Abstract
Loss of regenerative capacity is a normal part of aging. However, some organisms, such as the Mexican axolotl, retain striking regenerative capacity throughout their lives. Moreover, the development of age-related diseases is rare in this organism. In this review we will explore how axolotls are used as a model system to study regenerative processes, the exciting new technological advancements now available for this model, and how we can apply the lessons we learn from studying regeneration in the axolotl to understand, and potentially treat, age-related decline in humans.
Keywords: Aging, regeneration, Axolotl, cancer, scar formation, immune system, nerve signaling, technological advances
Introduction:
Regeneration is a part of life. Having the ability to replace old and damaged cells in all organ systems is essential not only for tissue homeostasis, but also for the prolonged survival of the organism. The ability to repair wounds is a property conserved by all organisms, as are many of the mechanisms to heal these injuries. However, the ability to regenerate more complex structures, such as limbs, is greatly variable among tetrapod species. It has been well-documented that these abilities to replace tissues during homeostasis and to heal acute injuries is negatively affected by aging in most organisms. However, some species are better at regenerating as adults than others.
It is thought that all tetrapods have the ability to regenerate complex structures such as organs or limbs as embryos, but many of these species, including mammals, lose this capacity as they develop into adult organisms. While others, including Urodele amphibians (salamanders and newts), are capable of regenerating throughout adulthood The Mexican axolotl (Ambystoma mexicanum) is an aquatic salamander that can regenerate multiple body parts including its limbs and internal organs such as its heart, brain, and lungs. In this review we will focus on the recent advancements in the axolotl model system for regeneration and discuss how aging effects regenerative processes in this model. We will also speculate how the study of regeneration in this model can be applied to better understanding, and potentially treating, aging pathologies in humans.
How aging affects the regenerative capacity in humans and the axolotl:
The aging process in humans and its impact on regeneration:
Humans go through multiple temporal stages throughout their lives, including embryogenesis and fetal development, childhood, adolescence, adulthood, midlife, mature adulthood, and ultimately death. Each stage is marked by important physiological changes. Some changes appear to be programed, such as the ones that occur via endocrine signaling [1], while others are in response to environmental factors such as nutrition [2] or normal cellular metabolism [3]. It is hard to pinpoint exactly when aging begins in humans, probably because so many genetic and environmental factors can affect this process (Reviewed in [4]). However multiple characteristics, including the loss of reproductive capacity, decreased metabolism, depletion of adult stem cell pools, increased cellular senescence, increased DNA damage, altered immune responses, increased time to heal injuries, and increased precedence of age-related diseases (cancer, heart disease, Alzheimer’s disease, etc.), have all been correlated with the aging process (reviewed in [4]). Elucidating the mechanisms underlying age-related pathologies will play a key role in developing therapies that improve our quality of life in the later years.
It has been well-documented that regenerative capacity declines with age in humans, and this decline can be linked to age-related pathologies in a variety of different organ systems. One potential reason for this decline is the changes that happen in the adult stem cell pools with age. For example, decreased intrinsic responsiveness of the stem cells or decreased environmental signals, such as those that come from the niche, have been documented in multiple somatic stem cell populations [5]. Additionally, decreased ability to self-renew, and altered potential to differentiate into progenitor populations is also observed in aging somatic stem cells [6]. Thus, this loss of somatic stem cell function not only leads to a decreased ability to replace or repair damaged tissues but would also results in the accumulation of potentially damaged or senescent cells that would also have a negative effect on the tissue environment, and overall health of the human.
Another reason for regenerative decline may pertain to changes in fibroblast activity. Although not well defined, fibroblasts are present in every organ system, and are particularly rich in the connective tissues. One of the most well recognized roles of fibroblasts rests in their ability to generate extracellular matrix molecules that constitutes the “glue” that holds tissues and organs together. However, fibroblasts are also known to retain positional memory, such that they remember where they are located relative to the different axes of the body, and even within a particular structure, such as the limb (reviewed in [7]). Most other cell types, termed pattern-following cells, in the body do not retain this memory; instead they respond to position-specific cues from the fibroblasts. These cues regulate gene expression in the pattern-following cells such that they respond in a location appropriate manner (reviewed in [7]). Thus, fibroblasts appear essential for tissue homeostasis in every major organ system. Given this importance, it is not surprising that the altered activity of fibroblasts during aging can have such a negative impact on the overall health in humans. Aberrant fibrotic activity is responsible for scarring in response to acute injury, which increases with age. Some of the major age-related chronic diseases, including heart disease, chronic lower respiratory diseases, kidney disease, and chronic liver disease and cirrhosis involve tissue fibrosis (reviewed in [8]). As we will describe in the following sections, fibroblasts play multiple, essential roles in wound healing and regeneration in the axolotl (reviewed in [8]). Thus, the axolotl is an excellent model to study the regulation of fibroblast activity during regenerative responses in adults.
Aging in the axolotl:
The axolotl life cycle occurs at a more rapid rate than humans. Embryogenesis takes approximately two weeks after which time the larval animals will hatch out from their eggs as free-swimming animals. These larvae will continue to develop in the following months where they will pattern limbs, develop lungs, and rapidly grow in size. At approximately 1 year after fertilization (9 months for males, 12 months for females), the animals become sexually mature although they retain many larval traits, such as the retention of gills and an aquatic habitat.
Just as in humans, axolotl fertility appears to diminish with age. Observations from our laboratory indicate that sexually mature females are most successful at mating for the first few years of life, while males retain fertility to a slightly older age. This pattern of decreasing fertility with age is not conserved amongst all species, including other amphibians, such as the red legged frog [9]. Interestingly however, in contrast to humans but similar to other amphibians, axolotl females have egg stem cells [10]. Thus, their decline in fertility appears to differ from the mechanisms that effect egg quality in aging humans.
