Abstract
Background.
Neurobiological differences linked to socioemotional and cognitive processing are well-documented in youth with disruptive behavior disorders (DBDs), especially youth with callous-unemotional (CU) traits. The current study expanded this literature by examining gray matter volume (GMV) differences among DBD youth with CU traits (DBDCU+), without CU traits (DBD-only), and typically developing (TD) youth.
Methods.
Data were from the first full sample release of the Adolescent Brain and Cognitive Development Study (M, age=9.49; 49% female). We tested whether GMVs of 11 regions of interest selected a priori (differentiated between our three groups: DBDCU+ (n=288), DBD-only (n=362), and TD (n=915). Models accounted for demographic confounders, ADHD, and intracranial volume. We examined two potential moderators of the relationship between GMVs and group membership: sex and clinically-significant anxiety (i.e., primary vs. secondary CU traits subtype).
Results.
DBDCU+ youth had lower right amygdala GMV and DBD-only youth had lower bilateral amygdala GMV relative to TD youth. DBDCU+ youth had lower bilateral hippocampal GMV and DBD-only youth had lower left hippocampal GMV relative to TD youth. DBDCU+ youth evidenced lower left insula GMV relative to TD youth. Finally, DBD-only youth had lower left superior frontal gyrus and lower right caudal anterior cingulate cortex GMV relative to TD youth. There was no moderation of associations between GMV and group membership by sex.
Conclusions.
Our findings implicate structural aberrations in both the amygdala and hippocampus in the etiology of DBDs, with minimal evidence for differences based on the presence or absence of CU traits.
Keywords: ABCD, amygdala, antisocial behavior, callous-unemotional traits, gray matter volume, hippocampus
Introduction
Childhood disruptive behavior disorder (DBD) problems, including aggression, rulebreaking, and violence cast a long shadow, predicting risk for persistent antisocial behavior, substance abuse, depression, and crime across the lifespan (1, 2). Clinically-significant DBDs are diagnosed as oppositional defiant disorder (ODD; irritability, anger, and defiant behavior) and conduct disorder (CD; behavior that violates the rights of others or age-appropriate norms or rules) (3). Prevalence estimates for CD and ODD range from 3% to 12% (4), making them common, with boys more likely to be affected than girls (5, 6). The financial implications of DBDs are significant, particularly in relation to greater use of educational, health, and psychosocial services (1), as well as the impact on crime (7). Furthermore, even best-practice psychosocial interventions bring about only modest reductions in DBDs (8). Thus, DBDs constitute a major public health concern owing to their prevalence, poor prognosis, and associated costs.
The presence of callous-unemotional (CU) traits appears to identify a qualitatively distinct subgroup of children with DBDs, who may require personalized treatment strategies (9). CU traits refer to low empathy and guilt, reduced emotional sensitivity to others, and apathy towards rules and school (10). Across development and beginning as young as age 3, CU traits predict severe and stable DBD symptoms (10, 11). Moreover, children with DBDs and high CU traits (DBDCU+) appear to show distinct etiological risk factors relative to children with DBD symptoms only (DBD-only) (10, 12). A promising avenue of research has explored neurobiological differences in brain regions linked to the socioemotional and cognitive processes that are impaired among children with DBDs and CU traits. This research is founded on several prominent neurobiological models, including the Violence Inhibition Mechanism model, which proposes that psychopathy (and, by extension, CU traits) arises from disruption to the learning systems involved in pairing emotionally aversive stimuli (e.g., distress of others) with outcomes (13-16). Empirical studies have thus examined the functioning of amygdala, which is implicated in emotion processing and fear conditioning (17), the insula and anterior cingulate cortex (ACC), linked with empathy (18), and the orbitofrontal cortex (OFC), associated with emotion regulation (19) in relation to DBDs and CU traits. The results of functional magnetic resonance imaging (fMRI) studies, particularly for DBDCU+ youth, implicate aberrant functioning of the amygdala (20, 21), insula (22, 23), and ACC (24) reactivity during emotion processing tasks and in OFC reactivity during contingency-based learning tasks relative to typically developing (TD) youth (25, 26, also see 27, and 28 for reviews).
To determine whether morphological differences underpin the functional brain and behavioral impairments associated with DBD and CU traits, studies have also explored regional gray-matter volumes (GMV) using structural magnetic resonance imaging (sMRI). An image-based meta-analysis of 13 studies established that relative to TD youth, children with DBDs exhibit reduced GMV in the amygdala, insula, OFC, superior frontal gyrus (SFG), and ACC (29). However, only 5 of the 13 studies included in that meta-analysis considered CU traits (29). Thus, it is plausible that unmeasured CU traits in past studies could contribute to the observed GMV differences between children with DBDs versus TD. Indeed, several studies published more recently than the meta-analysis offer a more complicated picture. For example, in a study of 134 adolescent arrestees, no associations were found between CU traits and CD symptoms and amygdala, insula, and OFC GMV, although CU traits were related to lower insula and amygdala gray matter concentration (30). In contrast, among youth recruited from the community including a subsample (n=77) with clinically-significant externalizing problems, reduced amygdala GMV was associated with higher CU traits (31). This mixed pattern of findings imply the need for further investigation of the GMV profiles of children based on DBD symptoms and/or CU traits. In addition, most studies of GMV and DBDs have focused on adolescents or samples with wide age ranges. Given that a host of physical, social, and neural changes relevant to CP and GMV development are heralded by the onset of puberty (32), an examination of GMV in late-childhood could provide insight into which children are most at risk for severe and persistent DBD symptoms and CU traits prior to the onset of puberty.
