Abstract
Objective:
To determine incidence and risk factors of post-sterilization hyphema in shelter cats.
Animals studied:
Retrospective medical record review of 1,204 cats and prospective screening of 195 cats.
Procedures:
The study consisted of 3 parts: (1) Survey responses were collected from 20 veterinarians, who perform high-quality high-volume spay-neuter (HQHVSN) in both shelter and public clinic settings. (2) Medical records of 1,204 cats were analyzed retrospectively over a 14-month time period. (3) Ophthalmic examinations, including tonometry, were performed prospectively on 195 cats before and after sterilization surgery over 8 weeks.
Results:
Nine of 20 surveyed veterinarians reported having witnessed hyphema in cats following sterilization surgery. Retrospective review of 1,204 medical record and prospective screening of 195 cats showed that 3 juvenile (<1 year of age) male cats (<2 kg) developed hyphema within 1 hour following surgery (0.2% incidence). In all 3 affected cats, anesthesia was induced with tiletamine/zolazepam (3 of 523 cats induced with this drug combination; 0.6% incidence), and hyphema resolved within 20 hours. Mean intraocular pressures as measured by Icare® TonoVet were (mean ± standard deviation) 11.5 ± 3.8 mmHg and 21.7 ± 4.6 mmHg for juvenile (<1 year of age) and adult (>1 year of age) cats, respectively.
Conclusions:
Survey responses and 3 observed cases confirm the existence of feline post-sterilization hyphema with an estimated incidence of 0.2%. The underlying mechanism for this occurrence remains unknown.
Keywords: cat, hyphema, shelter medicine, sterilization, tiletamine, zolazepam
1. INTRODUCTION
Discussions among veterinarians, who perform high-quality high-volume spay-neuter (HQHVSN) in both shelter and public clinic settings, have raised our awareness of a phenomenon where a few cats undergoing sterilization surgery develop mild, transient hyphema post-operatively. This observed blood in the anterior chamber of the eye appears to affect mostly juvenile cats under the age of 1 year, does not cause any discomfort, and tends to resolve within 24 hours. There is some speculation that post-sterilization hyphema may be a side-effect of specific anesthetic drugs, such as the combined use of tiletamine and zolazepam (Telazol®, Zoetis Inc., Kalamazoo, MI or Tilzolan®, Dechra Veterinary Products, Overland Park, KS). It is our impression that feline post-sterilization hyphema is largely unknown to veterinary ophthalmologists. The purposes of our study were (1) to describe feline post-sterilization hyphema, (2) to estimate its incidence, and (3) to determine any association with the use of specific anesthetic drugs.
2. METHODS
2.1. Survey
A closed-community forum, organized by the Association of Shelter Veterinarians (ASV) for veterinarians who perform high-quality high-volume spay-neuter (HQHVSN) in both shelter and public clinic settings, was surveyed about the occurrence of post-sterilization hyphema.
2.2. Review of medical records
Medical records of cats undergoing sterilization surgery at the shelter of the Capital Area Humane Society (Lansing, MI) from May 2016 to July 2017 were reviewed in order to estimate the incidence of post-operative hyphema as observed by the veterinary staff without detailed ophthalmic examination.
2.3. Prospective evaluation of cats undergoing sterilization surgery
The prospective part of our study was performed at the shelter of the Capital Area Humane Society (Lansing, MI) from June 2016 through July 2016, and it was approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC). The routine admission protocol for cats admitted to this shelter included a routine physical examination. If the date of birth was unknown, age was estimated based on tooth eruption and weight. Blood was collected to screen for the presence of feline immunodeficiency virus (FIV) antibodies and feline leukemia virus (FeLV) antigen (SNAP FIV/FeLV Combo Test, Idexx Laboratories, Westbrook, ME, or Viracheck® FeLV, Zoetis, Kalamazoo, MI); only cats that tested negative were included in the study. All cats received vaccinations and anti-parasitic treatment before sterilization surgery; typically these occurred within 1-2 days before surgery, with timing depending on an animal’s age, body weight, and health status at admission to the shelter. Cats were vaccinated routinely with feline rhinotracheitis-calici-panleukopenia modified live vaccine (Fel-O-Guard Plus 3, Boehringer Ingelheim Animal Health). Animals ≥4 months of age were also given rabies vaccine (Rabvac 3, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO). Adult cats received antiparasitic treatment by topical administration of imidacloprid-moxidectin combination (Advantage Multi® for Cats; Bayer HealthCare, LLC, Animal Health Division, Shawnee, KS) or selamectin (Revolution®; Zoetis Inc., Kalamazoo, MI). Antiparasitic treatment of juvenile cats consisted of oral administration of pyrantel pamoate suspension (10 mg/kg; Apexa™, MWI Animal Health, Boise, ID) and ponazuril (20 mg/kg; compounded by Roadrunner Pharmacy, Phoenix, AZ). Fecal samples were examined microscopically of cats with diarrhea or defecation outside the litterbox. Cats were treated if any parasites were noted on the fecal flotation or Giardia snap test (IDEXX Laboratories, Inc, Westbrook, ME). Routine urinalysis was performed in any cat with inappropriate urination outside the litterbox and consisted of urine specific gravity, centrifugation and sediment examination by microscopy, and Multistix® Reagent Strip (Siemens Medical Solutions USA, Inc, Malvern, PA). Only intact, healthy cats >2 months of age and >1 kg body weight underwent sterilization surgery under general anesthesia.