Although no data exists pertaining to the mortality rate of these organisms, the maximum life expectancy of an axolotl is estimated to be up to 25 years in captivity. Despite the lack of age-related research in this species, there are observable changes in the body structure of the animal with time. One of these changes is size; the axolotl is an indefinitely growing animal, continuing to increase in size throughout its life (Figure 1). Another is in the composition of its skeleton; during the larval stages of life, the axolotl skeleton is highly cartilaginous, and this cartilage is replaced by bone as the animal ages. Changes in tissue composition are also evident, such as the limb dermal layer thickening as the animal ages (Figure 2). Behavior also changes with age. Young larval animals exhibit more frequent locomotion and feeding compared to older animals, suggesting that their metabolism also decreases with age, as it does with humans.
Figure 1: Axolotl continue to grow after sexual maturity.

Live images of a hatchling axolotl (~3 weeks old), young juvenile (~3 months old), late juvenile (~5 months old), sexually mature adult (~1 year old), and a 3-year-old adult show the dramatic increase in size over time.
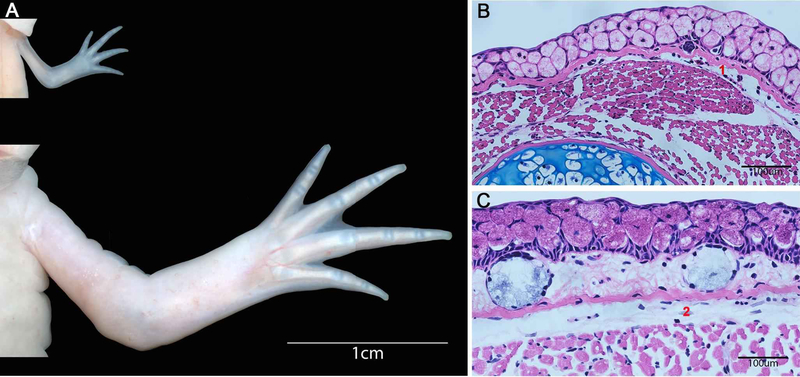
Figure 2: Changes in skin morphology in axolotl as they age.
A) Live images of the forelimbs of a young juvenile (top) and a sexually mature adult (bottom) staged animals. B) Histology staining of sections of the limbs in A showing the full thickness skin where “1” indicates the dermal layer from the juvenile staged limb, and “2” indicates the dermal layer on the adult staged limb.
Although the above-mentioned phenotypic changes have been associated to animals no more than 5 years old (mature adults), it is clear that axolotls age. Unfortunately, a precise comparison between aging in humans and the axolotl cannot be conducted due to the limited data relating to aging in the axolotl. However, as we will explore in the upcoming text, these organisms exhibit a striking resistance to age-related diseases and pathologies. It has been hypothesized that this resistance is due, in part, to their exceptional regenerative capacity [8]. Axolotl can regenerate a variety of different structures and organs and understanding the mechanisms of this regenerative capacity will likely help us better identify ways that aging can be slowed in humans, and how age-related diseases could be prevented and/or treated.
Basics of limb regeneration:
The process of limb regeneration has been the most extensively studied and characterized regenerative process in the axolotl, thus we will focus on limb regeneration here. The axolotl has the ability to regenerate complete limbs regardless the site of injury along the limb axis [11]. Regeneration is initiated by wounding, although not all wounds (such as a lateral limb wound) will result in the formation of a limb regenerate [12]. Within hours (although this timing increases with the age of the animal), a wound epithelium migrates and covers over the wound site. In the days following nerve fibers innervate this wound epithelium [13], and signaling feedback loops between the nerve and the wound epithelium establish a specialized signaling center known as the apical epithelial cap. This center then generates multiple signaling molecules that result in the dedifferentiation and proliferation of the underlying mature limb tissues into limb progenitor cells known as blastema cells [14–18]. Interactions in the wound between cells from the opposite axes of the limb establish the pattern of the missing limb structures [19]; and once this pattern is established, the cells re-differentiate into the missing limb structures [20].
Multiple studies on the axolotl have indicated that there are three basic requirements for regeneration of the limb; 1) the wound epithelium, 2) nerve signaling, and 3) the presence of cells from the different limb axes. One such study established a regenerative assay, known as the accessory limb model (ALM), which showed that the combination of these three components were sufficient for the generation of a limb (Figure 3) [21]. In this study, ectopic limbs were generated in lateral wounds on the side of the arm that had both a deviated nerve bundle and a skin graft from the opposite side of the limb axis [21]. The ALM has become a powerful regenerative assay that provides a platform to study each of these regeneration specific requirements in a “gain-of function” manner. Thus, the ALM and other regenerative assays will play an important role in our understanding of the basic biology of limb regeneration.
Figure 3: The Accessory Limb Model (ALM) is an assay to study limb regeneration.
A live image of an ectopic limb that has formed from making a wound site with a nerve on the anterior side of the limb on the RFP+ host animal (red) that was grafted with a blastema from a GFP+ donor animal (green).
Decline in regenerative ability with axolotl age:
Axolotl retain remarkable regenerative capacity throughout their lives. Despite their regenerative success diminishing with age, they still demonstrate regenerative capabilities that far exceed that of humans and other mammals. This age-related decline in regenerative ability in the axolotl could be contributed to and/or affected by a number of factors. 1) The rate of regeneration slows as the axolotl increases in age, from weeks in larval animals to months in sexually mature adults. This may be a consequence of the animal’s size, with smaller animals having less extremity girth and mass relative to adult animals leading to faster wound closure and tissue restoration rates (Figure 1). 2) With age, the skin of the axolotl thickens and losses flexibility, possibly making it more difficult to form a wound epithelium, as well as increase the likelihood of generating insufficient positional interactions for the generation of a complete limb structure at the site of injury [19]. Additional factors, such as circulating factors and hormones could also play a role in the timing of regeneration in aging animals, although this possibility has not yet been investigated.