In the current study, we sought to advance what is known about GMV differences, DBDs, and CU traits using data from the first full baseline release of the landmark Adolescent Brain and Cognitive Development (ABCD) Study, a longitudinal investigation of brain development and child health beginning at ages 9-10 years of age. Using the ABCD sample, we generated stringently-defined diagnostic subgroups of children classified based on DBD symptoms and CU traits (DBDCU+, DBD-only, and TD youth) that were larger in size than any other prior studies. The richness of the ABCD data also allowed us to account for salient demographic and psychiatric confounders to isolate whether differences in GMVs differentiated between youth on the basis of DBD symptoms of CU traits. First, we included age in models given that increases in GMV continue up to around age 12 (33). Second, we accounted for race and ethnicity to address the need for studies to explore brain-behavior associations in racially diverse samples (34) and because there is evidence that some “well-established” findings, including the link between amygdala hyporeactivity and CU traits do not replicate in African-American (35) and Hispanic (36) samples. Third, in light of evidence for structural and functional brain differences among children growing up in adverse contexts (37, 38), we accounted for parental education as a proxy for socioeconomic status. Fourth, we included child IQ scores given evidence for neuropsychological deficits among youth with DBDs (39). Fifth, we included sex as a covariate since males show higher rates of DBDs (6). Moreover, we also examined whether sex moderated associations between GMV and DBD groups since prior studies have identified sex-specific associations between brain structure and CU traits and DBD symptoms (40, 41). Sixth, to account for potential confounding effects of ADHD, which has been linked both to DBD severity (42) and specific brain morphological differences (43), we included ADHD diagnosis as a covariate. Finally, a burgeoning area of research indicates that DBDCU+ youth can be disambiguated further based on internalizing psychopathology. Specifically, research indicates heterogeneity among youth high on CU traits based on negative emotionality and heightened anxiety (“secondary CU traits”) versus dampened emotional reactivity and low levels of anxiety (“primary CU traits”) (44-47). As an exploratory analysis, we also therefore examined anxiety moderated associations between GMV and DBDCU+ group membership.
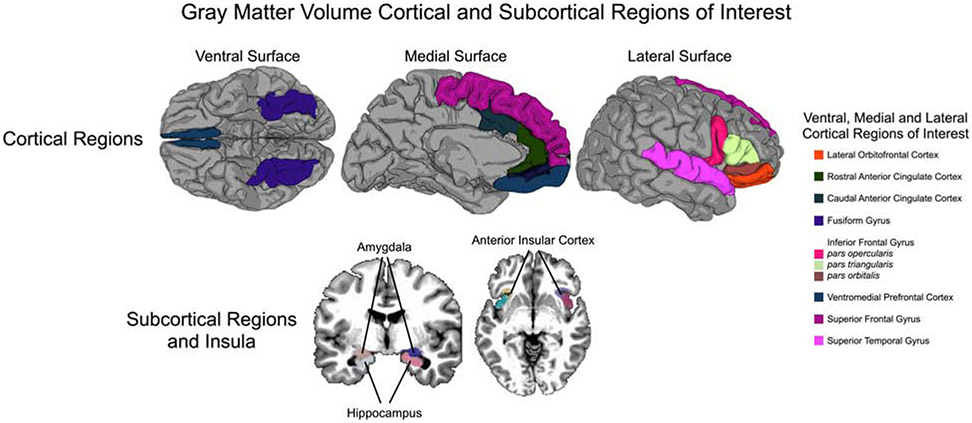
In the current study, we focused on cortical and subcortical regions of interest (ROIs) identified a priori if they featured in prominent theoretical models of the development of DBDs, CU traits, and psychopathy (13, 15, 16, 27), were reported as differing between DBD versus TD youth in an earlier image-based meta-analysis (29) and other recent studies (30, 31), or were available within the ABCD study (48). Accordingly, we explored GMVs of the following ROIs: amygdala, anterior cingulate cortex (caudal; ACC-C), anterior cingulate cortex (rostral; ACC-R), fusiform gyrus, hippocampus, inferior frontal gyrus (IFG), insula, orbitofrontal cortex (OFC), superior frontal gyrus (SFG), superior temporal gyrus (STG), and ventromedial prefrontal cortex (vmPFC) (see Figure 1). We hypothesized that relative to TD youth, youth with DBDs (i.e., with and without CU traits) would have significantly reduced GMV across these ROIs, but that GMV reductions would be particularly pronounced among DBDCU+ youth, especially youth with low anxiety (i.e., primary CU traits). We hypothesized that these effects would remain after accounting for the demographic and psychiatric confounders as detailed above.
Figure 1.
Gray Matter Volume Cortical and Subcortical Regions of Interest
Methods
Participants and procedures
Participants in this study are enrolled in the ongoing longitudinal ABCD Study (https://abcdstudy.org/) and included in the annual 2.0 data release (https://data-archive.nimh.nih.gov/abcd). The ABCD study recruited 11,874 healthy children, aged 9 to 10-years-old (mean age = 9.49 years) from across the United States (48% female; 57% Caucasian, 15% African American, 20% Hispanic, 8% Other), to be followed into early adulthood. The study aims to transform our understanding of brain development and links to substance use and other health outcomes (49). Participants across 21 study sites were recruited through public and private elementary schools (including charter schools) with sampling approaches intended to yield a final sample that closely approximates national sociodemographics (48). The human research protections programs and institutional review boards at universities participating in the ABCD project approved all experimental and consenting procedures, and all participants (assent) and their legal guardian provided written agreement to participate (consent). Additional ABCD study information is provided in Garavan, Bartsch (48).
Measures
Disruptive Behavior Disorders (DBD).
We quantified DBD using two measures. First, parents completed a self-administered computerized version of the Child Behavior Checklist (CBCL; 50). As part of the current study, we utilized the DSM-oriented conduct problems (α=.77) and oppositional defiant problems (α=.80) subscales of the CBCL. Second, parent’s completed a self-administered computerized version of the Schedule for Affective Disorders and Schizophrenia for school-age children (K-SADS-PL DSM-5; 51), which generates CD and ODD diagnoses in accordance with DSM diagnostic criteria in children and adolescents from 6 to 18 years of age. The traditional interview-based K-SADS are a reliable and widely-used measure of psychopathology in children and adolescents (51, 52). Importantly, research has demonstrated strong between current episode diagnoses using the computerized self-administered version and the paper-and-pencil version of the KSADS-5, with high percent agreement (range=88%–96%) and kappas in the good-to-excellent range (53, also see 54).