In preparation for sterilization surgery, animals were fasted for 2-4 hours before the procedure with access to water. All cats were premedicated by subcutaneous injection of 0.006 mg/kg buprenorphine hydrochloride (Roadrunner Pharmacy, Phoenix, AZ) and 0.05 mg/kg acepromazine maleate (VetOne®, MWI Animal Health, Boise, ID). In male juveniles with a body weight <2 kg general anesthesia was induced by intramuscular injection of 6.6 mg/kg tiletamine hydrochloride-zolazepam hydrochloride combination (Tilzolan®). Female juvenile and all adult cats received 5.5 mg/kg of intravenous ketamine hydrochloride (Zetamine™, VetOne®, MWI Animal Health, Boise, ID) combined with 0.275 mg/kg midazolam hydrochloride (Hospira, Lake Forest, IL) for induction of general anesthesia. Anesthesia was maintained with isoflurane (Fluriso™, MWI Animal Health, Boise, ID) and oxygen gas mixture administered either via facemask, in male juveniles with a body weight <2 kg, or via endotracheal tube, in all adults and female juveniles. The sterilization surgeries consisted of routine orchiectomies and ovariohysterectomies on males and females, respectively.1 All cats received a subcutaneous injection of 0.1 mg/kg meloxicam (Metacam®; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) for pain relief following surgery and were monitored closely until they had fully recovered from general anesthesia.
Ophthalmic examinations were performed on surgery days by a specifically trained veterinary student (APS), and findings were verified by a board-certified veterinary ophthalmologist (AMK). Both eyes of each cat were examined in the morning before surgery and again in the afternoons following full recovery from general anesthesia. No pharmacologic mydriasis was induced. Anterior segments were examined with diffuse and focal illumination using portable hand-held slit-lamp biomicroscopes (Kowa SL14; Kowa Company, Tokyo, Japan). Fundic examinations were performed by wide-view direct ophthalmoscopy (PanOptic™; Welch Allyn, Inc., Skaneateles, NY) and portable binocular indirect ophthalmoscopes (Keeler All Pupil II; Keeler Instruments, Broomall, PA) and condensing lenses (Pan Retinal 2.2D; Volk Optical, Mentor, OH). Intraocular pressures (IOP) were measured with a rebound tonometer (Icare® TonoVet; Icare Finland Oy, Vantaa, Finland). Presence of pupillary light reflexes, menace responses, and dazzle reflexes were also examined.
2.4. Data processing
The hyphema incidence data was processed by descriptive statistics. Intraocular pressures were compared between juvenile (<1 year of age) and adult cats (>1 year of age) by two-tailed unpaired t-test (Excel; Microsoft® Corporation, Redmond, WA).
3. RESULTS
3.1. Survey
A total of 20 survey responses were collected from veterinarians, who perform high-quality high-volume spay-neuter (HQHVSN) in both shelter and public clinic settings. Out of the 20 responses, 9 indicated having witnessed post-sterilization hyphema in cats at some point during their career. Of those 9 positive responses, 7 reported using tiletamine/zolazepam, 1 reported using propofol, and 1 reported using a ketamine/diazepam combination as part of their anesthetic protocol. Regarding delivery of maintenance inhalant anesthetic, 3/9 responses reported use of a facemask, 3/9 responses reported intubation, and 3/9 responses reported use of both a mask and intubation. All positive responses reported cases involving cats <1 year of age and 2/9 also reported cases involving cats >1 year. Most responses (8/9) reported the hyphema to be unilateral and transient.
