42. [DOI] [PubMed] [Google Scholar]
- 67.Phung S, Lee C, Hong C, Song M, Yi JK, Stevenson RG, et al. Effects of bioactive compounds on odontogenic differentiation and mineralization. J Dent Res. 2017; 96(1):107–15. [DOI] [PubMed] [Google Scholar]
- 68.Zhang N, Ma J, Melo MA, Weir MD, Bai Y, Xu HH. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent. 2015;43(2):225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng L, Zhang K, Zhang N, Melo MA, Weir MD, Zhou XD, et al. Developing a new generation of antimicrobial and bioactive dental resins. J Dent Res. 2017;96(8):855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreau JL, Weir MD, Giuseppetti AA, Chow LC, Antonucci JM, Xu HH. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J Biomed Mater Res B Appl Biomater. 2012;100(5):1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Houshmand B, Tabibzadeh Z, Motamedian SR, Kouhestani F. Effect of metformin on dental pulp stem cells attachment, proliferation and differentiation cultured on biphasic bone substitutes. Arch Oral Biol. 2018;95:44–50. [DOI] [PubMed] [Google Scholar]
- 72.Rao NS, Pradeep AR, Kumari M, Naik SB. Locally delivered 1% metformin gel in the treatment of smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2013;84(8):1165–71. [DOI] [PubMed] [Google Scholar]
- 73.Pradeep AR, Rao NS, Naik SB, Kumari M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2015;84(2):212–20. [DOI] [PubMed] [Google Scholar]