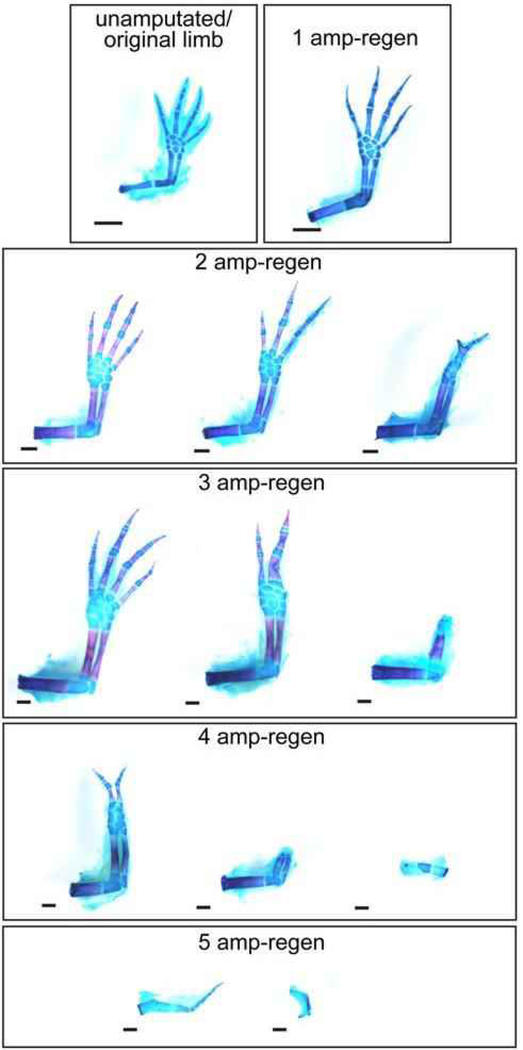
Additional factors including nerve signaling, repeated injuries, and metamorphosis effect the regenerative capacity of the axolotl. We will explore nerve related effects more thoroughly in the next section. In terms of repeated injuries, for many years it was thought that the axolotl had unlimited potential to regenerate its limbs. However, recently it was shown that regenerative capacity diminishes in almost half of the experimental axolotl after the repeated removal of the complete limb axis if amputated at the same plane along the proximal-distal limb axis (Figure 4) [22,23]. In this subset of animals, it was observed that repeated limb removal results in an increased fibrotic response and alters the morphology of the wound epithelium, which contributes to regenerative failure. What makes some axolotl resilient after repeated removal of the limb while others lose regenerative capacity is an important question that needs to be answered.
Figure 4: Decline of regenerative capacity in limbs repeatedly amputated at the same location.
Example whole-mount skeletal preparations of the resulting regenerates form from limbs that have been amputated in the same location 1, 2, 3, 4, or 5 times. Bryant et al. observed a marked decrease in regenerative potential with increasing number of amputations (republished with permission from [23]).
Metamorphosis can also have a negative effect on limb regeneration. In amphibians metamorphosis is activated through thyroid hormone signaling [24]. In frog species, which can regenerate as tadpoles, the ability to regenerate complete limbs diminishes when metamorphosis occurs [25]. Natural metamorphosis is rare in the axolotl, however the process can be induced using Thyroid hormone [26]. Although the axolotl maintains its ability to regenerate after metamorphosis, they appear to be more susceptible to patterning defects, commonly missing digits [27,28]. This issue is likely related to the changes in skin morphology, and its effect on wound healing. Interestingly, the life span of the axolotl is severely reduced after metamorphosis, where the metamorphosed animal commonly lives for only a year or two after the process has completed. However, since metamorphosis is not part on the normal life cycle of the axolotl, it will be hard to determine if impaired regeneration in this context is potentially age-related or due to the altered (and abnormal) physiology of metamorphosed axolotl.
Nerve function changes with age:
Diminished sensory and motor function is a hallmark of the aging process in humans and, as previously mentioned, nerve signaling is required for axolotl limb regeneration and wound healing. If nerve signaling is removed from an amputated limb stump, it will form scar tissue and fail to regenerate [29,30]. It has been well established that a threshold number of nerves must be present at the wound surface for limb regeneration to proceed [31].
The focus of current research has been to identify the neurotropic factors that are required for regeneration. Using the ALM assay described above, researchers have identified specific proteins that can substitute for nerve signaling and induce ectopic limb formation. Thus far, a combination of fibroblast growth factor (FGFs) and bone morphogenetic proteins (BMPs), or singularly Neuregulin-1 are sufficient to replace the nerve in the ALM and rescue regeneration of denervated axolotl limbs [14,18,32]. A combination of FGF2, FGF8, and BMP7 were also able to rescue some patterning during limb regeneration in the metamorphosed frog (Xenopus laevis), which would normally only produce a cartilaginous spike [33]. Such results could indicate that elevated levels of neurotropic factors are produced at sites of amputation in the axolotl, relative to the frog, in order for complete limb patterning to occur during regeneration.
Interestingly, there is evidence that axolotl neurons are affected by age. It was found that spinal cord extracts from older axolotls were diminished in their capacity to stimulate cell proliferation compared to extracts from their younger counterparts, indicating that the nerves are producing less of these neurotrophic factors over time [34]. Moreover, axolotl nerve regeneration is vital for limb regeneration, and as with many vertebrates, the ability to regrow neurons decreases with time. It was shown that repeated removal of limb buds produced miniaturized limbs that were less innervated than control limbs, indicating that when repeatedly challenged, nerve regeneration was reduced [22]. Therefore, the nervous system itself, which is crucial for limb regeneration, appears to be negatively affected by aging in the axolotl.
What can studying regeneration in the axolotl potentially tell us about minimizing aging pathologies in humans?
The immune system:
Chronic inflammation has been associated with the development of age-related diseases and frailty in humans. Age-related decline of the immune system is associated with a higher incidence of infections and decreased efficiency of immunizations. In both mammalian and amphibian models, the development of the immune system is correlated with a loss in regenerative ability [35].