Callous-Unemotional (CU) Traits.
We quantified CU traits using a measure derived and validated in a prior study using ABCD Study data (55) which includes one item from the CBCL (“lack of guilt after misbehaving”) (50) and three [reverse-scored] from the Strengths and Difficulties Questionnaire (SDQ; 56) (“is considerate of others’ feelings”, “is helpful if someone is hurt or upset”, “offers to help others”) (α=.73). In addition to a more traditional summed score approach, maximum a posteriori (MAP) scale scores were derived for CU traits, accounting for which items are endorsed by whom, providing person-specific factor scores for CU traits (55).
Group Classification.
We assigned children to groups using data from the full baseline sample (N=11,874). First, DBD was classified based on children having a T-score >66 on the CBCL’s DSM-oriented CP or oppositional defiant problems scales, or K-SADS CD or ODD diagnosis (n=1,561). From this overarching DBD group, youth were further categorized into two subgroups based on the presence of high versus low CU traits. DBD youth were classified with high CU traits if they received summed scores >=4 on the summed CU traits measure and CU MAP scores >=90th percentile (DBDCU+, n=288). DBD youth who endorsed no items on the measure of CU traits (i.e., summed scores=0), were classified into the DBD-only group (n=362). Finally, the designation of TD was applied to youth obtaining T-scores=50 across all CBCL scales and summed scores of zero on the measure of CU traits (n=915) (Table 1).
Table 1.
Sample characteristics for diagnostic criteria and study covariates across the total sample and by group.
|
TD (n=915) |
DBD-only (n=362) |
DBDCU+ (n=288) |
Total Sample (N=1565) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/% | SD | Range | M/% | SD | Range | M/% | SD | Range | M/% | SD | Range | |
| Diagnostic Criteria | ||||||||||||
| CBCL CP scale | 50.00a | 0 | 50–50 | 55.47b | 6.55 | 50–80 | 66.68c | 7.78 | 50–86 | 54.30 | 7.73 | 50–86 |
| CBCL ODD scale | 50.00a | 0 | 50–50 | 57.96b | 7.02 | 50–80 | 65.34c | 7.99 | 50–80 | 54.64 | 7.68 | 50–80 |
| CBCL ADHD scale | 50.00a | 0 | 50–50 | 56.39b | 7.14 | 50–80 | 61.91c | 8.53 | 50–80 | 53.23 | 5.63 | 50–80 |
| CBCL Anxiety scale | 50.00a | 0 | 50–50 | 57.67b | 8.82 | 50–94 | 58.31c | 9.00 | 50–97 | 53.30 | 6.94 | 50–97 |
| K-SADS CD diagnosis | 0%a | 14%b | 50%c | 9.2% | ||||||||
| K-SADS ODD diagnosis | 0%a | 25%b | 56%c | 25.3% | ||||||||
| K-SADS ADHD diagnosis | 0%a | 21 %b | 42%c | 12.8% | ||||||||
| K-SADS Anxiety diagnosis | 0%a | 5%b | 5%b | 2% | ||||||||
| CU traits MAP score | −.39a | .16 | −.65–−.11 | −.33b | .16 | −.65–.31 | 2.00c | .40 | 1.62–3.37 | .05 | .94 | −.65–3.37 |
| CU traits summed score | 0a | 0 | 0–0 | 0a | 0 | 0–0 | 4.83b | 1.07 | 4–8 | .87 | 1.91 | 0–8 |
| Covariates | ||||||||||||
| Sex (% Female) | 58%a | 42%b | 31%c | 49% | ||||||||
| Race (% African-American) | 17%a | 15%a | 20%a | 17% | ||||||||
| Ethnicity (% Hispanic) | 13%a | 11 %a | 9%a | 12% | ||||||||
| IQ | 50.06a | 11.45 | 21–89 | 47.37b | 11.63 | 21–89 | 45.19c | 11.57 | 21–83 | 48.53 | 11.67 | 21–89 |
| Parent Education | 16.70a | 2.76 | 3–21 | 16.66a | 2.37 | 6–21 | 16.35a | 2.78 | 6–21 | 16.62 | 2.68 | 3–21 |
| Intracranial Volume | 151.43a | 14.99 | 108.77–206.92 | 151.69a | 14.28 | 112.40–194.55 | 152.23a | 14.80 | 118.44–202.37 | 151.64 | 14.79 | 108.77–206.92 |
Note. Matching or absent subscripts indicate groups that did not differ significantly from each other. Mismatching subscripts indicate groups that differed from each other. CBCL=Child Behavior Checklist: CD=conduct disorder; DBD+CU = High DBD/High CU Traits; DBD-only = High DBD/Low CU Traits; TD = Typically Developing; MAP= maximum a posteriori; ODD=oppositional defiant disorder; Intracranial volumes are presented as 1/10,000 of their actual value for ease of readability.
Demographic and psychiatric confounders.