3.2. Review of medical records
During a 14-month time interval between May 2016 and July 2017, 2 cases of hyphema were identified out of 1,204 cats (636 males, 82 females) undergoing sterilization surgery (0.2% incidence). Of the 1,204 cats, 430 received tiletamine/zolazepam and 774 received ketamine/midazolam, and hyphema was observed in 2 juvenile male cats that received tiletamine/zolazepam.
3.3. Prospective evaluation of cats undergoing sterilization surgery
During the 8-week study period in June and July 2016 a total of 195 cats were examined, with one of them developing bilateral hyphema during recovery within 1 hour following surgery. Of the 195 cats, 96 received tiletamine/zolazepam and 99 received ketamine/midazolam, and hyphema was only observed in 1 animal that received tiletamine/zolazepam. The affected animal was a 3-month-old, male domestic short-haired cat with no preexisting ocular abnormalities. Although the hyphema was bilateral, the right eye was more severely affected with more blood in the anterior chamber (Fig. 1). Prior to surgery, the cat’s IOPs were 13 mmHg in both eyes. Following surgery, IOPs were essentially unchanged at 12 and 14 mmHg in left and right eye, respectively. There were no signs of trauma and no loss of ocular reflexes. The hyphema was completely resolved in both eyes by 20 hours following surgery with no visible lasting ocular abnormalities such as aqueous flare and cells.
FIGURE 1.
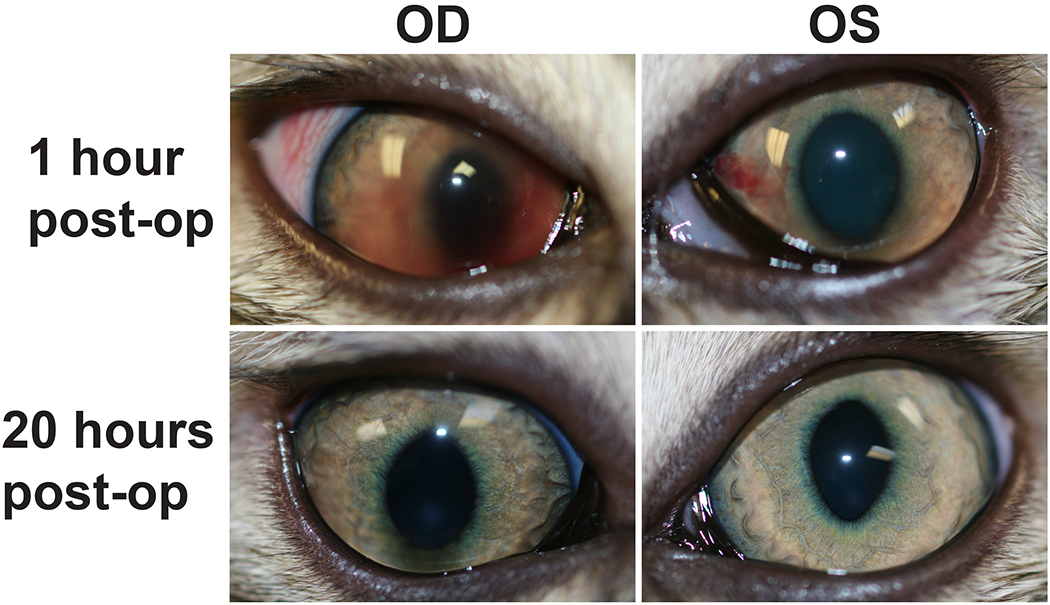
Bilateral hyphema in a 3-month-old, male domestic short-haired cat 1 hour following surgery (1 hour post-op). The hemorrhage was more diffuse and extensive in the right (OD) than left eye (OS) and was completely resolved by 20 hours post-op.
Table 1 summarizes all the cats examined with their baseline IOPs. Mean IOPs were significantly lower in juvenile (<1 year of age) compared to adult cats (>1 year of age): 11.5 ± 3.8 vs. 21.7 ± 4.6 mmHg (p<0.001).
TABLE 1.