Although the activity of the immune system in the aging axolotl has not been studied, inflammatory responses can have both positive and negative effects on regeneration. For example, the immune system appears to be suppressed in the regenerating limb, as allografts from transgenic animals are associated with little to no rejection during a regenerative response [20,36,37]. This might be due, in part, to a simpler adaptive immune system in the axolotl compared to mammals. Despite anecdotal evidence suggesting a suppressed immune response during regeneration, the presence of immune cells is also a requirement. For example, macrophages are essential for regeneration in both the axolotl heart and limb [38,39]. Macrophages could play multiple roles in the regenerate, potentially clearing out tissue and cell debris in the injured tissue and removing senescent cells from the regenerated tissue. Lastly, both pro- and anti-inflammatory signals are induced in the regenerating limb environment [38]. Thus, the balance between inhibitory and necessary immune responses for regeneration appears to be highly regulated and warrants further exploration.
Scar free healing:
It has been well documented that the ability for humans to heal cutaneous wounds diminishes with age. The process and end result of wound healing differs substantially between axolotl and mammals (reviewed in [40]). Mammals develop scars while axolotl have scar-free healing of cutaneous wounds throughout their lives. Healing wounds is a multistep process that starts with the formation of a blood clot, followed by an influx of inflammatory cells, re-epithelialization, migration and deposition of extracellular matrix (ECM) by fibroblasts, removal of cell debris, cell proliferation, and finally skin maturation [41].
Within this multistep framework of wound healing, several differences between humans and the axolotl can be noted. First, the axolotl is much faster at wound closure, where juvenile animals take only a few hours to cover a wound site with a migrating sheet of epidermis. Humans cover wounds by proliferating new epidermal cells, and this process can take days or even weeks, depending on wound size. In humans, the rate of epithelialization of a 2 × 2 cm split-thickness wound is significantly slower in elderly (>65 year) compared to young (18 to 55 year) patients [42]. A similar study has yet to be performed in the axolotl; however, anecdotal observations on axolotl of different ages/sizes indicates that wound epithelium formation is slower in older/larger animals. However, it is likely that the large difference in the size of the wounds in these animals plays at least a partial role in this disparity.
Secondly, collagen matrix development during wound healing also differs between humans and axolotl. Mammalian wounds start to express collagen, a major component of the skin, a few days after injury; while in the axolotl this takes over a week. Moreover, axolotl cells reorganize collagen fibers into a basket weave structure that is consistent with the structure in uninjured skin [43]. Humans, on the other hand, have minimal collagen reorganization and instead the collagen remains in thick linear bundles. Recent studies comparing cutaneous wound healing in the axolotl and humans identified a protein, SALL4, that was significantly enriched in the axolotl wounds and regulates collagen expression and deposition during scar-free healing [44]. These differences in ECM deposition and reorganization by fibroblasts are likely pivotal factors in determining the probability of scar formation in wounded tissue.
Low incidence of cancer:
The number of diagnosed cases of cancer is continually increasing in the human population [45]. As age is major risk factor for cancer development, it is not surprising that the general increase in human life expectancy has contributed significantly towards this phenomenon [45].
The mechanisms by which age contributes to the incidence of cancer in humans are unknown but likely to be influenced by environmental and behavioral factors as well as genetic predispositions. In the human genome for example, specific genes exhibit age-related changes in DNA methylation, and alterations in this pattern of methylation has been associated with a variety of cancers [46,47]. Unlike humans, axolotl exhibit an extremely low incidence of spontaneous tumorigenesis, with limited reports of neoplasm formation described in literature [48–52]. Interestingly, there does appear to be a positive correlation between the occurrence of neoplasm and age of onset (Table 1), as in the human population. Whether this, although infrequent, phenomena also correlates with age-related epigenetic alterations in the axolotl genome still needs to be determined. However, these animals may serve as a powerful model system in this context as well as providing insight into the factors and mechanisms that can naturally limit spontaneous tumorigenesis.
Table 1:
Accounts of spontaneous tumorigenesis in the axolotl.
The axolotl also appears to be resistant to carcinogens, with treatment of carcinogenic polycyclic hydrocarbons inducing neoplasm only after extended periods post treatment in 30% of the population. The outgrowths of this study exhibited characteristics of papillomata; although, they never progressed to carcinomata [54]. Age, however, did appear to correlate with disease latency, with increasing animal age at the time of treatment positively correlated with shorter latency times for neoplasm development in these animals. [54].
The regeneration permissive microenvironment of the axolotl is composed of dynamic acellular and cellular components that share striking similarities with tumors. Blastema cells, for example, exhibit typical cancer cell phenotypes including, but not limited to, increased proliferation [55], oncogene expression [56], activation of line-1 elements [57], and large scale chromatin modifications [58] relative to mature stump tissue. These highly proliferative, undifferentiated cells have short, unpolarized, disordered actin fibers constituting their cytoskeletons and are surrounded by a disorganized ECM [55], a characteristic feature of the tumor microenvironment (as reviewed by [59]). Similar to a cancerous environment the composition of the blastema ECM is enriched for fibronectin [60] and tenascin [55,60,61], which promote cell migration and proliferation (as reviewed by [62]). This upregulation in fibronectin and tenascin expression is conserved in other regenerating tissues as well, including the regenerating newt (Notophthalmus viridescens) limb [63] and mammalian ear punch regenerate [64]. The dynamic remodeling and degradation of the ECM in both oncogenic and regenerative microenvironments is associated with the expression of a variety of matrix metalloproteinases (MMPs) [65,66].
Interestingly, the above mentioned cellular and acellular changes documented in oncogenesis correlate with enhanced tumor progression, metastasis and thus poor clinical outcomes; while in regenerative growths they aid in the formation of a patterned, functional structure and are stably resolved in the new tissue. The cellular mechanisms producing these disparate outcomes from very similar microenvironments are unknown and are active areas of research in the field.