First, models were explored only accounting for intracranial volume but no other covariates (Model 1). Second, to establish specificity in the relationship between cortical and subcortical GMVs and group membership, we ran models that accounted for intracranial volume and demographic variables that are known to be related both to DBDs and CU traits, as well as brain structure and function: sex, race, age, parent education, and IQ (Model 2). Third, all models were explored accounting for intracranial volume, demographic variables, and ADHD diagnosis indexed via children being above the clinical-cutoff on the CBCL DSM-oriented ADHD subscale (Model 3; note that results were similar when children above the clinical cut-off for ADHD were excluded altogether, n=263, Model 4). Fourth, to address the primary versus secondary CU traits distinction, we examined whether there were GMV differences among the DBDCU+ group contingent on having a T-score >66 on the CBCL’s DSM-oriented anxiety problem scale, or receiving a K-SADS anxiety diagnosis (51, 52). Finally, although imaging parameters were made as similar as possible across imaging vendors within the ABCD study, some variation was unavoidable due to hardware and software constraints. Following ABCD guidelines, all models also accounted for nesting within families (i.e., sibling pairs) and for site differences. This additional step involved the CLUSTER correction procedures for sibling pairs and stratification sampling by study site in Mplus vs.7 (see Analytic Strategy).
Imaging Measures
Acquisition.
The ABCD Study is a collaborative effort, including a Coordinating Center, 21 data acquisition sites across the United States, and a Data Analysis and Informatics Center (DAIC). The ABCD DAIC performs centralized processing and analysis of MRI data from each modality, leveraging validated methods used in other large-scale studies (see 57).
sMRI preprocessing and brain segmentation.
Cortical surface reconstruction and subcortical segmentation was performed using FreeSurfer v5.3.0 and included skull stripping, N3 intensity inhomogeneity correction, white matter segmentation, initial mesh creation, correction of toplogical defects, generation of optimal white and pial surfaces, and nonlinear registration to a spherical surface-based atlas based on the alignment of sulcal-gyral patterns (58). More details of the preprocessing steps and brain segmentation procedures are provided in Supplemental Methods (and in more detail in 57).
sMRI Morphometric Analysis.
While a range of morphometric measures are available within the ABCD data, given our hypotheses, we focused specifically on gray matter volume. Image intensity measures include T1w, T2w, and T1w and T2w cortical contrast (normalized difference between gray and white matter intensity values). Intensity values were sampled at a distance of ±0.2 mm along the normal vector at each surface location and cortical contrast were calculated from the gray and white matter values. Averages were calculated for each cortical parcel in the default FreeSurfer parcellation scheme using unsmoothed, surface-based maps of morphometric and image intensity measures (58). Weighted averages were calculated for each fuzzy-cluster parcel defined based on genetic correlation of surface area using smoothed surface maps (~66 mm FWHM, matching the level of smoothing used for derivation of the fuzzy cluster parcels) (59). Averages of the unsmoothed intensity measures for the volumetric ROIs were calculated, in addition to the volume of each structure. ROIs were selected from those derived within the Freesurfer parcellation scheme made available within the ABCD study (see 57 for more information, as well as the Supplemental Methods and Table S1).
Analytic Strategy
To explore whether GMV ROIs predicted group membership (i.e., TD, DBDCU+, or DBD-only), we employed multinomial logistic regression analysis controlling for intracranial volume, demographic covariates, and ADHD diagnosis. In exploratory analysis, we examined whether regional GMV differences differentiated primary versus secondary DBDCU+ youth. We also explored whether sex moderated associations between GMV ROIs and group classifications, using the Wald χ2 statistic to assess unique contribution of interaction terms between sex and GMV ROIs. All models were specified using maximum likelihood estimation with robust standard errors (MLR) and using a Monte Carlo numerical integration algorithm (60). Complex sampling and recruitment procedures implemented in the ABCD study (e.g. cluster correction for sibling pairs, stratification by study site) were accounted for using the cluster option and Type=Complex command available in Mplus vs.7 (60).
Results
Descriptive statistics for study variables are presented in Table 1. Groups did not differ on the basis of race, ethnicity, parental education, or intrcranial volume. However, males were more likely to be in the DBDCU+ and DBD-only groups relative to the TD group. Second, IQ was higher among TD youth relative to both DBDCU+ and DBD-only youth. Third, rates of psychopathology were the highest among DBDCU+ youth relative to both TD youth and DBD-only youth, with the exception of K-SADS anxiety disorder diagnoses, which had equivalent rates among the DBDCU+ and DBD-only groups. These findings reinforced our strategy of using multinomial logistic regression analyses to examine associations between regional GMVs and DBD/CU traits subgroup membership with and without covariates included.
First, children with lower amygdala GMVs were more likely to have a DBD diagnosis than TD children. Specifically, we found that children in the DBDCU+ group had lower right amygdala volumes relative to TD youth and that children in the DBD-only group had lower bilateral amygdala volumes compared to TD youth, controlling for demographic covariates and ADHD (Tables 2 and S2). In contrast to our hypothesis, however, this effect was not more pronounced on the basis of CU traits. That is, there were no significant differences between the DBDCU+ group and DBD-only groups.
Table 2.
Confidence intervals and odd ratios for multinomial logistic regressions exploring whether GMVs or ROIs predict membership in DBDCU+, DBD-only, and TD groups accounting for demographic covariates and ADHD diagnosis.