Summary of pre-surgical mean baseline IOPs
| Age Groups | Number of Cats | Mean Age (±SD) (Months) | Mean IOP (±SD) (mmHg) |
|---|---|---|---|
| Juvenile cats | 179 | 2.5 ± 1.0 | 11.5 ± 3.8* |
| Adult cats | 16 | 16.9 ± 7.0 | 21.7 ± 4.6* |
| All cats | 195 | 3.7 ± 4.5 | 12.3 ± 4.7 |
Abbreviation: IOP, intraocular pressure; SD, standard deviation
significant difference in IOP between juvenile and adult cats (p < 0.001)
4. DISCUSSION
Survey responses and 3 observed cases confirm the existence of feline post-sterilization hyphema. When combining the numbers of retrospective and prospective studies, we estimate a post-sterilization hyphema incidence of 0.2% (3/1,399 cats). Although not all cats received a detailed ophthalmic examination, based on the available information, none of the affected animals had any preexisting ophthalmic or systemic conditions; and there was no ocular trauma. All 3 of our cases were juvenile male cats with a body weight <2 kg and anesthetized with tiletamine/zolazepam, supporting the possible association of post-sterilization hyphema with this drug combination and/or the age and sex of the animals. The incidence of post-sterilization hyphema in all our cats receiving tiletamine/zolazepam was 0.6% (3/523 cats). Our survey results indicate that feline post-sterilization hyphema can also occur with other anesthetics, such as propofol and ketamine/diazepam combination. To the best of our knowledge, there are no reports that suggest a link between any of these anesthetic induction agents and intraocular hemorrhage.
We have no explanation why feline post-sterilization hyphema occurs. Since we found no physical damage in the affected eyes and since the blood disappeared within 20 hours with no treatment and no lasting anterior uveitis, we think that the blood most likely entered the anterior chamber via the iridocorneal angle. This is a commonly observed phenomenon during intraocular surgery: blood can enter the anterior chamber retrogradely through the aqueous humor drainage pathways of the iridocorneal angle if the eye is hypotensive with IOP lower than episcleral venous pressure.2 We do not know why blood would enter the anterior chamber in cats during sterilization surgery since we found no indications of ocular hypotension when performing tonometry before and after the procedure. We also have no likely explanation how the use of anesthetic face mask could have resulted in hyphema. Even though we did not measure blood pressures, we think that it is unlikely for acute blood pressure spikes to have resulted in hyphema, especially since we did not observe any vascular damage and the blood disappeared within hours.
During our investigation of post-sterilization hyphema we determined mean IOPs in adult cats measured by Icare® TonoVet as 21.7 ± 4.6 mmHg, which is comparable to the previously published normal mean IOPs for adult cats: 20.7 ± 0.5 mmHg.3–5 Our significantly lower mean IOPs of 11.5 ± 3.8 mmHg in juvenile compared to adult cats also confirms previous reports of low IOPs in kittens because of the still ongoing maturation of anterior segment and aqueous humor outflow pathways.5,6
ACKNOWLEDGMENTS
The authors thank the staff of the Capital Area Humane Society for their technical support and the veterinarians who responded to the survey. The study was funded by the Michigan State University Graduate School Fellowship Funds and NIH grant R01-EY025752.
Footnotes
CONFLICTS OF INTEREST
There are no conflicts of interest related to this study.
REFERENCES
- 1.Association of Shelter Veterinarians’ Veterinary Task Force to Advance Spay-Neuter, Griffin B, Bushby PA, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs J Am Vet Med Assoc. 2016;249(2):165–188. [DOI] [PubMed] [Google Scholar]
- 2.Komáromy AM, Ramsey DT, Brooks DE, Ramsey CC, Kallberg ME, Andrew SE. Hyphema. Part I. Pathophysiologic considerations. Compend Contin Educ Pract Vet. 1999;21(11):1064–1069. [Google Scholar]
- 3.Rusanen E, Florin M, Hassig M, Spiess BM. Evaluation of a rebound tonometer (Tonovet) in clinically normal cat eyes. Vet Ophthalmol. 2010;13(1):31–36. [DOI] [PubMed] [Google Scholar]
- 4.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet® rebound tonometer in normal and glaucomatous cats. Vet Ophthalmol. 2013;16(2):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLellan GJ, Teixeira LB. Feline Glaucoma. Vet Clin North Am Small Anim Pract. 2015;45(6):1307–1333, vii. [DOI] [PubMed] [Google Scholar]
- 6.Adelman S, Shinsako D, Kiland JA, et al. The post-natal development of intraocular pressure in normal domestic cats (Felis catus) and in feline congenital glaucoma. Exp Eye Res. 2018;166:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