Heterochronic parabiosis and tissue grafting
Heterochronic parabiosis, the processes by which the circulatory systems of old and young animals are joined, has provided valuable insight into biological aging. This heterochronic pairing facilitates improved muscle and liver regenerative capacity in old mice, demonstrated by improved activation of resident-aged progenitor muscle and hepatic cells respectively, when paired with young mice; the young mice exhibit slightly reduced progenitor cell activation in this experiment [67]. These findings indicate that age-related systemic factors have a greater capacity to alter regenerative success than the autonomous capacity of progenitor cells themselves. The identity of the systemic factors responsible for these changes are not without controversy [68]; however, some evidence suggests that growth differentiation factor 11, at least in part, contributes positively to muscle regeneration and neurogenesis in aged mice [69,70].
Embryonic parabiosis has been successfully achieved in the Mexican axolotl [71] as well as the closely related species, the spotted salamander Ambystoma maculatum [72]; although, not for age related studies. A major hindrance to heterochronic parabiosis in the axolotl is the large size difference between young and old animals. However, if such a surgery could be successfully achieved and the connection maintained between young and old animals, it could provide valuable information about systemic factors that may influence both the regenerative process and homeostatic tissue maintenance with age. Grafting experiments, where by old or young tissue is grafted to oppositely aged counterparts, are far more amenable currently. Such experiments with muscle tissue have been done in murine models and demonstrated age related effects. Old muscle exhibited improved regenerative abilities when implanted into a young murine host, while the young graft was impaired in the old recipient mice. These findings indicate that systemic factors have a stronger effect, relative to tissue autonomous factors, in regulating muscle regenerative capacity [73]. It would be interesting to determine if similar effects would be exhibited in a highly regenerative species such as the axolotl. Muscle satellite cells are known to participate in limb regeneration [74] and hence the capacity of these progenitor cells in differently aged axolotl hosts may provide insights regarding their autonomous regenerative ability and the effect of systemic factors with age.
Technological advancements on the axolotl system to study regeneration and aging:
Historically, the repertoire of molecular techniques available for use in the axolotl was limited, with much emphasis being placed instead on phenomenological, embryonic and biochemical studies. The absence of a fully sequence genome was a major contributing factor to this limitation. However, over the last decade many technological advances in the genome and genome manipulations have improved the molecular tool kit available for this model organism.
One of the most pivotal advances in terms of such technologies has been the sequencing and assembly of the entire axolotl genome, a daunting feat given the large size of the genome - 32 Gb [75,76]. This data has already revealed that several coding and non-coding sequences with potential roles in limb regeneration are species-specific, and are absent in amniotes [75], and may aid in our understanding and evaluation of the genetic requirements for regeneration and the evolutionary retention or loss of this capability.
Despite the absence of a fully sequenced genome prior to 2018, transcriptional analysis was originally facilitated through large- and small-scale microarray studies (targeted towards known RNA sequences) as well as RNA-sequencing (using human and Xenopus data sets as reference libraries). Coupling micro-array and RNA-sequencing technologies with the now available genomic sequence of the axolotl will facilitate a far more complete understanding of gene expression during regeneration. This will be of vital importance in the context of aging, where transcriptional profiling as a function of age may provide insight into changes in regenerative capacity over time. As RNA-sequencing has been achieved at single cell resolution [77], the transcriptional contribution of different cell types, such as fibroblasts and immune cells, in the aging regenerative organ can also be evaluated.
In addition to single cell RNA sequencing, grafting experiments and/or transgenic animals have aided in our understanding of cell-linage contributions during regeneration. Grafting tissue in embryonic and post-embryonic staged axolotl can be done with ease, and transgenic lines provide a reliable means to mark donor tissue (as reviewed by [78]). Generation of transgenic animals has historically been performed via germ-line integration of exogenous DNA, generally a plasmid, injected into the axolotl egg [79]. Although this method is constrained by the long generation time of the axolotl, it has been used to establish a number of stable lines [36,80–83]. One transgenic line of value in aging studies is the Prrx1-driving tamoxifen inducible cre line which stably labels the connective tissue of the axolotl [83]. As fibroblasts play a key role in scar formation, which impedes regeneration, evaluating the specific functions of this cell population during regeneration may provide insights and explanations as to the changes in regenerative capacity with age, and the divergent outcomes documented in wound healing between regenerative and non-regenerative species.
More recently the transgenic system has been coupled with an induction system [82,84] and tissue-specific promoters [85], which will allow us to gain insight into the regulation of gene expression during regeneration. Furthermore, in vivo genome editing techniques have greatly improved in the axolotl and will continue to do so given the now-sequenced genome. Although transcriptional activator-like effector nucleases (TALENs) mediated genome editing has been demonstrated to work in the axolotl, the CRISPR-Cas 9 system is far more efficient [86] and has been successfully used to knock-in and -out genetic material within the organism [87–89]. Viral-directed genome modifications have also been established and have the advantage of allowing cell-type specific genome editing [16,90–93]. Any potential gene candidates, or regulatory elements thereof, that appear to contribute to regenerative failure in aging systems can be manipulated using the above-mentioned techniques in the axolotl, and this will allow for direct determination of their role in this process.
Conclusion:
Recent technological advancements and the acquisition of extensive genomic information in the Mexican axolotl makes it an exciting time to study the process of regeneration in this model system. One important point that we hope to illustrate here is that although axolotls demonstrate age related phenotypic changes, some of which are similar to those observed in humans, they are unique in that they retain their ability to regenerate multiple types of complex structures throughout their lives. Despite the regenerative ability of the axolotl slowing with age, it still far exceeds such capacities in mammals. Thus, these organisms appear to be resistant, to some degree, to the age-related mechanisms that drive regenerative decline in humans. It is also possible that their unique regenerative abilities are linked to their very low incidence of age-related pathologies. Although there is much to learn about how regeneration occurs in the axolotl, it is clear that multiple aspects of this important biological process will one day aid in the development of therapies for age-related pathologies in humans.
Acknowledgments
Funding Sources
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health [grant number 1R15HD092180-01A1].