| DBDCUa+ vs TD OR (95% Confidence Interval) |
DBDCU+a vs DBD- only OR (95% Confidence Interval) |
DBD-onlyb vs TD OR (95% Confidence Interval) |
|
|---|---|---|---|
| Left Hemisphere | |||
| Anterior Cingulate Cortex (Caudal) | 1.02 (.87, 1.20) | 1.06 (.90, 1.26) | 0.97 (.84, 1.11) |
| Anterior Cingulate Cortex (Rostral) | 1.00 (.84, 1.19) | 0.89 (.73, 1.08) | 1.11 (.96, 1.30) |
| Amygdala | 1.18 (.98, 1.43) | 0.92 (.75, 1.14) | 1.28** fdr (1.07, 1.52) |
| Fusiform Gyrus | 1.02 (.85, 1.21) | 1.09 (.90, 1.31) | 0.94 (.80, 1.09) |
| Hippocampus | 1.30*fdr (1.07, 1.59) | 1.07 (.87, 1.33) | 1.21* (1.02, 1.44) |
| Inferior Frontal Gyrus | 1.14 (.96, 1.36) | 1.06 (.88, 1.28) | 1.08 (.92, 1.25) |
| Insula | 1.21* (1.00, 1.47) | 1.09 (.79, 1.24) | 1.11 (.93, 1.32) |
| Orbitofrontal Cortex | 1.12 (.94, 1.39) | 0.99 (.73, 1.14) | 1.13 (.94, 1.35) |
| Superior Frontal Gyrus | 1.14 (.94, 1.39) | 0.91 (.73, 1.14) | 1.25* (1.03, 1.51) |
| Superior Temporal Gyrus | 0.89 (.74, 1.09) | 1.03 (.83, 1.27) | 0.87 (.73, 1.03) |
| Ventromedial Prefrontal Cortex | 1.02 (.86, 1.22) | 1.01 (.84, 1.22) | 1.01 (.88, 1.16) |
| Riaht Hemisphere | |||
| Anterior Cingulate Cortex (Caudal) | 1.14 (.97, 1.33) | 0.98 (.83, 1.16) | 1.16*(1.01, 1.34) |
| Anterior Cingulate Cortex (Rostral) | 1.18 (.99, 1.40) | 1.19 (.99, 1.43) | 0.99 (.86, 1.15) |
| Amygdala | 1.26* (1.04, 1.52) | 0.97 (.79, 1.20) | 1.30* fdr (1.10, 1.52) |
| Fusiform Gyrus | 1.04 (.85, 1.27) | 1.12 (.91, 1.39) | 0.93 (.79, 1.09) |
| Hippocampus | 1.26* (1.04, 1.53) | 1.09 (.89, 1.35) | 1.15 (.97, 1.37) |
| Inferior Frontal Gyrus | 1.06 (.88, 1.28) | 1.00 (.82, 1.21) | 1.06 (.91, 1.23) |
| Insula | 1.11 (.92, 1.35) | 1.08 (.88, 1.33) | 1.03 (.86, 1.23) |
| Orbitofrontal Cortex | 1.16 (.96, 1.39) | 1.11 (.91, 1.36) | 1.04 (.89, 1.22) |
| Superior Frontal Gyrus | 1.02 (.84, 1.23) | 0.94 (.76, 1.16) | 1.08 (.91, 1.29) |
| Superior Temporal Gyrus | 0.97 (.80, 1.17) | 0.95 (.77, 1.18) | 1.02 (.85, 1.22) |
| Ventromedial Prefrontal Cortex | 0.93 (.79, 1.10) | 0.93 (.78, 1.12) | 1.00 (.86, 1.15) |
Note. Significant findings are bolded.
p<.05
p<.01. DBD+CU = High DBD/High CU Traits; DBD-only = High DBD/Low CU Traits; TD = Typically Developing.
Reference category is DBDCU+ youth
Reference category is DBD-only youth.
Indicates that effect remains significant when FDR-corrected (67). Each column represents a separate multivariate logistic regression and presents odds ratios controlling for demographic covariates and ADHD diagnosis. Odds ratios are reported relative to the reference group. Odds ratios > 1 indicate increased GMV among the non-reference group relative to the reference group (i.e., lower GMV in the reference group). For models with no covariates and demographic covariates only, as well as results when children with an ADHD diagnosis excluded, see Supplemental 2).
Second, children with lower hippocampal volumes were more likely to be in the DBDCU+ (bilateral hippocampus) and DBD-only (left hippocampus) groups relative to TD youth (Tables 2 and S2). However DBDCU+ and DBD-only groups did not differ significantly from each other.
Third, DBDCU+ youth evidenced lower left insula GMV relative to TD youth, although there were no significant differences between DBDCU+ and DBD-only youth. Fourth, DBDCU+ youth also evidenced lower right OFC GMV relative to TD youth, although this difference was rendered non-significant after accounting for ADHD diagnosis (Tables 2 and S2). Finally, children with lower left SFG GMV were more likely to be in the DBD-only group relative to the TD group.
Beyond these differences, no other GMV differences were found, including no significant differences between DBDCU+ youth and DBD-only youth in any analyses. With regards to the primary versus secondary DBDCU+ distinction, no significant between-group differences emerged with one exception: within the overall DBDCU+ group, lower hippocampal volume predicted membership of the primary DBDCU+ group relative to both the TD and secondary DBDCU+ groups (Table S3). There were also no effects that were significantly moderated by sex (results available on request).
Discussion
The current study identified morphometric differences across several brain regions that were associated with DBDs and CU traits. We leveraged the large-scale ABCD baseline release to derive phenotypically-narrow groups, categorized as DBDCU+, DBD-only, and TD. In support of study hypotheses, youth with DBDs showed reduced amygdala GMVs relative to TD youth. Thus, atypical development of the amygdala, specifically a relative reduction in amygdala volume relative to total intracranial volume, is implicated in the emergence of DBDs, over and above demographic covariates and comorbid ADHD. One way that such aberrations in structure might impact the emergence of DBDs is via functional impairments in core socioemotional and learning processes that are known to be mediated via the amygdala (17). This inference, while not tested in the current study, is consistent with findings of fMRI studies showing disrupted functioning of the amygdala during the processing of cues or emotion among youth with DBDs (20, 21). In contrast to our hypotheses however, lower amygdala volume did not differentiate between DBDCU+ and DBD-only youth. This finding is consistent with prior reports that found no association between CU traits and amygdala GMV (30, 61), but inconsistent with other reports noting specificity in associations between CU traits and lower amygdala GMV (31). Nevertheless, in a meta-analysis of 13 studies, the most reliable finding was that reduced GMV of the left amygdala specifically differentiated youth with childhood-onset DBD problems from TD youth, which aligns with the age of our sample. Thus, reduced amygdala GMV may be a more general marker of risk for early-onset and life-course persistent forms of DBD symptomatology, which could also be a proxy for severity and represent what is being captured by measures of CU traits when studies do not also account for age-of onset of DBD symptoms.
Lower hippocampal volume also differentiated between DBD youth relative to TD youth, particularly DBDCU+ youth and a primary DBDCU+ subgroup. Our findings suggest that aberrant structural development of the hippocampus may underpin a DBD phenotype characterized specifically by low anxiety, callousness, and uncaring. Indeed, lower volume of the left hippocampus differentiated between primary DBDCU+ relative to secondary DBDCU+ youth. In support of this interpretation, prior studies have reported that reduced hippocampal GMV is associated with psychopathy among incarcerated adults (62) and psychopathic traits in an adult community sample (63), as well as differentiating between incarcerated youth with high psychopathic traits and healthy controls (64). As CU traits are purported to map onto the affective deficits associated with psychopathy (10, 44, 45), these results implicate structural aberrations of the hippocampus as underlying a DBD phenotype characterized by low empathy, low guilt, uncaring, and shallow affect.
Several other study findings warrant consideration. First, reduced left insula GMV differentiated between DBDCU+ youth relative to TD youth. This finding is challenging to interpret alongside prior studies that have reported mixed findings for the association between insula GMV and DBDCU+. Although lower insula GMV were shown to differentiate between youth with DBDs in a meta-analysis of 13 studies, this relationship did not appear to be driven by concurrent CU traits (29). Moreover, higher insula GMV predicted CU traits among youth with low DBD symptoms (30) and among male adolescents (40). Given that increases in GMV continue up to around age 12 (33), studies are needed to longitudinally assess large followed from early childhood through to adolescence to explore how developmental or pubertal stage moderates the nature of any association between insula GMV and DBD symptoms versus CU traits over time. Second, lower SFG GMV differentiated between youth in the DBD-only group relative to the TD group. In their meta-analysis, Rogers and DeBrito also reported that youth with DBDs evidenced reduced GMV in the SFG (29). Together, these findings suggest that atypical development of the SFG may be involved in the development of DBDs. One way that structural differences may manifest is via the purported role of the SFG in social cognition, including perspective-taking, which is disrupted among youth with DBDs (10). Future studies capable of assessing both functional and structural imaging, as well as behavioral measures, are needed to test this assertion. Finally, in contrast to hypotheses, we did not find GMV differences among DBDCU+ or DBD-only youth relative to TD youth in the ACC, fusiform gyrus, IFG, OFC, SFG, or vmPFC. Further, we found no significant moderation in the associations between GMV ROIs and groups on the basis of sex. That is, variability in regional GMVs did not explain group membership differently in girls versus boys.
Several limitations of the current study should be considered, which may help to explain some of the null findings. First, there is complexity in GMV development during the transition to adolescence, including evidence for substantial heterogeneity in developmental trajectories (32). Thus, the examination of individual differences in GMV and group membership at a single time-point of late-childhood, as necessitated by our cross-sectional design, may have masked important differences in how individual differences in GMV trajectories differentiate youth with DBDs/CU traits, as well as contributed to some of the inconsistent findings reported between studies, including ours, based on sample age or sex (32). Follow-up studies that take advantage of future waves of ABCD data collection are paramount for addressing how individual differences GMV change across development impact DBDs and CU traits. Second, although we accounted for various demographic covariates, developmental trajectories of GMV are influenced by other concomitant factors that also predict DBD symptoms and CU traits, including relationship quality and early life stress (32, 65), for which we did not account. Third, while we focused on regional GMV (i.e., function of both surface area and cortical thickness; 66), future studies using the ABCD dataset could examine questions that were beyond our scope, including examining environmental versus genetic influences on surface area and cortical thickness (cf., 66) that could differentially be related DBD symptoms or CU traits.
In conclusion, our findings suggest that reduced GMV of the amygdala and hippocampus may be indicative of risk for DBDs, with lower hippocampal volume a specific marker of risk for DBDCU+, particularly a primary DBDCU+ variant. Our findings add to a growing account of the structural abnormalities associated with DBDs, with some evidence for heterogeneity on the basis of CU traits and anxiety. Future studies conducted within a prospective longitudinal design can establish whether these GMV reductions represent predictive biomarkers of youth at risk for persistent antisocial behavior, which could inform personalized and mechanistic interventions to address neurobiologically-mediated deficits in socioemotional processing among DBD youth based on structural abnormalities.
Supplementary Material
Acknowledgements:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from https://dx.doi.org/10.15154/1412097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures Section: All authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Rebecca Waller, Department of Psychology, University of Pennsylvania, Philadelphia, PA, USA.
Samuel W. Hawes, Department of Psychology, Florida International University, Miami, FL, USA.
Amy L. Byrd, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Anthony S. Dick, Department of Psychology, Florida International University, Miami, FL, USA.
Matthew T. Sutherland, Department of Psychology, Florida International University, Miami, FL, USA.
Michael C. Riedel, Department of Physics, Florida International University, Miami, FL, USA.
Michael J. Tobia, Department of Physics, Florida International University, Miami, FL, USA.
Katherine L. Bottenhorn, Department of Psychology, Florida International University, Miami, FL, USA
Angela R. Laird, Department of Physics, Florida International University, Miami, FL, USA.
Raul Gonzalez, Department of Psychology, Florida International University, Miami, FL, USA.