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.van Heemst D: Insulin, IGF-1 and longevity. Aging Dis 2010;1:147–157. [PMC free article] [PubMed] [Google Scholar]
- 2.Shimokawa I, Trindade LS: Dietary restriction and aging in rodents: a current view on its molecular mechanisms. Aging Dis 2010;1:89–107. [PMC free article] [PubMed] [Google Scholar]
- 3.Afanas’ev I: Signaling and Damaging Functions of Free Radicals in Aging-Free Radical Theory, Hormesis, and TOR. Aging Dis 2010;1:75–88. [PMC free article] [PubMed] [Google Scholar]
- 4.Jin K: Modern Biological Theories of Aging. Aging Dis 2010;1:72–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DL, Rando TA: Emerging models and paradigms for stem cell ageing. Nat Cell Biol 2011;13:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J, Lee YD, Wagers AJ: Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat Med 2014;20:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieira WA, Mccusker CD: Hierarchical pattern formation during amphibian limb regeneration. BioSystems 2019;183:103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker C, Gardiner DM: The axolotl model for regeneration and aging research: A mini-review. Gerontology 2011;57:565–571. [DOI] [PubMed] [Google Scholar]
- 9.Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, et al. : Diversity of ageing across the tree of life. Nature 2014;505:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erler P, Sweeney A, Monaghan JR: Regulation of Injury-Induced Ovarian Regeneration by Activation of Oogonial Stem Cells. Stem Cells 2017;35:236–247. [DOI] [PubMed] [Google Scholar]
- 11.Goss RJ: Principles of Regeneration. Academic Press, 1969. [Google Scholar]
- 12.Vieira WA, Wells KM, Raymond MJ, Souza L De, Garcia E, Mccusker CD: FGF, BMP, and RA signaling are sufficient for the induction of complete limb regeneration from non-regenerating wounds on Ambystoma mexicanum limbs. Dev Biol 2019;451:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M, Inoue S: The nerve and the epidermal apical cap in regeneration of the forelimb of adult Triturus. J Exp Zool 1964;155:105–16. [DOI] [PubMed] [Google Scholar]
- 14.Makanae A, Mitogawa K, Satoh A: Co-operative Bmp- and Fgf-signaling inputs convert skin wound healing to limb formation in urodele amphibians. Dev Biol 2014;396:57–66. [DOI] [PubMed] [Google Scholar]
- 15.Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM: Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development 1996;122:3487–97. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Esteban CR, Raya M, Kawakami H, Martí M, Dubova I, et al. : Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev 2006;20:3232–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrberg J, Gardiner DM: Regulation of axolotl (Ambystoma mexicanum) limb blastema cell proliferation by nerves and BMP2 in organotypic slice culture. PLoS One 2015;10:e0123186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makanae A, Hirata A, Honjo Y, Mitogawa K, Satoh A: Nerve independent limb induction in axolotls. Dev Biol 2013; DOI: 10.1016/j.ydbio.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Bryant SV, French V, Bryant PJ: Distal regeneration and symmetry. Science (80- ) 1981;212:993–1002. [DOI] [PubMed] [Google Scholar]
- 20.McCusker CD, Gardiner DM: Positional Information Is Reprogrammed in Blastema Cells of the Regenerating Limb of the Axolotl (Ambystoma mexicanum). PLoS One 2013;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo T, Bryant SV, Gardiner DM: A stepwise model system for limb regeneration. Dev Biol 2004;270:135–145. [DOI] [PubMed] [Google Scholar]
- 22.Bryant DM, Sousounis K, Farkas JE, Bryant S, Thao N, Guzikowski AR, et al. : Repeated removal of developing limb buds permanently reduces appendage size in the highly-regenerative axolotl. Dev Biol 2017;424:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant DM, Sousounis K, Payzin-Dogru D, Bryant S, Sandoval AGW, Martinez Fernandez J, et al. : Identification of regenerative roadblocks via repeat deployment of limb regeneration in axolotls. npj Regen Med 2017;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galton VA: The role of thyroid hormone in amphibian metamorphosis. Trends Endocrinol Metab 1992; DOI: 10.1016/1043-2760(92)90020-2 [DOI] [PubMed] [Google Scholar]
- 25.Herrera-Rincon C, Golding AS, Moran KM, Harrison C, Martyniuk CJ, Guay JA, et al. : Brief Local Application of Progesterone via a Wearable Bioreactor Induces Long-Term Regenerative Response in Adult Xenopus Hindlimb. Cell Rep 2018;25:1593–1609.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page RB, Voss SR: Induction of metamorphosis in axolotls (Ambystoma mexicanum). Cold Spring Harb Protoc 2009;8:doi: 10.1101/pdb.prot5268. [DOI] [PubMed] [Google Scholar]
- 27.Monaghan JR, Stier AC, Michonneau F, Smith MD, Pasch B, Maden M, et al. : Experimentally induced metamorphosis in axolotls reduces regenerative rate and fidelity. Regeneration 2014;1:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demircan T, Ovezmyradov G, Yıldırım B, Keskin İ, İlhan AE, Fesçioğlu EC, et al. : Experimentally induced metamorphosis in highly regenerative axolotl (ambystoma mexicanum) under constant diet restructures microbiota. Sci Rep 2018;8 DOI: 10.1038/s41598-018-29373-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salley JD, Tassava RA: Responses of denervated adult newt limb stumps to reinnervation and reinjury. J Exp Zool 1981;215:183–189. [DOI] [PubMed] [Google Scholar]
- 30.Mescher AL, White GW, Brokaw JJ: Apoptosis in regenerating and denervated, nonregenerating urodele forelimbs. Wound Repair Regen 2000;8:110–116. [DOI] [PubMed] [Google Scholar]
- 31.Singer M: The nervous system and regeneration of the forelimb of adult Triturus. V. The influence of number of nerve fibers, including a quantitative study of limb innervation. J Exp Zool 1946;101:299–377. [DOI] [PubMed] [Google Scholar]
- 32.Farkas JE, Freitas PD, Bryant DM, Whited JL, Monaghan JR: Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development 2016;143:2724–2731. [DOI] [PubMed] [Google Scholar]
- 33.Mitogawa K, Makanae A, Satoh A: Hyperinnervation improves Xenopus laevis limb regeneration. Dev Biol 2018;433:276–286. [DOI] [PubMed] [Google Scholar]
- 34.Boilly B, Albert P: Blastema cell proliferation in vitro: effects of limb amputation on the mitogenic activity of spinal cord extracts. Biol Cell 1988;62:183–187. [PubMed] [Google Scholar]
- 35.Mescher AL, Neff AW: Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol 2005;93:39–66. [DOI] [PubMed] [Google Scholar]
- 36.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, et al. : Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 2009;460:60–65. [DOI] [PubMed] [Google Scholar]
- 37.Nacu E, Glausch M, Le HQ, Damanik FFR, Schuez M, Knapp D, et al. : Connective tissue cells, but not muscle cells, are involved in establishing the proximo-distal outcome of limb regeneration in the axolotl. Development 2013;140:513–518. [DOI] [PubMed] [Google Scholar]
- 38.Godwin JW, Pinto AR, Rosenthal NA: Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci 2013;110:9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godwin JW, Debuque R, Salimova E, Rosenthal NA: Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. npj Regen Med 2017;2:22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murawala P, Tanaka EM, Currie JD: Regeneration: The ultimate example of wound healing. Semin Cell Dev Biol 2012;23:954–962. [DOI] [PubMed] [Google Scholar]
- 41.Wallace HA, Zito PM: Wound Healing Phases. 2019. [PubMed] [Google Scholar]
- 42.Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A: Effect of age on wound healing in healthy human beings. Surgery 1992;112:293–297. [PubMed] [Google Scholar]
- 43.Seifert A, Monaghan J, Voss R, Maden M: Skin regeneration in adult axolotls: A blueprint for scar-free healing in vertebrates. PLoS One 2012;7:e32875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson JR, Gearhart MD, Honson DD, Reid TA, Gardner MK, Moriarity BS, et al. : A novel role for SALL4 during scar-free wound healing in axolotl. npj Regen Med 2016;1:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weir HK, Thompson TD, Soman A, Møller B: The Past, Present, and Future of Cancer Incidence in the United States : 1975 Through 2020. Cancer 2015;121:1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z, Taylor JA: Genome-wide age-related DNA methylation changes in blood and other tissues relate to histone modification, expression and cancer. Carcinogenesis 2014;35:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakyan VK, Down TA, Maslau S, Andrew T, Yang T, Beyan H, et al. : Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res 2010;20:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khudoley VV, Ellselv VV: Multiple Melanomas in the Axolotl Ambystoma mexlcanum 1. J Exp Zool 1979;63:101–103. [PubMed] [Google Scholar]
- 49.Harshbarger JC, Chang C, Delanney LE, Rose L, Green DE: Cutaneous mastocytomas in the neotenic caudate amphibians Ambystoma mexicanum ( axolotl ) and Ambystoma tigrinum ( tiger salamander ). J Cancer Res Clin Oncol 1999;125:187–192. [DOI] [PubMed] [Google Scholar]
- 50.Shioda C, Uchida K, Nakayama H: Pathological Features of Olfactory Neuroblastoma in an Axolotl ( Ambystoma mexicanum ). J Vet Med Sci 2011;73:1109–1111. [DOI] [PubMed] [Google Scholar]
- 51.Koyama H, Meyer-rochow VB: Carcinoma in the axolotl : Ambystoma mexicanum (Amphibia : Urodela ). Zool Anzeiger - A J Comp Zool 1989;223:26–32. [Google Scholar]
- 52.Brunst VV, Roque AL: A Spontaneous Teratoma in an Axoloti (Siredon mexicanuin). Cancer Res 1969;29:223–229. [PubMed] [Google Scholar]
- 53.Menger B, Vogt PM, Jacobsen ID, Allmeling C, Kuhbier JW, Mutschmann F, et al. : Resection of a Large Intra-Abdominal Tumor in the Mexican. Vet Surg 2010;39:232–233. [DOI] [PubMed] [Google Scholar]
- 54.Ingram AJ: The reactions to carcinogens in the axolotl ( Ambystoma mexicanum ) in relation to the ‘ regeneration field control ‘ hypothesis. J Embryol Exp Morphol 1971;26:425–441. [PubMed] [Google Scholar]
- 55.Mccusker CD, Athippozhy A, Diaz-castillo C, Fowlkes C, Gardiner DM, Voss SR: Positional plasticity in regenerating Amybstoma mexicanum limbs is associated with cell proliferation and pathways of cellular differentiation. BMC Dev Biol 2015;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart R, Rascón CA, Tian S, Nie J, Barry C, Chu LF, et al. : Comparative RNA-seq Analysis in the Unsequenced Axolotl: The Oncogene Burst Highlights Early Gene Expression in the Blastema. PLoS Comput Biol 2013;9:e1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu W, Kuo D, Nathanson J, Satoh A, Pao GM, Yeo GW, et al. : Retrotransposon long interspersed nucleotide element-1 (LINE-1) is activated during salamander limb regeneration. Dev Growth Differ 2012;54:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sosnik J, Vieira WA, Webster KA, Siegfried KR, McCusker CD: A new and improved algorithm for the quantification of chromatin condensation from microscopic data shows decreased chromatin condensation in regenerating axolotl limb cells. PLoS One 2017;12:e0185292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu P, Weaver VM, Werb Z: The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao N, Jhamb D, Milner DJ, Li B, Song F, Wang M, et al. : Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol 2009;7:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onda H, Goldhamer DJ, Tassava RA: An extracellular matrix molecule of newt and axolotl regenerating limb blastemas and embryonic limb buds: immunological relationship of MT1 antigen with tenascin. Development 1990;108:657–68. [DOI] [PubMed] [Google Scholar]
- 62.Rabinowitz JS, Robitaille AM, Wang Y, Ray CA, Thummel R, Gu H, et al. : Transcriptomic, proteomic, and metabolomic landscape of positional memory in the caudal fin of zebrafish. PNAS 2017;114:E717–E726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calve S, Odelberg SJ, Simon HG: A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol 2010;344:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gawriluk TR, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, et al. : Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun 2016;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gialeli C, Theocharis AD, Karamanos NK: Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16–27. [DOI] [PubMed] [Google Scholar]