References
- 1.Rivenbark JG, Odgers CL, Caspi A, Harrington H, Hogan S, Houts RM, Poulton R, Moffitt TE. The high societal costs of childhood conduct problems: evidence from administrative records up to age 38 in a longitudinal birth cohort. Journal of Child Psychology and Psychiatry. 2018;59:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fergusson DM, John Horwood L, Ridder EM. Show me the child at seven: the consequences of conduct problems in childhood for psychosocial functioning in adulthood. Journal of child psychology and psychiatry. 2005;46:837–849. [DOI] [PubMed] [Google Scholar]
- 3.Association AP: Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA, American Psychiatric Publishing; 2013. [Google Scholar]
- 4.Nock MK, Kazdin AE, Hiripi E, Kessler RC. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the National Comorbidity Survey Replication. Journal of Child Psychology and Psychiatry. 2007;48:703–713. [DOI] [PubMed] [Google Scholar]
- 5.Berkout OV, Young JN, Gross AM. Mean girls and bad boys: Recent research on gender differences in conduct disorder. Aggression and Violent Behavior. 2011;16:503–511. [Google Scholar]
- 6.Demmer DH, Hooley M, Sheen J, McGillivray JA, Lum JA. Sex differences in the prevalence of oppositional defiant disorder during middle childhood: a meta-analysis. Journal of abnormal child psychology. 2017;45:313–325. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DA. The cost of crime. Foundations and Trends® in Microeconomics. 2012;7:209–265. [Google Scholar]
- 8.Bakker M, Greven C, Buitelaar J, Glennon J. Practitioner Review: Psychological treatments for children and adolescents with conduct disorder problems–a systematic review and meta-analysis. Journal of child psychology and psychiatry. 2017;58:4–18. [DOI] [PubMed] [Google Scholar]
- 9.Hyde LW, Waller R, Burt SA. Commentary: Improving treatment for youth with callous-unemotional traits through the intersection of basic and applied science–reflections on Dadds et al.(2014). Journal of Child Psychology and Psychiatry. 2014;55:781–783. [DOI] [PubMed] [Google Scholar]
- 10.Frick PJ, Ray JV, Thornton LC, Kahn RE. Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of child Psychology and Psychiatry. 2014;55:532–548. [DOI] [PubMed] [Google Scholar]
- 11.Waller R, Shaw DS, Neiderhiser JM, Ganiban JM, Natsuaki MN, Reiss D, Trentacosta CJ, Leve LD, Hyde LW. Toward an understanding of the role of the environment in the development of early callous behavior. Journal of Personality. 2017;85:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waller R, Hyde LW. Callous-unemotional behaviors in early childhood: the development of empathy and prosociality gone awry. Current opinion in psychology. 2018;20:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. [DOI] [PubMed] [Google Scholar]
- 14.Blair RJR. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71:727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair R Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual differences. 1999;27:135–145. [Google Scholar]
- 16.Blair RJR. A cognitive developmental approach to morality: Investigating the psychopath. Cognition. 1995;57:1–29. [DOI] [PubMed] [Google Scholar]
- 17.LeDoux JE. Coming to terms with fear. Proceedings of the National Academy of Sciences. 2014;111:2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalska KJ, Zeffiro TA, Decety J. Brain response to viewing others being harmed in children with conduct disorder symptoms. Journal of child psychology and psychiatry. 2016;57:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in cognitive sciences. 2005;9:242–249. [DOI] [PubMed] [Google Scholar]
- 20.Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA psychiatry. 2014;71:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, van Beelen P, Popma A, Vermeiren RR, Colins OF. Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biological Psychiatry. 2017;82:283–293. [DOI] [PubMed] [Google Scholar]
- 22.Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, De Brito SA, Viding E. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Current Biology. 2013;23:901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of general psychiatry. 2012;69:814–822. [DOI] [PubMed] [Google Scholar]
- 24.Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, Pine DS, Decety J, Blair RJR. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of child psychology and psychiatry. 2013;54:900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwenck C, Ciaramidaro A, Selivanova M, Tournay J, Freitag CM, Siniatchkin M. Neural correlates of affective empathy and reinforcement learning in boys with conduct problems: fMRI evidence from a gambling task. Behavioural brain research. 2017;320:75–84. [DOI] [PubMed] [Google Scholar]
- 26.Veroude K, von Rhein D, Chauvin RJ, van Dongen EV, Mennes MJ, Franke B, Heslenfeld DJ, Oosterlaan J, Hartman CA, Hoekstra PJ. The link between callous-unemotional traits and neural mechanisms of reward processing: An fMRI study. Psychiatry Research: Neuroimaging. 2016;255:75–80. [DOI] [PubMed] [Google Scholar]
- 27.Blair R, Veroude K, Buitelaar J. Neuro-cognitive system dysfunction and symptom sets: a review of fMRI studies in youth with conduct problems. Neuroscience & Biobehavioral Reviews. 2016;91:69–90. [DOI] [PubMed] [Google Scholar]
- 28.Hyde LW, Shaw DS, Hariri AR. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: Review, integration, and directions for research. Developmental Review. 2013;33:168–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers JC, De Brito SA. Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA psychiatry. 2016;73:64–72. [DOI] [PubMed] [Google Scholar]
- 30.Cohn MD, Viding E, McCrory E, Pape L, van den Brink W, Doreleijers TA, Veltman DJ, Popma A. Regional gray matter volume and concentration in at-risk adolescents: Untangling associations with callous-unemotional traits and conduct disorder symptoms. Psychiatry Research: Neuroimaging. 2016;254:180–187. [DOI] [PubMed] [Google Scholar]
- 31.Cardinale EM, O'Connell K, Robertson EL, Meena LB, Breeden AL, Lozier LM, VanMeter JW, Marsh AA. Callous and uncaring traits are associated with reductions in amygdala volume among youths with varying levels of conduct problems. Psychological medicine. 2019;49:1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulkes L, Blakemore S-J. Studying individual differences in human adolescent brain development. Nature neuroscience. 2018:1. [DOI] [PubMed] [Google Scholar]
- 33.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861. [DOI] [PubMed] [Google Scholar]