- 66.Santosh N, Windsor LJ, Mahmoudi BS, Li B, Zhang W, Chernoff EA, et al. : Matrix Metalloproteinase Expression During Blastema Formation in Regeneration- Competent Versus Regeneration-Deficient Amphibian Limbs. Dev Dyn 2011;240:1127–1141. [DOI] [PubMed] [Google Scholar]
- 67.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weismann IL, Rando TA: Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–764. [DOI] [PubMed] [Google Scholar]
- 68.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, et al. : GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab 2015;22:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, et al. : Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013;153:828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, et al. : Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science (80- ) 2014;344:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mes-Hartree M, Armstrong JB: Evidence that the premature death mutation (p) in the Mexican axolotl (Ambystoma mexicanum) is not an autonomous cell lethal. J Embryol Exp Morphol 1980;60:295–302. [PubMed] [Google Scholar]
- 72.Kumar A, Delgado J-P, Gates PB, Neville G, Forge A, Brockes JP: The aneurogenic limb identifies developmental cell interactions underlying vertebrate limb regeneration. Proc Natl Acad Sci 2011;108:13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlson BM, Faulkner JA: Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Physiol 2017;256:C1262-C1266. [DOI] [PubMed] [Google Scholar]
- 74.Sandoval-Guzmán T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, et al. : Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 2014;14:174–187. [DOI] [PubMed] [Google Scholar]
- 75.Nowoshilow S, Schloissnig S, Fei J-F, Dahl A, Pang AWC, Pippel M, et al. : The axolotl genome and the evolution of key tissue formation regulators. Nature 2018;554:50–55. [DOI] [PubMed] [Google Scholar]
- 76.Smith JJ, Timoshevskaya N, Timoshevskiy VA, Keinath MC, Hardy D, Voss SR: A chromosome-scale assembly of the axolotl genome. Genome Res 2019;29:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leigh ND, Dunlap GS, Johnson K, Mariano R, Oshiro R, Wong AY, et al. : Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nat Commun 2018;9 DOI: 10.1038/s41467-018-07604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voss SR, Epperlein HH, Tanaka EM: Ambystoma mexicanum, the axolotl: A versatile amphibian model for regeneration, development, and evolution studies. Cold Spring Harb Protoc 2009;4 DOI: 10.1101/pdb.emo128 [DOI] [PubMed] [Google Scholar]
- 79.Khattak S, Richter T, Tanaka EM: Generation of transgenic axolotls (Ambystoma mexicanum). Cold Spring Harb Protoc 2009;4:doi: 10.1101/pdb.prot5264. [DOI] [PubMed] [Google Scholar]
- 80.Sobkow L, Epperlein HH, Herklotz S, Straube WL, Tanaka EM: A germline GFP transgenic axolotl and its use to track cell fate: Dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Dev Biol 2006;290:386–397. [DOI] [PubMed] [Google Scholar]
- 81.Currie JD, Kawaguchi A, Traspas RM, Schuez M, Chara O, Tanaka EM: Live Imaging of Axolotl Digit Regeneration Reveals Spatiotemporal Choreography of Diverse Article Live Imaging of Axolotl Digit Regeneration Reveals Spatiotemporal Choreography of Diverse Connective Tissue Progenitor Pools. Dev Cell 2016;39:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khattak S, Schuez M, Richter T, Knapp D, Haigo SL, Sandoval-Guzmán T, et al. : Germline transgenic methods for tracking cells and testing gene function during regeneration in the axolotl. Stem Cell Reports 2013;1:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerber T, Murawala P, Knapp D, Masselink W, Schuez M, Hermann S, et al. : Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science (80- ) 2018;362 DOI: 10.1126/science.aaq0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whited JL, Lehoczky JA, Tabin CJ: Inducible genetic system for the axolotl. Proc Natl Acad Sci 2012;109:13662–13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monaghan JR, Maden M: Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev Biol 2012;368:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fei JF, Schuez M, Tazaki A, Taniguchi Y, Roensch K, Tanaka EM: CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Reports 2014;3:444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flowers GP, Timberlake AT, Mclean KC, Monaghan JR, Crews CM: Highly efficient targeted mutagenesis in axolotl using Cas9 RNA-guided nuclease. Development 2014;141:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fei J-F, Lou WP-K, Knapp D, Murawala P, Gerber T, Taniguchi Y, et al. : Application and optimization of CRISPR–Cas9-mediated genome engineering in axolotl (Ambystoma mexicanum). 2018. DOI: 10.1038/s41596-018-0071-0 [DOI] [PubMed] [Google Scholar]
- 89.Fei J-F, Schuez M, Knapp D, Taniguchi Y, Drechsel DN, Tanaka EM: Efficient gene knockin in axolotl and its use to test the role of satellite cells in limb regeneration. Proc Natl Acad Sci 2017;114:12501–12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy S, Gardiner DM, Bryant SV: Vaccinia as a tool for functional analysis in regenerating limbs: Ectopic expression of Shh. Dev Biol 2000;218:199–205. [DOI] [PubMed] [Google Scholar]
- 91.Whited JL, Tsai SL, Beier KT, White JN, Piekarski N, Hanken J, et al. : Pseudotyped retroviruses for infecting axolotl in vivo and in vitro. Development 2013;140:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khattak S, Sandoval-Guzmán T, Stanke N, Protze S, Tanaka EM, Lindemann D: Foamy virus for efficient gene transfer in regeneration studies. BMC Dev Biol 2013;13 DOI: 10.1186/1471-213X-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bryant DM, Johnson K, DiTommaso T, Tickle T, Couger MB, Payzin-Dogru D, et al. : A tissue-mapped axolotl de novo transcriptome enables identification of limb regneration factors. Cell Rep 8385;18:762–776. [DOI] [PMC free article] [PubMed] [Google Scholar]