- 34.Falk E, Hyde L, Mitchell C, Faul J, Gonzalez R, Heitzeg M, Schulenberg J. Neuroscience meets population science: What is a representative brain. Proceedings of the National Academy of Sciences. 2013;110:17615–17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, Forbes EE. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical psychological science. 2016;4:527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dotterer HL, Hyde LW, Swartz JR, Hariri AR, Williamson DE. Amygdala reactivity predicts adolescent antisocial behavior but not callous-unemotional traits. Developmental cognitive neuroscience. 2017;24:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O. Family income, parental education and brain structure in children and adolescents. Nature neuroscience. 2015;18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. 2010;21:896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barker ED, Tremblay RE, van Lier PA, Vitaro F, Nagin DS, Assaad JM, Seguin JR. The neurocognition of conduct disorder behaviors: Specificity to physical aggression and theft after controlling for ADHD symptoms. Aggressive Behavior. 2011;37:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raschle NM, Menks WM, Fehlbaum LV, Steppan M, Smaragdi A, Gonzalez-Madruga K, Rogers J, Clanton R, Kohls G, Martinelli A, Bernhard A, Konrad K, Herpertz-Dahlmann B, Freitag CM, Fairchild G, De Brito SA, Stadler C. Callous-unemotional traits and brain structure: Sex-specific effects in anterior insula of typically-developing youths. NeuroImage: Clinical. 2018;17:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smaragdi A, Cornwell H, Toschi N, Riccelli R, Gonzalez-Madruga K, Wells A, Clanton R, Baker R, Rogers J, Martin-Key N. Sex differences in the relationship between conduct disorder and cortical structure in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2017;56:703–712. [DOI] [PubMed] [Google Scholar]
- 42.Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waschbusch DA, Biswas A, MacLean MG, Babinski DE, Karch KM. The delinquency outcomes of boys with ADHD with and without comorbidity. Journal of abnormal child psychology. 2011;39:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens MC, Haney-Caron E. Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. Journal of psychiatry & neuroscience: JPN. 2012;37:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn RE, Frick PJ, Youngstrom EA, Kogos Youngstrom J, Feeny NC, Findling RL. Distinguishing primary and secondary variants of callous-unemotional traits among adolescents in a clinic-referred sample. Psychological assessment. 2013;25:966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimonis ER, Frick PJ, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Development and Psychopathology. 2012;24:1091–1103. [DOI] [PubMed] [Google Scholar]
- 46.Lee Z, Salekin RT, Iselin A-MR. Psychopathic traits in youth: is there evidence for primary and secondary subtypes? Journal of Abnormal Child Psychology. 2010;38:381–393. [DOI] [PubMed] [Google Scholar]
- 47.Waller R, Hicks BM. Trajectories of alcohol and marijuana use among primary versus secondary psychopathy variants within an adjudicated adolescent male sample. Personality Disorders: Theory, Research, and Treatment. 2019;10:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein R, Heeringa S, Jernigan T, Potter A, Thompson W, Zahs D. Recruiting the ABCD Sample: Design Considerations and Procedures. Developmental Cognitive Neuroscience. 2018. [DOI] [PMC free article] [PubMed]
- 49.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, Pérez-Stable EJ, Riley WT, Bloch MH, Conway K. The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental cognitive neuroscience. 2017. [DOI] [PMC free article] [PubMed]
- 50.Achenbach T, Ruffle T. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in review. 2000;21:265–271. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman, Birmaher B, Brent DA, Rao U, Ryan ND: KIDDIE-SADS-present and lifetime version (K-SADS-PL) Instrument developed at Western Psychiatric Institute and Clinic, Pittsburgh, PA: 1996. [Google Scholar]
- 52.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 53.Kobak K, Kratochvil C, Stanger C, Kaufman J: Computerized screening of comorbidity in adolescents with substance or psychiatric disorders. in Poster presented at the 33rd annual anxiety disorders and depression conference, La Jolla, CA Retrieved from http://wwwtelepsychologynet/resources/KSADS_ADAAPosterv4pdf2013. [Google Scholar]
- 54.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Developmental cognitive neuroscience. 2018;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hawes SW, Waller R, Thompson WK, Hyde LW, Byrd AL, Burt AS, Klump KL, Gonzalez R. Assessing callous-unemotional traits: development of a brief, reliable measure in a large and diverse sample of preadolescent youth. Psychol Med. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodman R The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psyciatr. 1997;38:581–586. [DOI] [PubMed] [Google Scholar]
- 57.Hagler DJ, Hatton SN, Makowski C, Cornejo MD, Fair DA, Dick AS, Sutherland MT, Casey B, Barch DM, Harms MP. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. bioRxiv. 2018:457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischl B FreeSurfer. Neuroimage. 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C, Gutierrez E, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, Fennema-Notestine C, Jak AJ, Neale MC, Franz CE. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muthén LK, Muthén BO: Mplus user’s guide. Seventh edition, Los Angeles, CA: Muthén & Muthén; 1998-2012. [Google Scholar]
- 61.Sebastian CL, De Brito SA, McCrory EJ, Hyde ZH, Lockwood PL, Cecil CAM, Viding E. Gray Matter Volumes in Children with Conduct Problems and Varying Levels of Callous-Unemotional Traits. Journal of Abnormal Child Psychology. 2016;44:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of abnormal psychology. 2012;121:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cope LM, Shane MS, Segall JM, Nyalakanti PK, Stevens MC, Pearlson GD, Calhoun VD, Kiehl KA. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Research: Neuroimaging. 2012;204:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steele VR, Rao V, Calhoun VD, Kiehl KA. Machine learning of structural magnetic resonance imaging predicts psychopathic traits in adolescent offenders. Neuroimage. 2017;145:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tyborowska A, Volman I, Niermann HC, Pouwels JL, Smeekens S, Cillessen AH, Toni I, Roelofs K. Early-life and pubertal stress differentially modulate gray matter development in human adolescents. Scientific reports. 2018;8:9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or gray matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.